Mesonephric-like Adenocarcinoma of the Uterine Corpus: Genomic and Immunohistochemical Profiling with Comprehensive Clinicopathological Analysis of 17 Consecutive Cases from a Single Institution
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection and Clinicopathological Data Collection
2.2. NGS
2.3. Array Comparative Genomic Hybridization (CGH)
2.4. MSI Testing
2.5. PD-L1 22C3 Pharmdx IHC
2.6. MMR IHC
2.7. IHC Interpretation
2.8. Statistical Analysis
3. Results
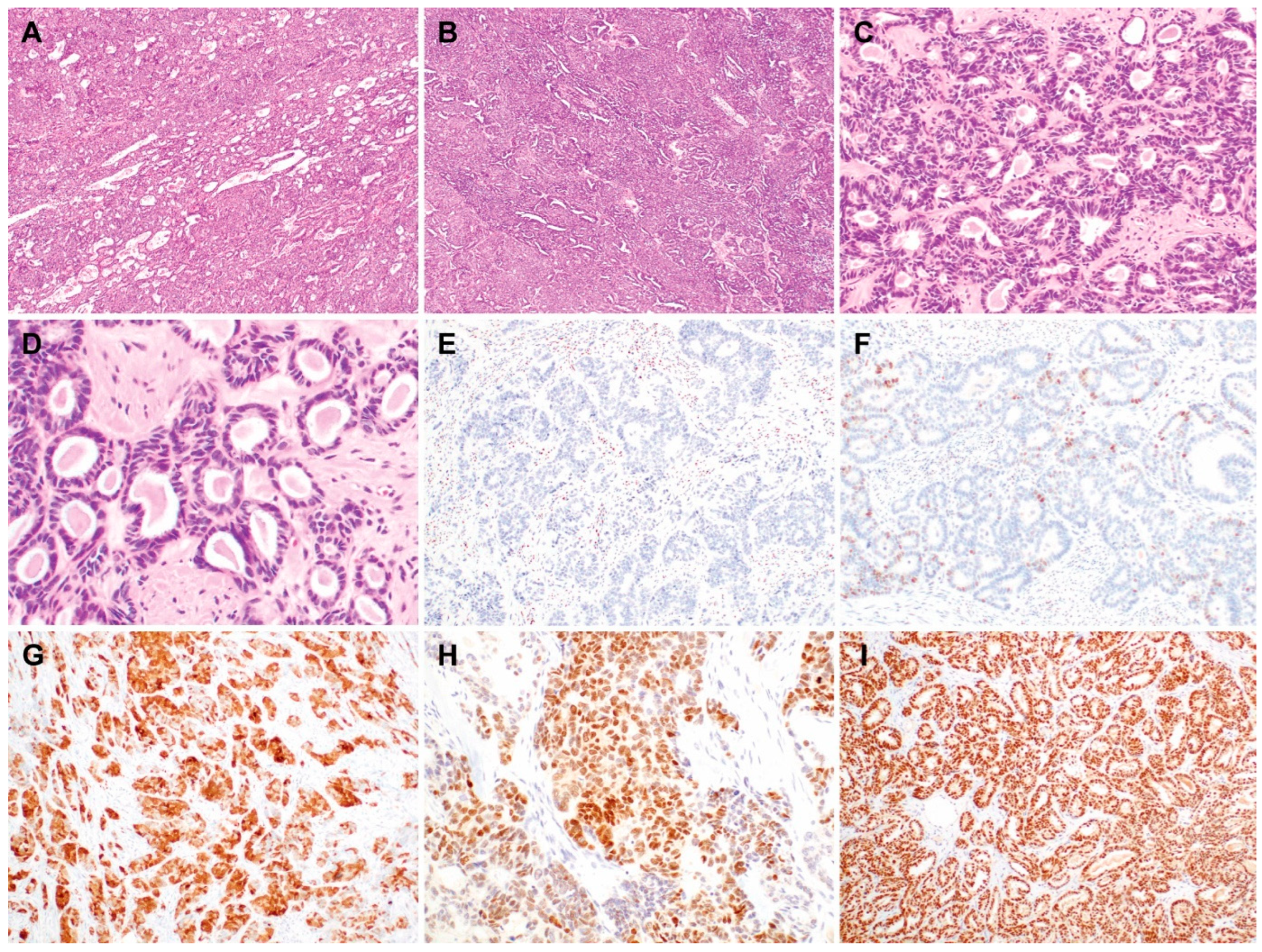
3.1. Clinicopathological Characteristics
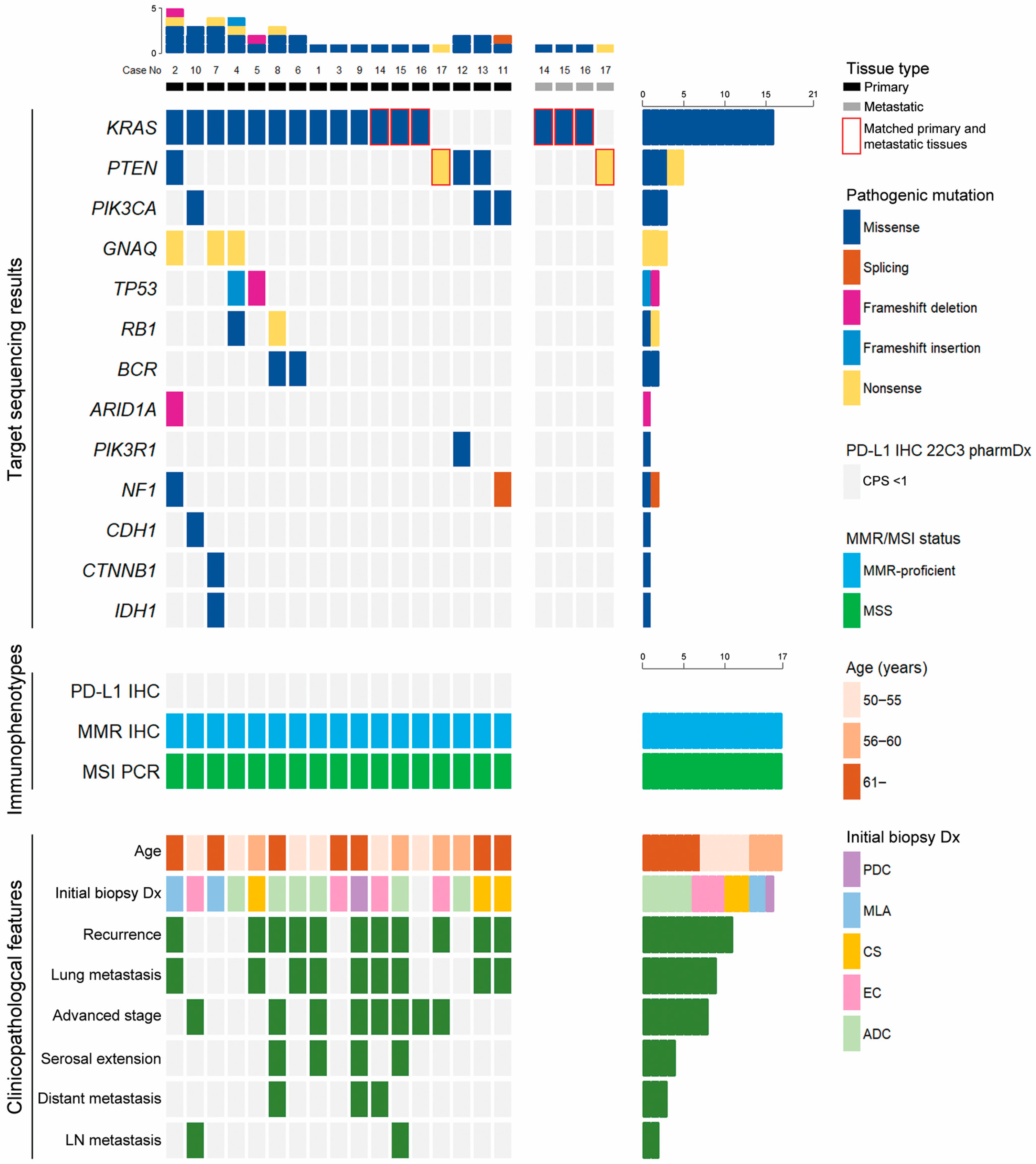
3.2. NGS Results
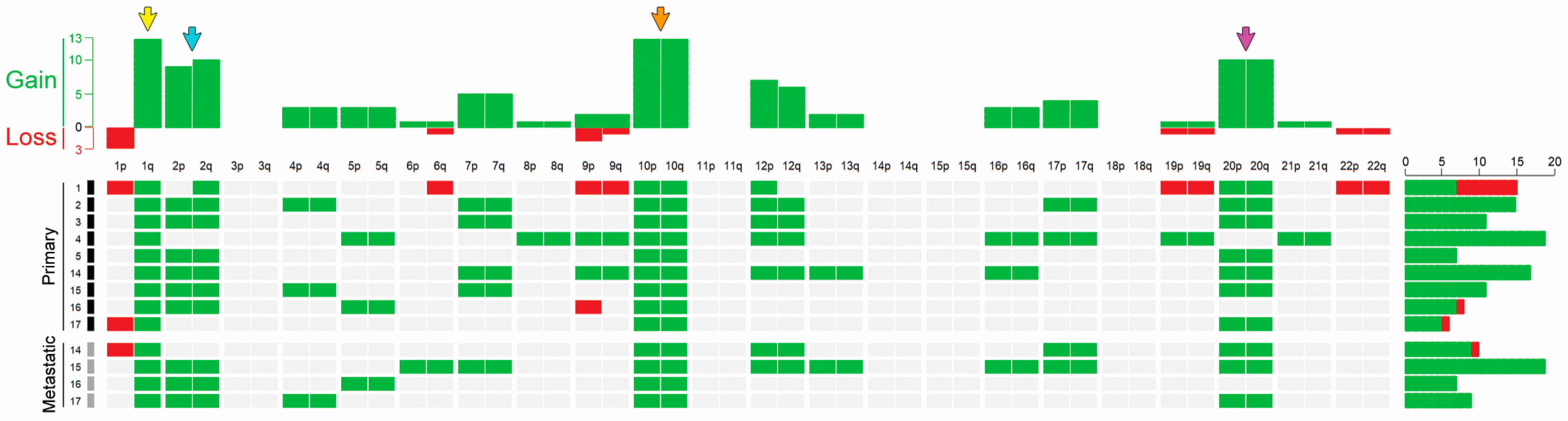
3.3. CGH Results
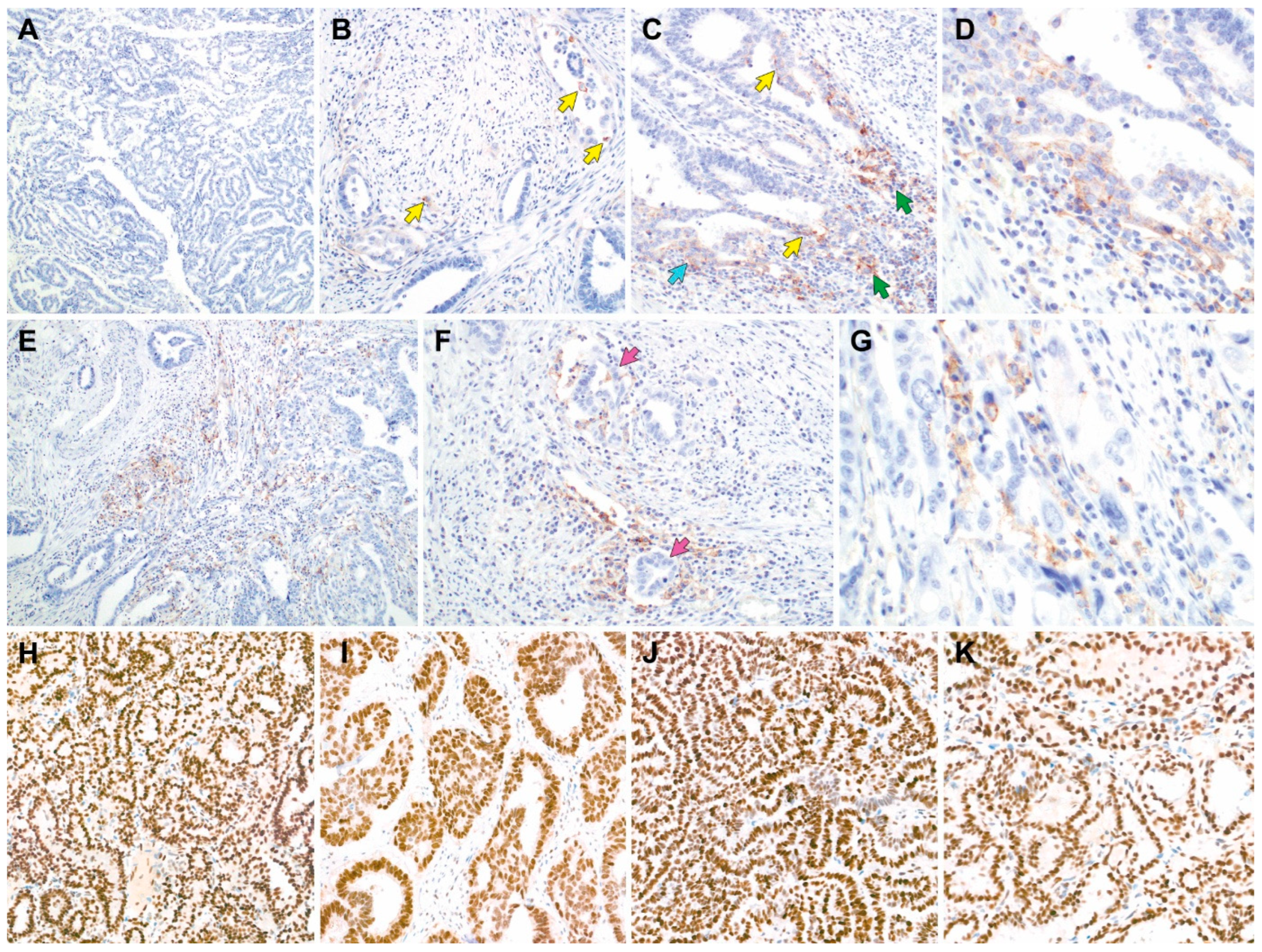
3.4. PD-L1 Expression, MMR Protein Expression, MSI Status, and Tumor Mutational Burden
3.5. Clinicopathological and Prognostic Significance of Recurrent Pathogenic Mutations
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laliscia, C.; Gadducci, A.; Coccia, N.; Mattioni, R.; Fuentes, T.; Caretto, M.; Pistolesi, S.; Puccini, P.; Perrone, F.; Morganti, R.; et al. Lymph-vascular space involvement and/or p53 overexpression correlated with the clinical outcome of early-stage endometrial cancer patients treated with adjuvant vaginal brachytherapy. Anticancer Res. 2023, 43, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, E.; Kim, H.S. Mesonephric-like carcinosarcoma of the uterine corpus: Clinicopathological, molecular and prognostic characteristics in comparison with uterine mesonephric-like adenocarcinoma and conventional endometrial carcinosarcoma. Cancer Genom. Proteom. 2022, 19, 747–760. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, H.; Yeo, M.K.; Won, K.Y.; Kim, Y.S.; Han, G.H.; Kim, H.S.; Na, K. Mesonephric-like adenocarcinoma of the uterine corpus: Comprehensive analyses of clinicopathological, molecular, and prognostic characteristics with retrospective review of 237 endometrial carcinoma cases. Cancer Genom. Proteom. 2022, 19, 526–539. [Google Scholar] [CrossRef]
- Henley, S.J.; Ward, E.M.; Scott, S.; Ma, J.; Anderson, R.N.; Firth, A.U.; Thomas, C.C.; Islami, F.; Weir, H.K.; Lewis, D.R.; et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer 2020, 126, 2225–2249. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- WHO Classification of Tumors Editorial Board (Ed.) WHO Classification of Tumours: Female Genital Tumours, 5th ed.; WHO: Lyon, France, 2020. [Google Scholar]
- Amant, F.; Mirza, M.R.; Koskas, M.; Creutzberg, C.L. Cancer of the corpus uteri. Int. J. Gynaecol. Obs. 2018, 143, 37–50. [Google Scholar] [CrossRef]
- Howitt, B.E.; Nucci, M.R. Mesonephric proliferations of the female genital tract. Pathology 2018, 50, 141–150. [Google Scholar] [CrossRef]
- McFarland, M.; Quick, C.M.; McCluggage, W.G. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: Report of a series of mesonephric-like adenocarcinomas. Histopathology 2016, 68, 1013–1020. [Google Scholar] [CrossRef]
- Koh, H.H.; Park, E.; Kim, H.S. Mesonephric-like adenocarcinoma of the ovary: Clinicopathological and molecular characteristics. Diagnostics 2022, 12, 326. [Google Scholar] [CrossRef]
- Gagan, J.; Van Allen, E.M. Next-generation sequencing to guide cancer therapy. Genome Med. 2015, 7, 80. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N.; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Goulder, A.; Gaillard, S.L. Molecular classification of endometrial cancer: Entering an era of precision medicine. J. Gynecol. Oncol. 2022, 33, e47. [Google Scholar] [CrossRef] [PubMed]
- Bosse, T.; Nout, R.A.; McAlpine, J.N.; McConechy, M.K.; Britton, H.; Hussein, Y.R.; Gonzalez, C.; Ganesan, R.; Steele, J.C.; Harrison, B.T.; et al. Molecular Classification of Grade 3 Endometrioid Endometrial Cancers Identifies Distinct Prognostic Subgroups. Am. J. Surg. Pathol. 2018, 42, 561–568. [Google Scholar] [CrossRef]
- Ma, T.; Chai, M.; Shou, H.; Ru, G.; Zhao, M. Mesonephric-like adenocarcinoma of uterine corpus: A clinicopathological and targeted genomic profiling study in a single institution. Front. Oncol. 2022, 12, 911695. [Google Scholar] [CrossRef]
- Arslanian, E.; Singh, K.; James Sung, C.; Quddus, M.R. Somatic mutation analysis of mesonephric-like adenocarcinoma and associated putative precursor lesions: Insight into pathogenesis and potential molecular treatment targets. Gynecol. Oncol. Rep. 2022, 42, 101049. [Google Scholar] [CrossRef]
- Buckle, I.; Guillerey, C. Inhibitory receptors and immune checkpoints regulating natural killer cell responses to cancer. Cancers 2021, 13, 4263. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Q.; Zhu, Y.; Zhang, S.; Peng, Q.; Strickland, A.L.; Zheng, W.; Zhou, F. PD-L1 expression in endometrial serous carcinoma and its prognostic significance. Cancer Manag. Res. 2021, 13, 9157–9165. [Google Scholar] [CrossRef]
- Hudson, K.; Cross, N.; Jordan-Mahy, N.; Leyland, R. The extrinsic and intrinsic roles of PD-L1 and its receptor PD-1: Implications for immunotherapy treatment. Front. Immunol. 2020, 11, 568931. [Google Scholar] [CrossRef]
- Mamat Yusof, M.N.; Chew, K.T.; Kampan, N.; Abd Aziz, N.H.; Md Zin, R.R.; Tan, G.C.; Shafiee, M.N. PD-L1 expression in endometrial cancer and its association with clinicopathological features: A systematic review and meta-analysis. Cancers 2022, 14, 3911. [Google Scholar] [CrossRef]
- Mo, Z.; Liu, J.; Zhang, Q.; Chen, Z.; Mei, J.; Liu, L.; Yang, S.; Li, H.; Zhou, L.; You, Z. Expression of PD-1, PD-L1 and PD-L2 is associated with differentiation status and histological type of endometrial cancer. Oncol. Lett. 2016, 12, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Sungu, N.; Yildirim, M.; Desdicioglu, R.; Basaran Aydogdu, O.; Kilicarslan, A.; Tatli Dogan, H.; Kilic Yazgan, A.; Akyol, M.; Erdogan, F. Expression of Immunomodulatory Molecules PD-1, PD-L1, and PD-L2, and their Relationship with Clinicopathologic Characteristics in Endometrial Cancer. Int. J. Gynecol. Pathol. 2019, 38, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Rasco, D.; Vogelzang, N.J.; Brose, M.S.; Cohn, A.L.; Mier, J.; Di Simone, C.; Hyman, D.M.; Stepan, D.E.; Dutcus, C.E.; et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 711–718. [Google Scholar] [CrossRef]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Kommoss, F.; Grevenkamp, F.; Karnezis, A.; et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- van den Heerik, A.; Horeweg, N.; Nout, R.A.; Lutgens, L.; van der Steen-Banasik, E.M.; Westerveld, G.H.; van den Berg, H.A.; Slot, A.; Koppe, F.L.A.; Kommoss, S.; et al. PORTEC-4a: International randomized trial of molecular profile-based adjuvant treatment for women with high-intermediate risk endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 2002–2007. [Google Scholar] [CrossRef]
- Jamieson, A.; Barroilhet, L.M.; McAlpine, J.N. Molecular classification in endometrial cancer: Opportunities for precision oncology in a changing landscape. Cancer 2022, 128, 2853–2857. [Google Scholar] [CrossRef]
- Yamashita, H.; Nakayama, K.; Ishikawa, M.; Nakamura, K.; Ishibashi, T.; Sanuki, K.; Ono, R.; Sasamori, H.; Minamoto, T.; Iida, K.; et al. Microsatellite instability is a biomarker for immune checkpoint inhibitors in endometrial cancer. Oncotarget 2018, 9, 5652–5664. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Jung, J.; Kang, Y.; Lee, Y.J.; Kim, E.; Ahn, B.; Lee, E.; Kim, J.Y.; Lee, J.H.; Lee, Y.; Kim, C.H.; et al. Comparison of the mismatch repair system between primary and metastatic colorectal cancers using immunohistochemistry. J. Pathol. Transl. Med. 2017, 51, 129–136. [Google Scholar] [CrossRef]
- Jumaah, A.S.; Al-Haddad, H.S.; Salem, M.M.; McAllister, K.A.; Yasseen, A.A. Mismatch repair deficiency and clinicopathological characteristics in endometrial carcinoma: A systematic review and meta-analysis. J. Pathol. Transl. Med. 2021, 55, 202–211. [Google Scholar] [CrossRef]
- Ukkola, I.; Nummela, P.; Pasanen, A.; Kero, M.; Lepisto, A.; Kytola, S.; Butzow, R.; Ristimaki, A. Detection of microsatellite instability with Idylla MSI assay in colorectal and endometrial cancer. Virchows Arch. 2021, 479, 471–479. [Google Scholar] [CrossRef]
- Seo, Y.; Park, E.; Kim, H.S. Cytological features of mesonephric-like adenocarcinoma of the uterine corpus. Diagn. Cytopathol. 2023, 51, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Bae, G.E.; Kim, J.; Kim, H.S. Mesonephric-like differentiation of endometrial endometrioid carcinoma: Clinicopathological and molecular characteristics distinct from those of uterine mesonephric-like adenocarcinoma. Diagnostics 2021, 11, 1450. [Google Scholar] [CrossRef]
- Kim, H.N.; Jang, J.; Heo, Y.J.; Kim, B.; Jung, H.; Jang, Y.; Kang, S.Y.; Kim, S.T.; Lee, J.; Kang, W.K.; et al. PD-L1 expression in gastric cancer determined by digital image analyses: Pitfalls and correlation with pathologist interpretation. Virchows Arch. 2020, 476, 243–250. [Google Scholar] [CrossRef]
- Sohn, J.; Lee, Y.; Kim, H.S. Endometrioid carcinomas of the ovaries and endometrium involving endocervical polyps: Comprehensive clinicopathological analyses. Diagnostics 2022, 12, 2339. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Kim, H.S. Mesonephric adenocarcinoma of the vagina harboring TP53 mutation. Diagnostics 2022, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; An, M.; Klempner, S.J.; Lee, H.; Kim, K.M.; Sa, J.K.; Cho, H.J.; Hong, J.Y.; Lee, T.; Min, Y.W.; et al. Determinants of response and intrinsic resistance to PD-1 blockade in microsatellite instability-high gastric cancer. Cancer Discov. 2021, 11, 2168–2185. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Na, K.; Kim, S.W.; Kim, H.S. Dedifferentiated mesonephric-like adenocarcinoma of the uterine corpus. Anticancer Res. 2021, 41, 2719–2726. [Google Scholar] [CrossRef]
- Na, K.; Kim, H.S. Clinicopathologic and molecular characteristics of mesonephric adenocarcinoma arising from the uterine body. Am. J. Surg. Pathol. 2019, 43, 12–25. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.S. Mesonephric-like Adenocarcinoma of the Uterine Corpus: Comparison Between Mismatch Repair Protein Immunostaining and Microsatellite Instability Testing. Anticancer Res. 2023, 43, 1785–1795. [Google Scholar] [CrossRef]
- Heo, Y.J.; Kim, B.; Kim, H.; Kim, S.; Jang, M.S.; Kim, K.M. PD-L1 expression in paired biopsies and surgical specimens in gastric adenocarcinoma: A digital image analysis study. Pathol. Res. Pract. 2021, 218, 153338. [Google Scholar] [CrossRef]
- McCluggage, W.G. Mesonephric-like Adenocarcinoma of the Female Genital Tract: From Morphologic Observations to a Well-characterized Carcinoma with Aggressive Clinical Behavior. Adv. Anat. Pathol. 2022, 29, 208–216. [Google Scholar] [CrossRef]
- Mirkovic, J.; Olkhov-Mitsel, E.; Amemiya, Y.; Al-Hussaini, M.; Nofech-Mozes, S.; Djordjevic, B.; Kupets, R.; Seth, A.; McCluggage, W.G. Mesonephric-like adenocarcinoma of the female genital tract: Novel observations and detailed molecular characterisation of mixed tumours and mesonephric-like carcinosarcomas. Histopathology 2023, 82, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Kwon, M.J.; Ra, Y.J.; Lee, H.S.; Kim, H.S.; Nam, E.S.; Cho, S.J.; Park, H.R.; Min, S.K.; Seo, J.; et al. Significance of druggable targets (PD-L1, KRAS, BRAF, PIK3CA, MSI, and HPV) on curatively resected esophageal squamous cell carcinoma. Diagn. Pathol. 2020, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018, 24, 1449–1458. [Google Scholar] [CrossRef]
- Huang, R.S.P.; Haberberger, J.; Murugesan, K.; Danziger, N.; Hiemenz, M.; Severson, E.; Duncan, D.L.; Ramkissoon, S.H.; Ross, J.S.; Elvin, J.A.; et al. Clinicopathologic and genomic characterization of PD-L1-positive uterine cervical carcinoma. Mod. Pathol. 2021, 34, 1425–1433. [Google Scholar] [CrossRef]
- Stelloo, E.; Jansen, A.M.L.; Osse, E.M.; Nout, R.A.; Creutzberg, C.L.; Ruano, D.; Church, D.N.; Morreau, H.; Smit, V.; van Wezel, T.; et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann. Oncol. 2017, 28, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.C.; Nucci, M.R.; Ritterhouse, L.L.; Howitt, B.E.; Sholl, L.M. Unusual mismatch repair immunohistochemical patterns in endometrial carcinoma. Am. J. Surg. Pathol. 2016, 40, 909–916. [Google Scholar] [CrossRef]
- Mirkovic, J.; McFarland, M.; Garcia, E.; Sholl, L.M.; Lindeman, N.; MacConaill, L.; Dong, F.; Hirsch, M.; Nucci, M.R.; Quick, C.M.; et al. Targeted genomic profiling reveals recurrent KRAS mutations in mesonephric-like adenocarcinomas of the female genital tract. Am. J. Surg. Pathol. 2018, 42, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Kolin, D.L.; Costigan, D.C.; Dong, F.; Nucci, M.R.; Howitt, B.E. A combined morphologic and molecular approach to retrospectively identify KRAS-mutated mesonephric-like adenocarcinomas of the endometrium. Am. J. Surg. Pathol. 2019, 43, 389–398. [Google Scholar] [CrossRef]
- da Silva, E.M.; Fix, D.J.; Sebastiao, A.P.M.; Selenica, P.; Ferrando, L.; Kim, S.H.; Stylianou, A.; Da Cruz Paula, A.; Pareja, F.; Smith, E.S.; et al. Mesonephric and mesonephric-like carcinomas of the female genital tract: Molecular characterization including cases with mixed histology and matched metastases. Mod. Pathol. 2021, 34, 1570–1587. [Google Scholar] [CrossRef]
- Kim, H.; Na, K.; Bae, G.E.; Kim, H.S. Mesonephric-like adenocarcinoma of the uterine corpus: Comprehensive immunohistochemical analyses using markers for mesonephric, endometrioid and serous tumors. Diagnostics 2021, 11, 2042. [Google Scholar] [CrossRef] [PubMed]
- Mustachio, L.M.; Chelariu-Raicu, A.; Szekvolgyi, L.; Roszik, J. Targeting KRAS in cancer: Promising therapeutic strategies. Cancers 2021, 13, 1204. [Google Scholar] [CrossRef]
- Sideris, M.; Emin, E.I.; Abdullah, Z.; Hanrahan, J.; Stefatou, K.M.; Sevas, V.; Emin, E.; Hollingworth, T.; Odejinmi, F.; Papagrigoriadis, S.; et al. The role of KRAS in endometrial cancer: A mini-review. Anticancer Res. 2019, 39, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Ring, K.L.; Yates, M.S.; Schmandt, R.; Onstad, M.; Zhang, Q.; Celestino, J.; Kwan, S.Y.; Lu, K.H. Endometrial cancers with activating KRAS mutations have activated estrogen signaling and paradoxical response to MEK inhibition. Int. J. Gynecol. Cancer 2017, 27, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Mirkovic, J.; Sholl, L.M.; Garcia, E.; Lindeman, N.; MacConaill, L.; Hirsch, M.; Dal Cin, P.; Gorman, M.; Barletta, J.A.; Nucci, M.R.; et al. Targeted genomic profiling reveals recurrent KRAS mutations and gain of chromosome 1q in mesonephric carcinomas of the female genital tract. Mod. Pathol. 2015, 28, 1504–1514. [Google Scholar] [CrossRef]
- Kim, H.; Yoon, N.; Woo, H.Y.; Lee, E.J.; Do, S.I.; Na, K.; Kim, H.S. Atypical mesonephric hyperplasia of the uterus harbors pathogenic mutation of Kirsten rat sarcoma 2 viral oncogene homolog (KRAS) and gain of chromosome 1q. Cancer Genom. Proteom. 2020, 17, 813–826. [Google Scholar] [CrossRef]
- Horn, L.C.; Hohn, A.K.; Krucken, I.; Stiller, M.; Obeck, U.; Brambs, C.E. Mesonephric-like adenocarcinomas of the uterine corpus: Report of a case series and review of the literature indicating poor prognosis for this subtype of endometrial adenocarcinoma. J. Cancer Res. Clin. Oncol. 2020, 146, 971–983. [Google Scholar] [CrossRef]
- Devarashetty, S.; Chennapragada, S.S.; Mansour, R. Not your typical adenocarcinoma: A case of mesonephric adenocarcinoma of the cervix with fibroblast growth factor receptor 2 (FGFR2) mutation. Cureus 2022, 14, e25098. [Google Scholar] [CrossRef]
- Makker, V.; Taylor, M.H.; Oaknin, A.; Casado Herraez, A.; Orlowski, R.; Dutta, L.; Ren, M.; Zale, M.; O’Malley, D.M. Characterization and Management of Adverse Reactions in Patients with Advanced Endometrial Carcinoma Treated with Lenvatinib Plus Pembrolizumab. Oncologist 2021, 26, e1599–e1608. [Google Scholar] [CrossRef]
- Makker, V.; Taylor, M.H.; Aghajanian, C.; Oaknin, A.; Mier, J.; Cohn, A.L.; Romeo, M.; Bratos, R.; Brose, M.S.; DiSimone, C.; et al. Lenvatinib Plus Pembrolizumab in Patients with Advanced Endometrial Cancer. J. Clin. Oncol. 2020, 38, 2981–2992. [Google Scholar] [CrossRef] [PubMed]
- Kommoss, F.K.; Karnezis, A.N.; Kommoss, F.; Talhouk, A.; Taran, F.A.; Staebler, A.; Gilks, C.B.; Huntsman, D.G.; Kramer, B.; Brucker, S.Y.; et al. L1CAM further stratifies endometrial carcinoma patients with no specific molecular risk profile. Br. J. Cancer 2018, 119, 480–486. [Google Scholar] [CrossRef]
- Loukovaara, M.; Pasanen, A.; Butzow, R. Molecular classification of endometrial carcinoma: A clinically oriented review. J. Clin. Pathol. 2022, 75, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Travaglino, A.; Raffone, A.; Gencarelli, A.; Mollo, A.; Guida, M.; Insabato, L.; Santoro, A.; Zannoni, G.F.; Zullo, F. TCGA classification of endometrial cancer: The place of carcinosarcoma. Pathol. Oncol. Res. 2020, 26, 2067–2073. [Google Scholar] [CrossRef]
- Arciuolo, D.; Travaglino, A.; Raffone, A.; Raimondo, D.; Santoro, A.; Russo, D.; Varricchio, S.; Casadio, P.; Inzani, F.; Seracchioli, R.; et al. TCGA molecular prognostic groups of endometrial carcinoma: Current knowledge and future perspectives. Int. J. Mol. Sci. 2022, 23, 11684. [Google Scholar] [CrossRef]
- Pors, J.; Segura, S.; Chiu, D.S.; Almadani, N.; Ren, H.; Fix, D.J.; Howitt, B.E.; Kolin, D.; McCluggage, W.G.; Mirkovic, J.; et al. Clinicopathologic characteristics of mesonephric adenocarcinomas and mesonephric-like adenocarcinomas in the gynecologic tract: A multi-institutional study. Am. J. Surg. Pathol. 2021, 45, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Pors, J.; Ho, J.; Prentice, L.; Thompson, E.; Cochrane, D.; Gibbard, E.; Huntsman, D.; Gilks, B.; Hoang, L.N. c-KIT analysis and targeted molecular sequencing of mesonephric carcinomas of the female genital tract. Am. J. Surg. Pathol. 2020, 44, 495–502. [Google Scholar] [CrossRef]
- Euscher, E.D.; Bassett, R.; Duose, D.Y.; Lan, C.; Wistuba, I.; Ramondetta, L.; Ramalingam, P.; Malpica, A. Mesonephric-like carcinoma of the endometrium: A subset of endometrial carcinoma with an aggressive behavior. Am. J. Surg. Pathol. 2020, 44, 429–443. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.I.; Kim, N.R.; Kim, H.; Kim, H.S.; Chung, H.H.; Kim, J.W.; Lee, C.; Lee, M. Prognostic significance of L1CAM expression in addition to ProMisE in endometrial cancer. Gynecol. Oncol. 2023, 174, 231–238. [Google Scholar] [CrossRef]
- Asano, H.; Hatanaka, K.C.; Matsuoka, R.; Dong, P.; Mitamura, T.; Konno, Y.; Kato, T.; Kobayashi, N.; Ihira, K.; Nozaki, A.; et al. L1CAM predicts adverse outcomes in patients with endometrial cancer undergoing full lymphadenectomy and adjuvant chemotherapy. Ann. Surg. Oncol. 2020, 27, 2159–2168. [Google Scholar] [CrossRef]
- Costigan, D.C.; Dong, F.; Nucci, M.R.; Howitt, B.E. Clinicopathologic and immunohistochemical correlates of CTNNB1 mutated endometrial endometrioid carcinoma. Int. J. Gynecol. Pathol. 2020, 39, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Klat, J.; Mladenka, A.; Dvorackova, J.; Bajsova, S.; Simetka, O. L1CAM as a negative prognostic factor in endometrioid endometrial adenocarcinoma FIGO stage IA-IB. Anticancer Res. 2019, 39, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Kim, G.N.; Fellman, B.M.; Urbauer, D.L.; Mills, G.B.; Zhang, W.; Broaddus, R.R. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod. Pathol. 2017, 30, 1032–1041. [Google Scholar] [CrossRef]
- Lin, D.I.; Shah, N.; Tse, J.Y.; Killian, J.K.; Hemmerich, A.; Edgerly, C.; Haberberger, J.; Severson, E.A.; Huang, R.S.P.; Ramkissoon, S.H.; et al. Molecular profiling of mesonephric and mesonephric-like carcinomas of cervical, endometrial and ovarian origin. Gynecol. Oncol. Rep. 2020, 34, 100652. [Google Scholar] [CrossRef]
- Leon-Castillo, A.; Gilvazquez, E.; Nout, R.; Smit, V.T.; McAlpine, J.N.; McConechy, M.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; et al. Clinicopathological and molecular characterisation of ’multiple-classifier’ endometrial carcinomas. J. Pathol. 2020, 250, 312–322. [Google Scholar] [CrossRef]
- Cavaliere, A.F.; Perelli, F.; Zaami, S.; Piergentili, R.; Mattei, A.; Vizzielli, G.; Scambia, G.; Straface, G.; Restaino, S.; Signore, F. Towards personalized medicine: Non-coding RNAs and endometrial cancer. Healthcare 2021, 9, 965. [Google Scholar] [CrossRef] [PubMed]
- Vallone, C.; Rigon, G.; Gulia, C.; Baffa, A.; Votino, R.; Morosetti, G.; Zaami, S.; Briganti, V.; Catania, F.; Gaffi, M.; et al. Non-coding RNAs and endometrial cancer. Genes 2018, 9, 187. [Google Scholar] [CrossRef]
- Piergentili, R.; Gullo, G.; Basile, G.; Gulia, C.; Porrello, A.; Cucinella, G.; Marinelli, E.; Zaami, S. Circulating miRNAs as a tool for early diagnosis of endometrial cancer: Implications for the fertility-sparing process: Clinical, biological, and legal aspects. Int. J. Mol. Sci. 2023, 24, 11356. [Google Scholar] [CrossRef]
- Piergentili, R.; Basile, G.; Nocella, C.; Carnevale, R.; Marinelli, E.; Patrone, R.; Zaami, S. Using ncRNAs as tools in cancer diagnosis and treatment: The way towards personalized medicine to improve patients’ health. Int. J. Mol. Sci. 2022, 23, 9353. [Google Scholar] [CrossRef]
- Saw, P.E.; Xu, X.; Chen, J.; Song, E.W. Non-coding RNAs: The new central dogma of cancer biology. Sci. China Life Sci. 2021, 64, 22–50. [Google Scholar] [CrossRef] [PubMed]
- Piergentili, R.; Zaami, S.; Cavaliere, A.F.; Signore, F.; Scambia, G.; Mattei, A.; Marinelli, E.; Gulia, C.; Perelli, F. Non-coding RNAs as prognostic markers for endometrial cancer. Int. J. Mol. Sci. 2021, 22, 3151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Ren, C.; Yao, Y.; Wang, Q.; Li, F.; Li, Y.; Jiang, A.; Wang, G. Identifying prognostic biomarkers in endometrial carcinoma based on ceRNA network. J. Cell Biochem. 2020, 121, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Delangle, R.; De Foucher, T.; Larsen, A.K.; Sabbah, M.; Azais, H.; Bendifallah, S.; Darai, E.; Ballester, M.; Mehats, C.; Uzan, C.; et al. The use of microRNAs in the management of endometrial cancer: A meta-analysis. Cancers 2019, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, J.; Sabbah, M.; Castela, M.; Mehats, C.; Uzan, C.; Canlorbe, G. Clinical value and molecular function of circulating microRNAs in endometrial cancer regulation: A systematic review. Cells 2022, 11, 1836. [Google Scholar] [CrossRef] [PubMed]
| Case No | Age (Years) | Initial Bx Dx | Surgical Procedure | Initial Hysterectomy Dx | Initial Serosal Extension | Initial LN Metastasis | Initial Distant Metastasis | Initial Stage | Post-Operative Treatment | Post-Treatment Recurrence | Initial or Recurrent Lung Metastasis | RFS (Months) | Survival Status | OS (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | ADC | TH, BSO | MLA | Yes | No | No | IIIB | CCRT | Yes | Yes | 1.1 | Dead | 28.2 |
| 2 | 65 | MLA | TH, BSO, PLND, PALND | MLA | No | No | No | IB | RT | Yes | Yes | 51.6 | Alive | 63.6 |
| 3 | 70 | EC-G3 | TH, BSO, PLND, PALND, PRT | MLA | No | No | No | IB | RT | No | No | 66.5 | Alive | 66.5 |
| 4 | 53 | ADC/DDx | TH, BSO, PLND, PALNS | MLA | No | No | No | IA | No | No | No | 63.5 | Alive | 63.5 |
| 5 | 60 | CS | TH, BSO, PLND, PALND | CS | No | No | No | IA | CTx | Yes | Yes | 8.0 | Dead | 25.1 |
| 6 | 52 | ADC | RH, BSO, PLND, PALND | EC-G1 | No | No | No | IB | No | Yes | Yes | 8.9 | Dead | 85.6 |
| 7 | 61 | MLA | TH, BSO, PLND, PALND | MLA | No | No | No | IB | CTx | No | No | 63.1 | Alive | 63.1 |
| 8 | 65 | ADC/DDx | RH, RSO, PRT | MLA | Yes | No | Yes | IVB | CCRT | Yes | No | 1.3 | Alive | 53.8 |
| 9 | 61 | PDC | RH, BSO, PALND, COL, PRT, OMT, APP | MLA | Yes | No | Yes | IVB | CTx | Yes | Yes | 9.2 | Dead | 32.7 |
| 10 | 52 | EC-G2 | TH, BSO, PLND, PRT | MLA | No | Yes | No | IIIC | CCRT | No | No | 39.3 | Alive | 39.3 |
| 11 | 67 | CS | TH, BSO, PLND, PRT | CS | No | No | No | IB | No | Yes | Yes | 13.4 | Alive | 14.5 |
| 12 | 59 | ADC/DDx | TH, BSO, PLND, PALNS | MLA | No | No | No | IA | No | No | No | 60.0 | Alive | 60.0 |
| 13 | 75 | CS | TH, BSO, PALNS | CS | No | No | No | IB | No | Yes | Yes | 15.6 | Dead | 17.3 |
| 14 | 55 | EC-G2 | TH, BSO, OMT | EC-G2 | No | No | Yes | IVB | CCRT | Yes | Yes | 15.7 | Alive | 94.6 |
| 15 | 57 | ADC/DDx | RH, BSO, PLND, PALND, APP | CS | Yes | Yes | No | IIIC | CCRT | Yes | Yes | 12.1 | Alive | 65.4 |
| 16 | 52 | NA | TH, BSO, PLND, PALND, OMT, APP | MLA | No | No | No | IIIB | CCRT | No | No | 45.1 | Alive | 45.1 |
| 17 | 60 | EC-G2 | TH, BSO, PLND, PALND | EC-G3 | No | Yes | No | IIIB | CCRT | Yes | No | 16.1 | Dead | 35.3 |
| Case No | Tumor Location | SNV | CNV | |||||
|---|---|---|---|---|---|---|---|---|
| Gene | Sequence Change | Amino Acid Change | VAF (%) | Type | Gene Gain | Gene Loss | ||
| 1 | Uterus | KRAS | c.34G>T | p.G12C | 40.5 | MS | NTRK1, ARID1B, JAK2, GNAQ, RET, HNF1A, SRC, GNAS | JAK1, CTNNB1, PIK3CA, ROS1 |
| 2 | Uterus | KRAS | c.34G>T | p.G12C | 84.7 | MS | NTRK1, DDR2, ALK, ERBB4, IDH1, RET, FGFR2, MDM2, SRC | None |
| PTEN | c.804C>A | p.D268E | 20.6 | MS | ||||
| NF1 | c.4942A>G | p.T1648A | 42.2 | MS | ||||
| ARID1A | c.863_875del | p.Q288Pfs*71 | 60.3 | FS | ||||
| GNAQ | c.303C>A | p.Y101* | 7.9 | NS | ||||
| 3 | Uterus | KRAS | c.34G>T | p.G12C | 62.2 | MS | NTRK1, DDR2, ALK, ERBB4, FGFR2, RET, SRC, TOP1, GNAS | None |
| 4 | Uterus | KRAS | c.35G>T | p.G12V | 71.8 | MS | NTRK1, HNF1A, CSF1R, CDKN2A, GNAQ, PTCH1, JAK3 | None |
| TP53 | c.210_211insG | p.P71fs*78 | 47.6 | FS | ||||
| RB1 | c.1861C>A | p.R621S | 52.5 | MS | ||||
| GNAQ | c.303C>A | p.Y101* | 6.6 | NS | ||||
| 5 | Uterus | KRAS | c.37G>T | p.G13C | 78.4 | MS | NTRK1, DDR2, ALK, ERBB4, CSF1R, CDKN2A, GNAQ, FGFR2, TOP1, GNAS | None |
| TP53 | c.216delC | p.V73fs | 72.3 | FS | ||||
| 6 | Uterus | KRAS | c.35G>A | p.G12D | 97.4 | MS | NA | NA |
| BCR | c.3316G>A | p.D1106N | 9.8 | MS | ||||
| 7 | Uterus | KRAS | c.35G>T | p.G12V | 39.9 | MS | NA | NA |
| CTNNB1 | c.134C>T | p.S45F | 4.1 | MS | ||||
| IDH1 | c.623A>G | p.Y208C | 53 | MS | ||||
| GNAQ | c.303C>A | p.Y101* | 7.9 | NS | ||||
| 8 | Uterus | KRAS | c.53G>C | p.G12A | 65.9 | MS | NA | NA |
| RB1 | c.1666C>T | p.R556* | 28.1 | NS | ||||
| BCR | c.3316G>A | p.D1106N | 10.6 | MS | ||||
| 9 | Uterus | KRAS | c.35G>T | p.G12V | 58 | MS | NA | NA |
| 10 | Uterus | KRAS | c.35G>A | p.G12D | 61.2 | MS | NA | NA |
| PIK3CA | c.1633G>A | p.E545K | 41.3 | MS | ||||
| CDH1 | c.2638G>A | p.E880K | 6.8 | MS | ||||
| 11 | Uterus | PIK3CA | c.317G>T | p.G106V | 49.2 | MS | NA | NA |
| NF1 | c.2991-1G>C | NA | 21.1 | SS | ||||
| 12 | Uterus | PTEN | c.804C>A | p.D268E | 17.9 | MS | NA | NA |
| PIK3R1 | c.1690A>G | p.N564D | 6.4 | MS | ||||
| 13 | Uterus | PTEN | c.70G>C | p.D24H | 95.5 | MS | NA | NA |
| PIK3CA | c.2740G>A | p.G914R | 94.8 | MS | ||||
| 14-1 | Uterus | KRAS | c.35G>T | p.G12V | 56.56 | MS | NTRK1, DNMT3A, RET, FGFR2, RAB35, POLE | JAK1 |
| 14-2 | Lung | KRAS | c.35G>T | p.G12V | 39.31 | MS | NTRK1, DDR2, ABL2, FGFR2, ERBB3, POLE | None |
| 15-1 | Uterus | KRAS | c.35G>A | p.G12D | 83.25 | MS | NTRK1, MDM4, AKT3, ALK, DNMT3A, MYCN, ERBB4, RET, FGFR2 | None |
| 15-2 | LN | KRAS | c.35G>A | p.G12D | 45.73 | MS | ALK, DNMT3A, MAP3K4, FGFR2 | None |
| 16-1 | Uterus | KRAS | c.35G>A | p.G12D | 12.52 | MS | DDR2, SMAD4 | JAK2 |
| 16-2 | Ovary | KRAS | c.35G>A | p.G12D | 34.27 | MS | NTRK1, DDR2, ABL2, DNMT3A, FGFR2 | None |
| 17-1 | Uterus | PTEN | c.892C>T | p.Q298* | 32.56 | NS | DDR2, ABL2, MDM4 | None |
| 17-2 | LN | PTEN | c.892C>T | p.Q298* | 28.09 | NS | NTRK1, DDR2 | None |
| Case No | MSI Status | PD-L1 22C3 Pharmdx CPS | MMR IHC | TMB (per Mb) |
|---|---|---|---|---|
| 1 | MSS | 0 | MMR-proficient | 3.28 |
| 2 | MSS | 0 | MMR-proficient | 3.99 |
| 3 | MSS | 0 | MMR-proficient | 4.05 |
| 4 | MSS | 0 | MMR-proficient | 4.15 |
| 5 | MSS | 0 | MMR-proficient | 4.38 |
| 6 | MSS | 0 | MMR-proficient | 2.70 |
| 7 | NA | 0 | MMR-proficient | NA |
| 8 | NA | 0 | MMR-proficient | NA |
| 9 | MSS | 0 | MMR-proficient | 3.44 |
| 10 | MSS | 0 | MMR-proficient | 3.47 |
| 11 | MSS | 0.5 | MMR-proficient | 3.51 |
| 12 | MSS | 0.5 | MMR-proficient | 3.54 |
| 13 | MSS | 0 | MMR-proficient | 3.57 |
| 14 | MSS | 0 | MMR-proficient | 3.67 |
| 15 | MSS | 0.1 | MMR-proficient | 4.15 |
| 16 | MSS | 0 | MMR-proficient | 4.72 |
| 17 | MSS | 0 | MMR-proficient | 3.38 |
| Characteristic | KRAS | PTEN | PIK3CA | PTEN or PIK3CA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild Type | Mutant | p-Value | Wild Type | Mutant | p-Value | Wild Type | Mutant | p-Value | Wild Type | Mutant | p-Value | ||
| Age (years) | <60 | 1 (12.5) | 7 (87.5) | 0.66 | 7 (87.5) | 1 (12.5) | 0.66 | 7 (87.5) | 1 (12.5) | 1.00 | 6 (75.0) | 2 (25.0) | 0.74 |
| ≥60 | 3 (33.3) | 6 (66.7) | 6 (66.7) | 3 (33.3) | 7 (77.8) | 2 (22.2) | 5 (55.6) | 4 (44.4) | |||||
| Initial serosal extension | No | 4 (30.8) | 9 (69.2) | 0.55 | 9 (69.2) | 4 (30.8) | 0.55 | 10 (76.9) | 3 (23.1) | 0.76 | 7 (53.8) | 6 (46.2) | 0.28 |
| Yes | 0 (0.0) | 4 (100.0) | 4 (100.0) | 0 (0.0) | 4 (100.0) | 0 (0.0) | 4 (100.0) | 0 (0.0) | |||||
| Initial lymph node metastasis | No | 4 (26.7) | 11 (73.3) | 1.00 | 11 (73.3) | 4 (26.7) | 1.00 | 13 (86.7) | 2 (13.3) | 0.77 | 10 (66.7) | 5 (33.3) | 1.00 |
| Yes | 0 (0.0) | 2 (100.0) | 2 (100.0) | 0 (0.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | |||||
| Initial distant metastasis | No | 4 (28.6) | 10 (71.4) | 0.76 | 10 (71.4) | 4 (28.6) | 0.76 | 11 (78.6) | 3 (21.4) | 0.96 | 8 (57.1) | 6 (42.9) | 0.46 |
| Yes | 0 (0.0) | 3 (100.0) | 3 (100.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | |||||
| Initial or recurrent lung metastasis | No | 2 (25.0) | 6 (75.0) | 1.00 | 6 (75.0) | 2 (25.0) | 1.00 | 7 (87.5) | 1 (12.5) | 1.00 | 5 (62.5) | 3 (37.5) | 1.00 |
| Yes | 2 (22.2) | 7 (77.8) | 7 (77.8) | 2 (22.2) | 7 (77.8) | 2 (22.2) | 6 (66.7) | 3 (33.3) | |||||
| Initial stage | I | 3 (33.3) | 6 (66.7) | 0.49 | 6 (66.7) | 3 (33.3) | 0.49 | 7 (77.8) | 2 (22.2) | 0.67 | 5 (55.6) | 4 (44.4) | 0.37 |
| II | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| III | 1 (20.0) | 4 (80.0) | 4 (80.0) | 1 (20.0) | 4 (80.0) | 1 (20.0) | 3 (60.0) | 2 (40.0) | |||||
| IV | 0 (0.0) | 3 (100.0) | 3 (100.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | |||||
| Post-treatment recurrence | No | 1 (16.7) | 5 (83.3) | 1.00 | 5 (83.3) | 1 (16.7) | 1.00 | 5 (83.3) | 1 (16.7) | 1.00 | 4 (66.7) | 2 (33.3) | 1.00 |
| Yes | 3 (27.3) | 8 (72.7) | 8 (72.7) | 3 (27.3) | 9 (81.8) | 2 (18.2) | 7 (63.6) | 4 (36.4) | |||||
| Characteristic | Recurrence-Free Survival | Overall Survival | |||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||
| p-Value | p-Value | HR (95% CI) | p-Value | p-Value | HR (95% CI) | ||
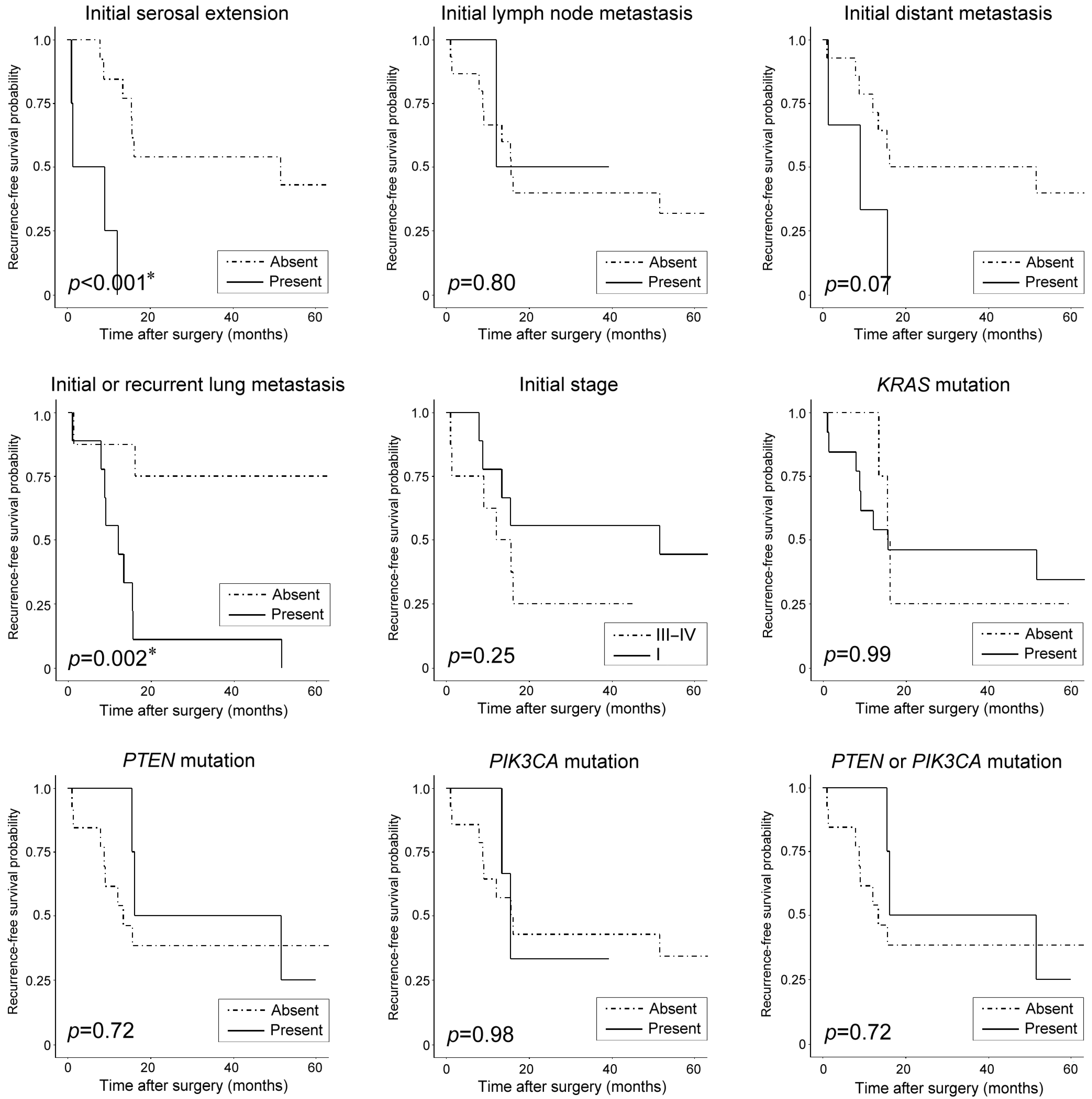
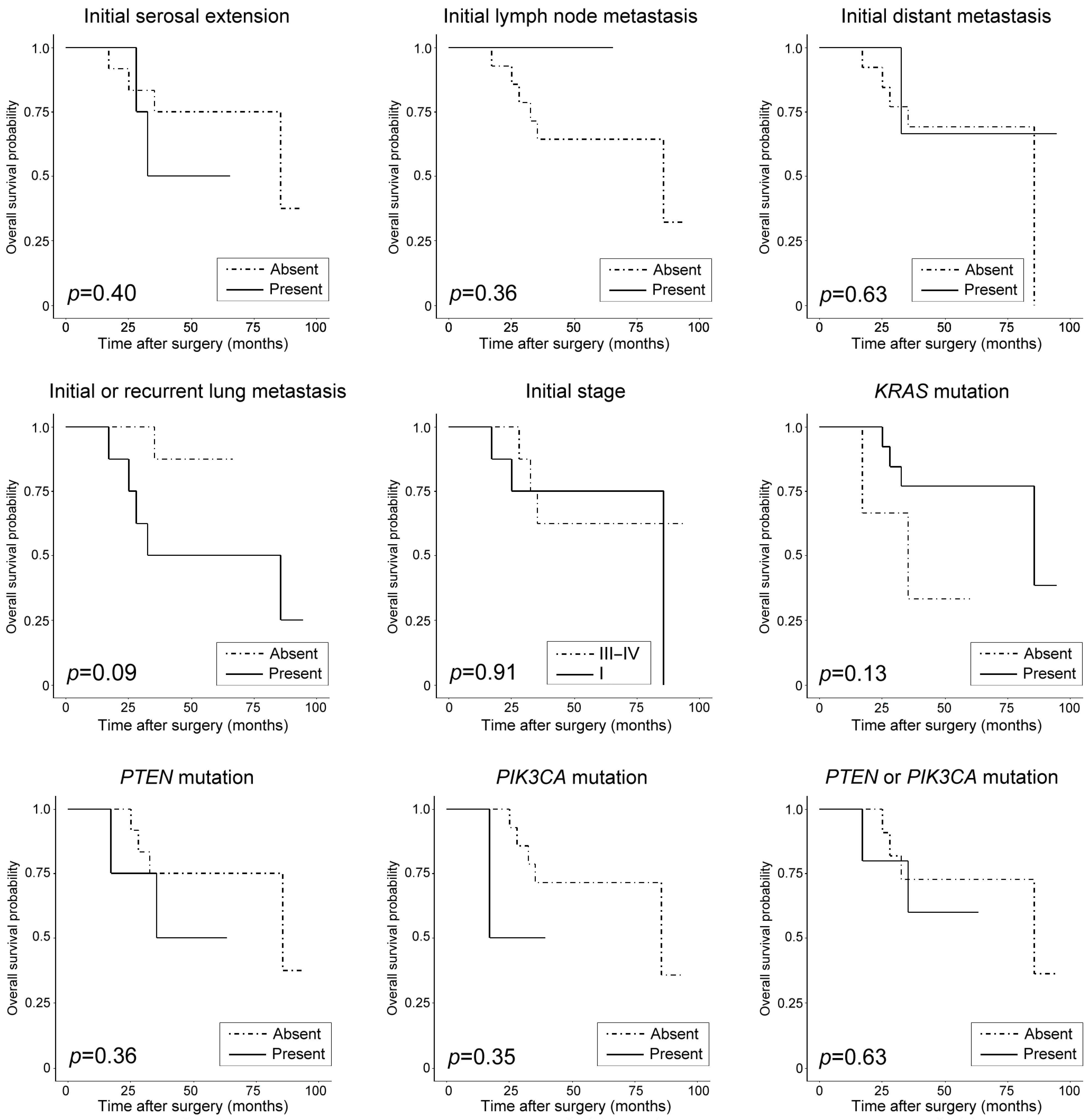
| Initial serosal extension | Present | <0.001 * | 0.037 * | 6.30 (1.12–35.45) | 0.40 | NA | NA |
| Absent | |||||||
| Initial lymph node metastasis | Present | 0.80 | NA | NA | 0.36 | NA | NA |
| Absent | |||||||
| Initial distant metastasis | Present | 0.07 | NA | NA | 0.63 | NA | NA |
| Absent | |||||||
| Initial or recurrent lung metastasis | Present | 0.002 * | 0.02 * | 7.31 (1.38–38.75) | 0.09 | NA | NA |
| Absent | |||||||
| Initial stage | III−IV | 0.25 | NA | NA | 0.91 | NA | NA |
| I−II | |||||||
| KRAS mutation | Present | 0.99 | NA | NA | 0.13 | NA | NA |
| Absent | |||||||
| PTEN mutation | Present | 0.72 | NA | NA | 0.36 | NA | NA |
| Absent | |||||||
| PIK3CA mutation | Present | 0.98 | NA | NA | 0.35 | NA | NA |
| Absent | |||||||
| PTEN or PIK3CA mutation | Present | 0.72 | NA | NA | 0.63 | NA | NA |
| Absent | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, H.-H.; Park, E.; Kim, H.-S. Mesonephric-like Adenocarcinoma of the Uterine Corpus: Genomic and Immunohistochemical Profiling with Comprehensive Clinicopathological Analysis of 17 Consecutive Cases from a Single Institution. Biomedicines 2023, 11, 2269. https://doi.org/10.3390/biomedicines11082269
Koh H-H, Park E, Kim H-S. Mesonephric-like Adenocarcinoma of the Uterine Corpus: Genomic and Immunohistochemical Profiling with Comprehensive Clinicopathological Analysis of 17 Consecutive Cases from a Single Institution. Biomedicines. 2023; 11(8):2269. https://doi.org/10.3390/biomedicines11082269
Chicago/Turabian StyleKoh, Hyun-Hee, Eunhyang Park, and Hyun-Soo Kim. 2023. "Mesonephric-like Adenocarcinoma of the Uterine Corpus: Genomic and Immunohistochemical Profiling with Comprehensive Clinicopathological Analysis of 17 Consecutive Cases from a Single Institution" Biomedicines 11, no. 8: 2269. https://doi.org/10.3390/biomedicines11082269
APA StyleKoh, H.-H., Park, E., & Kim, H.-S. (2023). Mesonephric-like Adenocarcinoma of the Uterine Corpus: Genomic and Immunohistochemical Profiling with Comprehensive Clinicopathological Analysis of 17 Consecutive Cases from a Single Institution. Biomedicines, 11(8), 2269. https://doi.org/10.3390/biomedicines11082269





