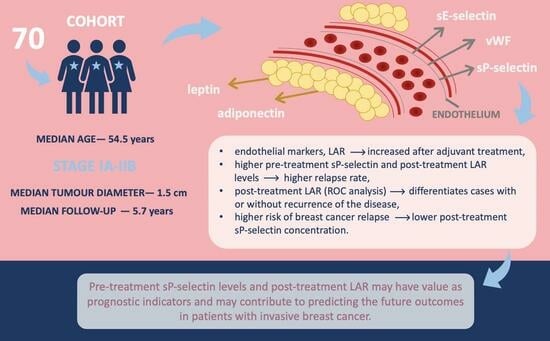
Complex Analysis of Endothelial Markers as Potential Prognostic Indicators in Luminal Invasive Breast Carcinoma Patients: Outcomes of a Six-Year Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrolment
2.2. Tumor Characteristics
2.3. Follow-Up
2.4. Ethics Statement
2.5. Treatment Requirements
2.6. Sample Collection and Analysis
2.6.1. Leptin-to-Adiponectin Ratio
2.6.2. Serum sE-Selectin Measurements
2.6.3. Serum sP-Selectin Analysis
2.6.4. Von Willebrand Factor Antigen Measurements
2.6.5. Immunohistochemistry (IHC) Analysis
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. LAR Levels Prior to and after Treatment in Relation to the Types of Therapy
3.3. Patients’ Treatment in Relation to Their Pre- and Post-Treatment sE-Selectin Concentrations
3.4. Relationship between sP-Selectin Concentrations before and after Treatment
3.5. vWF Concentrations Prior to and after Treatment in Relation to the Types of Therapy
3.6. Association between Endothelial Markers and Pre-Treatment LAR Values
3.7. Association between Endothelial Markers and Post-Treatment LAR Values
3.8. Correlation Analysis of Clinical Parameters before and after Treatment
3.9. Association of the Analysed Parameters with DFS in Linear Regression
3.10. Survival Analysis Regarding Pre- and Post-Treatment LAR and Endothelial Markers
3.11. Univariate and Multivariate Cox Proportional Hazards Regression Models Applied to Determine Prognostic Values of Tested Parameters
| Variable | Multivariate | Univariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Pre-Treatment LAR valueLowHigh | Medians | 11.32 | 0.0371 | 1.09 | 0.8838 |
| (1.16–110.81) | (0.33–3.58) | ||||
| ROC cut-off points | 2.28 | 0.4423 | 1.11 | 0.8643 | |
| (0.28–18.80) | (0.33–3.81) | ||||
| Post-Treatment LAR valueLowHigh | Medians | 10.32 | 0.0274 | 4.7 | 0.0477 |
| (1.30–82.12) | (1.02–21.78) | ||||
| ROC cut-off points | 10.32 | 0.0274 | 4.7 | 0.0477 | |
| (1.30–82.12) | (1.02–21.78) | ||||
| Pre-Treatment sE-Selectin ConcentrationLowHigh | Medians | 0.52 | 0.3958 | 0.55 | 0.3377 |
| (0.12–2.33) | (0.16–1.87) | ||||
| ROC cut-off points | 0.18 | 0.0334 | 0.39 | 0.1376 | |
| (0.04–0.88) | (0.12–1.35) | ||||
| Post-Treatment sE-Selectin ConcentrationLowHigh | Medians | 0.99 | 0.9849 | 0.81 | 0.7233 |
| (0.25–3.87) | (0.25–2.65) | ||||
| ROC cut-off points | 0.99 | 0.9849 | 0.81 | 0.7233 | |
| (0.25–3.87) | (0.25–2.65) | ||||
| Pre-Treatment sP-Selectin ConcentrationLowHigh | Medians | 3.31 | 0.1808 | 4.48 | 0.0555 |
| (0.57–19.10) | (0.97–20.82) | ||||
| ROC cut-off points | 5.92 | 0.1162 | 7.55 | 0.0543 | |
| (0.64–54.48) | (0.96–59.19) | ||||
| Post-Treatment sP-Selectin ConcentrationLowHigh | Medians | 0.58 | 0.5649 | 1.66 | 0.4257 |
| (0.09–3.65) | (0.47–5.77) | ||||
| ROC cut-off points | 2.81 | 0.1948 | 2.75 | 0.1081 | |
| (0.59–13.41) | (0.80–9.48) | ||||
| Pre-Treatment vWF ConcentrationLowHigh | Medians | 0.43 | 0.2115 | 0.39 | 0.1343 |
| (0.11–1.62) | (0.11–1.34) | ||||
| ROC cut-off points | 0.22 | 0.088 | 0.26 | 0.0841 | |
| (0.04–1.26) | (0.06–1.20) | ||||
| Post-Treatment vWF ConcentrationLowHigh | Medians | 2.58 | 0.2546 | 1.82 | 0.3396 |
| (0.51–13.17) | (0.53–6.22) | ||||
| ROC cut-off points | 6.26 | 0.0681 | 2.16 | 0.2029 | |
| (0.87–44.94) | (0.66–7.09) | ||||
4. Discussion
4.1. Endothelial Markers before and after Treatment Depending on the Type of Treatment
4.2. Endothelial Markers’ Relationship with LAR Concentrations before and after Treatment
4.3. Regression Model Analysis of Research Variables as Prognostic Indicators
4.4. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Denderen, B.J.; Thompson, E.W. Cancer: The to and fro of tumour spread. Nature 2013, 493, 487–488. [Google Scholar] [PubMed]
- Xu, B.; Hu, X.; Feng, J.; Geng, C.; Jin, F.; Li, H.; Li, M.; Li, Q.; Liao, N.; Liu, D.; et al. Chinese expert consensus on the clinical diagnosis and treatment of advanced breast cancer (2018). Cancer 2020, 126, 3867–3882. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J. Extending Survival with Chemotherapy in Metastatic Breast Cancer. Oncologist 2005, 10, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Mu, P. Targeting Breast Cancer Metastasis. Breast Cancer 2015, 9 (Suppl. S1), 23–34. [Google Scholar] [PubMed]
- Guo, Y.; Arciero, C.A.; Jiang, R.; Behera, M.; Peng, L.; Li, X. Different Breast Cancer Subtypes Show Different Metastatic Patterns: A Study from A Large Public Database. Asian Pac. J. Cancer Prev. 2020, 21, 3587–3593. [Google Scholar] [CrossRef]
- Gong, T.-T.; Wu, Q.-J.; Wang, Y.-L.; Ma, X.-X. Circulating adiponectin, leptin and adiponectin-leptin ratio and endometrial cancer risk: Evidence from a meta-analysis of epidemiologic studies. Int. J. Cancer 2015, 137, 1967–1978. [Google Scholar] [CrossRef]
- Agostinis-Sobrinho, C.; Vicente, S.E.C.F.; Norkiene, S.; Rauckienė-Michaelsson, A.; Kievisienė, J.; Dubey, V.P.; Razbadauskas, A.; Lopes, L.; Santos, R. Is the Leptin/Adiponectin Ratio a Better Diagnostic Biomarker for Insulin Resistance than Leptin or Adiponectin Alone in Adolescents? Children 2022, 9, 1193. [Google Scholar] [CrossRef]
- Borsig, L.; Laubli, H. Cell Adhesion during Tumorigenesis and Metastasis. In Encyclopedia of Cancer, 3rd ed.; Boffetta, P., Hainaut, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 307–314. [Google Scholar]
- Drnasfield, I.; Stocks, S.C.; Haslett, C. Regulation of cell adhesion molecule expression and function associated with neutrophil apoptosis. Blood 1995, 85, 3264–3273. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Lee, S.; YChoi, H.; Cho, S.G. The Impact of Adhesion Molecules on the In Vitro Culture and Differentiation of Stem Cells. Biotechnol. J. 2018, 13, 1700575. [Google Scholar]
- Ley, K. Functions of selectins. In Mammalian Carbohydate Recognition System; Springer: Berlin/Heidelberg, Germany, 2001; Volume 33, pp. 177–200. [Google Scholar] [CrossRef]
- Tinoco, R.; Otero, D.C.; Takahashi, A.A.; Bradley, L.M. PSGL-1: A new player in the immune checkpoint landscape. Trends Immunol. 2017, 38, 323–335. [Google Scholar] [CrossRef]
- Alves, C.S.; Burdick, M.M.; Thomas, S.N.; Pawar, P.; Konstantopoulos, K. The dual role of CD44 as a functional P-selectin ligand and fibrin receptor in colon carcinoma cell adhesion. Am. J. Physiol.-Cell Physiol. 2008, 294, C907–C916. [Google Scholar] [CrossRef] [PubMed]
- Mannori, G.; Crottet, P.; Cecconi, O.; Hanasaki, K.; Aruffo, A.; Nelson, R.M.; Varki, A.; Bevilacqua, M.P. Differential colon cancer cell adhesion to E-, P-, and L-selectin: Role of mucin-type glycoproteins. Cancer Res. 1995, 55, 4425–4431. [Google Scholar] [PubMed]
- Gout, S.; Morin, C.; Houle, F.; Huot, J. Death receptor-3, a new E-Selectin counter-receptor that confers migration and survival advantages to colon carcinoma cells by triggering p38 and ERK MAPK activation. Cancer Res. 2006, 66, 9117–9124. [Google Scholar] [CrossRef] [PubMed]
- Schrijver, I.T.; Kemperman, H.; Roest, M.; Kesecioglu, J.; de Lange, D.W. Soluble P-selectin as a Biomarker for Infection and Survival in Patients with a Systemic Inflammatory Response Syndrome on the Intensive Care Unit. Biomark. Insights 2017, 12, 1177271916684823. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.H.; Coupland, L.A.; Freeman, C.; Chong, B.H.; Parish, C.R. Activation of tumour cell ECM degradation by thrombin-activated platelet membranes: Potentially a P-selectin and GPIIb/IIIa-dependent process. Clin. Exp. Metastasis 2015, 32, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Coupland, L.A.; Chong, B.H.; Parish, C.R. Platelets and P-selectin control tumor cell metastasis in an organ-specific manner and independently of NK cells. Cancer Res. 2012, 72, 4662–4671. [Google Scholar] [CrossRef]
- Franchini, M.; Frattini, F.; Crestani, S.; Bonfanti, C.; Lippi, G. von Willebrand factor and cancer: A renewed interest. Thromb. Res. 2013, 13, 290–292. [Google Scholar] [CrossRef]
- Dhami, S.P.S.; Patmore, S.; Comerford, C.; Byrne, C.M.; Cavanagh, B.; Castle, J.; Kirwan, C.C.; Kenny, M.; Schoen, I.; O’Donnell, J.S.; et al. Breast cancer cells mediate endothelial cell activation, promoting von Willebrand factor release, tumor adhesion, and transendothelial migration. J. Thromb. Haemost. 2022, 20, 2350–2365. [Google Scholar] [CrossRef]
- Rhone, P.; Zarychta, E.; Bielawski, K.; Ruszkowska-Ciastek, B. Pre-surgical level of von Willebrand factor as an evident indicator of breast cancer recurrence. Cancer Biomark. 2020, 29, 359–372. [Google Scholar] [CrossRef]
- Nalabolu, M.R.; Palasamudram, K.; Jamil, K. Adiponectin and leptin molecular actions and clinical significance in breast cancer. Int. J. Hematol. Oncol. Stem Cell Res. 2014, 8, 31–40. [Google Scholar]
- Dal Maso, L.; Augustin, L.S.; Karalis, A.; Talamini, R.; Franceschi, S.; Trichopoulos, D.; Mantzoros, C.S.; La Vecchia, C. Circulating adiponectin and endometrial cancer risk. J. Clin. Endocrinol. Metab. 2004, 89, 1160–1163. [Google Scholar]
- Goktas, S.; Yilmaz, M.I.; Caglar, K.; Sonmez, A.; Kilic, S.; Bedir, S. Prostate cancer and adiponectin. Urology 2005, 65, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Słomian, G.J.; Nowak, D.; Buczkowska, M.; Głogowska-Gruszka, A.; Słomian, S.P.; Roczniak, W.; Janyga, S.; Nowak, P. The role of adiponectin and leptin in the treatment of ovarian cancer patients. Endokrynol. Pol. 2019, 70, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Iwan-Zietek, I.; Ruszkowska-Ciastek, B.; Michalska, M.; Overskaug, E.; Goralczyk, K.; Dabrowiecki, S.; Róść, D. Association of adiponectin and leptin-to-adiponectin ratio with the function of platelets in morbidly obese patients. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2016, 67, 555–561. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.D.; Gao, D.; Redfern, A.; Thompson, E.W. Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat. Rev. Cancer 2019, 19, 716–732. [Google Scholar] [CrossRef]
- Park, M.; Kim, D.; Ko, S.; Kim, A.; Mo, K.; Yoon, H. Breast Cancer Metastasis: Mechanisms and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 6806. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, R.; Tang, J.; Wu, X.; Zhu, L.; Huang, H.; Chen, H.; Xiao, M.; Luo, H.; Zheng, H.; et al. Prognostic Observational Analysis of BMI, Leptin, and Adiponectin in Children With Acute Lymphocytic Leukemia Undergoing Remission-Induction Chemotherapy. Front. Pediatr. 2022, 10, 797836. [Google Scholar] [CrossRef]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar]
- Christodoulatos, G.S.; Spyrou, N.; Kadillari, J.; Psallida, S.; Dalamaga, M. The role of adipokines in breast cancer: Current evidence and perspectives. Curr. Obes. Rep. 2019, 8, 413–433. [Google Scholar]
- Siemińska, L.; Borowski, A.; Marek, B.; Nowak, M.; Kajdaniuk, D.; Warakomski, J.; Kos-Kudła, B. Serum concentrations of adipokines in men with prostate cancer and benign prostate hyperplasia. Endokrynol. Pol. 2018, 69, 120–127. [Google Scholar]
- Morad, V.; Abrahamsson, A.; Dabrosin, C. Estradiol affects extracellular leptin:adiponectin ratio in human breast tissue in vivo. J. Clin. Endocrinol. Metab. 2014, 99, 3460–3467. [Google Scholar] [CrossRef]
- Kang, S.A.; Blache, C.A.; Bajana, S.; Hasan, N.; Kamal, M.; Morita, Y.; Gupta, V.; Tsolmon, B.; Suh, K.S.; Gorenstein, D.G.; et al. The effect of soluble E-selectin on tumor progression and metastasis. BMC Cancer 2016, 16, 331. [Google Scholar]
- Ramcharan, K.S.; Lip, G.Y.; Stonelake, P.S.; Blann, A.D. Effect of standard chemotherapy and antiangiogenic therapy on plasma markers and endothelial cells in colorectal cancer. Br. J. Cancer 2014, 111, 1742–1749. [Google Scholar] [CrossRef]
- Mann, A.P.; Tanaka, T. E-selectin: Its role in cancer and potential as a biomarker. Transl. Med. 2011, S1, 002. [Google Scholar]
- Muz, B.; Abdelghafer, A.; Markovic, M.; Yavner, J.; Melam, A.; Salama, N.N.; Azab, A.K. Targeting E-selectin to Tackle Cancer Using Uproleselan. Cancers 2021, 13, 335. [Google Scholar] [CrossRef]
- Mills, P.J.; Ancoli-Israel, S.; Parker, B.; Natarajan, L.; Hong, S.; Jain, S.; Sadler, G.R.; von Känel, R. Predictors of inflammation in response to anthracycline-based chemotherapy for breast cancer. Brain Behav. Immun. 2008, 22, 98–104. [Google Scholar] [CrossRef]
- Gil-Bazo, I.; Catalán Goni, V.; Alonso Gutiérrez, A.; Rodríguez Rodríguez, J.; Páramo Fernández, J.A.; de la Cámara Gómez, J.; Hernández Lizoain, J.L.; García-Foncillas López, J. Impact of surgery and chemotherapy on von Willebrand factor and vascular endothelial growth factor levels in colorectal cancer. Clin. Transl. Oncol. Off. Public Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2005, 7, 150–155. [Google Scholar] [CrossRef]
- Giri, S.; Bose, J.C.; Chandrasekar, A.; Tiwary, B.K.; Gajalakshmi, P.; Chatterjee, S. Increased Plasma Nitrite and von Willebrand Factor Indicates Early Diagnosis of Vascular Diseases in Chemotherapy Treated Cancer Patients. Cardiovasc. Toxicol. 2019, 19, 36–47. [Google Scholar] [CrossRef]
- Liu, J.; Lin, P.C.; Zhou, B.P. Inflammation fuels tumor progress and metastasis. Curr. Pharm. Des. 2015, 21, 3032–3040. [Google Scholar] [CrossRef]
- Maślanka, K. The role of platelets in inflammatory processes. J. Transfus. Med. 2014, 7, 102–109. [Google Scholar]
- Weller, A.; Isenmann, S.; Vestweber, D. Cloning of the mouse endothelial selectins. Expression of both E- and P-selectin is inducible by tumor necrosis factor alpha. J. Biol. Chem. 1992, 267, 15176–15183. [Google Scholar] [CrossRef]
- Woollard, K.J.; Chin-Dusting, J. P-selectin antagonism in inflammatory disease. Curr. Pharm. Des. 2010, 16, 4113–4118. [Google Scholar] [CrossRef]
- Ay, C.; Simanek, R.; Vormittag, R.; Dunkler, D.; Alguel, G.; Koder, S.; Kornek, G.; Marosi, C.; Wagner, O.; Zielinski, C.; et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: Results from the Vienna Cancer and Thrombosis Study (CATS). Blood 2008, 112, 2703–2708. [Google Scholar] [CrossRef]
- den Berg, Y.W.; Osanto, S.; Reitsma, P.H.; Versteeg, H.H. The relationship between tissue factor and cancer progression: Insights from bench and bedside. Blood 2012, 119, 924–932. [Google Scholar]
- Ferroni, P.; Roselli, M.; Martini, F.; D’Alessandro, R.; Mariotti, S.; Basili, S.; Spila, A.; Aloe, S.; Palmirotta, R.; Maggini, A.; et al. Prognostic value of soluble P-selectin levels in colorectal cancer. Int. J. Cancer 2004, 111, 404–408. [Google Scholar] [CrossRef]
- Graf, M.E.; Sookthai, D.; Johnson, T.; Schübel, R.; González Maldonado, S.; Pletsch-Borba, L.; Katzke, V.; Bugert, P.; Hoffmeister, M.; Kaaks, R.; et al. Pre-diagnostic plasma concentrations of Fibrinogen, sGPIIb/IIIa, sP-selectin, sThrombomodulin, Thrombopoietin in relation to cancer risk: Findings from a large prospective study. Int. J. Cancer 2018, 143, 2659–2667. [Google Scholar]
- Alexandrakis, M.G.; Passam, F.H.; Sfiridaki, K.; Kafarakis, P.; Karydi, E.; Liapi, D.; Perisinakis, K.; Kyriakou, D.S. Clinical significance of circulating endothelial adhesion molecules (sE-selectin and sICAM) in untreated multiple myeloma patients. Clin. Chim. Acta 2004, 349, 39–43. [Google Scholar] [CrossRef]
- Diaz, E.S.; Karlan, B.Y.; Li, A.J. Obesity-associated adipokines correlate with survival in epithelial ovarian cancer. Gynecol. Oncol. 2013, 129, 353–357. [Google Scholar] [CrossRef]
- Terraube, V.; Pendu, R.; Baruch, D.; Gebbink, M.F.; Meyer, D.; Lenting, P.J.; Denis, C.V. Increased metastatic potential of tumor cells in von Willebrand factor-deficient mice. J. Thromb. Haemost. JTH 2006, 4, 519–526. [Google Scholar] [CrossRef]
- Tigges, U.; Hyer, E.G.; Scharf, J.; Stallcup, W.B. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development 2008, 35, 523–532. [Google Scholar] [CrossRef]
- Pépin, M.; Kleinjan, A.; Hajage, D.; Büller, H.R.; Di Nisio, M.; Kamphuisen, P.W.; Salomon, L.; Veyradier, A.; Stepanian, A.; Mahé, I. ADAMTS-13 and von Willebrand factor predict venous thromboembolism in patients with cancer. J. Thromb. Haemost. 2016, 14, 306–315. [Google Scholar] [CrossRef] [PubMed]
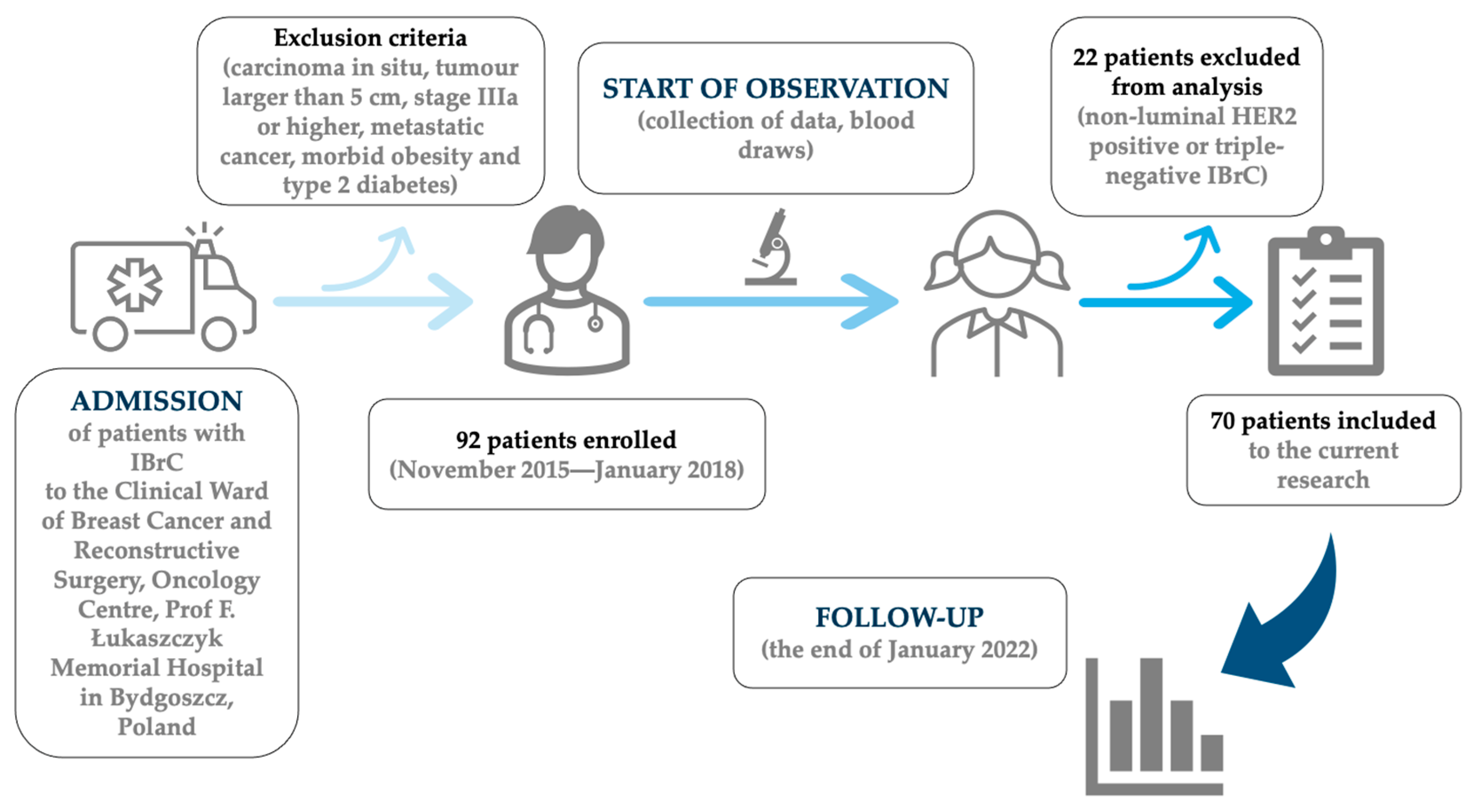





| Demographic and Clinical Data | Overall (n = 70) | Patients without Progression (n = 59) | Patients with Progression (n = 11) |
|---|---|---|---|
| n (%) | |||
| Age | |||
| <55 years | 35 (50%) | 30 (51%) | 5 (45.5%) |
| >55 years | 35 (50%) | 29 (49%) | 6 (54.5%) |
| Menopausal status | |||
| Pre-menopausal | 26 (37%) | 22 (37%) | 4 (36%) |
| Post-menopausal | 44 (63%) | 37 (63%) | 7 (64%) |
| BMI (kg/m2) | |||
| Normal (18.5 ≤ 24.99) | 34 (48.6%) | 27 (46%) | 7 (64%) |
| Overweight (25 ≤ 29.99) | 23 (32.8%) | 22 (37%) | 1 (9%) |
| Obese (>30) | 13 (18.6%) | 10 (17%) | 3 (27%) |
| Parity status | |||
| 0 | 6 (9%) | 3 (5%) | 3 (27.2%) |
| 1–2 | 50 (71%) | 46 (78%) | 4 (36.4%) |
| 3 and more | 14 (20%) | 10 (17%) | 4 (36.4%) |
| Localization of tumor | |||
| Right breast | 36 (51%) | 31 (53%) | 5 (45.5%) |
| Left breast | 34 (49%) | 28 (47%) | 6 (54.5%) |
| Lymph node status | |||
| N0 | 53 (76%) | 46 (78%) | 7 (64%) |
| N1 | 17 (24%) | 13 (22%) | 4 (36%) |
| Histological type | |||
| IDC | 61 (87%) | 51 (86%) | 10 (91%) |
| ILC | 9 (13%) | 8 (14%) | 1 (9%) |
| TNM staging classification | |||
| T1 | 48 (69%) | 44 (75%) | 4 (36%) |
| T2 | 22 (31%) | 15 (25%) | 7 (64%) |
| Grade according to Elston–Ellis | |||
| 1 + 2 | 61 (87%) | 53 (90%) | 8 (73%) |
| 3 | 9 (13%) | 6 (10%) | 3 (27%) |
| Molecular type | |||
| Luminal A (HR+/HER2−/Ki-67 < 20%) | 50 (71%) | 46 (78%) | 4 (36%) |
| Luminal B (HR+/HER2−/Ki-67 ≥ 20%) | 16 (23%) | 10 (17%) | 6 (55%) |
| Luminal B HER2+ (HR+ HER2+) | 4 (6%) | 3 (5%) | 1 (9%) |
| Stage | |||
| I | 35 (50%) | 33 (56%) | 2 (18%) |
| II | 35 (50%) | 26 (44%) | 9 (82%) |
| Progesterone receptor (PR) | |||
| Negative | 5 (7%) | 3 (5%) | 2 (18%) |
| Positive | 65 (93%) | 56 (95%) | 9 (82%) |
| E-cadherin | |||
| Negative | 5 (7%) | 5 (8%) | 0 |
| Positive | 65 (93%) | 54 (92%) | 11 (100%) |
| Ki-67 | |||
| <20% | 50 (71%) | 45 (76%) | 5 (45.5%) |
| ≥20% | 20 (29%) | 14 (24%) | 6 (54.5%) |
| Feature/Number of Patients | Pre-Treatment LAR Value | Post-Treatment LAR Value | p-Value |
|---|---|---|---|
| Surgery | p = 0.2260 | p = 0.8974 | |
| BCS + Radiotherapy—BCT | 0.35 | 0.83 | 0.0192 |
| 56 | 0.11/0.59 | 0.33/1.39 | |
| Mastectomy | 0.47 | 0.8 | 0.0005 |
| 14 | 0.21/0.78 | 0.40/1.35 | |
| Chemotherapy | p = 0.1458 | p = 0.3883 | |
| Anthracycline based | 0.28 | 0.73 | 0.0089 |
| 23 | 0.12/0.60 | 0.24/1.23 | |
| Non-anthracycline | 0.61 | 1.05 | 0.4652 |
| 4 | 0.15/1.48 | 0.43/2.17 | |
| No | 0.47 | 0.83 | 0.0014 |
| 43 | 0.21/0.83 | 0.46/1.35 | |
| Endocrine therapy * | p = 0.5473 | p = 0.1923 | |
| Tamoxifen | 0.48 | 0.71 | 0.0708 |
| 40 | 0.22/0.79 | 0.36/1.22 | |
| Inhibitor aromatase | 0.4 | 1.15 | 0.0042 |
| 17 | 0.24/0.56 | 0.52/1.42 | |
| Tamoxifen and inhibitor aromatase | 0.61 | 1.17 | 0.0280 |
| 7 | 0.18/1.02 | 0.84/1.93 |
| Feature/Number of Patients | Pre-Treatment sE-Selectin Concentration (ng/mL) | Post-Treatment sE-Selectin Concentration (ng/mL) | p-Value |
|---|---|---|---|
| Surgery | p = 0.0840 | p = 0.7225 | |
| BCS + Radiotherapy—BCT | 35.36 | 147.23 | <0.0001 |
| 56 | 29.20/45.60 | (59.43) | |
| Mastectomy | 28.59 | 153.85 | 0.0015 |
| 14 | 21.95/36.51 | (62.49) | |
| Chemotherapy | p = 0.0125 | p = 0.0081 | |
| Anthracycline | 29.7 | 128.42 | 0.0001 |
| 23 | 21.72/35.56 | 77.02/143.96 | |
| Non-anthracycline | 37.89 | 171.61 | 0.0679 |
| 4 | 36.69/51.95 | 159.55/205.32 | |
| No | 35.36 | 180.58 | <0.0001 |
| 43 | 28.31/47.40 | 92.56/202.70 | |
| Endocrine therapy * | p = 0.4964 | p = 0.1460 | |
| Tamoxifen | 35.36 | 133.44 | <0.0001 |
| 40 | 26.89/45.10 | 81.38/187.20 | |
| Inhibitor aromatase | 32.5 | 165.55 | 0.0003 |
| 17 | 30.20/62.50 | 135.93/224.24 | |
| Tamoxifen and inhibitor aromatase | 33.79 | 179.26 | 0.0180 |
| 7 | 21.54/38.90 | 128.42/229.48 |
| Feature/Number of Patients | Pre-Treatment sP-Selectin Concentration (ng/mL) | Post-Treatment sP-Selectin Concentration (ng/mL) | p-Value |
|---|---|---|---|
| Surgery | p = 0.3709 | p = 0.4295 | |
| BCS + Radiotherapy—BCT | 253.95 | 1687.86 | <0.0001 |
| 56 | 190.40/344.10 | 1051.14/2447.81 | |
| Mastectomy | 342.5 | 2071.25 | 0.0015 |
| 14 | 184.30/383.25 | 1617.68/2508.57 | |
| Chemotherapy | p = 0.4316 | p = 0.9237 | |
| Anthracycline | 266.95 | 1864.48 | 0.0001 |
| 23 | 177.5/344.10 | (781.04) | |
| Non-anthracycline | 299.3 | 1607.3 | 0.0679 |
| 4 | 188.63/439.60 | (739.12) | |
| No | 263.15 | 1886.39 | <0.0001 |
| 43 | 193.05/383.25 | (881.77) | |
| Endocrine therapy * | p = 0.2307 | p = 0.0015 | |
| Tamoxifen | 286 | 1859.1 | <0.0001 |
| 40 | 197.65/380.35 | (820.91) | |
| Inhibitor aromatase | 276.65 | 1947.98 | 0.0003 |
| 17 | 192.15/359.40 | (965.53) | |
| Tamoxifen and inhibitor aromatase | 247.4 | 2055.24 | 0.0180 |
| 7 | 177.50/344.10 | (729.06) |
| Feature/Number of Patients | Pre-Treatment vWF Concentration (mU/mL) | Post-Treatment vWF Concentration (mU/mL) | p-Value |
|---|---|---|---|
| Surgery | p = 0.9095 | p = 0.2041 | |
| BCS + Radiotherapy—BCT | 575.61 | 2254.19 | <0.0001 |
| 56 | (240.3) | 1473.88/2928.03 | |
| Mastectomy | 567.21 | 1633.26 | 0.0012 |
| 14 | (270.85) | 1299.04/2730.81 | |
| Chemotherapy | p = 0.6517 | p = 0.0486 | |
| Anthracycline | 582.5 | 2579.4 | <0.0001 |
| 23 | (219.47) | 1902.00/3242.13 | |
| Non-anthracycline | 745.85 | 1665.7 | 0.0679 |
| 4 | (140.01) | 1578.01/1950.54 | |
| No | 553.2 | 1802.17 | <0.0001 |
| 43 | (260.08) | 1290.09/2798.43 | |
| Endocrine therapy * | p = 0.3784 | p = 0.5300 | |
| Tamoxifen | 569.9 | 2038.18 | <0.0001 |
| 40 | 439.00/737.70 | 1290.09/2865.13 | |
| Inhibitor aromatase | 700 | 1873.39 | 0.0003 |
| 17 | 500.00/811.80 | 1564.76/2898.46 | |
| Tamoxifen and inhibitor aromatase | 569.9 | 2392.3 | 0.0180 |
| 7 | 111.00/600.00 | 920.97/3919.44 |
| Pre-Treatment LAR Low Value (<0.27) n = 23 | Pre-Treatment LAR Moderate Value (0.27–0.65) n = 25 | Pre-Treatment LAR High Value (>0.65) n = 22 | p-Value | |
|---|---|---|---|---|
| Pre-Treatment | 29.76 | 32.91 | 40.95 | 0.1391 |
| sE-selectin Concentration (ng/mL) | 25.25/36.87 | 27.61/48.05 | 32.11/46.00 | |
| Post-Treatment | 131.55 | 165.17 | 179.26 | 0.2829 |
| sE-selectin Concentration (ng/mL) | 81.13/172.55 | 84.31/224.24 | 116.55/192.51 | |
| Pre-Treatment | 252.1 | 308.08 | 247.4 | 0.532 |
| sP-selectin Concentration (ng/mL) | 177.50/327.95 | 215.75/378.08 | 210.60/383.25 | |
| Post-Treatment | 1916.87 | 1941.61 | 1870.24 | 0.9607 |
| sP-selectin Concentration (ng/mL) | (821.54) | (821.33) | (866.22) | |
| Pre-Treatment vWF | 553.23 | 538.06 | 653.55 | 0.2312 |
| Concentration (mU/mL) | (224.74) | (246.07) | (246.01) | |
| Post-Treatment vWF | 2098.39 | 2123.11 | 2261.09 | 0.8478 |
| Concentration (mU/mL) | (816.75) | (1168.85) | (960.44) |
| Post-Treatment LAR Low Value (<0.60) n = 25 | Post-Treatment LAR Moderate Value (0.60–1.04) n = 19 | Post-Treatment LAR High Value (>1.04) n = 26 | p-Value | |
|---|---|---|---|---|
| Pre-Treatment | 32.38 | 38.76 | 32.5 | 0.4678 |
| sE-selectin Concentration (ng/mL) | 25.67/47.30 | 28.76/45.64 | 25.25/39.96 | |
| Post-Treatment | 137.19 | 153.89 | 155.77 | 0.5101 |
| sE-selectin Concentration (ng/mL) | (64.26) | (58.67) | (56.28) | |
| Pre-Treatment | 242.35 | 249.38 | 327.95 | 0.0528 |
| sP-selectin Concentration (ng/mL) | 158.35/318.00 | 194.03/310.78 | 237.75/380.35 | |
| Post-Treatment | 1597.57 | 2097.78 | 1687.86 | 0.6921 |
| sP-selectin Concentration (ng/mL) | 986.10/2500.01 | 1513.27/2444.38 | 1224.87/2550.92 | |
| Pre-Treatment vWF | 673.95 | 500 | 569.9 | 0.1947 |
| Concentration (mU/mL) | 461.00/773.35 | 400.00/647.90 | 382.10/811.80 | |
| Post-Treatment vWF | 2456.8 | 2101.08 | 2148.9 | 0.8757 |
| Concentration (mU/mL) | 1532.14/3178.53 | 1581.64/2617.41 | 1449.11/2949.19 |
| Model 1 | Model 2 | Model 3 | Model 4 | ||
|---|---|---|---|---|---|
| Pre-Treatment LAR value | Beta | 0.0374 | −0.0181 | −0.0202 | −0.0009 |
| p-value | 0.7656 | 0.9144 | 0.9042 | 0.9949 | |
| Post-Treatment LAR value | Beta | −0.0331 | −0.0831 | 0.0073 | −0.0835 |
| p-value | 0.7972 | 0.5616 | 0.9602 | 0.5151 | |
| Pre-Treatment sE-selectin | Beta | 0.0839 | 0.054 | 0.1127 | 0.0619 |
| Concentration (ng/mL) | p-value | 0.5023 | 0.6929 | 0.4046 | 0.6048 |
| Post-Treatment sE-selectin | Beta | 0.0748 | 0.0539 | 0.0283 | 0.0742 |
| Concentration (ng/mL) | p-value | 0.5627 | 0.6864 | 0.8324 | 0.5313 |
| Pre-Treatment sP-selectin | Beta | 0.0213 | 0.0525 | 0.0306 | 0.0171 |
| Concentration (ng/mL) | p-value | 0.8635 | 0.6928 | 0.8187 | 0.8831 |
| Post-Treatment sP-selectin | Beta | −0.1619 | −0.1566 | −0.2576 | −0.2437 |
| Concentration (ng/mL) | p-value | 0.2076 | 0.2357 | 0.0504 | 0.0583 |
| Pre-Treatment vWF | Beta | 0.1682 | 0.1496 | 0.1186 | 0.0641 |
| Concentration (mU/mL) | p-value | 0.1651 | 0.2377 | 0.3519 | 0.5632 |
| Post-Treatment vWF | Beta | −0.0292 | −0.0236 | −0.0406 | −0.0551 |
| Concentration (mU/mL) | p-value | 0.8125 | 0.8519 | 0.7476 | 0.6252 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowska, K.; Rhone, P.; Koziorzemska, P.; Formanowicz, D.; Ruszkowska-Ciastek, B. Complex Analysis of Endothelial Markers as Potential Prognostic Indicators in Luminal Invasive Breast Carcinoma Patients: Outcomes of a Six-Year Observational Study. Biomedicines 2023, 11, 2246. https://doi.org/10.3390/biomedicines11082246
Kwiatkowska K, Rhone P, Koziorzemska P, Formanowicz D, Ruszkowska-Ciastek B. Complex Analysis of Endothelial Markers as Potential Prognostic Indicators in Luminal Invasive Breast Carcinoma Patients: Outcomes of a Six-Year Observational Study. Biomedicines. 2023; 11(8):2246. https://doi.org/10.3390/biomedicines11082246
Chicago/Turabian StyleKwiatkowska, Katarzyna, Piotr Rhone, Paulina Koziorzemska, Dorota Formanowicz, and Barbara Ruszkowska-Ciastek. 2023. "Complex Analysis of Endothelial Markers as Potential Prognostic Indicators in Luminal Invasive Breast Carcinoma Patients: Outcomes of a Six-Year Observational Study" Biomedicines 11, no. 8: 2246. https://doi.org/10.3390/biomedicines11082246
APA StyleKwiatkowska, K., Rhone, P., Koziorzemska, P., Formanowicz, D., & Ruszkowska-Ciastek, B. (2023). Complex Analysis of Endothelial Markers as Potential Prognostic Indicators in Luminal Invasive Breast Carcinoma Patients: Outcomes of a Six-Year Observational Study. Biomedicines, 11(8), 2246. https://doi.org/10.3390/biomedicines11082246