Plumbagin: A Promising In Vivo Antiparasitic Candidate against Schistosoma mansoni and In Silico Pharmacokinetic Properties (ADMET)
Abstract
:1. Introduction
2. Materials and Methods
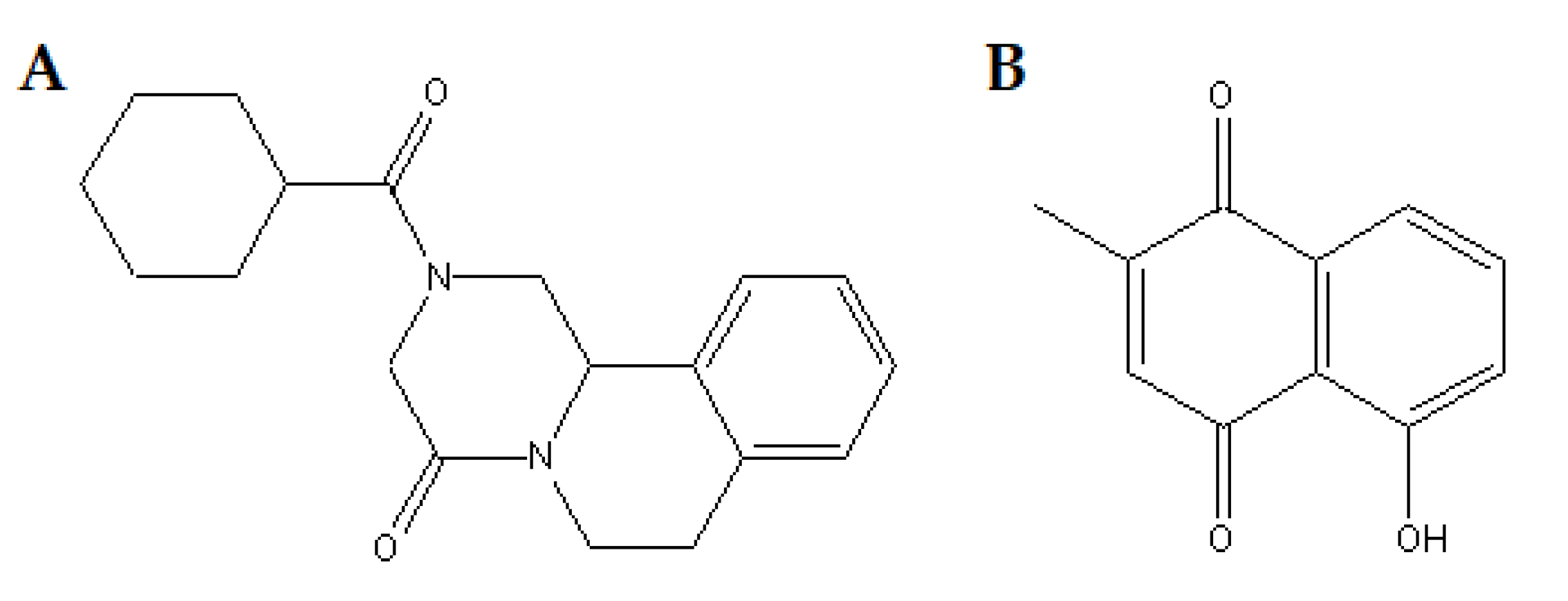
2.1. Drugs
2.2. Ethical and Animal Considerations
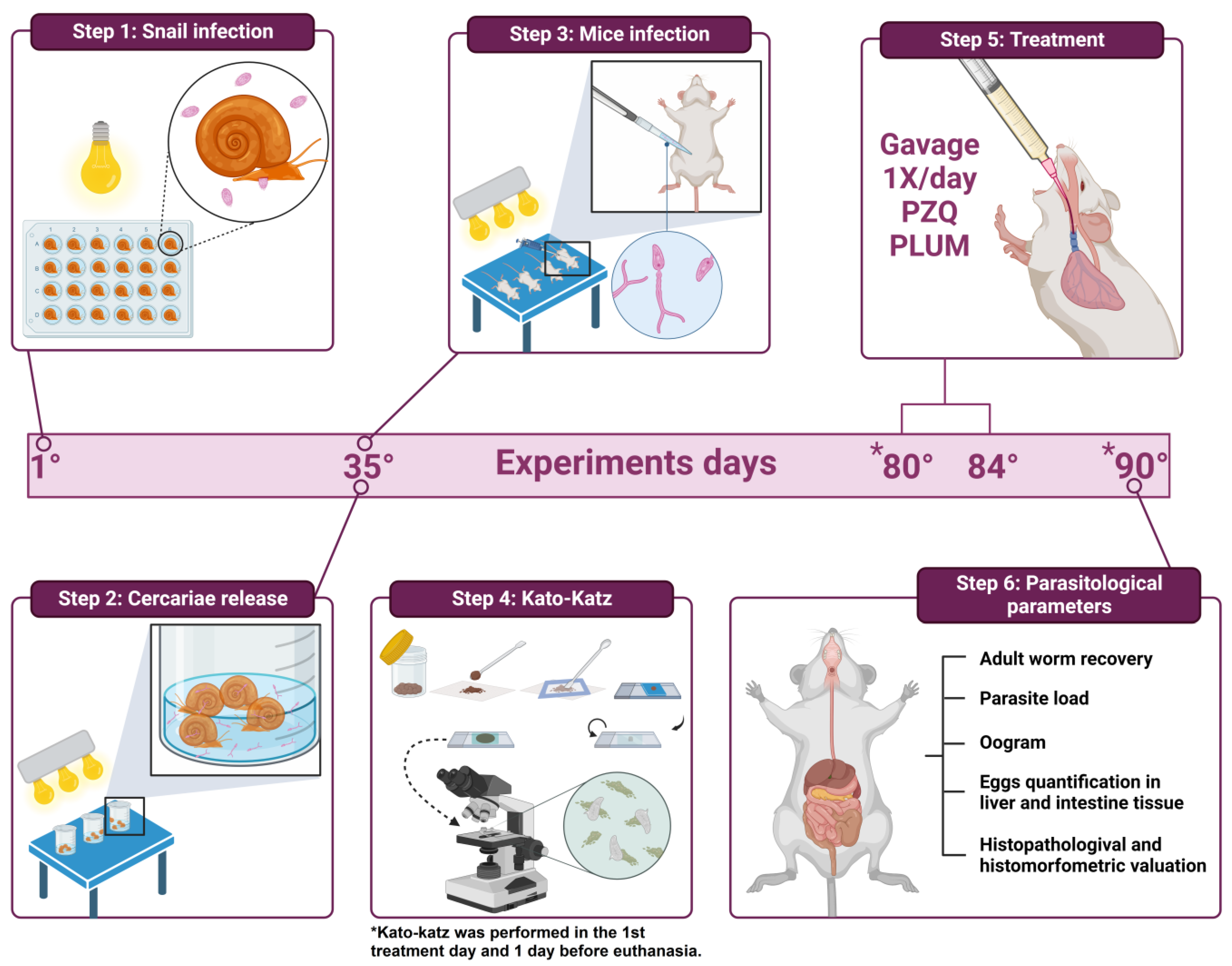
2.2.1. Infection of Biomphalaria glabrata with Schistosoma mansoni Miracidia and Cercarial Shedding
2.2.2. Infection of Mice and Experimental Groups
- G1 = animals that received only sterile saline solution;
- G2 = animals treated with PZQ;
- G3, G4, and G5 = animals treated with PLUM.
2.3. Parasitological Parameters (Figure 2 Step 6)
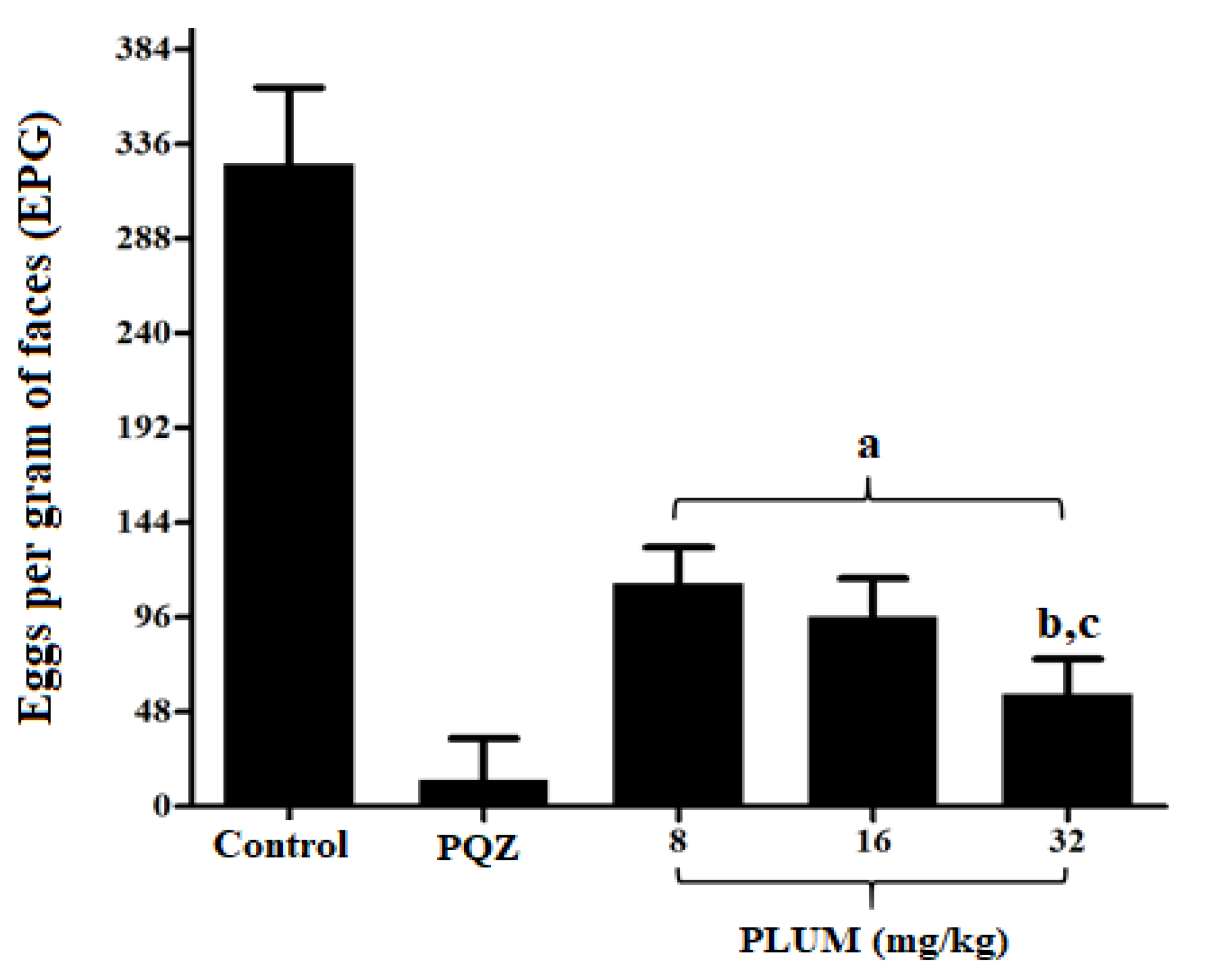
2.3.1. Eggs per Gram of Feces
2.3.2. Worm Recovery
2.3.3. Oogram Pattern
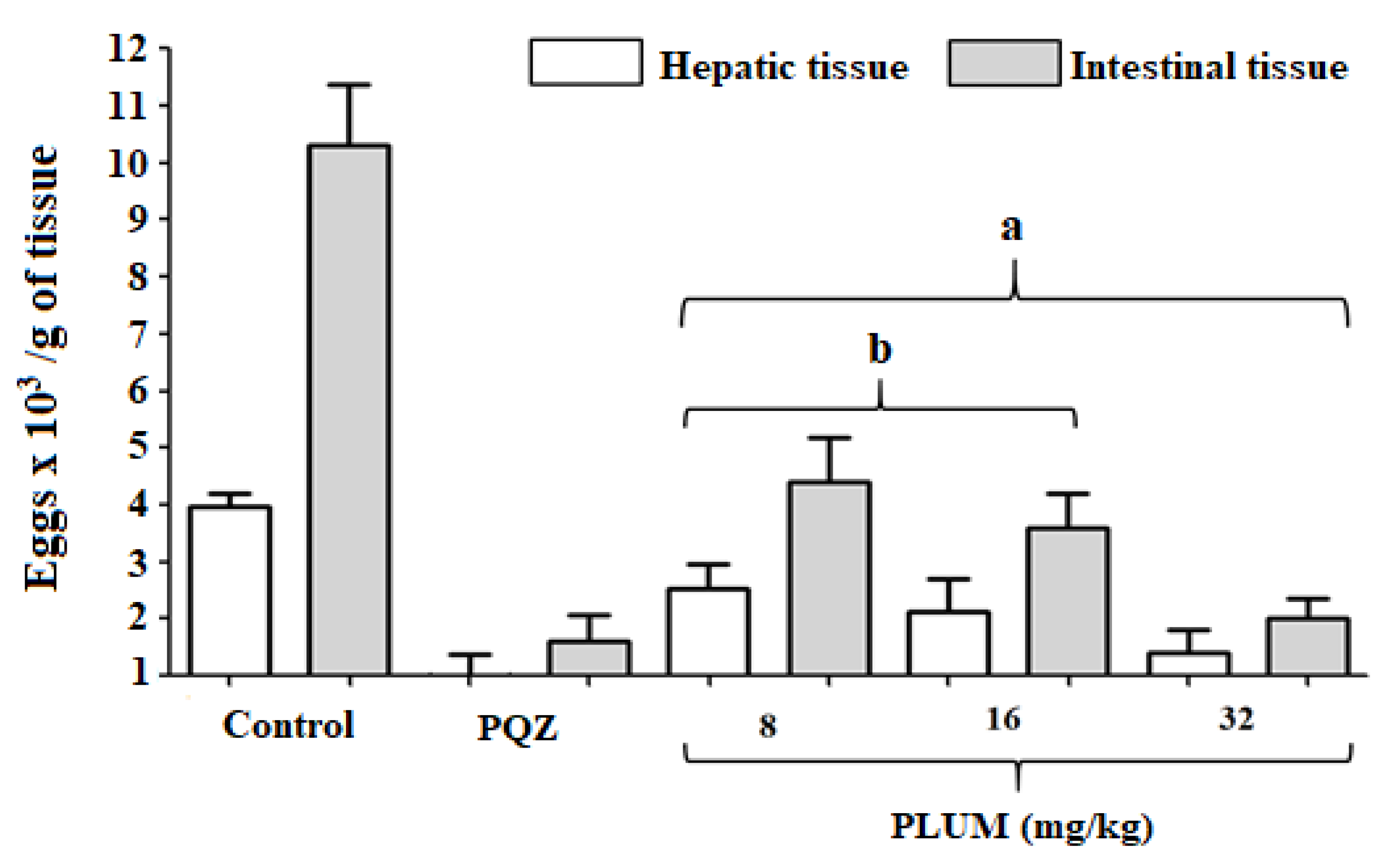
2.3.4. Quantification of Eggs in the Liver and Intestinal Tissue
2.3.5. Histomorphological and Histomorphometric Evaluation of Hepatic Granulomas
2.4. In Silico Evaluation of Pharmacokinetic Parameters: Absorption, Distribution, Metabolism, Excretion, Toxicity (ADMET) and Prediction of Oral Bioavailability
2.5. Statistical Analyzes
3. Results and Discussion
3.1. Effect of PLUM on Schistosoma mansoni
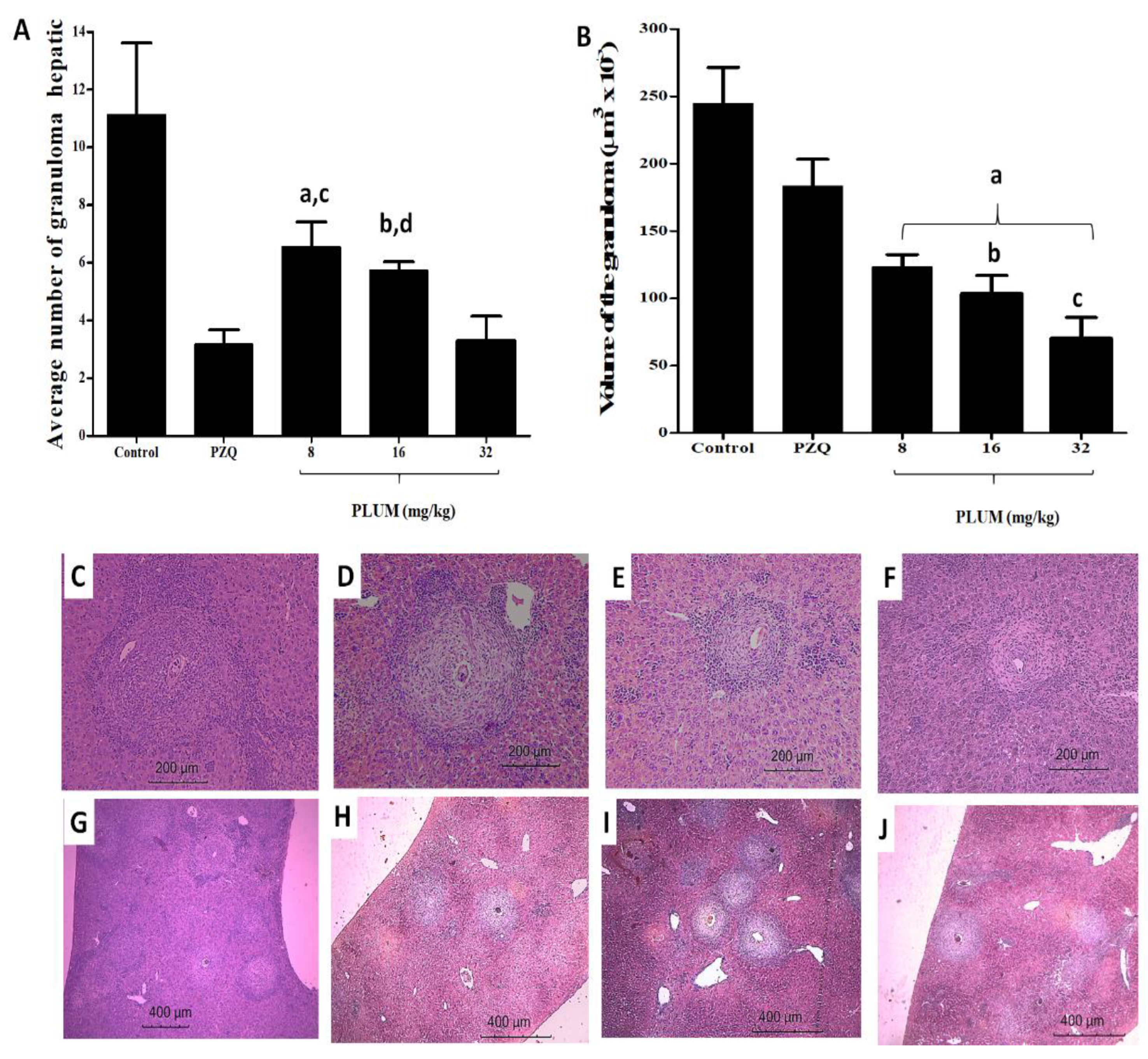
3.2. Effects of PLUM on the Numerical and Volumetric of Schistosomal Hepatic Granulomas
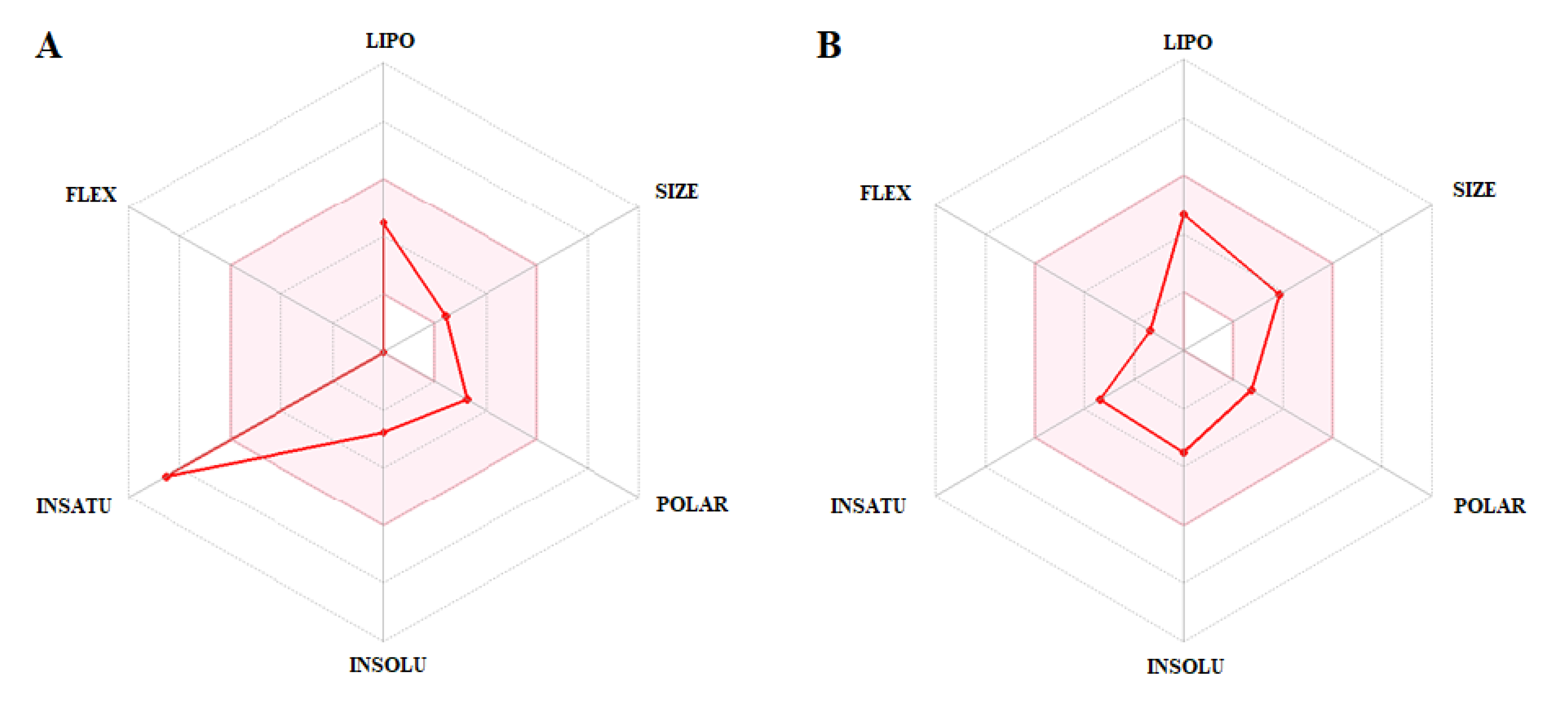
3.3. In Silico Evaluation of Pharmacokinetic Parameters: Absorption, Distribution, Metabolism, Excretion, Toxicity (ADMET) and Prediction of Oral Bioavailability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Schistosomiasis. Fact sheet detail. 2023. Available online: http://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 27 June 2023).
- Aruleba, R.T.; Adekiya, T.A.; Oyinloye, B.E.; Masamba, P.; Mbatha, L.S.; Pretorius, A.; Kappo, A.P. PZQ Therapy: How Close are we in the Development of Effective Alternative Anti-schistosomal Drugs? Infect. Disord. Drug. Targets. 2019, 19, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Chuah, C.; Gobert, G.N.; Latif, B.; Heo, C.C.; Leow, C.Y. Schistosomiasis in Malaysia: A review. Acta Trop. 2019, 190, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Katz, N. The Discovery of Schistosomiasis mansoni in Brazil. Acta Trop. 2008, 108, 69–71. [Google Scholar] [CrossRef]
- Person, B.; Rollinson, D.; Ali, S.M.; Mohammed, U.A.; A’kadir, F.M.; Kabole, F.; Knopp, S. Evaluation of a Urogenital Schistosomiasis Behavioural Intervention Among Students from Rural Schools in Unguja and Pemba Islands, Zanzibar. Acta Trop. 2021, 220, 105960. [Google Scholar] [CrossRef]
- Gonçalves, M.M.; Barreto, M.G.; Peralta, R.H.; Gargioni, C.; Gonçalves, T.; Igreja, R.P.; Soares, M.S.; Peralta, J.M. Immunoassays as an Auxiliary Tool for the Serodiagnosis of Schistosoma Mansoni Infection in Individuals with Low Intensity of Egg Elimination. Acta Trop. 2006, 100, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Paz, W.S.; Duthie, M.S.; Jesus, A.R.; Araújo, K.C.G.M.; Santos, A.D.; Bezerra-Santos, M. Population-Based, Spatiotemporal Modeling of Social Risk Factors and Mortality from Schistosomiasis in Brazil Between 1999 and 2018. Acta Trop. 2021, 218, 105897. [Google Scholar] [CrossRef]
- Mwanga, J.R.; Lwambo, N.J.; Rumisha, S.F.; Vounatsou, P.; Utzinger, J. Dynamics of People’s Socio-Economic Status in the Face of Schistosomiasis Control Interventions in Ukerewe District, Tanzania. Acta Trop. 2013, 128, 399–406. [Google Scholar] [CrossRef]
- Utzinger, J.; N’goran, E.K.; Caffrey, C.R.; Keiser, J. From Innovation to Application: Social-Ecological Context, Diagnostics, Drugs and Integrated Control of Schistosomiasis. Acta Trop. 2011, 120, 121–137. [Google Scholar] [CrossRef]
- Gazzinelli, A.; Velasquez-Melendez, G.; Crawford, S.B.; LoVerde, P.T.; Correa-Oliveira, R.; Kloos, H. Socioeconomic determinants of schistosomiasis in a poor rural area in Brazil. Acta Trop. 2006, 99, 260–271. [Google Scholar] [CrossRef]
- Silva, J.C.S.; Lins, C.R.B.; Lacerda, S.S.; Ramos, R.E.M.; Araújo, H.D.A.; Melo-Júnior, M.R.; Alves, L.C.; Brayner, F.A.; Nunes, I.S.; Melo, F.L.; et al. In vitro and In vivo Effects of P-MAPA Immunomodulator on Schistosomiasis. Acta Trop. 2021, 218, 105909. [Google Scholar] [CrossRef]
- Dias, H.S.; Domingues, A.L.; Cordeiro, F.T.; Jucá, N.; Lopes, E.P. Associating Portal Congestive Gastropathy and Hepatic Fibrosis in Hepatosplenic Mansoni Schistosomiasis. Acta Trop. 2013, 126, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Ayé, P.; Phongluxa, K.; Vonghachack, Y.; Sayasone, S.; Oroth, R.; Odermatt, P. Patients with Severe Schistosomiasis Mekongi Morbidity Demonstrating Ongoing Transmission in Southern Lao People’s Democratic Republic. Acta Trop. 2020, 204, 105323. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, L.D.P.; Fontes, D.A.F.; Aguilera, C.S.B.; Timóteo, T.R.R.; Ângelos, M.A.; Silva, L.C.P.B.B.; Melo, C.G.; Rolim, L.A.; Silva, R.M.F.; Neto, P.J.R. Schistosomiasis: Drugs Used and Treatment Strategies. Acta Trop. 2017, 176, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.S.; Bernardes, M.V.A.S.; Melo, F.L.; Sá, M.P.B.O.; Carvalho, B.M. Praziquantel Versus Praziquantel Associated with Immunomodulators in Mice Infected with Schistosoma mansoni: A Systematic Review and Meta-Analysis. Acta Trop. 2020, 204, 105359. [Google Scholar] [CrossRef]
- Soares, R.N.; Ximenes, E.C.P.A.; Araújo, S.B.; Silva, R.L.D.; Souza, V.M.O.; Coelho, L.C.B.B.; Freitas Neto, J.L.; Rolim Neto, P.J.; Araújo, H.D.A.; Aires, A.L.; et al. Evaluation of β-lapachone-methyl-β-cyclodextrin Inclusion Complex Prepared by Spray Drying and its Application Against Different Developmental Stages of Schistosoma mansoni in Murine Model. Chem. Biol. Interact. 2023, 373, 110374. [Google Scholar] [CrossRef]
- Webster, B.L.; Diaw, O.T.; Seye, M.M.; Faye, D.S.; Stothard, J.R.; Sousa-Figueiredo, J.C.; Rollinson, D. Praziquantel Treatment of School Children from Single and Mixed Infection Foci of Intestinal and Urogenital Schistosomiasis Along the Senegal River Basin: Monitoring Treatment Success and Re-infection Patterns. Acta Trop. 2013, 128, 292–302. [Google Scholar] [CrossRef]
- Pica-Mattoccia, L.; Doenhoff, M.J.; Valle, C.; Basso, A.; Troiani, A.R.; Liberti, P.; Festucci, A.; Guidi, A.; Cioli, D. Genetic Analysis of Decreased Praziquantel Sensitivity in a Laboratory Strain of Schistosoma mansoni. Acta Trop. 2009, 111, 82–85. [Google Scholar] [CrossRef]
- Vale, N.; Gouveia, M.J.; Rinaldi, G.; Brindley, P.J.; Gärtner, F.; Costa, J.M.C. Praziquantel for Schistosomiasis: Single-Drug Metabolism Revisited, Mode of Action, and Resistance. Antimicrob. Agents Chemother. 2017, 61, e02582-e16. [Google Scholar] [CrossRef]
- Leonardo, L.; Rivera, P.; Saniel, O.; Solon, J.A.; Chigusa, Y.; Villacorte, E.; Chua, J.C.; Moendeg, K.; Manalo, D.; Crisostomo, B.; et al. New Endemic Foci of Schistosomiasis Infections in the Philippines. Acta Trop. 2015, 141, 354–360. [Google Scholar] [CrossRef]
- Conceição, J.R.; Lopes, C.P.G.; Ferreira, E.I.; Epiphanio, S.; Giarolla, J. Neglected Tropical Diseases and Systemic Racism Especially in Brazil: From Socio-Economic Aspects to the Development of New Drugs. Acta Trop. 2022, 235, 106654. [Google Scholar] [CrossRef]
- Rollinson, D.; Knopp, S.; Levitz, S.; Stothard, J.R.; Tchuenté, L.-A.T.; Garba, A.; Mohammed, K.A.; Schur, N.; Person, B.; Colley, D.G.; et al. Time to set the Agenda for Schistosomiasis Elimination. Acta Trop. 2013, 128, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Abou-El-Naga, I.F. Towards Elimination of Schistosomiasis After 5000 Years of Endemicity in Egypt. Acta Trop. 2018, 181, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J. Natural Products with Antischistosomal Activity. Future Med. Chem. 2015, 7, 801–820. [Google Scholar] [CrossRef]
- El-Beshbishi, S.N.; Saleh, N.E.; Abd El-Mageed, S.A.; El-Nemr, H.E.E.; Abdalla, H.A.; Shebl, A.M.; Taman, A. Effect of Omega-3 Fatty Acids Administered as Monotherapy or Combined with Artemether on Experimental Schistosoma mansoni Infection. Acta Trop. 2019, 194, 62–68. [Google Scholar] [CrossRef]
- Araújo, H.D.A.; Aires, A.L.; Soares, C.L.R.; Brito, T.G.S.; Nascimento, W.M.; Martins, M.C.B.; Silva, T.G.; Brayner, F.A.; Alves, L.C.; Silva, N.H.; et al. Usnic Acid Potassium Salt from Cladonia substellata (Lichen): Synthesis, Cytotoxicity and In Vitro Anthelmintic Activity and Ultrastructural Analysis Against Adult Worms of Schistosoma mansoni. Acta Trop. 2019, 192, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Araújo, H.D.A.; Santos, V.H.B.; Brayner, F.A.; Alves, L.C.; Silva, N.H.; Albuquerque, M.C.P.A.; Aires, A.L.; Lima, V.L.M. In vitro Activity of Usnic Acid Potassium Salt Against Different Developmental Stages of Schistosoma mansoni: An Ultrastructural Study. Acta Trop. 2020, 201, 105159. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.A.M.F.; Aires, A.L.; Soares, C.L.R.; Siqueira, W.N.; Lima, M.V.; Martins, M.C.B.; Albuquerque, M.C.P.A.; Silva, T.G.; Brayner, F.A.; Alves, L.C.; et al. Schistosomicidal Effect of Divaricatic Acid From Canoparmelia texana (Lichen): In Vitro Evaluation and Ultrastructural Analysis Against Adult Worms of Schistosoma mansoni. Acta Trop. 2021, 222, 106044. [Google Scholar] [CrossRef]
- Padhye, S.; Dandawate, P.; Yusufi, M.; Ahmad, A.; Sarkar, F.H. Perspectives on Medicinal Properties of Plumbagin and its Analogs. Med. Res. Rev. 2012, 32, 1131–1158. [Google Scholar] [CrossRef]
- Fowler, P.; Meurer, K.; Honarvar, N.; Kirkland, D. A Review of the Genotoxic Potential of 1,4-Naphthoquinone. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 834, 6–17. [Google Scholar] [CrossRef]
- Basnet, B.B.; Liu, L.; Zhao, W.; Liu, R.; Ma, K.; Bao, L.; Ren, J.; Wei, X.; Yu, H.; Wei, J.; et al. New 1, 2-Naphthoquinone-Derived Pigments From the Mycobiont of Lichen Trypethelium eluteriae Sprengel. Nat. Prod. Res. 2019, 33, 2044–2050. [Google Scholar] [CrossRef]
- Ahmadi, E.S.; Tajbakhsh, A.; Iranshahy, M.; Asili, J.; Kretschmer, N.; Shakeri, A.; Sahebkar, A. Naphthoquinone Derivatives Isolated from Plants: Recent Advances in Biological Activity. Mini Rev. Med. Chem. 2020, 20, 2019–2035. [Google Scholar] [CrossRef] [PubMed]
- Ravichandiran, P.; Masłyk, M.; Sheet, S.; Janeczko, M.; Premnath, D.; Kim, A.R.; Park, B.H.; Han, M.K.; Yoo, D.J. Synthesis and Antimicrobial Evaluation of 1,4-Naphthoquinone Derivatives as Potential Antibacterial Agents. Chemistry Open. 2019, 8, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Wellington, K.W.; Nyoka, N.B.; McGaw, L.J. Investigation of the Antibacterial and Antifungal Activity of Thiolated Naphthoquinones. Drug Dev. Res. 2019, 80, 386–394. [Google Scholar] [CrossRef]
- Manickam, M.; Boggu, P.R.; Cho, J.; Nam, Y.J.; Lee, S.J.; Jung, S.H. Investigation of Chemical Reactivity of 2-Alkoxy-1, 4-Naphthoquinones and their Anticancer Activity. Bioorg. Med. Chem. Lett. 2018, 28, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Milackova, I.; Prnova, M.S.; Majekova, M.; Sotnikova, R.; Stasko, M.; Kovacikova, L.; Banerjee, S.; Veverka, M.; Stefek, M. 2-Chloro-1,4-Naphthoquinone Derivative of Quercetin as an Inhibitor of Aldose Reductase and Anti-inflammatory Agent. J. Enzyme Inhib. Med. Chem. 2015, 30, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.; Polonik, S. 1,4-Naphthoquinones: Some Biological Properties and Application. Chem. Pharm. Bull. 2020, 68, 46–57. [Google Scholar] [CrossRef]
- El-Beshbishi, S.N.; El Bardicy, S.; Tadros, M.; Ayoub, M.; Taman, A. Biological Activity of Artemisinin-Naphthoquine Phosphate on Schistosoma haematobium Stages and the Vector Bulinus truncatus. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 320–325. [Google Scholar] [CrossRef] [PubMed]
- França, W.W.M.; Silva, A.M.; Diniz, E.G.M.; Silva, H.A.M.F.; Pereira, D.R.; Melo, A.M.M.A.; Coelho, L.C.B.B.; Albuquerque, M.C.P.A.; Araújo, H.D.A.; Aires, A.L. Toxic, Cytotoxic and Genotoxic Effect of Plumbagin in the Developmental Stages of Biomphalaria glabrata (Say, 1818-intermediate host) and cercaricidal activity against the infectious agent of schistosomiasis mansoni. Pest Manag. Sci. 2022, 78, 5172–5183. [Google Scholar] [CrossRef]
- Stalin, A.; Dhivya, P.; Lin, D.; Feng, Y.; Asharaja, A.C.; Gandhi, M.R.; Kannan, B.S.; Kandhasamy, S.; Reegan, A.D.; Chen, Y. Synthesis, Molecular Docking and Mosquitocidal Efficacy of Lawsone and its Derivatives Against the Dengue Vector Aedes aegypti L. (Diptera: Culicidae). Med. Chem. 2022, 18, 170–180. [Google Scholar] [CrossRef]
- Pertino, M.W.; de la Torre, A.F.; Schmeda-Hirschmann, G.; Vega, C.; Rolón, M.; Coronel, C.; Rojas de Arias, A.; Leal López, K.; Carranza-Rosales, P.; Viveros Valdez, E. Synthesis, Trypanocidal and Anti-Leishmania Activity of New Triazole-Lapachol and Norlapachol Hybrids. Bioorg. Chem. 2020, 103, 104122. [Google Scholar] [CrossRef]
- Ortiz-Pérez, E.; Rivera, G.; Salas, C.O.; Zarate-Ramos, J.J.; Trofymchuk, O.S.; Hernandez-Soberanis, L.; Perales-Flores, J.D.; Vázquez, K. Natural and Synthetic Naphthoquinones as Potential Anti-Infective Agents. Curr. Top. Med. Chem. 2021, 21, 2046–2069. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, D.V.C.; Tavares, G.S.V.; Pereira, I.A.G.; Oliveira-da-Silva, J.A.; Ramos, F.F.; Lage, D.P.; Machado, A.S.; Carvalho, L.M.; Reis, T.A.R.; Carvalho, A.M.R.S.; et al. Flau-A, a Naphthoquinone Derivative, is a Promising Therapeutic Candidate Against Visceral Leishmaniasis: A preliminary Study. Exp. Parasitol. 2022, 233, 108205. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.F.A.; Borgati, T.F.; Souza, L.C.R.; Tagliati, C.A.; Oliveira, A.B. In Silico, In Vitro, and In Vivo Evaluation of Natural Bignoniaceous Naphthoquinones in Comparison with Atovaquone Targeting the Selection of Potential Antimalarial Candidates. Toxicol. Appl. Pharmacol. 2020, 401, 115074. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.L.R.; Portes, J.A.; Araújo, M.H.; Silva, J.L.S.; Rennó, M.N.; Netto, C.D.; Silva, A.J.M.; Costa, P.R.R.; Souza, W.; Seabra, S.H.; et al. Further Evidence that Naphthoquinone Inhibits Toxoplasma gondii growth In Vitro. Parasitol. Int. 2015, 64, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Mata-Santos, T.; Pinto, N.F.; Mata-Santos, H.A.; Moura, K.G.; Carneiro, P.F.; Carvalho, T.S.; Del Rio, K.P.; Pinto, M.C.F.R.; Martins, L.R.; Fenalti, J.M. Anthelmintic Activity of Lapachol, β-lapachone and its Derivatives Against Toxocara canis Larvae. Rev. Inst. Med. Trop. São Paulo. 2015, 57, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Mata-Santos, T.; Mata-Santos, H.A.; Carneiro, P.F.; Moura, K.C.G.; Fenalti, J.M.; Klafke, G.B.; Cruz, L.A.X.; Martins, L.H.R.; Pinto, N.F.; Pinto, M.C.; et al. Toxocara canis: Anthelmintic Activity of Quinone Derivatives in Murine Toxocarosis. Parasitology. 2016, 143, 507–517. [Google Scholar] [CrossRef]
- Alferova, V.A.; Shuvalov, M.V.; Korshun, V.A.; Tyurin, A.P. Naphthoquinone-Derived Polyol Macrolides From Natural Sources. Russ. Chem. Bull. 2019, 68, 955–966. [Google Scholar] [CrossRef]
- Aires, A.L.; Ximenes, E.C.; Silva, R.A.; Barbosa, V.X.; Góes, A.J.; Peixoto, C.A.; Souza, V.M.; Albuquerque, M.C.P.A. Ultrastructural Analysis of β-lapachone-Induced Surface Membrane Damage in Male Adult Schistosoma mansoni BH Strain Worms. Exp Parasitol. 2014, 142, 83–90. [Google Scholar] [CrossRef]
- Aires, A.L.; Ximenes, E.C.; Barbosa, V.X.; Góes, A.J.; Souza, V.M.; Albuquerque, M.C.P.A. β-Lapachone: A Naphthoquinone with Promising Antischistosomal Properties in Mice. Phytomedicine. 2014, 21, 261–267. [Google Scholar] [CrossRef]
- Wang, T.; Qiao, H.; Zhai, Z.; Zhang, J.; Tu, J.; Zheng, X.; Qian, N.; Zhou, H.; Lu, E.; Tang, T. Plumbagin Ameliorates Collagen-Induced Arthritis by Regulating Treg/Th17 Cell Imbalances and Suppressing Osteoclastogenesis. Front Immunol. 2019, 9, 3102. [Google Scholar] [CrossRef]
- Tsao, Y.C.; Chang, Y.J.; Wang, C.H.; Chen, L. Discovery of Isoplumbagin as a Novel NQO1 Substrate and Anti-Cancer Quinone. Int. J. Mol. Sci. 2020, 21, 4378. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Ghosh, S.; Shaw, R.; Patra, M.M.; Calcuttawala, F.; Mukherjee, N.; Gupta, S.K.D. Mycobacterium tuberculosis Thymidylate Synthase (ThyX) is a Target for Plumbagin, a Natural Product with Antimycobacterial Activity. PLoS ONE. 2020, 15, e0228657. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Wang, W.; Zhang, J.; Fu, Y.; Liu, Q.; Li, X.; Wang, T.; Zhang, Q. Exploitation of the Antifungal and Antibiofilm Activities of Plumbagin Against Cryptococcus neoformans. Biofouling 2022, 38, 558–574. [Google Scholar] [CrossRef] [PubMed]
- Peres, R.B.; Batista, M.M.; Bérenger, A.L.R.; Camillo, F.D.C.; Figueiredo, M.R.; Soeiro, M.N.C. Antiparasitic Activity of Plumbago auriculata Extracts and Its Naphthoquinone Plumbagin against Trypanosoma cruzi. Pharmaceutics. 2023, 15, 1535. [Google Scholar] [CrossRef]
- Biradar, Y.S.; Bodupally, S.; Padh, H. Evaluation of antiplasmodial properties in 15 selected traditional medicinal plants from India. J. Integr. Med. 2020, 18, 80–85. [Google Scholar] [CrossRef]
- Minsakorn, S.; Nuplod, K.; Puttarak, P.; Chawengkirttikul, R.; Panyarachun, B.; Ngamniyom, A.; Charoenkul, T.; Jaisa-Aad, M.; Panyarachun, P.; Anuracpreeda, P. The Anthelmintic Effects of Medicinal Plant Extracts Against Paramphistome Parasites, Carmyerius spatiosus. Acta Parasitol. 2019, 64, 566–574. [Google Scholar] [CrossRef]
- Awasthi, B.P.; Kathuria, M.; Pant, G.; Kumari, N.; Mitra, K. Plumbagin, a Plant-Derived Naphthoquinone Metabolite Induces Mitochondria-Mediated Apoptosis-like Cell Death in Leishmania donovani: An Ultrastructural and Physiological Study. Apoptosis 2016, 21, 941–953. [Google Scholar] [CrossRef]
- Gupta, A.C.; Mohanty, S.; Saxena, A.; Maurya, A.K.; Bawankule, D.U. Plumbagin, a Vitamin K3 Analogue Ameliorate Malaria Pathogenesis by Inhibiting Oxidative Stress and Inflammation. Inflammopharmacology 2018, 26, 983–991. [Google Scholar] [CrossRef]
- Rani, R.; Sethi, K.; Kumar, S.; Varma, R.S.; Kumar, R. Natural Naphthoquinones and Their Derivatives as Potential Drug Molecules Against Trypanosome Parasites. Chem. Biol. Drug Des. 2022, 100, 786–817. [Google Scholar] [CrossRef]
- Lorsuwannarat, N.; Piedrafita, D.; Chantree, P.; Sansri, V.; Songkoomkrong, S.; Bantuchai, S.; Sangpairot, K.; Kueakhai, P.; Changklungmoa, N.; Chaichanasak, P.; et al. The In Vitro Anthelmintic Effects of Plumbagin on Newly Excysted and 4-Weeks-old Juvenile Parasites of Fasciola gigantica. Exp. Parasit. 2014, 136, 5–13. [Google Scholar] [CrossRef]
- Saowakon, N.; Lorsuwannarat, N.; Changklungmoa, N.; Wanichanon, C.; Sobhon, P. Paramphistomum cervi: The In Vitro Effect of Plumbagin on Motility, Survival and Tegument Structure. Exp. Parasitol. 2013, 133, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Lorsuwannarat, N.; Saowakon, N.; Ramasoota, P.; Wanichanon, C.; Sobhon, P. The Anthelmintic Effect of Plumbagin on Schistosoma mansoni. Exp. Parasitol. 2013, 133, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Coultas, K.A. Identification of Plumbagin and Sanguinarine as Effective Chemotherapeutic Agents for Treatment of Schistosomiasis. Int. J. Parasitol. Drugs Drug. Resist. 2013, 3, 28–34. [Google Scholar] [CrossRef]
- Bakery, H.H.; Allam, G.A.; Abuelsaad, A.S.A.; Abdel-Latif, M.; Elkenawy, A.E.; Khalil, R.G. Anti-inflammatory, Antioxidant, Anti-fibrotic and Schistosomicidal Properties of Plumbagin in Murine Schistosomiasis. Parasite Immunol. 2022, 44, e12945. [Google Scholar] [CrossRef]
- Araújo, H.D.A.; Silva, H.A.M.F.; Siqueira, W.N.; Santos, V.H.B.; Lima, M.V.; Silva Júnior, J.G.; Silva, N.H.; Albuquerque, M.C.P.A.; Melo, A.M.M.A.; Aires, A.L.; et al. Sublethal Concentrations of Usnic Acid Potassium Salt Impairs Physiological Parameters of Biomphalaria glabrata (Say, 1818) (Pulmonata: Planorbidae) Infected and not Infected with Schistosoma mansoni. Acta Trop. 2021, 222, 106067. [Google Scholar] [CrossRef] [PubMed]
- Olivier, l.; Stirewalt, M.A. An Efficient Method for Exposure of Mice to Cercariae of Schistosoma mansoni. J. Parasitol. 1952, 38, 19–23. [Google Scholar] [CrossRef]
- Sumsakul, W.; Plengsuriyakarn, T.; Na-Bangchang, K. Pharmacokinetics, Toxicity, and Cytochrome P450 Modulatory Activity of Plumbagin. BMC Pharmacol. Toxicol. 2016, 17, 50. [Google Scholar] [CrossRef]
- Katz, N. A Simple Device for Quantitative Stool Thick-Smear Technique in Schistosomiasis Mansoni. Rev. Inst. Med. Trop. São Paulo. 1972, 14, 397–400. [Google Scholar]
- Smithers, S.R.; Terry, R.J. The Infection of Laboratory Hosts with Cercariae of Schistosoma mansoni and the Recovery of the Adult Worms. Parasitology. 1965, 55, 695–700. [Google Scholar] [CrossRef]
- Tendler, M.; Pinto, R.M.; Lima, A.O.; Gebara, G.; Katz, N. Schistosoma mansoni: Vaccination with Adult Worm Antigens. Int. J. Parasitol. 1986, 16, 347–352. [Google Scholar] [CrossRef]
- Pellegrino, J.; Oliveira, C.A.; Faria, J.; Cunha, A.S. New Approach to the Screeningof Drugs in Experimental Schistosomiasis Mansoni in Mice. Am. J. Trop. Med. Hyg. 1962, 11, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Cheever, A.W. Conditions Affecting the Accuracy of Potassium Hydroxidedigestion Techniques for Counting Schistosoma mansoni Eggs in Tissues. Bull. World Health Organ. 1968, 39, 328–331. [Google Scholar] [PubMed]
- Aires, A.L.; Araújo, H.D.A.; Galvão, A.M.; Araújo, S.B.; Silva, R.L.; Anjos, Z.P.; Maia, M.B.S.; Souza, V.M.O.; Albuquerque, M.C.P.A. Schistosomicidal, Hepatoprotective and Antioxidant Activities of the N-acetyl-L-cysteine and/or Praziquantel in Experimental Acute Mansonic Schistosomiasis. 3 Biotech. 2023, 13, 215. [Google Scholar] [CrossRef]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J.Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Turner, H.C.; Bettis, A.A.; Dunn, J.C.; Whitton, J.M.; Hollingsworth, T.D.; Fleming, F.M.; Anderson, R.M. Economic Considerations for Moving Beyond the Kato-Katz Technique for Diagnosing Intestinal Parasites As We Move Towards Elimination. Trends Parasitol. 2017, 33, 435–443. [Google Scholar] [CrossRef]
- Cools, P.; Vlaminck, J.; Albonico, M.; Ame, S.; Ayana, M.; Antonio, B.P.J.; Cringoli, G.; Dana, D.; Keiser, J.; Maurelli, M.P.; et al. Diagnostic Performance of a Single and Duplicate Kato-Katz, Mini-FLOTAC, FECPAKG2 and qPCR for the Detection and Quantification of Soil-Transmitted Helminths in Three Endemic Countries. PLoS Negl. Trop. Dis. 2019, 13, e0007446. [Google Scholar] [CrossRef]
- Garba, A.; Lamine, M.S.; Barkiré, N.; Djibo, A.; Sofo, B.; Gouvras, A.N.; Labbo, R.; Sebangou, H.; Webster, J.P.; Fenwick, A.; et al. Efficacy and Safety of Two Closely Spaced Doses of Praziquantel Against Schistosoma haematobium and S. mansoni and Re-Infection Patterns in School-Aged Children in Niger. Acta Trop. 2013, 128, 334–344. [Google Scholar] [CrossRef]
- Cruz, C.S.; França, W.W.M.; Arújo, H.D.A.; Ximenes, E.C.P.A.; Souza, V.M.; Albuquerque, M.C.P.A.; Aires, A.L.; Costa, V.M.A. In Vitro and In Vivo Evaluation of Bacillus clausii Against Schistosoma mansoni. Acta Trop. 2022, 235, 106669. [Google Scholar] [CrossRef]
- El-Beshbishi, S.N.; Taman, A.; El-Malky, M.; Azab, M.S.; El-Hawary, A.K.; El-Tantawy, D.A. First Insight Into the Effect of Single Oral Dose Therapy with Artemisinin-Naphthoquine Phosphate Combination in a Mouse Model of Schistosoma mansoni Infection. Int. J. Parasitol. 2013, 43, 521–530. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Soares, I.A.O.; Rao, G.S.; Badoco, F.R.; Furtado, R.A.; Correa, M.B.; Tavares, D.C.; Cunha, W.R.; Magalhães, L.G. Antiparasitic Activity of Menadione (Vitamin K3) Against Schistosoma mansoni in BABL/c Mice. Acta Trop. 2017, 167, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Thakor, N.; Janathia, B. Plumbagin: A Potential Candidate for Future Research and Development. Curr. Pharm. Biotechnol. 2021, 23, 1800–1812. [Google Scholar] [CrossRef] [PubMed]
- Panic, G.; Vargas, M.; Scandale, I.; Keiser, J. Activity Profile of an FDA-Approved Compound Library against Schistosoma mansoni. PLoS Negl. Trop. Dis. 2015, 31, e0003962. [Google Scholar] [CrossRef]
- Nogueira, R.A.; Lira, M.G.S.; Licá, I.C.L.; Frazão, G.C.C.G.; Santos, V.A.F.; Filho, A.C.C.M.; Rodrigues, J.G.M.; Miranda, G.S.; Carvalho, R.C.; Nascimento, F.R.F. Praziquantel: An Update on the Mechanism of its Action Against Schistosomiasis and New Therapeutic Perspectives. Mol. Biochem. Parasitol 2022, 252, 111531. [Google Scholar] [CrossRef]
- Seif El-Din, S.H.; Al-Hroob, A.M.; Ebeid, F.A. Schistosoma mansoni: N-acetylcysteine Downregulates Oxidative Stress and Enhances the Antischistosomal Activity of Artemether in Mice. Exp. Parasitol. 2011, 128, 230–235. [Google Scholar] [CrossRef]
- Steinmeier, J.; Kube, S.; Karger, G.; Ehrke, E.; Dringen, R. β-Lapachone Induces Acute Oxidative Stress in Rat Primary Astrocyte Cultures that is Terminated by the NQO1- Inhibitor Dicoumarol. Neurochem Res. 2020, 45, 2442–2455. [Google Scholar] [CrossRef]
- Kuntz, A.N.; Davioud-Charvet, E.; Sayed, A.A.; Califf, L.L.; Dessolin, J.; Arnér, E.S.J.; Williams, D.L. Thioredoxin Glutathione Reductase From Schistosoma mansoni: An Essential Parasite Enzyme and a Key Drug Target. PLoS Med. 2007, 4, e206. [Google Scholar] [CrossRef]
- Tripathi, T.; Chetri, P.B. Potent Inhibitors of Thioredoxin Glutathione Reductase: Grail of Anti-Schistosome Drug within Reach? ACS Infect. Dis. 2020, 6, 893–895. [Google Scholar] [CrossRef]
- Mati, V.L.; Melo, A.L. Current Applications of Oogram Methodology in Experimental Schistosomiasis; Fecundity of Female Schistosoma mansoni and Egg Release in the Intestine of AKR/J Mice Following Immunomodulatory Treatment with Pentoxifylline. J. Helminthol. 2013, 87, 115–124. [Google Scholar] [CrossRef]
- Beshay, E.V.N.; Rady, A.A.; Afifi, A.F.; Mohamed, A.H. Schistosomicidal, Antifibrotic and Antioxidant Effects of Cucurbita pepo L. Seed Oil and Praziquantel Combined Treatment for Schistosoma mansoni Infection in a Mouse Model. J. Helminthol. 2019, 93, 286–294. [Google Scholar] [CrossRef]
- Penido, M.L.O.; Coelho, P.M.Z.; Mello, R.T.; Piló-Veloso, D.; Oliveira, M.C.; Kusel, J.R.; Nelson, D.L. Antischistosomal Activity of Aminoalkanethiols, Aminoalkanethiosulfuric Acids and the Corresponding Disulfides. Acta Trop. 2008, 108, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, S.A.P.; Oliveira, R.N.; Mendes, T.M.F.; Santos, K.R.; Boaventura Jr, S.; Garcia, V.L.; Jeraldo, V.L.S.; Allegretti, S.M. In Vitro and In Vivo Evaluation of Six Artemisinin Derivatives Against Schistosoma mansoni. Parasitol Res. 2019, 118, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Tavares, N.C.; Mourão, M.M. Parasitemia Evaluation in Mice Infected with Schistosoma mansoni. Bio Protoc. 2021, 11, e4017. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.; Fallon, P.G. Schistosoma “Eggs-iting” the Host: Granuloma Formation and Egg Excretion. Front. Immunol. 2018, 9, 2492. [Google Scholar] [CrossRef]
- Barbosa, C.S.; Gomes, E.C.S.; Campos, J.V.; Oliveira, F.J.M.; Mesquita, M.C.S.; Oliveira, E.C.A.; Domingues, A.L.C. Morbidity of Mansoni Schistosomiasis in Pernambuco-Brazil: Analysis on the Temporal Evolution of Deaths, Hospital Admissions and Severe Clinical Forms (1999–2014). Acta Trop. 2016, 164, 10–16. [Google Scholar] [CrossRef]
- Silva, D.V.S.P.; Nascimento, P.H.B.; Rocha, J.V.R.; Marques, D.S.C.; Brayner, F.A.; Alves, L.C.; Araújo, H.D.A.; Cruz Filho, I.J.; Albuquerque, M.C.P.A.; Lima, M.C.A.; et al. In vitro Activity, Ultrastructural Analysis and In Silico Pharmacokinetic Properties (ADMET) of Thiazole Compounds Against Adult Worms of Schistosoma mansoni. Acta Trop. 2023, 7, 106965. [Google Scholar] [CrossRef]
- Rocha, J.A.; Rego, N.C.S.; Carvalho, B.T.S.; Silva, F.I.; Sousa, J.A.; Ramos, R.M.; Passos, I.N.G.; Moraes, J.; Leite, J.R.S.A.; Lima, F.C.A. Computational Quantum Chemistry, Molecular Docking, and ADMET Predictions of Imidazole Alkaloids of Pilocarpus microphyllus with Schistosomicidal Properties. PLoS ONE. 2018, 13, 1–23. [Google Scholar] [CrossRef]
| Experimental Groups | Average Worm Burden | % Of Eggs per Developmental Stage | |||||
|---|---|---|---|---|---|---|---|
| Total | Reduction (%) | Female | Reduction (%) | Immature * | Mature | Dead | |
| Control | 32.14 ± 2.73 | - | 14.29 ± 1.6 | - | 53.99 ± 5.81 | 41.39 ± 5.88 | 4.57 ± 0.61 |
| PZQ (50 mg/kg) | 0.8 ± 0.44 a | 97.51 | 0.4 ± 0.54 a | 97.2 | 0.0 ± 0.0 g | 2.37 ± 1.4 | 97.63 ± 1.4 h |
| PLUM | |||||||
| 8 mg/kg | 17.13 ± 1.72 a | 46.7 | 8.0 ± 1.63 a | 44.01 | 29.67 ± 3.2 g | 32.33 ± 5.31 | 38.33 ± 4.41 h |
| 16 mg/kg | 14.38 ± 1.84 a,b | 55.25 | 6.75 ± 0.89 a | 52.76 | 25.57 ± 3.64 g | 35.0 ± 55 | 39.43 ± 6.29 h |
| 32 mg/kg | 8.87 ± 1.12 a,c,d | 72.4 | 4.12 ± 0.99 a,e,f | 71.16 | 18.83 ± 4.53 g | 37.0 ± 6.54 | 44.17 ± 7.41 h |
| Parameters | PLUM | PZQ | Unit |
|---|---|---|---|
| Absorption | |||
| Water solubility | −2.65 | −4.00 | Numeric (log mol/L) |
| Caco2 permeability | 1.19 | 1.75 | Numeric (log Papp in 10−6 cm/s) |
| Intestinal absorption | 96.25 | 93.42 | Numeric (%Absorbed) |
| Skin Permeability | −2.93 | −3.14 | Numeric (log Kp) |
| P-glycoprotein substrate | No | Yes | Categorical (Yes/No) |
| P-glycoprotein I inhibitor | No | No | Categorical (Yes/No) |
| P-glycoprotein II inhibitor | No | No | Categorical (Yes/No) |
| Distribution | |||
| VDssa | 0.14 | 0.52 | Numeric (log L/kg) |
| Fraction unbound | 0.48 | 0.15 | Numeric (Fu) |
| BBB permeability | 0.47 | 0.46 | Numeric (log BB) |
| CNS permeability | −2.82 | −1.78 | Numeric (log PS) |
| Metabolism | |||
| CYP2D6 substrate | No | No | Categorical (Yes/No) |
| CYP3A4 substrate | No | Yes | Categorical (Yes/No) |
| CYP1A2 inhibitor | Yes | No | Categorical (Yes/No) |
| CYP2C19 inhibitor | No | Yes | Categorical (Yes/No) |
| CYP2C9 inhibitor | No | No | Categorical (Yes/No) |
| CYP2D6 inhibitor | No | No | Categorical (Yes/No) |
| CYP3A4 inhibitor | No | No | Categorical (Yes/No) |
| Excretion | |||
| Total clearance | 0.14 | 1.05 | Numeric (log mL/min/kg) |
| Renal OCT2 substrate | No | Yes | Categorical (Yes/No) |
| Toxicity | |||
| AMES toxicity | Yes | No | Categorical (Yes/No) |
| Maximum tolerated dose | 0.40 | −0.23 | Numeric (log mg/kg/day) |
| hERG I inhibitor | No | No | Categorical (Yes/No) |
| hERG II inhibitor | No | No | Categorical (Yes/No) |
| Oral rat acute toxicity | 1.63 | 2.26 | Numeric (mol/kg) |
| Oral rat chronic toxicity | 2.55 | 1.11 | Numeric (log mg/kg_bw/day) |
| Hepatotoxicity | No | No | Categorical (Yes/No) |
| Skin sensitization | No | No | Categorical (Yes/No) |
| T. Pyriformis toxicity | 0.71 | 1.31 | Numeric (log µg/L) |
| Minnow toxicity | 1.75 | 1.56 | Numeric (log mM) |
| Oral bioavailability | |||
| Lipinski’s rule | 0 | 0 | Violation (numeric) |
| Veber’s rule | 0 | 0 | Violation (numeric) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.M.N.; França, W.W.M.; Santos, V.H.B.; Souza, R.A.F.; Silva, A.M.; Diniz, E.G.M.; Aguiar, T.W.A.; Rocha, J.V.R.; Souza, M.A.A.; Nascimento, W.R.C.; et al. Plumbagin: A Promising In Vivo Antiparasitic Candidate against Schistosoma mansoni and In Silico Pharmacokinetic Properties (ADMET). Biomedicines 2023, 11, 2340. https://doi.org/10.3390/biomedicines11092340
Silva LMN, França WWM, Santos VHB, Souza RAF, Silva AM, Diniz EGM, Aguiar TWA, Rocha JVR, Souza MAA, Nascimento WRC, et al. Plumbagin: A Promising In Vivo Antiparasitic Candidate against Schistosoma mansoni and In Silico Pharmacokinetic Properties (ADMET). Biomedicines. 2023; 11(9):2340. https://doi.org/10.3390/biomedicines11092340
Chicago/Turabian StyleSilva, Lucas M. N., Wilza W. M. França, Victor H. B. Santos, Renan A. F. Souza, Adriana M. Silva, Emily G. M. Diniz, Thierry W. A. Aguiar, João V. R. Rocha, Mary A. A. Souza, Wheverton R. C. Nascimento, and et al. 2023. "Plumbagin: A Promising In Vivo Antiparasitic Candidate against Schistosoma mansoni and In Silico Pharmacokinetic Properties (ADMET)" Biomedicines 11, no. 9: 2340. https://doi.org/10.3390/biomedicines11092340
APA StyleSilva, L. M. N., França, W. W. M., Santos, V. H. B., Souza, R. A. F., Silva, A. M., Diniz, E. G. M., Aguiar, T. W. A., Rocha, J. V. R., Souza, M. A. A., Nascimento, W. R. C., Lima Neto, R. G., Cruz Filho, I. J., Ximenes, E. C. P. A., Araújo, H. D. A., Aires, A. L., & Albuquerque, M. C. P. A. (2023). Plumbagin: A Promising In Vivo Antiparasitic Candidate against Schistosoma mansoni and In Silico Pharmacokinetic Properties (ADMET). Biomedicines, 11(9), 2340. https://doi.org/10.3390/biomedicines11092340








