The Interaction of Gut Microbiota and Heart Failure with Preserved Ejection Fraction: From Mechanism to Potential Therapies
Abstract
1. Introduction
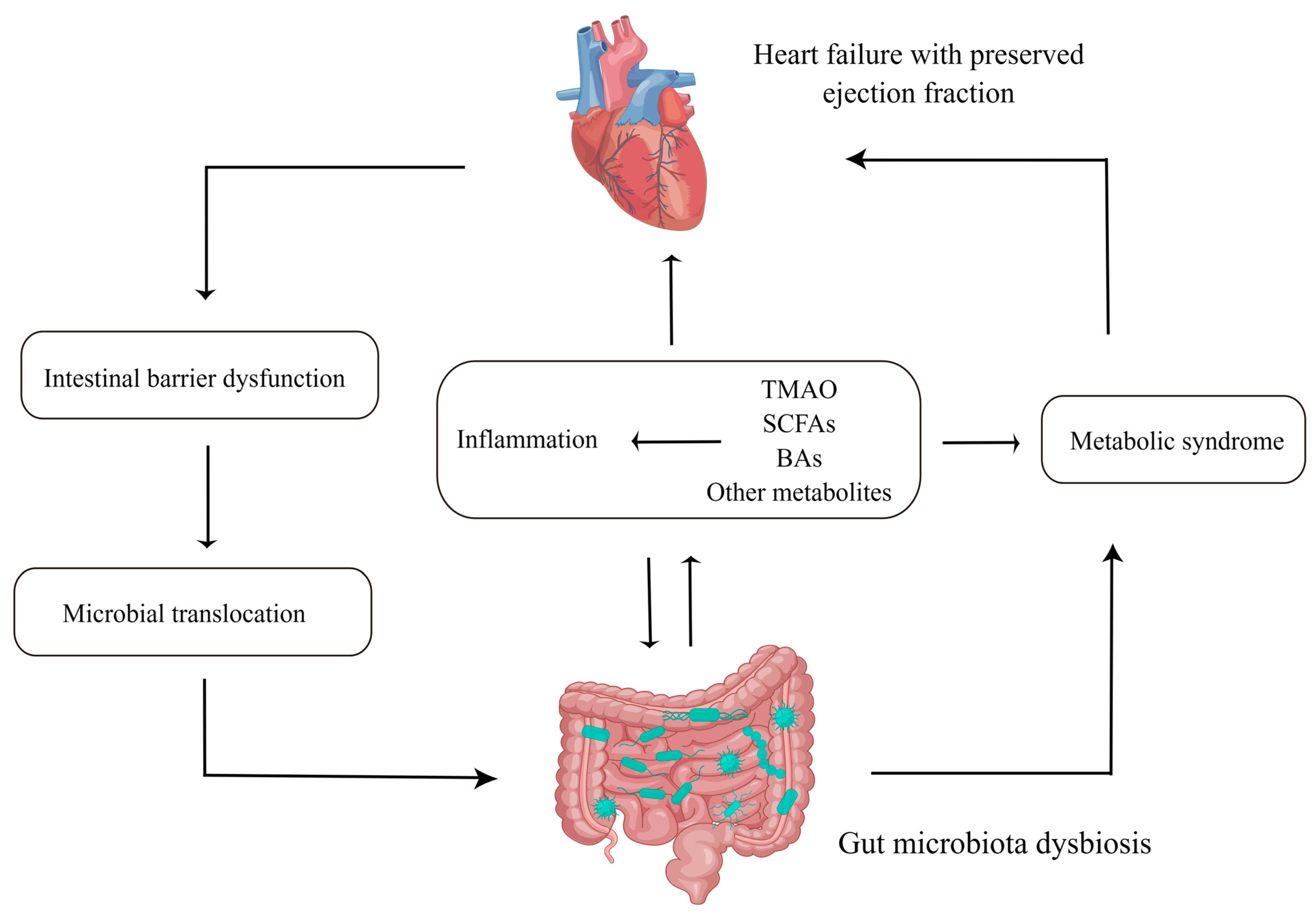
2. The Features of GMB in HFpEF
3. The Mechanisms by Which GMB Interacts with HFpEF
3.1. Intestinal Barrier Dysfunction and Microbial Translocation
3.2. Inflammation
3.3. Metabolites
3.3.1. Trimethylamine N-oxide (TMAO)
3.3.2. SCFAs
3.3.3. Bile Acids (BAs)
3.3.4. Other Metabolites
4. Metabolic Syndrome (MetS), HFpEF and GMB
5. Potential Therapies
5.1. Dietary Intervention
5.2. Antibiotics
5.3. Probiotics and Prebiotics
5.4. Fecal Microbiota Transplantation (FMT)
5.5. Exercise Training
5.6. Targeting Metabolites and Inflammation
5.7. Traditional Chinese Medicine (TCM)
6. Prospect
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miranda-Silva, D.; Lima, T.; Rodrigues, P.; Leite-Moreira, A.; Falcao-Pires, I. Mechanisms underlying the pathophysiology of heart failure with preserved ejection fraction: The tip of the iceberg. Heart Fail. Rev. 2021, 26, 453–478. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef]
- Huang, Z.; Mei, X.; Jiang, Y.; Chen, T.; Zhou, Y. Gut Microbiota in Heart Failure Patients With Preserved Ejection Fraction (GUMPTION Study). Front. Cardiovasc. Med. 2021, 8, 803744. [Google Scholar] [CrossRef] [PubMed]
- Beale, A.L.; O’Donnell, J.A.; Nakai, M.E.; Nanayakkara, S.; Vizi, D.; Carter, K.; Dean, E.; Ribeiro, R.V.; Yiallourou, S.; Carrington, M.J.; et al. The Gut Microbiome of Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2021, 10, e020654. [Google Scholar] [CrossRef]
- Avalos-Fernandez, M.; Alin, T.; Metayer, C.; Thiebaut, R.; Enaud, R.; Delhaes, L. The respiratory microbiota alpha-diversity in chronic lung diseases: First systematic review and meta-analysis. Respir. Res. 2022, 23, 214. [Google Scholar] [CrossRef]
- Kaburova, A.; Drapkina, O.; Yudin, S.; Koretsky, S.; Makarov, V.; Pokrovskaya, M.; Kraevoy, S.; Shoybonov, B.; Efimova, I. Relationship between gut microbiota and markers of myocardial fibrosis in with chronic heart failure with preserved ejection fraction. Cardiovasc. Ther. Prev. 2021, 20, 2834. [Google Scholar] [CrossRef]
- Kaburova, A.N.; Drapkina, O.; Uydin, S.M.; Vishnyakova, M.V.; Pokrovskaya, M.; Koretsky, S.; Abramenko, A.; Chermensky, G.V.; Efimova, I.A.; Makarov, V. The link between left ventricular extracellular volume determined by T1 myocardial mapping and gut microbiota composition in individuals with heart failure and preserved ejection fraction. Eur. Heart J. 2020, 41, ehaa946-0864. [Google Scholar] [CrossRef]
- Hummel, S.; Bassis, C.; Marolt, C.; Konerman, M.; Schmidt, T. Gut microbiome differs between heart failure with preserved ejection fraction and age-matched controls. J. Am. Coll. Cardiol. 2019, 73, 750. [Google Scholar] [CrossRef]
- Omote, K.; Verbrugge, F.H.; Borlaug, B.A. Heart Failure with Preserved Ejection Fraction: Mechanisms and Treatment Strategies. Annu. Rev. Med. 2022, 73, 321–337. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Voors, A.A.; de Boer, R.A.; Solomon, S.D.; van Veldhuisen, D.J. Heart failure with preserved ejection fraction: From mechanisms to therapies. Eur. Heart J. 2018, 39, 2780–2792. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Aquilani, R.; Testa, C.; Baiardi, P.; Angioletti, S.; Boschi, F.; Verri, M.; Dioguardi, F. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC Heart Fail. 2016, 4, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Sandek, A.; Bauditz, J.; Swidsinski, A.; Buhner, S.; Weber-Eibel, J.; von Haehling, S.; Schroedl, W.; Karhausen, T.; Doehner, W.; Rauchhaus, M.; et al. Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H. Current progress of research on intestinal bacterial translocation. Microb. Pathog. 2021, 152, 104652. [Google Scholar] [CrossRef]
- Zhou, X.; Li, J.; Guo, J.; Geng, B.; Ji, W.; Zhao, Q.; Li, J.; Liu, X.; Liu, J.; Guo, Z.; et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome 2018, 6, 66. [Google Scholar] [CrossRef]
- Niebauer, J.; Volk, H.D.; Kemp, M.; Dominguez, M.; Schumann, R.R.; Rauchhaus, M.; Poole-Wilson, P.A.; Coats, A.J.S.; Anker, S.D. Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 1999, 353, 1838–1842. [Google Scholar] [CrossRef]
- Fukui, H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar] [CrossRef]
- Peschel, T.; Schönauer, M.; Thiele, H.; Anker, S.; Schuler, G.; Niebauer, J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur. J. Heart Fail. 2003, 5, 609–614. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef]
- Cianci, R.; Franza, L.; Borriello, R.; Pagliari, D.; Gasbarrini, A.; Gambassi, G. The Role of Gut Microbiota in Heart Failure: When Friends Become Enemies. Biomedicines 2022, 10, 2712. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, F.A.; Ambrosini, S.; Mohammed, S.A.; Kraler, S.; Luscher, T.F.; Costantino, S.; Paneni, F. Inflammation in Metabolic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 742178. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.; Lindner, D.; Kasner, M.; Zietsch, C.; Savvatis, K.; Escher, F.; von Schlippenbach, J.; Skurk, C.; Steendijk, P.; Riad, A.; et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ. Heart Fail. 2011, 4, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Rodolico, D.; Hill, J.A. Metabolic inflammation in heart failure with preserved ejection fraction. Cardiovasc. Res. 2021, 117, 423–434. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Orlenko, A.; Zhao, L.; Basso, M.D.; Cvijic, M.E.; Li, Z.; Spires, T.E.; Yarde, M.; Wang, Z.; Seiffert, D.A.; et al. Multiple Plasma Biomarkers for Risk Stratification in Patients With Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2020, 75, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Nair, N. Epidemiology and pathogenesis of heart failure with preserved ejection fraction. Rev. Cardiovasc. Med. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Zach, V.; Bahr, F.L.; Edelmann, F. Suppression of Tumourigenicity 2 in Heart Failure with Preserved Ejection Fraction. Card. Fail. Rev. 2020, 6, e02. [Google Scholar] [CrossRef]
- DuBrock, H.M.; AbouEzzeddine, O.F.; Redfield, M.M. High-sensitivity C-reactive protein in heart failure with preserved ejection fraction. PLoS ONE 2018, 13, e0201836. [Google Scholar] [CrossRef]
- Paulus, W.J.; Zile, M.R. From Systemic Inflammation to Myocardial Fibrosis: The Heart Failure With Preserved Ejection Fraction Paradigm Revisited. Circ. Res. 2021, 128, 1451–1467. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef]
- Li, C.; Qin, D.; Hu, J.; Yang, Y.; Hu, D.; Yu, B. Inflamed adipose tissue: A culprit underlying obesity and heart failure with preserved ejection fraction. Front. Immunol. 2022, 13, 947147. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xie, M.; Li, Q.; Xu, X.; Ou, W.; Zhang, Y.; Xiao, H.; Yu, H.; Zheng, Y.; Liang, Y.; et al. Targeting Mitochondria-Inflammation Circuit by beta-Hydroxybutyrate Mitigates HFpEF. Circ. Res. 2021, 128, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, L.; Pitzer, A.L.; Li, X.; Li, P.L.; Zhang, Y. Contribution of redox-dependent activation of endothelial Nlrp3 inflammasomes to hyperglycemia-induced endothelial dysfunction. J. Mol. Med. 2016, 94, 1335–1347. [Google Scholar] [CrossRef]
- Yang, H.J.; Kong, B.; Shuai, W.; Zhang, J.J.; Huang, H. Knockout of MD1 contributes to sympathetic hyperactivity and exacerbates ventricular arrhythmias following heart failure with preserved ejection fraction via NLRP3 inflammasome activation. Exp. Physiol. 2020, 105, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, J.; Xu, Y.; Liu, J.; Ye, J.; Wang, Z.; Ye, D.; Feng, Y.; Xu, S.; Pan, W.; et al. Selective Inhibition of NLRP3 Inflammasome Reverses Pressure Overload-Induced Pathological Cardiac Remodeling by Attenuating Hypertrophy, Fibrosis, and Inflammation. Int. Immunopharmacol. 2021, 99, 108046. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e4114. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Conraads, V.M.; Bosmans, J.M.; Schuerwegh, A.J.; Goovaerts, I.; De Clerck, L.S.; Stevens, W.J.; Bridts, C.H.; Vrints, C.J. Intracellular monocyte cytokine production and CD 14 expression are up-regulated in severe vs mild chronic heart failure. J. Heart Lung Transplant. 2005, 24, 854–859. [Google Scholar] [CrossRef]
- Sandek, A.; Bjarnason, I.; Volk, H.D.; Crane, R.; Meddings, J.B.; Niebauer, J.; Kalra, P.R.; Buhner, S.; Herrmann, R.; Springer, J.; et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int. J. Cardiol. 2012, 157, 80–85. [Google Scholar] [CrossRef]
- Branchereau, M.; Burcelin, R.; Heymes, C. The gut microbiome and heart failure: A better gut for a better heart. Rev. Endocr. Metab. Disord. 2019, 20, 407–414. [Google Scholar] [CrossRef]
- Yu, L.; Feng, Z. The Role of Toll-Like Receptor Signaling in the Progression of Heart Failure. Mediat. Inflamm. 2018, 2018, 9874109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Yu, Z.L.; Chen, J.Y.; Li, X.Y.; Wang, Z.P.; Wu, M.; Liu, L.T. The crosstalk between NLRP3 inflammasome and gut microbiome in atherosclerosis. Pharmacol. Res. 2022, 181, 106289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Li, B.; Luo, Y.; Gong, Y.; Jin, X.; Zhang, J.; Zhou, Y.; Zhuo, X.; Wang, Z.; et al. Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc. Res. 2022, 118, 785–797. [Google Scholar] [CrossRef]
- Cho, C.E.; Caudill, M.A. Trimethylamine-N-Oxide: Friend, Foe, or Simply Caught in the Cross-Fire? Trends Endocrinol. Metab. 2017, 28, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Fernandez, M.L. Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease. Curr. Atheroscler. Rep. 2021, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Simo, C.; Garcia-Canas, V. Dietary bioactive ingredients to modulate the gut microbiota-derived metabolite TMAO. New opportunities for functional food development. Food Funct. 2020, 11, 6745–6776. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Guo, F.; Qiu, X.; Tan, Z.; Li, Z.; Ouyang, D. Plasma trimethylamine n-oxide is associated with renal function in patients with heart failure with preserved ejection fraction. BMC Cardiovasc. Disord. 2020, 20, 394. [Google Scholar] [CrossRef]
- Dong, Z.; Zheng, S.; Shen, Z.; Luo, Y.; Hai, X. Trimethylamine N-Oxide is Associated with Heart Failure Risk in Patients with Preserved Ejection Fraction. Lab. Med. 2021, 52, 346–351. [Google Scholar] [CrossRef]
- Kinugasa, Y.; Nakamura, K.; Kamitani, H.; Hirai, M.; Yanagihara, K.; Kato, M.; Yamamoto, K. Trimethylamine N-oxide and outcomes in patients hospitalized with acute heart failure and preserved ejection fraction. ESC Heart Fail. 2021, 8, 2103–2110. [Google Scholar] [CrossRef]
- Salzano, A.; Israr, M.Z.; Yazaki, Y.; Heaney, L.M.; Kanagala, P.; Singh, A.; Arnold, J.R.; Gulsin, G.S.; Squire, I.B.; McCann, G.P.; et al. Combined use of trimethylamine N-oxide with BNP for risk stratification in heart failure with preserved ejection fraction: Findings from the DIAMONDHFpEF study. Eur. J. Prev. Cardiol. 2020, 27, 2159–2162. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Shrestha, K.; Borowski, A.G.; Wu, Y.; Troughton, R.W.; Klein, A.L.; Hazen, S.L. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J. Card. Fail. 2015, 21, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zheng, X.; Feng, M.; Li, D.; Zhang, H. Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide Contributes to Cardiac Dysfunction in Western Diet-Induced Obese Mice. Front. Physiol. 2017, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Sun, Z.; Xie, S.; Hu, Z.; Lan, Q.; Sun, Y.; Yuan, L.; Zhai, C. Intestinal Flora Derived Metabolites Affect the Occurrence and Development of Cardiovascular Disease. J. Multidiscip. Healthc. 2022, 15, 2591–2603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Ke, B.; Du, J. TMAO: How gut microbiota contributes to heart failure. Transl. Res. 2021, 228, 109–125. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, F.; Tang, R.; Bao, C.; Zhou, Q.; Ye, K.; Shen, Y.; Liu, C.; Hong, C.; Yang, K.; et al. Gut Microbial Metabolite Trimethylamine N-Oxide Aggravates Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2022, 66, 452–460. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Yang, F.; Zhao, R.; Pan, X.; Liang, J.; Tian, L.; Li, X.; Liu, L.; Xing, Y.; et al. Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front. Pharmacol. 2019, 10, 1360. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Hu, T.; Wu, Q.; Yao, Q.; Jiang, K.; Yu, J.; Tang, Q. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res. Rev. 2022, 81, 101706. [Google Scholar] [CrossRef]
- Panagia, M.; He, H.; Baka, T.; Pimentel, D.R.; Croteau, D.; Bachschmid, M.M.; Balschi, J.A.; Colucci, W.S.; Luptak, I. Increasing mitochondrial ATP synthesis with butyrate normalizes ADP and contractile function in metabolic heart disease. NMR Biomed. 2020, 33, e4258. [Google Scholar] [CrossRef]
- Carley, A.N.; Maurya, S.K.; Fasano, M.; Wang, Y.; Selzman, C.H.; Drakos, S.G.; Lewandowski, E.D. Short-Chain Fatty Acids Outpace Ketone Oxidation in the Failing Heart. Circulation 2021, 143, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Hori, D.; Flavahan, S.; Steppan, J.; Flavahan, N.A.; Berkowitz, D.E.; Pluznick, J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016, 48, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Karoor, V.; Strassheim, D.; Sullivan, T.; Verin, A.; Umapathy, N.S.; Dempsey, E.C.; Frank, D.N.; Stenmark, K.R.; Gerasimovskaya, E. The Short-Chain Fatty Acid Butyrate Attenuates Pulmonary Vascular Remodeling and Inflammation in Hypoxia-Induced Pulmonary Hypertension. Int. J. Mol. Sci. 2021, 22, 9916. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.M. Sodium Butyrate Controls Cardiac Hypertrophy in Experimental Models of Rats. Cardiovasc. Toxicol. 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Y.; Qi, B.; Cui, X.; Dong, X.; Wang, Y.; Han, X.; Li, F.; Shen, D.; Zhang, X.; et al. Association of Small Intestinal Bacterial Overgrowth With Heart Failure and Its Prediction for Short-Term Outcomes. J. Am. Heart Assoc. 2021, 10, e015292. [Google Scholar] [CrossRef]
- Mollar, A.; Marrachelli, V.G.; Nunez, E.; Monleon, D.; Bodi, V.; Sanchis, J.; Navarro, D.; Nunez, J. Bacterial metabolites trimethylamine N-oxide and butyrate as surrogates of small intestinal bacterial overgrowth in patients with a recent decompensated heart failure. Sci. Rep. 2021, 11, 6110. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Gruner, N.; Mattner, J. Bile Acids and Microbiota: Multifaceted and Versatile Regulators of the Liver-Gut Axis. Int. J. Mol. Sci. 2021, 22, 1397. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Vasavan, T.; Ferraro, E.; Ibrahim, E.; Dixon, P.; Gorelik, J.; Williamson, C. Heart and bile acids—Clinical consequences of altered bile acid metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, N.I.; Mohamed, A.S.; Sheikh Abdul Kadir, S.H.; Othman, M.H.D. Overview of Bile Acids Signaling and Perspective on the Signal of Ursodeoxycholic Acid, the Most Hydrophilic Bile Acid, in the Heart. Biomolecules 2018, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.S.; Hanafi, N.I.; Sheikh Abdul Kadir, S.H.; Md Noor, J.; Abdul Hamid Hasani, N.; Ab Rahim, S.; Siran, R. Ursodeoxycholic acid protects cardiomyocytes against cobalt chloride induced hypoxia by regulating transcriptional mediator of cells stress hypoxia inducible factor 1alpha and p53 protein. Cell Biochem. Funct. 2017, 35, 453–463. [Google Scholar] [CrossRef]
- Liu, X.; Fassett, J.; Wei, Y.; Chen, Y. Regulation of DDAH1 as a Potential Therapeutic Target for Treating Cardiovascular Diseases. Evid. Based Complement. Altern. Med. 2013, 2013, 619207. [Google Scholar] [CrossRef]
- Li, X.; Han, K.Q.; Shi, Y.N.; Men, S.Z.; Li, S.; Sun, M.H.; Dong, H.; Lu, J.J.; Ma, L.J.; Zhao, M.; et al. Effects and mechanisms of ursodeoxycholic acid on isoprenaline-Induced myocardial fibrosis in mice. Zhonghua Yi Xue Za Zhi 2017, 97, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Chuang, C.C.; Hemmelgarn, B.T.; Best, T.M. Heart failure with preserved ejection fraction: Defining the function of ROS and NO. J. Appl. Physiol. 2015, 119, 944–951. [Google Scholar] [CrossRef]
- Eblimit, Z.; Thevananther, S.; Karpen, S.J.; Taegtmeyer, H.; Moore, D.D.; Adorini, L.; Penny, D.J.; Desai, M.S. TGR5 activation induces cytoprotective changes in the heart and improves myocardial adaptability to physiologic, inotropic, and pressure-induced stress in mice. Cardiovasc. Ther. 2018, 36, e12462. [Google Scholar] [CrossRef]
- Guo, C.; Xie, S.; Chi, Z.; Zhang, J.; Liu, Y.; Zhang, L.; Zheng, M.; Zhang, X.; Xia, D.; Ke, Y.; et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity 2016, 45, 802–816. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef]
- Inagaki, T.; Moschetta, A.; Lee, Y.K.; Peng, L.; Zhao, G.; Downes, M.; Yu, R.T.; Shelton, J.M.; Richardson, J.A.; Repa, J.J.; et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 3920–3925. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, S.; Mencarelli, A.; Chini, M.G.; Distrutti, E.; Renga, B.; Bifulco, G.; Baldelli, F.; Donini, A.; Fiorucci, S. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS ONE 2011, 6, e25637. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Cappella, A.; Gagliano, N.; Sfondrini, L.; Stacchiotti, A. Polyphenols-Gut-Heart: An Impactful Relationship to Improve Cardiovascular Diseases. Antioxidants 2022, 11, 1700. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, N.; Kan, J.; Tang, S.; Sun, R.; Wang, Z.; Chen, M.; Liu, J.; Jin, C. Polyphenols from Arctium lappa L. ameliorate doxorubicin-induced heart failure and improve gut microbiota composition in mice. J. Food Biochem. 2022, 46, e13731. [Google Scholar] [CrossRef] [PubMed]
- Palombaro, M.; Raoul, P.; Cintoni, M.; Rinninella, E.; Pulcini, G.; Aspromonte, N.; Ianiro, G.; Gasbarrini, A.; Mele, M.C. Impact of Diet on Gut Microbiota Composition and Microbiota-Associated Functions in Heart Failure: A Systematic Review of In Vivo Animal Studies. Metabolites 2022, 12, 1271. [Google Scholar] [CrossRef] [PubMed]
- Dyck, G.J.B.; Raj, P.; Zieroth, S.; Dyck, J.R.B.; Ezekowitz, J.A. The Effects of Resveratrol in Patients with Cardiovascular Disease and Heart Failure: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 904. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Tang, Z.; Li, B.; Yu, J.; Li, W.; Liu, Z.; Tian, C. Resveratrol against Cardiac Fibrosis: Research Progress in Experimental Animal Models. Molecules 2021, 26, 6860. [Google Scholar] [CrossRef]
- Tuerhongjiang, G.; Guo, M.; Qiao, X.; Lou, B.; Wang, C.; Wu, H.; Wu, Y.; Yuan, Z.; She, J. Interplay Between Gut Microbiota and Amino Acid Metabolism in Heart Failure. Front. Cardiovasc. Med. 2021, 8, 752241. [Google Scholar] [CrossRef]
- Chen, W.S.; Wang, C.H.; Cheng, C.W.; Liu, M.H.; Chu, C.M.; Wu, H.P.; Huang, P.C.; Lin, Y.T.; Ko, T.; Chen, W.H.; et al. Elevated plasma phenylalanine predicts mortality in critical patients with heart failure. ESC Heart Fail. 2020, 7, 2884–2893. [Google Scholar] [CrossRef]
- Gawalko, M.; Agbaedeng, T.A.; Saljic, A.; Muller, D.N.; Wilck, N.; Schnabel, R.; Penders, J.; Rienstra, M.; van Gelder, I.; Jespersen, T.; et al. Gut microbiota, dysbiosis and atrial fibrillation. Arrhythmogenic mechanisms and potential clinical implications. Cardiovasc. Res. 2022, 118, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Nemet, I.; Saha, P.P.; Gupta, N.; Zhu, W.; Romano, K.A.; Skye, S.M.; Cajka, T.; Mohan, M.L.; Li, L.; Wu, Y.; et al. A Cardiovascular Disease-Linked Gut Microbial Metabolite Acts via Adrenergic Receptors. Cell 2020, 180, 862–877.e822. [Google Scholar] [CrossRef]
- Romano, K.A.; Nemet, I.; Prasad Saha, P.; Haghikia, A.; Li, X.S.; Mohan, M.L.; Lovano, B.; Castel, L.; Witkowski, M.; Buffa, J.A.; et al. Gut Microbiota-Generated Phenylacetylglutamine and Heart Failure. Circ. Heart Fail. 2022, 16, e009972. [Google Scholar] [CrossRef]
- Zhao, M.; Wei, H.; Li, C.; Zhan, R.; Liu, C.; Gao, J.; Yi, Y.; Cui, X.; Shan, W.; Ji, L.; et al. Gut microbiota production of trimethyl-5-aminovaleric acid reduces fatty acid oxidation and accelerates cardiac hypertrophy. Nat. Commun. 2022, 13, 1757. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Matsushita, K.; Lazo, M.; Bello, N.; Blumenthal, R.S.; Gerstenblith, G.; Nambi, V.; Ballantyne, C.M.; Solomon, S.D.; Selvin, E.; et al. Obesity and Subtypes of Incident Cardiovascular Disease. J. Am. Heart Assoc. 2016, 5, e003921. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Kajio, H. Abdominal Obesity Is Associated With an Increased Risk of All-Cause Mortality in Patients With HFpEF. J. Am. Coll. Cardiol. 2017, 70, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Paulus, W.J. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016, 134, 73–90. [Google Scholar] [CrossRef] [PubMed]
- von Bibra, H.; Strohle, A.; St John Sutton, M.; Worm, N. Dietary therapy in heart failure with preserved ejection fraction and/or left ventricular diastolic dysfunction in patients with metabolic syndrome. Int. J. Cardiol. 2017, 234, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Yap, E.P.; Kp, M.M.J.; Ramachandra, C.J. Targeting the Metabolic-Inflammatory Circuit in Heart Failure with Preserved Ejection Fraction. Curr. Heart Fail. Rep. 2022, 19, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montero, C.; Fraile-Martinez, O.; Gomez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; Garcia-Honduvilla, N.; Asunsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Tektonidis, T.G.; Akesson, A.; Gigante, B.; Wolk, A.; Larsson, S.C. A Mediterranean diet and risk of myocardial infarction, heart failure and stroke: A population-based cohort study. Atherosclerosis 2015, 243, 93–98. [Google Scholar] [CrossRef]
- Tektonidis, T.G.; Akesson, A.; Gigante, B.; Wolk, A.; Larsson, S.C. Adherence to a Mediterranean diet is associated with reduced risk of heart failure in men. Eur. J. Heart Fail. 2016, 18, 253–259. [Google Scholar] [CrossRef]
- Kouvari, M.; Chrysohoou, C.; Aggelopoulos, P.; Tsiamis, E.; Tsioufis, K.; Pitsavos, C.; Tousoulis, D. Mediterranean diet and prognosis of first-diagnosed Acute Coronary Syndrome patients according to heart failure phenotype: Hellenic Heart Failure Study. Eur. J. Clin. Nutr. 2017, 71, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Ruiz-Leon, A.M.; Sierra-Perez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef] [PubMed]
- Conraads, V.M.; Jorens, P.G.; De Clerck, L.S.; Van Saene, H.K.; Ieven, M.M.; Bosmans, J.M.; Schuerwegh, A.; Bridts, C.H.; Wuyts, F.; Stevens, W.J.; et al. Selective intestinal decontamination in advanced chronic heart failure: A pilot trial. Eur. J. Heart Fail. 2004, 6, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as deep modulators of gut microbiota: Between good and evil. Gut 2016, 65, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef]
- Troseid, M.; Andersen, G.O.; Broch, K.; Hov, J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef]
- Winkel, P.; Hilden, J.; Hansen, J.F.; Kastrup, J.; Kolmos, H.J.; Kjoller, E.; Jensen, G.B.; Skoog, M.; Lindschou, J.; Gluud, C.; et al. Clarithromycin for stable coronary heart disease increases all-cause and cardiovascular mortality and cerebrovascular morbidity over 10years in the CLARICOR randomised, blinded clinical trial. Int. J. Cardiol. 2015, 182, 459–465. [Google Scholar] [CrossRef]
- Heianza, Y.; Zheng, Y.; Ma, W.; Rimm, E.B.; Albert, C.M.; Hu, F.B.; Rexrode, K.M.; Manson, J.E.; Qi, L. Duration and life-stage of antibiotic use and risk of cardiovascular events in women. Eur. Heart J. 2019, 40, 3838–3845. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Gan, X.T.; Ettinger, G.; Huang, C.X.; Burton, J.P.; Haist, J.V.; Rajapurohitam, V.; Sidaway, J.E.; Martin, G.; Gloor, G.B.; Swann, J.R.; et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ. Heart Fail. 2014, 7, 491–499. [Google Scholar] [CrossRef]
- Costanza, A.C.; Moscavitch, S.D.; Faria Neto, H.C.; Mesquita, E.T. Probiotic therapy with Saccharomyces boulardii for heart failure patients: A randomized, double-blind, placebo-controlled pilot trial. Int. J. Cardiol. 2015, 179, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.M.; Shihata, W.A.; Jama, H.A.; Tsyganov, K.; Ziemann, M.; Kiriazis, H.; Horlock, D.; Vijay, A.; Giam, B.; Vinh, A.; et al. Deficiency of Prebiotic Fiber and Insufficient Signaling Through Gut Metabolite-Sensing Receptors Leads to Cardiovascular Disease. Circulation 2020, 141, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Vlasov, A.A.; Shperling, M.I.; Terkin, D.A.; Bystrova, O.V.; Osipov, G.A.; Salikova, S.P.; Grinevich, V.B. Effect of Prebiotic Complex on Gut Microbiota and Endotoxemia in Female Rats with Modeled Heart Failure. Bull. Exp. Biol. Med. 2020, 168, 435–438. [Google Scholar] [CrossRef]
- Johnson, L.P.; Walton, G.E.; Psichas, A.; Frost, G.S.; Gibson, G.R.; Barraclough, T.G. Prebiotics Modulate the Effects of Antibiotics on Gut Microbial Diversity and Functioning in Vitro. Nutrients 2015, 7, 4480–4497. [Google Scholar] [CrossRef] [PubMed]
- Yelin, I.; Flett, K.B.; Merakou, C.; Mehrotra, P.; Stam, J.; Snesrud, E.; Hinkle, M.; Lesho, E.; McGann, P.; McAdam, A.J.; et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat. Med. 2019, 25, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Yeoh, B.S.; Chassaing, B.; Xiao, X.; Saha, P.; Aguilera Olvera, R.; Lapek, J.D., Jr.; Zhang, L.; Wang, W.B.; Hao, S.; et al. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell 2018, 175, 679–694.e622. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A.; Sadowsky, M.J. Understanding the mechanisms of faecal microbiota transplantation. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 508–516. [Google Scholar] [CrossRef]
- Rinott, E.; Youngster, I.; Yaskolka Meir, A.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Fava, F.; Scholz, M.U.; et al. Effects of Diet-Modulated Autologous Fecal Microbiota Transplantation on Weight Regain. Gastroenterology 2021, 160, 158–173.e110. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojarvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e616. [Google Scholar] [CrossRef]
- Pandey, A.; Patel, K.V.; Vaduganathan, M.; Sarma, S.; Haykowsky, M.J.; Berry, J.D.; Lavie, C.J. Physical Activity, Fitness, and Obesity in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2018, 6, 975–982. [Google Scholar] [CrossRef]
- Hieda, M.; Sarma, S.; Hearon, C.M., Jr.; MacNamara, J.P.; Dias, K.A.; Samels, M.; Palmer, D.; Livingston, S.; Morris, M.; Levine, B.D. One-Year Committed Exercise Training Reverses Abnormal Left Ventricular Myocardial Stiffness in Patients With Stage B Heart Failure With Preserved Ejection Fraction. Circulation 2021, 144, 934–946. [Google Scholar] [CrossRef]
- Pandey, A.; Parashar, A.; Kumbhani, D.; Agarwal, S.; Garg, J.; Kitzman, D.; Levine, B.; Drazner, M.; Berry, J. Exercise training in patients with heart failure and preserved ejection fraction: Meta-analysis of randomized control trials. Circ. Heart Fail. 2015, 8, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Luo, X.; Wang, Q.; Wang, W.; Sun, R.; Zhang, X.; Yu, J. The effect of exercise training and physiotherapy on diastolic function, exercise capacity and quality of life in patients with heart failure with preserved ejection fraction: A systematic review and meta-analysis. Kardiol. Pol. 2021, 79, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhai, W.; Yang, C.; Li, Z.; Mao, L.; Zhao, M.; Wu, X. The Relationship among Physical Activity, Intestinal Flora, and Cardiovascular Disease. Cardiovasc. Ther. 2021, 2021, 3364418. [Google Scholar] [CrossRef] [PubMed]
- Ostman, C.; Smart, N.A.; Morcos, D.; Duller, A.; Ridley, W.; Jewiss, D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2017, 16, 110. [Google Scholar] [CrossRef]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar] [CrossRef]
- Wang, G.; Kong, B.; Shuai, W.; Fu, H.; Jiang, X.; Huang, H. 3,3-Dimethyl-1-butanol attenuates cardiac remodeling in pressure-overload-induced heart failure mice. J. Nutr. Biochem. 2020, 78, 108341. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef]
- Organ, C.L.; Li, Z.; Sharp, T.E., 3rd; Polhemus, D.J.; Gupta, N.; Goodchild, T.T.; Tang, W.H.W.; Hazen, S.L.; Lefer, D.J. Nonlethal Inhibition of Gut Microbial Trimethylamine N-oxide Production Improves Cardiac Function and Remodeling in a Murine Model of Heart Failure. J. Am. Heart Assoc. 2020, 9, e016223. [Google Scholar] [CrossRef]
- Roberts, A.B.; Gu, X.; Buffa, J.A.; Hurd, A.G.; Wang, Z.; Zhu, W.; Gupta, N.; Skye, S.M.; Cody, D.B.; Levison, B.S.; et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat. Med. 2018, 24, 1407–1417. [Google Scholar] [CrossRef]
- Challa, A.A.; Lewandowski, E.D. Short-Chain Carbon Sources: Exploiting Pleiotropic Effects for Heart Failure Therapy. JACC Basic Transl. Sci. 2022, 7, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Guida, F.; Paparo, L.; Trinchese, G.; Aitoro, R.; Avagliano, C.; Fiordelisi, A.; Napolitano, F.; Mercurio, V.; Sala, V.; et al. The novel butyrate derivative phenylalanine-butyramide protects from doxorubicin-induced cardiotoxicity. Eur. J. Heart Fail. 2019, 21, 519–528. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Schefold, J.C.; Jankowska, E.A.; Springer, J.; Vazir, A.; Kalra, P.R.; Sandek, A.; Fauler, G.; Stojakovic, T.; Trauner, M.; et al. Ursodeoxycholic acid in patients with chronic heart failure: A double-blind, randomized, placebo-controlled, crossover trial. J. Am. Coll. Cardiol. 2012, 59, 585–592. [Google Scholar] [CrossRef]
- Miragoli, M.; Kadir, S.H.; Sheppard, M.N.; Salvarani, N.; Virta, M.; Wells, S.; Lab, M.J.; Nikolaev, V.O.; Moshkov, A.; Hague, W.M.; et al. A protective antiarrhythmic role of ursodeoxycholic acid in an in vitro rat model of the cholestatic fetal heart. Hepatology 2011, 54, 1282–1292. [Google Scholar] [CrossRef]
- Chung, J.; An, S.H.; Kang, S.W.; Kwon, K. Ursodeoxycholic Acid (UDCA) Exerts Anti-Atherogenic Effects by Inhibiting RAGE Signaling in Diabetic Atherosclerosis. PLoS ONE 2016, 11, e0147839. [Google Scholar] [CrossRef]
- Fang, S.; Suh, J.M.; Reilly, S.M.; Yu, E.; Osborn, O.; Lackey, D.; Yoshihara, E.; Perino, A.; Jacinto, S.; Lukasheva, Y.; et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med. 2015, 21, 159–165. [Google Scholar] [CrossRef]
- Kolijn, D.; Pabel, S.; Tian, Y.; Lodi, M.; Herwig, M.; Carrizzo, A.; Zhazykbayeva, S.; Kovacs, A.; Fulop, G.A.; Falcao-Pires, I.; et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Galpha oxidation. Cardiovasc. Res. 2021, 117, 495–507. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Arena, R.; Biondi-Zoccai, G.; Canada, J.M.; Oddi, C.; Abouzaki, N.A.; Jahangiri, A.; Falcao, R.A.; Kontos, M.C.; Shah, K.B.; et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am. J. Cardiol. 2014, 113, 321–327. [Google Scholar] [CrossRef]
- Lin, T.L.; Lu, C.C.; Lai, W.F.; Wu, T.S.; Lu, J.J.; Chen, Y.M.; Tzeng, C.M.; Liu, H.T.; Wei, H.; Lai, H.C. Role of gut microbiota in identification of novel TCM-derived active metabolites. Protein Cell 2021, 12, 394–410. [Google Scholar] [CrossRef]
- Liu, S.; Pi, Z.; Liu, Z.; Song, F.; Liu, S. Fecal metabolomics based on mass spectrometry to investigate the mechanism of qishen granules against isoproterenol-induced chronic heart failure in rats. J. Sep. Sci. 2020, 43, 4305–4313. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yue, S.; Yang, Z.; Feng, W.; Meng, X.; Wang, A.; Peng, C.; Wang, C.; Yan, D. Oral hydroxysafflor yellow A reduces obesity in mice by modulating the gut microbiota and serum metabolism. Pharmacol. Res. 2018, 134, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef] [PubMed]

| Sample Size | Features of GMB Composition | Key Finding | Alpha Diversity | Beta Diversity | Reference |
|---|---|---|---|---|---|
| n = 30 HFpEF n = 30 controls | The characteristic bacterial community of HFpEF group was Enterococcus, Lactobacillus | An increase of pro-inflammatory microbiome and a decreased anti-inflammatory microbiome | Lower Chao index in HFpEF but no difference of Shannon index and Simpson index | Significant difference of composition between HFpEF and controls | [4] |
| n = 26 HFpEF n = 67 controls | The ratio of Firmicutes to Bacteroidetes tended to be lower (no statistical significance); significant differences in the abundance of specific bacterial populations | A depletion of the producers of short-chain fatty acids, especially Ruminococcus | Lower Chao index in HFpEF but no difference of Shannon index | Significant difference of composition between HFpEF and controls | [5] |
| n = 42 HFpEF | Firmicutes, Bacteroidetes and Proteobacteria have the highest abundance | GMB is associated with myocardial fibrosis indicators (C-terminal propeptide of procollagen type I, N-terminal propeptide of pro-collagen type III) | - | - | [7] |
| n = 42 HFpEF | The relative abundance of the most prevalent phyla was Bacteroidetes, Firmicutes and Proteobacteria | GMB is associated with the left ventricular extracellular volume | - | - | [8] |
| n = 27 HFpEF n = 30 age-matched controls | 24 OTUs were differentially present between HFpEF and controls; the abundance of Prevotella was significantly reduced in HFpEF | The GMB differs between HFpEF and age-matched controls | - | - | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Jiang, Y.; Xu, H.; Zhou, Y. The Interaction of Gut Microbiota and Heart Failure with Preserved Ejection Fraction: From Mechanism to Potential Therapies. Biomedicines 2023, 11, 442. https://doi.org/10.3390/biomedicines11020442
Yu W, Jiang Y, Xu H, Zhou Y. The Interaction of Gut Microbiota and Heart Failure with Preserved Ejection Fraction: From Mechanism to Potential Therapies. Biomedicines. 2023; 11(2):442. https://doi.org/10.3390/biomedicines11020442
Chicago/Turabian StyleYu, Wei, Yufeng Jiang, Hui Xu, and Yafeng Zhou. 2023. "The Interaction of Gut Microbiota and Heart Failure with Preserved Ejection Fraction: From Mechanism to Potential Therapies" Biomedicines 11, no. 2: 442. https://doi.org/10.3390/biomedicines11020442
APA StyleYu, W., Jiang, Y., Xu, H., & Zhou, Y. (2023). The Interaction of Gut Microbiota and Heart Failure with Preserved Ejection Fraction: From Mechanism to Potential Therapies. Biomedicines, 11(2), 442. https://doi.org/10.3390/biomedicines11020442






