Recent Advances in Microbiota-Associated Metabolites in Heart Failure
Abstract
1. Introduction
2. Heart Failure Global Burden and Quality of Life
3. Gut Microbiota Species Implicated in Heart Failure
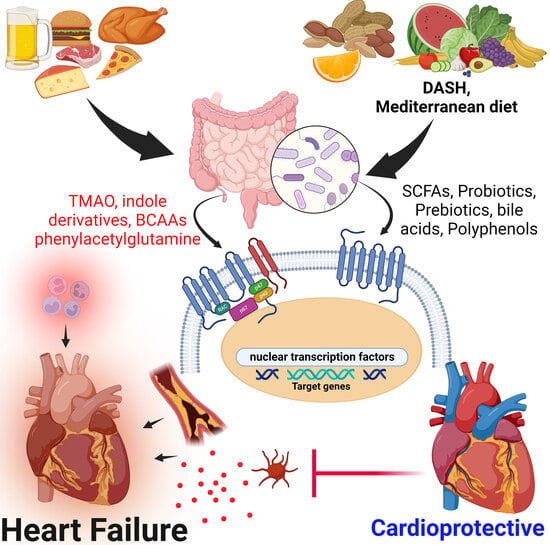
4. Mechanisms of Gut Microbiota Metabolites Implicated in Heart Failure
4.1. Beneficial Effects of Gut Microbiota-Derived Metabolites in Heart Failure Pathophysiology
4.2. Gut Metabolites Implicated in Heart Failure
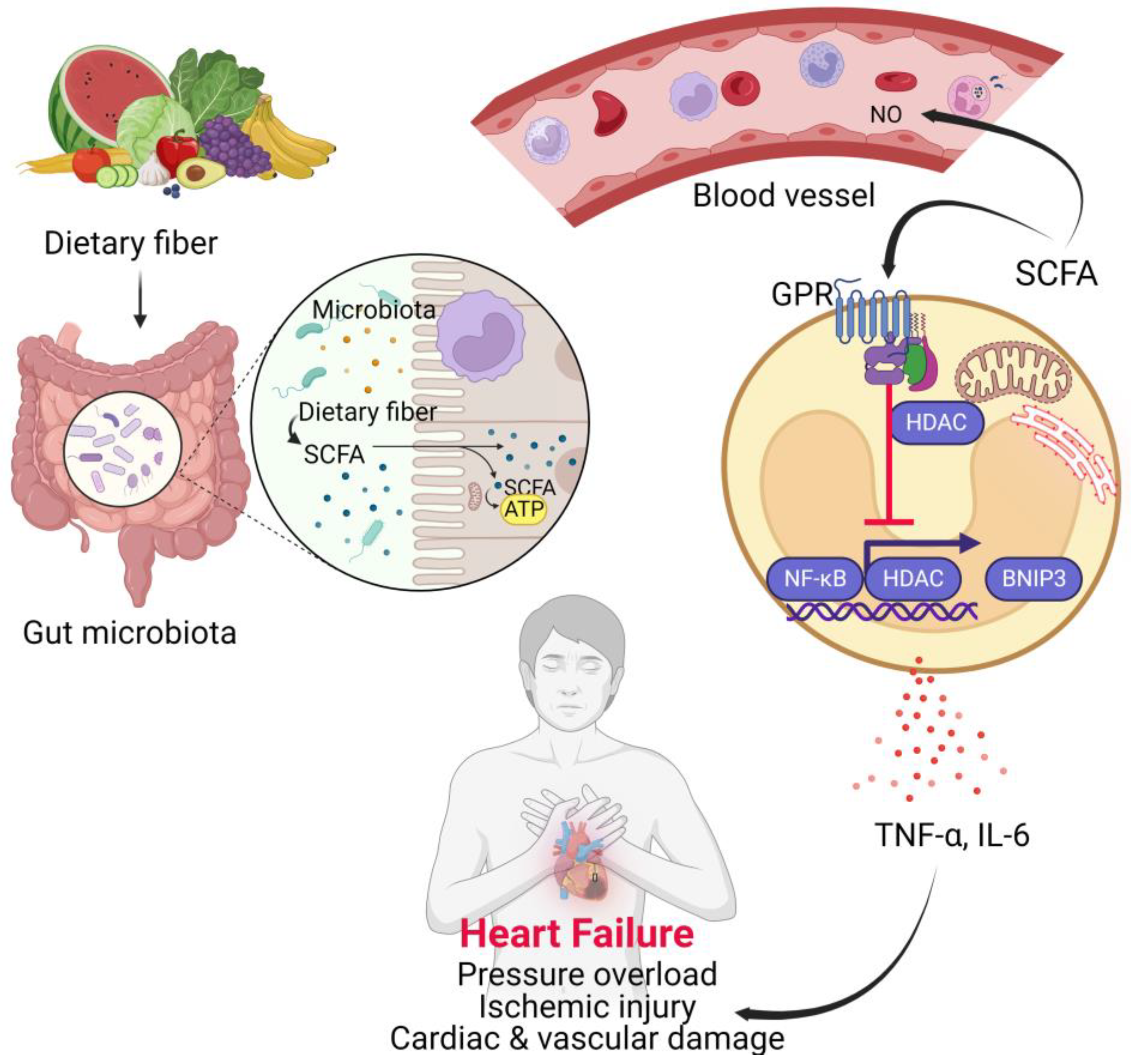
4.3. Short Chain Fatty Acids (SCFAs)
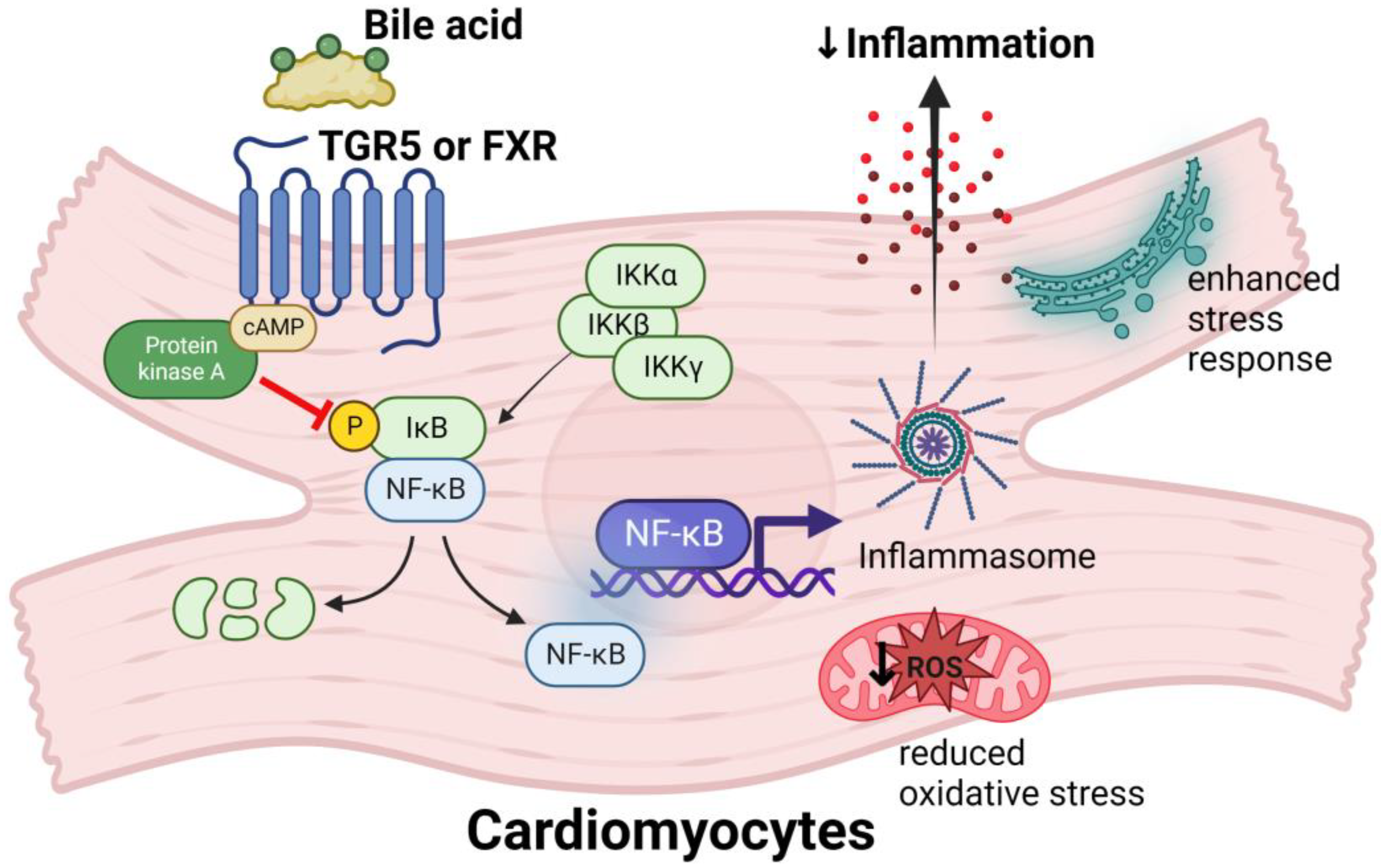
4.4. Bile Acids
4.5. Branched-Chain Amino Acids
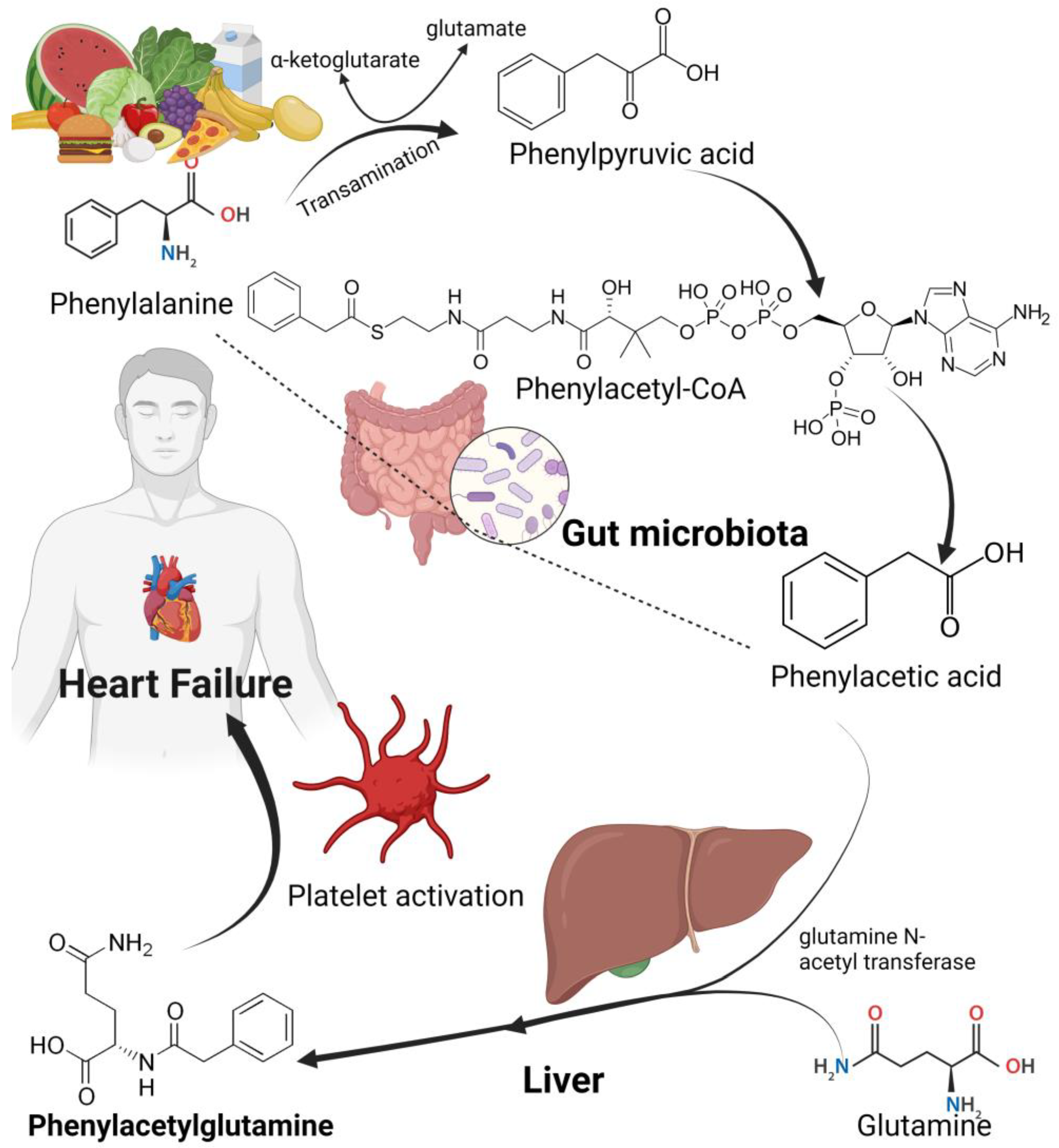
4.6. Phenylacetylglutamine
4.7. Tryptophan and Indole Derivatives
4.8. Trimethylamine N-Oxide (TMAO)
5. Beneficial Dietary Interventions and Therapy to Modulate the Gut Microbiota in Heart Failure and Other Cardiovascular Diseases
6. Gut Microbiota Benefits on Other Organs/Systems
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guha, S.; Harikrishnan, S.; Ray, S.; Sethi, R.; Ramakrishnan, S.; Banerjee, S.; Bahl, V.K.; Goswami, K.C.; Banerjee, A.K.; Shanmugasundaram, S.; et al. CSI Position Statement on Management of Heart Failure in India. Indian. Heart J. 2018, 70, S1–S72. [Google Scholar] [CrossRef] [PubMed]
- Naraen, A.; Duvva, D.; Rao, A. Heart Failure and Cardiac Device Therapy: A Review of Current National Institute of Health and Care Excellence and European Society of Cardiology Guidelines. Arrhythm. Electrophysiol. Rev. 2023, 12, e21. [Google Scholar] [CrossRef] [PubMed]
- Norhammar, A.; Bodegard, J.; Vanderheyden, M.; Tangri, N.; Karasik, A.; Maggioni, A.P.; Sveen, K.A.; Taveira-Gomes, T.; Botana, M.; Hunziker, L.; et al. Prevalence, Outcomes and Costs of a Contemporary, Multinational Population with Heart Failure. Heart 2023, 109, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L. Epidemiology of Heart Failure. Circ. Res. 2021, 128, 1421–1434. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Ke, B.; Du, J. TMAO: How Gut Microbiota Contributes to Heart Failure. Transl. Res. 2021, 228, 109–125. [Google Scholar] [CrossRef]
- Hemmati, M.; Kashanipoor, S.; Mazaheri, P.; Alibabaei, F.; Babaeizad, A.; Asli, S.; Mohammadi, S.; Gorgin, A.H.; Ghods, K.; Yousefi, B.; et al. Importance of Gut Microbiota Metabolites in the Development of Cardiovascular Diseases (CVD). Life Sci. 2023, 329, 121947. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Ren, S.; Ding, Y.; Remex, N.S.; Bhuiyan, M.S.; Qu, J.; Tang, X. Gut Microbiota and Microbiota-Derived Metabolites in Cardiovascular Diseases. Chin. Med. J. 2023. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, X.; Song, Z.; Dai, Z.; Luo, K.; Chen, B.; Zhou, Z.; Cui, Y.; Feng, B.; Zhu, Z.; et al. Targeting Gut Microbiota-Derived Kynurenine to Predict and Protect the Remodeling of the Pressure-Overloaded Young Heart. Sci. Adv. 2023, 9, eadg7417. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, Y.; Zhu, P.; Nasser, M.I.; Zhang, X.; Zhao, M. Implication of Gut Microbiota in Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 5394096. [Google Scholar] [CrossRef]
- Qian, B.; Zhang, K.; Li, Y.; Sun, K. Update on Gut Microbiota in Cardiovascular Diseases. Front. Cell Infect. Microbiol. 2022, 12, 1059349. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K. Role of Gut Microbiota and Their Metabolites on Atherosclerosis, Hypertension and Human Blood Platelet Function: A Review. Nutrients 2021, 13, 144. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Zhong, W.; Shu, J.; Abu Much, A.; Lotan, D.; Grupper, A.; Younis, A.; Dai, H. Burden of Heart Failure and Underlying Causes in 195 Countries and Territories from 1990 to 2017. Eur. J. Prev. Cardiol. 2021, 28, 1682–1690. [Google Scholar] [CrossRef]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global Burden of Heart Failure: A Comprehensive and Updated Review of Epidemiology. Cardiovasc. Res. 2022, 118, 3272–3287. [Google Scholar] [CrossRef]
- Seferović, P.M.; Vardas, P.; Jankowska, E.A.; Maggioni, A.P.; Timmis, A.; Milinković, I.; Polovina, M.; Gale, C.P.; Lund, L.H.; Lopatin, Y.; et al. The Heart Failure Association Atlas: Heart Failure Epidemiology and Management Statistics 2019. Eur. J Heart Fail. 2021, 23, 906–914. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the Impact of Heart Failure in the United States: A Policy Statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Cook, C.; Cole, G.; Asaria, P.; Jabbour, R.; Francis, D.P. The Annual Global Economic Burden of Heart Failure. Int. J. Cardiol. 2014, 171, 368–376. [Google Scholar] [CrossRef]
- Johansson, I.; Joseph, P.; Balasubramanian, K.; McMurray, J.J.V.; Lund, L.H.; Ezekowitz, J.A.; Kamath, D.; Alhabib, K.; Bayes-Genis, A.; Budaj, A.; et al. Health-Related Quality of Life and Mortality in Heart Failure: The Global Congestive Heart Failure Study of 23,000 Patients From 40 Countries. Circulation 2021, 143, 2129–2142. [Google Scholar] [CrossRef]
- Warraich, H.J.; Kitzman, D.W.; Whellan, D.J.; Duncan, P.W.; Mentz, R.J.; Pastva, A.M.; Benjamin Nelson, M.; Upadhya, B.; Reeves, G.R. Physical Function, Frailty, Cognition, Depression and Quality-of-Life in Hospitalized Adults ≥60 Years with Acute Decompensated Heart Failure with Preserved versus Reduced Ejection Fraction: Insights from the REHAB-HF Trial. Circ. Heart Fail. 2018, 11, e005254. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Lubetkin, E.I.; Barile, J.P.; Horner-Johnson, W.; DeMichele, K.; Stark, D.S.; Zack, M.M.; Thompson, W.W. Quality-Adjusted Life Years (QALY) for 15 Chronic Conditions and Combinations of Conditions Among US Adults Aged 65 and Older. Med. Care 2018, 56, 740–746. [Google Scholar] [CrossRef]
- McGrath, R.; Al Snih, S.; Markides, K.; Hall, O.; Peterson, M. The Burden of Health Conditions for Middle-Aged and Older Adults in the United States: Disability-Adjusted Life Years. BMC Geriatr. 2019, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Adane, E.; Atnafu, A.; Aschalew, A.Y. The Cost of Illness of Hypertension and Associated Factors at the University of Gondar Comprehensive Specialized Hospital Northwest Ethiopia, 2018. Clin. Outcomes Res. 2020, 12, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ademi, Z.; Ackerman, I.N.; Zomer, E.; Liew, D. Productivity-Adjusted Life-Years: A New Metric for Quantifying Disease Burden. PharmacoEconomics 2021, 39, 271–273. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed. Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef]
- Pasini, E.; Aquilani, R.; Testa, C.; Baiardi, P.; Angioletti, S.; Boschi, F.; Verri, M.; Dioguardi, F. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC Heart Fail. 2016, 4, 220–227. [Google Scholar] [CrossRef]
- Mamic, P.; Heidenreich, P.A.; Hedlin, H.; Tennakoon, L.; Staudenmayer, K.L. Hospitalized Patients with Heart Failure and Common Bacterial Infections: A Nationwide Analysis of Concomitant Clostridium Difficile Infection Rates and In-Hospital Mortality. J. Card. Fail. 2016, 22, 891–900. [Google Scholar] [CrossRef]
- Sun, W.; Du, D.; Fu, T.; Han, Y.; Li, P.; Ju, H. Alterations of the Gut Microbiota in Patients With Severe Chronic Heart Failure. Front. Microbiol. 2022, 12, 813289. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kirabo, A. Salt and Gut Microbiota in Heart Failure. Curr. Hypertens. Rep. 2023, 25, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhao, S.; Tian, J.; Liu, X. Significance of Gut Microbiota and Short-Chain Fatty Acids in Heart Failure. Nutrients 2022, 14, 3758. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Lupu, V.V.; Adam Raileanu, A.; Mihai, C.M.; Morariu, I.D.; Lupu, A.; Starcea, I.M.; Frasinariu, O.E.; Mocanu, A.; Dragan, F.; Fotea, S. The Implication of the Gut Microbiome in Heart Failure. Cells 2023, 12, 1158. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef]
- Westermann, D.; Lindner, D.; Kasner, M.; Zietsch, C.; Savvatis, K.; Escher, F.; von Schlippenbach, J.; Skurk, C.; Steendijk, P.; Riad, A.; et al. Cardiac Inflammation Contributes to Changes in the Extracellular Matrix in Patients with Heart Failure and Normal Ejection Fraction. Circ. Heart Fail. 2011, 4, 44–52. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Rodolico, D.; Hill, J.A. Metabolic Inflammation in Heart Failure with Preserved Ejection Fraction. Cardiovasc. Res. 2021, 117, 423–434. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Orlenko, A.; Zhao, L.; Basso, M.D.; Cvijic, M.E.; Li, Z.; Spires, T.E.; Yarde, M.; Wang, Z.; Seiffert, D.A.; et al. Multiple Plasma Biomarkers for Risk Stratification in Patients With Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2020, 75, 1281–1295. [Google Scholar] [CrossRef]
- Zach, V.; Bähr, F.L.; Edelmann, F. Suppression of Tumourigenicity 2 in Heart Failure With Preserved Ejection Fraction. Card. Fail. Rev. 2020, 6, e02. [Google Scholar] [CrossRef]
- DuBrock, H.M.; AbouEzzeddine, O.F.; Redfield, M.M. High-Sensitivity C-Reactive Protein in Heart Failure with Preserved Ejection Fraction. PLoS ONE 2018, 13, e0201836. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Zile, M.R. From Systemic Inflammation to Myocardial Fibrosis. Circ. Res. 2021, 128, 1451–1467. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Cardiac Fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, L.; Pitzer, A.L.; Li, X.; Li, P.-L.; Zhang, Y. Contribution of Redox-Dependent Activation of Endothelial Nlrp3 Inflammasomes to Hyperglycemia-Induced Endothelial Dysfunction. J. Mol. Med. 2016, 94, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; Kong, B.; Shuai, W.; Zhang, J.-J.; Huang, H. Knockout of MD1 Contributes to Sympathetic Hyperactivity and Exacerbates Ventricular Arrhythmias Following Heart Failure with Preserved Ejection Fraction via NLRP3 Inflammasome Activation. Exp. Physiol. 2020, 105, 966–978. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, J.; Xu, Y.; Liu, J.; Ye, J.; Wang, Z.; Ye, D.; Feng, Y.; Xu, S.; Pan, W.; et al. Selective Inhibition of NLRP3 Inflammasome Reverses Pressure Overload-Induced Pathological Cardiac Remodeling by Attenuating Hypertrophy, Fibrosis, and Inflammation. Int. Immunopharmacol. 2021, 99, 108046. [Google Scholar] [CrossRef]
- Roy, J.; Cyert, M.S. Identifying New Substrates and Functions for an Old Enzyme: Calcineurin. Cold Spring Harb. Perspect. Biol. 2020, 12, a035436. [Google Scholar] [CrossRef]
- Gelpi, R.J.; Gao, S.; Zhai, P.; Yan, L.; Hong, C.; Danridge, L.M.A.; Ge, H.; Maejima, Y.; Donato, M.; Yokota, M.; et al. Genetic Inhibition of Calcineurin Induces Diastolic Dysfunction in Mice with Chronic Pressure Overload. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1814–H1819. [Google Scholar] [CrossRef][Green Version]
- Frey, N.; Olson, E.N. Cardiac Hypertrophy: The Good, the Bad, and the Ugly. Annu. Rev. Physiol. 2003, 65, 45–79. [Google Scholar] [CrossRef]
- He, X.; Du, T.; Long, T.; Liao, X.; Dong, Y.; Huang, Z.-P. Signaling Cascades in the Failing Heart and Emerging Therapeutic Strategies. Signal. Transduct. Target. Ther. 2022, 7, 134. [Google Scholar] [CrossRef]
- Luo, Q.; Hu, Y.; Chen, X.; Luo, Y.; Chen, J.; Wang, H. Effects of Gut Microbiota and Metabolites on Heart Failure and Its Risk Factors: A Two-Sample Mendelian Randomization Study. Front. Nutr. 2022, 9, 899746. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, F.; Or-Rashid, M.H.; Mamun, A.A.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ying, J.; Ma, H.; Cui, H. Microbiota-Related Metabolites Fueling the Understanding of Ischemic Heart Disease. iMeta 2023, 2, e94. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-H.; Hu, Z.-Q.; Hara, Y.; Shimamura, T. Inhibition of Penicillinase by Epigallocatechin Gallate Resulting in Restoration of Antibacterial Activity of Penicillin against Penicillinase-Producing Staphylococcus Aureus. Antimicrob. Agents Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef]
- Yi, S.; Wang, W.; Bai, F.; Zhu, J.; Li, J.; Li, X.; Xu, Y.; Sun, T.; He, Y. Antimicrobial Effect and Membrane-Active Mechanism of Tea Polyphenols against Serratia Marcescens. World J. Microbiol. Biotechnol. 2014, 30, 451–460. [Google Scholar] [CrossRef]
- Nirengi, S.; Amagasa, S.; Homma, T.; Yoneshiro, T.; Matsumiya, S.; Kurosawa, Y.; Sakane, N.; Ebi, K.; Saito, M.; Hamaoka, T. Daily Ingestion of Catechin-Rich Beverage Increases Brown Adipose Tissue Density and Decreases Extramyocellular Lipids in Healthy Young Women. Springerplus 2016, 5, 1363. [Google Scholar] [CrossRef]
- Han, X.; Guo, J.; You, Y.; Yin, M.; Liang, J.; Ren, C.; Zhan, J.; Huang, W. Vanillic Acid Activates Thermogenesis in Brown and White Adipose Tissue. Food Funct. 2018, 9, 4366–4375. [Google Scholar] [CrossRef]
- Andrade, J.M.O.; Paraíso, A.F.; de Oliveira, M.V.M.; Martins, A.M.E.; Neto, J.F.; Guimarães, A.L.S.; de Paula, A.M.; Qureshi, M.; Santos, S.H.S. Resveratrol Attenuates Hepatic Steatosis in High-Fat Fed Mice by Decreasing Lipogenesis and Inflammation. Nutrition 2014, 30, 915–919. [Google Scholar] [CrossRef]
- Casadesus, G.; Shukitt-Hale, B.; Stellwagen, H.M.; Zhu, X.; Lee, H.-G.; Smith, M.A.; Joseph, J.A. Modulation of Hippocampal Plasticity and Cognitive Behavior by Short-Term Blueberry Supplementation in Aged Rats. Nutr. Neurosci. 2004, 7, 309–316. [Google Scholar] [CrossRef]
- van Praag, H.; Lucero, M.J.; Yeo, G.W.; Stecker, K.; Heivand, N.; Zhao, C.; Yip, E.; Afanador, M.; Schroeter, H.; Hammerstone, J.; et al. Plant-Derived Flavanol (-)Epicatechin Enhances Angiogenesis and Retention of Spatial Memory in Mice. J. Neurosci. 2007, 27, 5869–5878. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Li, H.; Christman, L.M.; Li, R.; Gu, L. Synergic Interactions between Polyphenols and Gut Microbiota in Mitigating Inflammatory Bowel Diseases. Food Funct. 2020, 11, 4878–4891. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Khan, N.; Andrés-Lacueva, C.; Urpí-Sardá, M.; Vázquez-Agell, M.; Lamuela-Raventós, R.M.; Estruch, R. Dihydroxylated Phenolic Acids Derived from Microbial Metabolism Reduce Lipopolysaccharide-Stimulated Cytokine Secretion by Human Peripheral Blood Mononuclear Cells. Br. J. Nutr. 2009, 102, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.K.; Soileau, J.L.; Ribnicky, D.; Wang, Z.Q.; Raskin, I.; Poulev, A.; Majewski, M.; Cefalu, W.T.; Gettys, T.W. Quercetin Transiently Increases Energy Expenditure but Persistently Decreases Circulating Markers of Inflammation in C57BL/6J Mice Fed a High-Fat Diet. Metabolism 2008, 57, S39–S46. [Google Scholar] [CrossRef]
- Hedayati, N.; Yaghoobi, A.; Salami, M.; Gholinezhad, Y.; Aghadavood, F.; Eshraghi, R.; Aarabi, M.-H.; Homayoonfal, M.; Asemi, Z.; Mirzaei, H.; et al. Impact of Polyphenols on Heart Failure and Cardiac Hypertrophy: Clinical Effects and Molecular Mechanisms. Front. Cardiovasc. Med. 2023, 10, 1174816. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Jia, Q.; Li, H.; Zhou, H.; Zhang, X.; Zhang, A.; Xie, Y.; Li, Y.; Lv, S.; Zhang, J. Role and Effective Therapeutic Target of Gut Microbiota in Heart Failure. Cardiovasc. Ther. 2019, 2019, 5164298. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Li, D.Y.; Hazen, S.L. Dietary Metabolism, the Gut Microbiome, and Heart Failure. Nat. Rev. Cardiol. 2019, 16, 137–154. [Google Scholar] [CrossRef]
- Kaye, D.M.; Shihata, W.A.; Jama, H.A.; Tsyganov, K.; Ziemann, M.; Kiriazis, H.; Horlock, D.; Vijay, A.; Giam, B.; Vinh, A.; et al. Deficiency of Prebiotic Fiber and Insufficient Signaling Through Gut Metabolite-Sensing Receptors Leads to Cardiovascular Disease. Circulation 2020, 141, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Hori, D.; Flavahan, S.; Steppan, J.; Flavahan, N.A.; Berkowitz, D.E.; Pluznick, J.L. Microbial Short Chain Fatty Acid Metabolites Lower Blood Pressure via Endothelial G Protein-Coupled Receptor 41. Physiol. Genom. 2016, 48, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T.; Nawab, Z.M. Acetate Relaxation of Isolated Vascular Smooth Muscle. Kidney Int. 1987, 32, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Nutting, C.W.; Islam, S.; Daugirdas, J.T. Vasorelaxant Effects of Short Chain Fatty Acid Salts in Rat Caudal Artery. Am. J. Physiol.-Heart Circ. Physiol. 1991, 261, H561–H567. [Google Scholar] [CrossRef]
- Mortensen, F.V.; Nielsen, H.; Mulvany, M.J.; Hessov, I. Short Chain Fatty Acids Dilate Isolated Human Colonic Resistance Arteries. Gut 1990, 31, 1391–1394. [Google Scholar] [CrossRef]
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.M.; Richards, E.M.; Pepine, C.J.; et al. Imbalance of Gut Microbiome and Intestinal Epithelial Barrier Dysfunction in Patients with High Blood Pressure. Clin Sci. 2018, 132, 701–718. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive Effect of Short-Chain Fatty Acids on Production of Proinflammatory Mediators by Neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Peh, A.; O’Donnell, J.A.; Broughton, B.R.S.; Marques, F.Z. Gut Microbiota and Their Metabolites in Stroke: A Double-Edged Sword. Stroke 2022, 53, 1788–1801. [Google Scholar] [CrossRef]
- Sorriento, D.; Santulli, G.; Fusco, A.; Anastasio, A.; Trimarco, B.; Iaccarino, G. Intracardiac Injection of AdGRK5-NT Reduces Left Ventricular Hypertrophy by Inhibiting NF-ΚB–Dependent Hypertrophic Gene Expression. Hypertension 2010, 56, 696–704. [Google Scholar] [CrossRef]
- Ritchie, M.E. Nuclear Factor-ΚB Is Selectively and Markedly Activated in Humans With Unstable Angina Pectoris. Circulation 1998, 98, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Valen, G. Signal Transduction through Nuclearfactor Kappa B in Ischemia-Reperfusion and Heartfailure. Basic. Res. Cardiol. 2004, 99, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Siednienko, J.; Jankowska, E.A.; Banasiak, W.; Gorczyca, W.A.; Ponikowski, P. Nuclear Factor-KappaB Activity in Peripheral Blood Mononuclear Cells in Cachectic and Non-Cachectic Patients with Chronic Heart Failure. Int. J. Cardiol. 2007, 122, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Frantz, S.; Stoerk, S.; Ok, S.; Wagner, H.; Angermann, C.E.; Ertl, G.; Bauersachs, J. Effect of Chronic Heart Failure on Nuclear Factor Kappa B in Peripheral Leukocytes. Am. J. Cardiol. 2004, 94, 671–673. [Google Scholar] [CrossRef]
- Shaw, J.; Zhang, T.; Rzeszutek, M.; Yurkova, N.; Baetz, D.; Davie, J.R.; Kirshenbaum, L.A. Transcriptional Silencing of the Death Gene BNIP3 by Cooperative Action of NF-ΚB and Histone Deacetylase 1 in Ventricular Myocytes. Circ. Res. 2006, 99, 1347–1354. [Google Scholar] [CrossRef]
- Gordon, J.W.; Shaw, J.A.; Kirshenbaum, L.A. Multiple Facets of NF-ΚB in the Heart. Circ. Res. 2011, 108, 1122–1132. [Google Scholar] [CrossRef]
- Birks, E.J.; Yacoub, M.H. The Role of Nitric Oxide and Cytokines in Heart Failure. Coron. Artery Dis. 1997, 8, 389–402. [Google Scholar] [CrossRef]
- Murashige, D.; Jang, C.; Neinast, M.; Edwards, J.J.; Cowan, A.; Hyman, M.C.; Rabinowitz, J.D.; Frankel, D.S.; Arany, Z. Comprehensive Quantification of Fuel Use by the Failing and Nonfailing Human Heart. Science 2020, 370, 364–368. [Google Scholar] [CrossRef]
- Palm, C.L.; Nijholt, K.T.; Bakker, B.M.; Westenbrink, B.D. Short-Chain Fatty Acids in the Metabolism of Heart Failure—Rethinking the Fat Stigma. Front. Cardiovasc. Med. 2022, 9, 915102. [Google Scholar] [CrossRef]
- Panagia, M.; He, H.; Baka, T.; Pimentel, D.R.; Croteau, D.; Bachschmid, M.M.; Balschi, J.A.; Colucci, W.S.; Luptak, I. Increasing Mitochondrial ATP Synthesis with Butyrate Normalizes ADP and Contractile Function in Metabolic Heart Disease. NMR Biomed. 2020, 33, e4258. [Google Scholar] [CrossRef]
- Tazoe, H.; Otomo, Y.; Kaji, I.; Tanaka, R.; Karaki, S.-I.; Kuwahara, A. Roles of Short-Chain Fatty Acids Receptors, GPR41 and GPR43 on Colonic Functions. J. Physiol. Pharmacol. 2008, 59 (Suppl. S2), 251–262. [Google Scholar] [PubMed]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-Chain Fatty Acids and Ketones Directly Regulate Sympathetic Nervous System via G Protein-Coupled Receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, J.; Wu, W.; Zhu, Y.; Liu, X. The Role of Bile Acids in Cardiovascular Diseases: From Mechanisms to Clinical Implications. Aging Dis. 2023, 14, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile Acids and the Gut Microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef]
- Mamic, P.; Chaikijurajai, T.; Tang, W.H.W. Gut Microbiome—A Potential Mediator of Pathogenesis in Heart Failure and Its Comorbidities: State-of-the-Art Review. J. Mol. Cell Cardiol. 2021, 152, 105–117. [Google Scholar] [CrossRef]
- Callender, C.; Attaye, I.; Nieuwdorp, M. The Interaction between the Gut Microbiome and Bile Acids in Cardiometabolic Diseases. Metabolites 2022, 12, 65. [Google Scholar] [CrossRef]
- Grüner, N.; Mattner, J. Bile Acids and Microbiota: Multifaceted and Versatile Regulators of the Liver–Gut Axis. Int. J. Mol. Sci. 2021, 22, 1397. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Hanafi, N.I.; Sheikh Abdul Kadir, S.H.; Md Noor, J.; Abdul Hamid Hasani, N.; Ab Rahim, S.; Siran, R. Ursodeoxycholic Acid Protects Cardiomyocytes against Cobalt Chloride Induced Hypoxia by Regulating Transcriptional Mediator of Cells Stress Hypoxia Inducible Factor 1α and P53 Protein. Cell Biochem. Funct. 2017, 35, 453–463. [Google Scholar] [CrossRef]
- Liu, X.; Fassett, J.; Wei, Y.; Chen, Y. Regulation of DDAH1 as a Potential Therapeutic Target for Treating Cardiovascular Diseases. Evid. Based Complement. Altern. Med. 2013, 2013, e619207. [Google Scholar] [CrossRef]
- Zuo, L.; Chuang, C.-C.; Hemmelgarn, B.T.; Best, T.M. Heart Failure with Preserved Ejection Fraction: Defining the Function of ROS and NO. J. Appl. Physiol. 2015, 119, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Eblimit, Z.; Thevananther, S.; Karpen, S.J.; Taegtmeyer, H.; Moore, D.D.; Adorini, L.; Penny, D.J.; Desai, M.S. TGR5 Activation Induces Cytoprotective Changes in the Heart and Improves Myocardial Adaptability to Physiologic, Inotropic, and Pressure-Induced Stress in Mice. Cardiovasc. Ther. 2018, 36, e12462. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Xie, S.; Chi, Z.; Zhang, J.; Liu, Y.; Zhang, L.; Zheng, M.; Zhang, X.; Xia, D.; Ke, Y.; et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity 2016, 45, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Purcell, N.H.; Tang, G.; Yu, C.; Mercurio, F.; DiDonato, J.A.; Lin, A. Activation of NF-ΚB Is Required for Hypertrophic Growth of Primary Rat Neonatal Ventricular Cardiomyocytes. Proc. Natl. Acad. Sci. USA 2001, 98, 6668–6673. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The Complexity of NF-ΚB Signaling in Inflammation and Cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-KappaB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Chen, Z.J.; Parent, L.; Maniatis, T. Site-Specific Phosphorylation of IκBα by a Novel Ubiquitination-Dependent Protein Kinase Activity. Cell 1996, 84, 853–862. [Google Scholar] [CrossRef]
- Guan, X.; Sun, Z. The Role of Intestinal Flora and Its Metabolites in Heart Failure. Infect. Drug Resist. 2023, 16, 51–64. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Matthan, N.R. Nutrition and Gastrointestinal Microbiota, Microbial-Derived Secondary Bile Acids, and Cardiovascular Disease. Curr. Atheroscler. Rep. 2020, 22, 47. [Google Scholar] [CrossRef]
- Sun, H.; Olson, K.C.; Gao, C.; Prosdocimo, D.A.; Zhou, M.; Wang, Z.; Jeyaraj, D.; Youn, J.-Y.; Ren, S.; Liu, Y.; et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation 2016, 133, 2038–2049. [Google Scholar] [CrossRef]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lu, G.; Ren, S.; Chen, J.; Wang, Y. Catabolism of Branched-Chain Amino Acids in Heart Failure: Insights from Genetic Models. Pediatr. Cardiol. 2011, 32, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Branched-Chain Amino Acids in Health and Disease: Metabolism, Alterations in Blood Plasma, and as Supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef]
- Sorimachi, K. Evolutionary Changes Reflected by the Cellular Amino Acid Composition. Amino Acids 1999, 17, 207–226. [Google Scholar] [CrossRef]
- Dai, Z.-L.; Zhang, J.; Wu, G.; Zhu, W.-Y. Utilization of Amino Acids by Bacteria from the Pig Small Intestine. Amino Acids 2010, 39, 1201–1215. [Google Scholar] [CrossRef]
- Davila, A.-M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.-H.; Sanz, Y.; Tomé, D. Re-Print of “Intestinal Luminal Nitrogen Metabolism: Role of the Gut Microbiota and Consequences for the Host”. Pharmacol. Res. 2013, 69, 114–126. [Google Scholar] [CrossRef]
- Chen, H.; Nie, Q.; Hu, J.; Huang, X.; Yin, J.; Nie, S. Multiomics Approach to Explore the Amelioration Mechanisms of Glucomannans on the Metabolic Disorder of Type 2 Diabetic Rats. J. Agric. Food Chem. 2021, 69, 2632–2645. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, Y.; Shi, M.; Tian, M.; Ji, J.; Liao, X.; Hu, X.; Chen, F. Dietary Luffa cylindrica (L.) Roem Promotes Branched-Chain Amino Acid Catabolism in the Circulation System via Gut Microbiota in Diet-Induced Obese Mice. Food Chem. 2020, 320, 126648. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Forte, M.; Frati, G.; Sadoshima, J. New Insights Into the Role of MTOR Signaling in the Cardiovascular System. Circ. Res. 2018, 122, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Volpe, M.; Sadoshima, J. Mammalian Target of Rapamycin Signaling in Cardiac Physiology and Disease. Circ. Res. 2014, 114, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR Signaling in Growth and Metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef]
- Kapahi, P.; Chen, D.; Rogers, A.N.; Katewa, S.D.; Li, P.W.-L.; Thomas, E.L.; Kockel, L. With TOR, Less Is More: A Key Role for the Conserved Nutrient-Sensing TOR Pathway in Aging. Cell Metab. 2010, 11, 453–465. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. Regulation of MTORC1 and Its Impact on Gene Expression at a Glance. J. Cell Sci. 2013, 126, 1713–1719. [Google Scholar] [CrossRef]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. MTOR Is a Key Modulator of Ageing and Age-Related Disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- Zhang, D.; Contu, R.; Latronico, M.V.G.; Zhang, J.; Rizzi, R.; Catalucci, D.; Miyamoto, S.; Huang, K.; Ceci, M.; Gu, Y.; et al. MTORC1 Regulates Cardiac Function and Myocyte Survival through 4E-BP1 Inhibition in Mice. J. Clin. Invest. 2010, 120, 2805–2816. [Google Scholar] [CrossRef]
- Wu, X.; Cao, Y.; Nie, J.; Liu, H.; Lu, S.; Hu, X.; Zhu, J.; Zhao, X.; Chen, J.; Chen, X.; et al. Genetic and Pharmacological Inhibition of Rheb1-MTORC1 Signaling Exerts Cardioprotection against Adverse Cardiac Remodeling in Mice. Am. J. Pathol. 2013, 182, 2005–2014. [Google Scholar] [CrossRef]
- Shioi, T.; McMullen, J.R.; Tarnavski, O.; Converso, K.; Sherwood, M.C.; Manning, W.J.; Izumo, S. Rapamycin Attenuates Load-Induced Cardiac Hypertrophy in Mice. Circulation 2003, 107, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Noureldein, M.H.; Eid, A.A. Gut Microbiota and MTOR Signaling: Insight on a New Pathophysiological Interaction. Microb. Pathog. 2018, 118, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.K.; Jansman, A.J.M.; Benis, N.; Ramiro-Garcia, J.; Schokker, D.; Kruijt, L.; Stolte, E.H.; Taverne-Thiele, J.J.; Smits, M.A.; Wells, J.M. Dietary Protein Sources Differentially Affect Microbiota, MTOR Activity and Transcription of MTOR Signaling Pathways in the Small Intestine. PLoS ONE 2017, 12, e0188282. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Zelzer, S.; Prüller, F.; Schnedl, W.J.; Weghuber, D.; Enko, D.; Bergsten, P.; Haybaeck, J.; Meinitzer, A. Branched-Chain Amino Acids Are Associated with Cardiometabolic Risk Profiles Found Already in Lean, Overweight and Obese Young. J. Nutr. Biochem. 2016, 32, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Gilstrap, L.G.; Wang, T.J. Biomarkers and Cardiovascular Risk Assessment for Primary Prevention: An Update. Clin. Chem. 2012, 58, 72–82. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Lopaschuk, G.D. Branched-Chain Amino Acid Metabolism in the Failing Heart. Cardiovasc. Drugs Ther. 2023, 37, 413–420. [Google Scholar] [CrossRef]
- McGarrah, R.W.; White, P.J. Branched-Chain Amino Acids in Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 77–89. [Google Scholar] [CrossRef]
- Du, C.; Liu, W.-J.; Yang, J.; Zhao, S.-S.; Liu, H.-X. The Role of Branched-Chain Amino Acids and Branched-Chain α-Keto Acid Dehydrogenase Kinase in Metabolic Disorders. Front. Nutr. 2022, 9, 932670. [Google Scholar] [CrossRef]
- Romano, K.A.; Nemet, I.; Prasad Saha, P.; Haghikia, A.; Li, X.S.; Mohan, M.L.; Lovano, B.; Castel, L.; Witkowski, M.; Buffa, J.A.; et al. Gut Microbiota-Generated Phenylacetylglutamine and Heart Failure. Circ. Heart Fail. 2023, 16, e009972. [Google Scholar] [CrossRef]
- Fu, H.; Kong, B.; Zhu, J.; Huang, H.; Shuai, W. Phenylacetylglutamine Increases the Susceptibility of Ventricular Arrhythmias in Heart Failure Mice by Exacerbated Activation of the TLR4/AKT/MTOR Signaling Pathway. Int. Immunopharmacol. 2023, 116, 109795. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, B.; Sun, Y.; Deng, H.; Wang, H.; Qiao, Z. Alteration of the Gut Microbiota and Metabolite Phenylacetylglutamine in Patients with Severe Chronic Heart Failure. Front. Cardiovasc. Med. 2023, 9, 1076806. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Brunengraber, H. Glutamate, a Window on Liver Intermediary Metabolism. J. Nutr. 2000, 130, 991S–994S. [Google Scholar] [CrossRef] [PubMed]
- Moldave, K.; Meister, A. Participation: Of Phenylacetyl-Adenylate in the Enzymic Synthesis of Phenylacetylglutamine. Biochim. Biophys. Acta 1957, 25, 434–435. [Google Scholar] [CrossRef]
- Nemet, I.; Saha, P.P.; Gupta, N.; Zhu, W.; Romano, K.A.; Skye, S.M.; Cajka, T.; Mohan, M.L.; Li, L.; Wu, Y.; et al. A Cardiovascular Disease-Linked Gut Microbial Metabolite Acts via Adrenergic Receptors. Cell 2020, 180, 862–877.e22. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Fan, Q.; Yang, Q.; Pan, R.; Zhuang, L.; Tao, R. Phenylacetylglutamine as a Risk Factor and Prognostic Indicator of Heart Failure. ESC Heart Fail. 2022, 9, 2645–2653. [Google Scholar] [CrossRef]
- Fang, C.; Zuo, K.; Fu, Y.; Li, J.; Wang, H.; Xu, L.; Yang, X. Dysbiosis of Gut Microbiota and Metabolite Phenylacetylglutamine in Coronary Artery Disease Patients With Stent Stenosis. Front. Cardiovasc. Med. 2022, 9, 832092. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, Y.; Fang, C.; Liu, X.; Dong, Y.; Xu, L.; Chen, M.; Zuo, K.; Wang, L. Prognostic Value of Plasma Phenylalanine and Gut Microbiota-Derived Metabolite Phenylacetylglutamine in Coronary in-Stent Restenosis. Front. Cardiovasc. Med. 2022, 9, 944155. [Google Scholar] [CrossRef]
- Poesen, R.; Claes, K.; Evenepoel, P.; de Loor, H.; Augustijns, P.; Kuypers, D.; Meijers, B. Microbiota-Derived Phenylacetylglutamine Associates with Overall Mortality and Cardiovascular Disease in Patients with CKD. J. Am. Soc. Nephrol. 2016, 27, 3479–3487. [Google Scholar] [CrossRef]
- Yu, F.; Li, X.; Feng, X.; Wei, M.; Luo, Y.; Zhao, T.; Xiao, B.; Xia, J. Phenylacetylglutamine, a Novel Biomarker in Acute Ischemic Stroke. Front. Cardiovasc. Med. 2021, 8, 798765. [Google Scholar] [CrossRef]
- Yu, F.; Feng, X.; Li, X.; Luo, Y.; Wei, M.; Zhao, T.; Xia, J. Gut-Derived Metabolite Phenylacetylglutamine and White Matter Hyperintensities in Patients With Acute Ischemic Stroke. Front. Aging Neurosci. 2021, 13, 675158. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Zhao, Z.; Song, X.; Qu, H.; Liu, H. Phenylacetylglutamine Is Associated with the Degree of Coronary Atherosclerotic Severity Assessed by Coronary Computed Tomographic Angiography in Patients with Suspected Coronary Artery Disease. Atherosclerosis 2021, 333, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, F.; Brunkwall, L.; Smith, E.; Orho-Melander, M.; Nilsson, P.M.; Fernandez, C.; Melander, O. The Gut Microbiota-Related Metabolite Phenylacetylglutamine Associates with Increased Risk of Incident Coronary Artery Disease. J. Hypertens. 2020, 38, 2427–2434. [Google Scholar] [CrossRef]
- Reichard, C.A.; Naelitz, B.D.; Wang, Z.; Jia, X.; Li, J.; Stampfer, M.J.; Klein, E.A.; Hazen, S.L.; Sharifi, N. Gut Microbiome-Dependent Metabolic Pathways and Risk of Lethal Prostate Cancer: Prospective Analysis of a PLCO Cancer Screening Trial Cohort. Cancer Epidemiol. Biomark. Prev. 2022, 31, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Sasso, J.M.; Ammar, R.M.; Tenchov, R.; Lemmel, S.; Kelber, O.; Grieswelle, M.; Zhou, Q.A. Gut Microbiome–Brain Alliance: A Landscape View into Mental and Gastrointestinal Health and Disorders. ACS Chem. Neurosci. 2023, 14, 1717–1763. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.; Farzi, A.; Zhu, W. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef]
- Trøseid, M.; Andersen, G.Ø.; Broch, K.; Hov, J.R. The Gut Microbiome in Coronary Artery Disease and Heart Failure: Current Knowledge and Future Directions. EBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef]
- Gao, H.; Liu, S. Role of Uremic Toxin Indoxyl Sulfate in the Progression of Cardiovascular Disease. Life Sci. 2017, 185, 23–29. [Google Scholar] [CrossRef]
- Li, C.; Chang, J.; Wang, Y.; Pan, G. Indole-3-Propionic Acid, a Product of Intestinal Flora, Inhibits the HDAC6/NOX2 Signaling and Relieves Doxorubicin-Induced Cardiomyocyte Damage. Folia Morphol. 2023. [Google Scholar] [CrossRef]
- Gesper, M.; Nonnast, A.B.H.; Kumowski, N.; Stoehr, R.; Schuett, K.; Marx, N.; Kappel, B.A. Gut-Derived Metabolite Indole-3-Propionic Acid Modulates Mitochondrial Function in Cardiomyocytes and Alters Cardiac Function. Front. Med. 2021, 8, 648259. [Google Scholar] [CrossRef]
- Mutengo, K.H.; Masenga, S.K.; Mweemba, A.; Mutale, W.; Kirabo, A. Gut Microbiota Dependant Trimethylamine N-Oxide and Hypertension. Front. Physiol. 2023, 14, 1075641. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Qian, Z.; Yin, J.; Xu, W.; Zhou, X. The Role of Intestinal Microbiota in Cardiovascular Disease. J. Cell Mol. Med. 2019, 23, 2343–2350. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Xin, F.-Z.; Zhou, D.; Xue, Y.-Q.; Liu, X.-L.; Yang, R.-X.; Pan, Q.; Fan, J.-G. Trimethylamine N-Oxide Attenuates High-Fat High-Cholesterol Diet-Induced Steatohepatitis by Reducing Hepatic Cholesterol Overload in Rats. World J. Gastroenterol. 2019, 25, 2450–2462. [Google Scholar] [CrossRef] [PubMed]
- Gatarek, P.; Kaluzna-Czaplinska, J. Trimethylamine N-Oxide (TMAO) in Human Health. EXCLI J. 2021, 20, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [PubMed]
- Boini, K.M.; Hussain, T.; Li, P.-L.; Koka, S. Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction. Cell Physiol. Biochem. 2017, 44, 152–162. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Q.; Jiang, H. Gut Microbiota in Atherosclerosis: Focus on Trimethylamine N-oxide. APMIS 2020, 128, 353–366. [Google Scholar] [CrossRef]
- Montezano, A.C.; Touyz, R.M. Molecular Mechanisms of Hypertension—Reactive Oxygen Species and Antioxidants: A Basic Science Update for the Clinician. Can. J. Cardiol. 2012, 28, 288–295. [Google Scholar] [CrossRef]
- Sinha, N.; Dabla, P.K. Oxidative Stress and Antioxidants in Hypertension-a Current Review. Curr. Hypertens. Rev. 2015, 11, 132–142. [Google Scholar] [CrossRef]
- He, W.; Luo, Y.; Liu, J.-P.; Sun, N.; Guo, D.; Cui, L.-L.; Zheng, P.-P.; Yao, S.-M.; Yang, J.-F.; Wang, H. Trimethylamine N-Oxide, a Gut Microbiota-Dependent Metabolite, Is Associated with Frailty in Older Adults with Cardiovascular Disease. Clin. Interv. Aging 2020, 15, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Vajdi, M.; Asghari-Jafarabadi, M. Gut Microbiota-Associated Metabolite Trimethylamine N-Oxide and the Risk of Stroke: A Systematic Review and Dose–Response Meta-Analysis. Nutr. J. 2020, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Tu, R.; Xia, J. Stroke and Vascular Cognitive Impairment: The Role of Intestinal Microbiota Metabolite TMAO. CNS Neurol. Disord. Drug Targets 2023, 22, 102–121. [Google Scholar]
- Sindhu, R.K.; Goyal, A.; Algın Yapar, E.; Cavalu, S. Bioactive Compounds and Nanodelivery Perspectives for Treatment of Cardiovascular Diseases. Appl. Sci. 2021, 11, 11031. [Google Scholar] [CrossRef]
- Domínguez-López, I.; Arancibia-Riveros, C.; Marhuenda-Muñoz, M.; Tresserra-Rimbau, A.; Toledo, E.; Fitó, M.; Ros, E.; Estruch, R.; Lamuela-Raventós, R.M. Association of Microbiota Polyphenols with Cardiovascular Health in the Context of a Mediterranean Diet. Food Res. Int. 2023, 165, 112499. [Google Scholar] [CrossRef]
- Wickman, B.E.; Enkhmaa, B.; Ridberg, R.; Romero, E.; Cadeiras, M.; Meyers, F.; Steinberg, F. Dietary Management of Heart Failure: DASH Diet and Precision Nutrition Perspectives. Nutrients 2021, 13, 4424. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Di Raimondo, D.; Casuccio, A.; Velardo, M.; Salamone, G.; Cataldi, M.; Corpora, F.; Restivo, V.; Pecoraro, R.; Della Corte, V.; et al. Mediterranean Diet Adherence and Congestive Heart Failure: Relationship with Clinical Severity and Ischemic Pathogenesis. Nutrition 2020, 70, 110584. [Google Scholar] [CrossRef]
- Compare, D.; Coccoli, P.; Rocco, A.; Nardone, O.M.; De Maria, S.; Cartenì, M.; Nardone, G. Gut—Liver Axis: The Impact of Gut Microbiota on Non Alcoholic Fatty Liver Disease. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 471–476. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean Diet and Health Status: Active Ingredients and Pharmacological Mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef]
- Rinott, E.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J.; et al. The Effects of the Green-Mediterranean Diet on Cardiometabolic Health Are Linked to Gut Microbiome Modifications: A Randomized Controlled Trial. Genome Med. 2022, 14, 29. [Google Scholar] [CrossRef]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Franco-de-Moraes, A.C.; de Almeida-Pititto, B.; da Rocha Fernandes, G.; Gomes, E.P.; da Costa Pereira, A.; Ferreira, S.R.G. Worse Inflammatory Profile in Omnivores than in Vegetarians Associates with the Gut Microbiota Composition. Diabetol. Metab. Syndr. 2017, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quintero, M.J.; Delgado, J.; Medina-Vera, D.; Becerra-Muñoz, V.M.; Queipo-Ortuño, M.I.; Estévez, M.; Plaza-Andrades, I.; Rodríguez-Capitán, J.; Sánchez, P.L.; Crespo-Leiro, M.G.; et al. Beneficial Effects of Essential Oils from the Mediterranean Diet on Gut Microbiota and Their Metabolites in Ischemic Heart Disease and Type-2 Diabetes Mellitus. Nutrients 2022, 14, 4650. [Google Scholar] [CrossRef] [PubMed]
- López-Miranda, J.; Pérez-Jiménez, F.; Ros, E.; De Caterina, R.; Badimón, L.; Covas, M.I.; Escrich, E.; Ordovás, J.M.; Soriguer, F.; Abiá, R.; et al. Olive Oil and Health: Summary of the II International Conference on Olive Oil and Health Consensus Report, Jaén and Córdoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Voloshyna, I.; Hussaini, S.M.; Reiss, A.B. Resveratrol in Cholesterol Metabolism and Atherosclerosis. J. Med. Food 2012, 15, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.-L.; Jun, M.; Jeong, W.-S. Role of Resveratrol in Regulation of Cellular Defense Systems against Oxidative Stress. Biofactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Bayram, B.; Ozcelik, B.; Grimm, S.; Roeder, T.; Schrader, C.; Ernst, I.M.A.; Wagner, A.E.; Grune, T.; Frank, J.; Rimbach, G. A Diet Rich in Olive Oil Phenolics Reduces Oxidative Stress in the Heart of SAMP8 Mice by Induction of Nrf2-Dependent Gene Expression. Rejuvenation Res. 2012, 15, 71–81. [Google Scholar] [CrossRef]
- Adamberg, K.; Kolk, K.; Jaagura, M.; Vilu, R.; Adamberg, S. The Composition and Metabolism of Faecal Microbiota Is Specifically Modulated by Different Dietary Polysaccharides and Mucin: An Isothermal Microcalorimetry Study. Benef. Microbes 2018, 9, 21–34. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.-Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M.; et al. Responses of Gut Microbiota and Glucose and Lipid Metabolism to Prebiotics in Genetic Obese and Diet-Induced Leptin-Resistant Mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef]
- Ashique, S.; Mishra, N.; Garg, A.; Sibuh, B.Z.; Taneja, P.; Rai, G.; Djearamane, S.; Wong, L.S.; Al-Dayan, N.; Roychoudhury, S.; et al. Recent Updates on Correlation between Reactive Oxygen Species and Synbiotics for Effective Management of Ulcerative Colitis. Front. Nutr. 2023, 10, 1126579. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, A.; Zakrzewska, Z.; Schab, M.; Zawartka, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prevention and Health Benefits of Prebiotics, Probiotics and Postbiotics in Acute Lymphoblastic Leukemia. Microorganisms 2023, 11, 1775. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Pechenyak, B.; Vyas, U.; Ranganathan, P.; Weinberg, A.; Liang, P.; Mallappallil, M.C.; Norin, A.J.; Friedman, E.A.; Saggi, S.J. Randomized Controlled Trial of Strain-Specific Probiotic Formulation (Renadyl) in Dialysis Patients. BioMed Res. Int. 2014, 2014, e568571. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.-C.; Strassburger, K.; Nowotny, B.; Kolb, H.; Nowotny, P.; Burkart, V.; Zivehe, F.; Hwang, J.-H.; Stehle, P.; Pacini, G.; et al. Intake of Lactobacillus Reuteri Improves Incretin and Insulin Secretion in Glucose-Tolerant Humans: A Proof of Concept. Diabetes Care 2015, 38, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The Pros, Cons, and Many Unknowns of Probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Pourrajab, B.; Naderi, N.; Janani, L.; Mofid, V.; Hajahmadi, M.; Dehnad, A.; Shidfar, F. Comparison of Probiotic Yogurt and Ordinary Yogurt Consumption on Serum Pentraxin3, NT-ProBNP, OxLDL, and ApoB100 in Patients with Chronic Heart Failure: A Randomized, Triple-Blind, Controlled Trial. Food Funct. 2020, 11, 10000–10010. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Bråthen Kristoffersen, A.; Hausken, T. Efficacy of Faecal Microbiota Transplantation for Patients with Irritable Bowel Syndrome in a Randomised, Double-Blind, Placebo-Controlled Study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef]
- Aira, A.; Rubio, E.; Vergara Gómez, A.; Fehér, C.; Casals-Pascual, C.; González, B.; Morata, L.; Rico, V.; Soriano, A. RUTI Resolution After FMT for Clostridioides Difficile Infection: A Case Report. Infect. Dis. Ther. 2021, 10, 1065–1071. [Google Scholar] [CrossRef]
- Fujimoto, K.; Kimura, Y.; Allegretti, J.R.; Yamamoto, M.; Zhang, Y.-Z.; Katayama, K.; Tremmel, G.; Kawaguchi, Y.; Shimohigoshi, M.; Hayashi, T.; et al. Functional Restoration of Bacteriomes and Viromes by Fecal Microbiota Transplantation. Gastroenterology 2021, 160, 2089–2102.e12. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Li, B.; Luo, Y.; Gong, Y.; Jin, X.; Zhang, J.; Zhou, Y.; Zhuo, X.; Wang, Z.; et al. Gut Microbiota Dysbiosis Promotes Age-Related Atrial Fibrillation by Lipopolysaccharide and Glucose-Induced Activation of NLRP3-Inflammasome. Cardiovasc. Res. 2022, 118, 785–797. [Google Scholar] [CrossRef]
- Tiwari, P.; Dwivedi, R.; Bansal, M.; Tripathi, M.; Dada, R. Role of Gut Microbiota in Neurological Disorders and Its Therapeutic Significance. J. Clin. Med. 2023, 12, 1650. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C. The Microbiota-Immune Axis as a Central Mediator of Gut-Brain Communication. Neurobiol. Dis. 2020, 136, 104714. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, D.; Cichy, W.; Przysławski, J.; Drzymała-Czyż, S. The Role of Microbiota and Enteroendocrine Cells in Maintaining Homeostasis in the Human Digestive Tract. Adv. Med. Sci. 2021, 66, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, T.; Itzhaki, R.F.; Balin, B.J.; Miklossy, J.; Barron, A.E. Role of Microbes in the Development of Alzheimer’s Disease: State of the Art—An International Symposium Presented at the 2017 IAGG Congress in San Francisco. Front. Genet. 2018, 9, 362. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Kang, Y.; Kang, X.; Zhang, H.; Liu, Q.; Yang, H.; Fan, W. Gut Microbiota and Parkinson’s Disease: Implications for Faecal Microbiota Transplantation Therapy. ASN Neuro 2021, 13, 17590914211016216. [Google Scholar] [CrossRef]
- Maurice, C.F.; Haiser, H.J.; Turnbaugh, P.J. Xenobiotics Shape the Physiology and Gene Expression of the Active Human Gut Microbiome. Cell 2013, 152, 39–50. [Google Scholar] [CrossRef]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The Gut-Skin Axis in Health and Disease: A Paradigm with Therapeutic Implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef]
- Levkovich, T.; Poutahidis, T.; Smillie, C.; Varian, B.J.; Ibrahim, Y.M.; Lakritz, J.R.; Alm, E.J.; Erdman, S.E. Probiotic Bacteria Induce a “Glow of Health”. PLoS ONE 2013, 8, e53867. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Sun, Q.; Wei, J.; Li, B.; Qiu, Y.; Liu, K.; Shao, D.; Ma, Z. Targeting the Pulmonary Microbiota to Fight against Respiratory Diseases. Cells 2022, 11, 916. [Google Scholar] [CrossRef]
- Yin, Y.; Sichler, A.; Ecker, J.; Laschinger, M.; Liebisch, G.; Höring, M.; Basic, M.; Bleich, A.; Zhang, X.-J.; Kübelsbeck, L.; et al. Gut Microbiota Promote Liver Regeneration through Hepatic Membrane Phospholipid Biosynthesis. J. Hepatol. 2023, 78, 820–835. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masenga, S.K.; Povia, J.P.; Lwiindi, P.C.; Kirabo, A. Recent Advances in Microbiota-Associated Metabolites in Heart Failure. Biomedicines 2023, 11, 2313. https://doi.org/10.3390/biomedicines11082313
Masenga SK, Povia JP, Lwiindi PC, Kirabo A. Recent Advances in Microbiota-Associated Metabolites in Heart Failure. Biomedicines. 2023; 11(8):2313. https://doi.org/10.3390/biomedicines11082313
Chicago/Turabian StyleMasenga, Sepiso K., Joreen P. Povia, Propheria C. Lwiindi, and Annet Kirabo. 2023. "Recent Advances in Microbiota-Associated Metabolites in Heart Failure" Biomedicines 11, no. 8: 2313. https://doi.org/10.3390/biomedicines11082313
APA StyleMasenga, S. K., Povia, J. P., Lwiindi, P. C., & Kirabo, A. (2023). Recent Advances in Microbiota-Associated Metabolites in Heart Failure. Biomedicines, 11(8), 2313. https://doi.org/10.3390/biomedicines11082313