Enhancing Immunogenicity in Metastatic Melanoma: Adjuvant Therapies to Promote the Anti-Tumor Immune Response
Abstract
1. Introduction
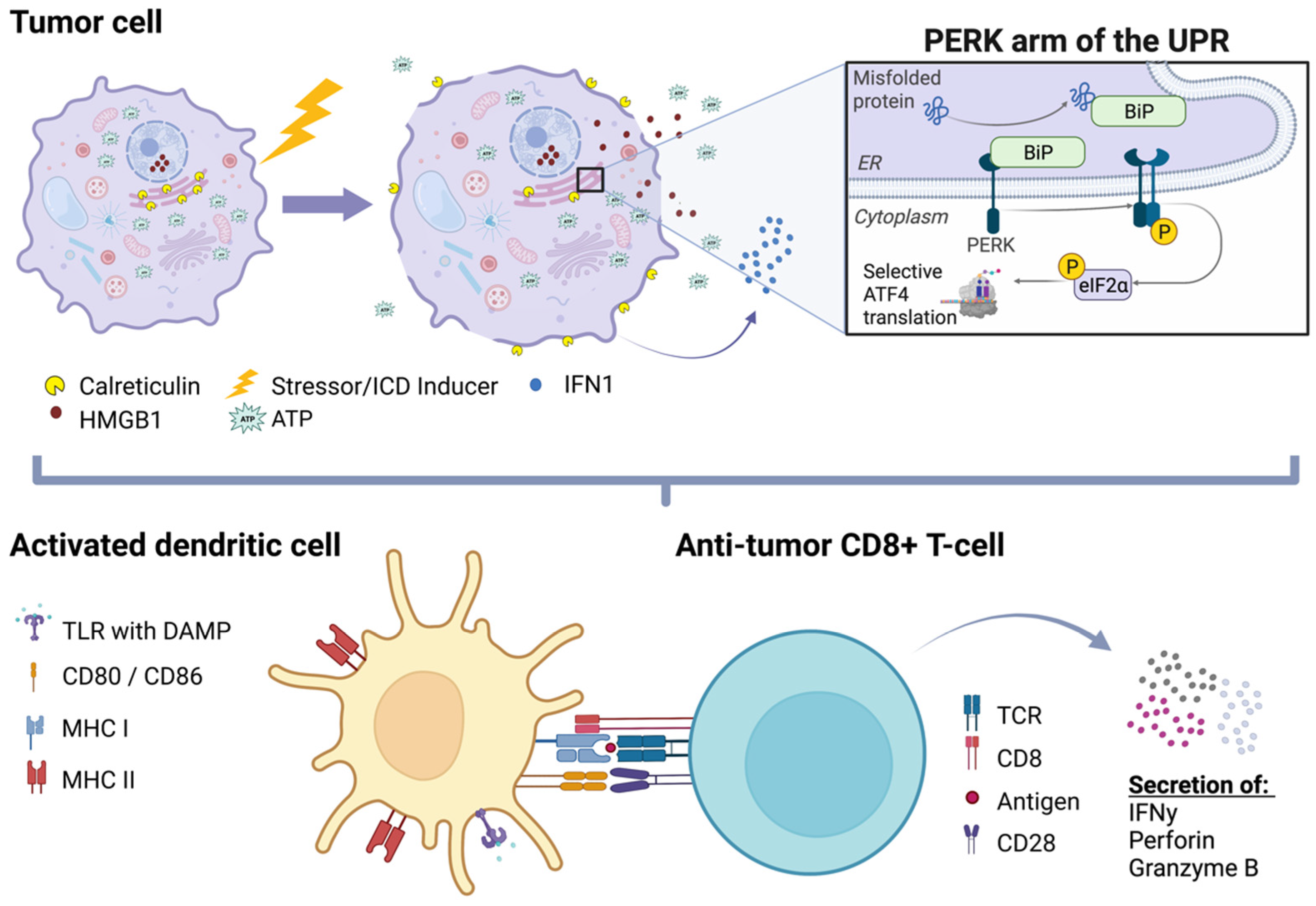
2. Immunogenic Cell Death (ICD)
3. Targets of ICD
3.1. Transcriptional Activators and Epigenetic Targets
3.2. ICD Signaling Pathway Targets
4. Novel Pro-Immunogenic Adjuvant Therapies
4.1. Nanovesicles for Local Targeting of Chemical ICD-Inducers
4.2. Oncolytic Viruses as Cancer Vaccines
4.3. Carbon Ion Radiotherapy
4.4. Photodynamic and Photothermal Therapy
4.5. Focused Ultrasound
4.6. Sonodynamic Therapy
4.7. Magnetic Hyperthermia
| ICD Therapy Platform | Mechanism | References |
|---|---|---|
| Nanovesicles | Platform that utilizes targeting ligands to deliver cargo to tumor site; mitigate systemic adverse effects; acidic pH or NIR can trigger cargo release; allow co-loading of cargo with other sensitizers/ICD-inducers; leads to increased DC activation | [97,99] |
| Carbon ion radiotherapy (CIRT) | Carbon ion (12C6+) beam radiation is directed at the tumor site; enhances CD4+ and CD8+ tumor infiltration; decreases myeloid-derived suppressor cell infiltration of the TIME | [120,121] |
| Photodynamic/photothermal therapy | Photosensitizer is excited by a light source to generate local hyperthermia (photothermal therapy) or ROS (photodynamic therapy) at the tumor site | [123] |
| Focused ultrasound (FUS) | Leads to non-specific tissue destruction and inflammation (HIFU) or to mild hyperthermia with increased heat shock protein expression and induction of cellular stress responses (LOFU); combination with nanovesicles and microbubbles leads to increased cell membrane permeability and improved drug delivery | [132,133,134,135,136,140] |
| Sonodynamic therapy | Combination therapy of FUS with a sensitizing agent; generates ROS and promotes tumor cell damage, apoptosis, and necrosis | [145] |
| Magnetic hyperthermia | Nanovesicle-mediated, localized hyperthermia induces necrotic ICD; increases expression of chemoattractant and TLR pathway markers | [147,148] |
| Nanosecond pulsed electric fields | Electric pulses enhance membrane permeability and trigger cellular stress responses (autophagy, necrosis, and apoptosis) | [149,150] |
| Plasma-derived oxidants | Increases presence of ROS/RNS | [151,152] |
4.8. Nanosecond Pulsed Electric Fields
4.9. Plasma-Derived Pro-Oxidant Treatment Modalities
5. Limitations of Current Therapies
6. Low-Energy Focused Ultrasound (LOFU) for Immune-Priming of ICD-Inducing Therapies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Chocarro, L.; Bocanegra, A.; Blanco, E.; Fernández-Rubio, L.; Arasanz, H.; Echaide, M.; Garnica, M.; Ramos, P.; Piñeiro-Hermida, S.; Vera, R.; et al. Cutting-Edge: Preclinical and Clinical Development of the First Approved Lag-3 Inhibitor. Cells 2022, 11, 2531. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Messersmith, H.; Kaur, V.; Kirkwood, J.M.; Kudchadkar, R.; McQuade, J.L.; Provenzano, A.; Swami, U.; Weber, J.; Alluri, K.C.; et al. Systemic Therapy for Melanoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 3947–3970. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Hodi, F.S.; Weber, J.S.; Allison, J.P.; Urba, W.J.; Robert, C.; O′Day, S.J.; Hoos, A.; Humphrey, R.; Berman, D.M.; et al. Development of ipilimumab: A novel immunotherapeutic approach for the treatment of advanced melanoma. Ann. N. Y. Acad. Sci. 2013, 1291, 1–13. [Google Scholar] [CrossRef]
- Barone, A.; Hazarika, M.; Theoret, M.R.; Mishra-Kalyani, P.; Chen, H.; He, K.; Sridhara, R.; Subramaniam, S.; Pfuma, E.; Wang, Y.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Patients with Unresectable or Metastatic Melanoma. Clin. Cancer Res. 2017, 23, 5661–5665. [Google Scholar] [CrossRef]
- Beaver, J.A.; Theoret, M.R.; Mushti, S.; He, K.; Libeg, M.; Goldberg, K.; Sridhara, R.; McKee, A.E.; Keegan, P.; Pazdur, R. FDA Approval of Nivolumab for the First-Line Treatment of Patients with BRAF(V600) Wild-Type Unresectable or Metastatic Melanoma. Clin. Cancer Res. 2017, 23, 3479–3483. [Google Scholar] [CrossRef]
- Sun, J.; Zager, J.S.; Eroglu, Z. Encorafenib/binimetinib for the treatment of BRAF-mutant advanced, unresectable, or metastatic melanoma: Design, development, and potential place in therapy. OncoTargets Ther. 2018, 11, 9081–9089. [Google Scholar] [CrossRef]
- Ascierto, P.A.; McArthur, G.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef]
- Webster, R.M.; Mentzer, S.E. The malignant melanoma landscape. Nat. Rev. Drug Discov. 2014, 13, 491–492. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P.; et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N. Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.; Kirkwood, J.M. Re-evaluating the role of dacarbazine in metastatic melanoma: What have we learned in 30 years? Eur. J. Cancer 2004, 40, 1825–1836. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105–2116. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Suciu, S.; Santinami, M.; Testori, A.; Kruit, W.H.; Marsden, J.; Punt, C.J.; Salès, F.; Gore, M.; MacKie, R.; et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: Final results of EORTC 18991, a randomised phase III trial. Lancet 2008, 372, 117–126. [Google Scholar] [CrossRef]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.J.; Atkins, M.B.; Kirkwood, J.M.; Agarwala, S.S.; Clark, J.I.; Ernstoff, M.S.; Fecher, L.; Gajewski, T.F.; Gastman, B.; Lawson, D.H.; et al. An update on the Society for Immunotherapy of Cancer consensus statement on tumor immunotherapy for the treatment of cutaneous melanoma: Version 2.0. J. Immunother. Cancer 2018, 6, 44. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Arlauckas, S.P.; Garris, C.S.; Kohler, R.H.; Kitaoka, M.; Cuccarese, M.F.; Yang, K.S.; Miller, M.A.; Carlson, J.C.; Freeman, G.J.; Anthony, R.M.; et al. In vivo imaging reveals a tumor-associated macrophage–mediated resistance pathway in anti–PD-1 therapy. Sci. Transl. Med. 2017, 9, eaal3604. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Tang, H.; Liang, Y.; Anders, R.A.; Taube, J.M.; Qiu, X.; Mulgaonkar, A.; Liu, X.; Harrington, S.M.; Guo, J.; Xin, Y.; et al. PD-L1 on host cells is essential for PD-L1 blockade–mediated tumor regression. J. Clin. Investig. 2018, 128, 580–588. [Google Scholar] [CrossRef]
- Lin, H.; Wei, S.; Hurt, E.M.; Green, M.D.; Zhao, L.; Vatan, L.; Szeliga, W.; Herbst, R.; Harms, P.W.; Fecher, L.A.; et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade–mediated tumor regression. J. Clin. Investig. 2018, 128, 805–815. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Lee, S.J.; Hodi, F.S.; Rao, U.N.; Cohen, G.I.; Hamid, O.; Hutchins, L.F.; Sosman, J.A.; Kluger, H.M.; Eroglu, Z.; et al. Phase III study of adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma: North American Intergroup E1609. J. Clin. Oncol. 2020, 38, 567–575. [Google Scholar] [CrossRef]
- Almutairi, A.R.; McBride, A.; Slack, M.; Erstad, B.L.; Abraham, I. Potential immune-related adverse events associated with monotherapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: A systematic review and meta-analysis. Front. Oncol. 2020, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Khattak, A.; Carlino, M.S.; et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: A secondary analysis of a randomized clinical trial. JAMA Oncol. 2020, 6, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, Z.; Zhang, W.; Zhang, W.; Buzdin, A.; Mu, X.; Yan, Q.; Zhao, X.; Chang, H.-H.; Duhon, M.; et al. FDA-approved and emerging next generation predictive biomarkers for immune checkpoint inhibitors in cancer patients. Front. Oncol. 2021, 11, 683419. [Google Scholar] [CrossRef] [PubMed]
- Marzagalli, M.; Ebelt, N.D.; Manuel, E.R. Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin. Cancer Biol. 2019, 59, 236–250. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol. Rev. 2000, 177, 134–140. [Google Scholar] [CrossRef]
- Hussein, M.R. Dendritic cells and melanoma tumorigenesis: An insight. Cancer Biol. Ther. 2005, 4, 501–505. [Google Scholar] [CrossRef]
- Bennaceur, K.; Chapman, J.; Brikci-Nigassa, L.; Sanhadji, K.; Touraine, J.-l.; Portoukalian, J. Dendritic cells dysfunction in tumour environment. Cancer Lett. 2008, 272, 186–196. [Google Scholar] [CrossRef]
- Tucci, M.; Stucci, S.; Passarelli, A.; Giudice, G.; Dammacco, F.; Silvestris, F. The immune escape in melanoma: Role of the impaired dendritic cell function. Expert Rev. Clin. Immunol. 2014, 10, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Consoli, F.; Vescovi, R.; Bugatti, M.; Vermi, W. Human plasmacytoid dendritic cells and cutaneous melanoma. Cells 2020, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Miloud, T.; Hämmerling, G.J.; Garbi, N. Review of murine dendritic cells: Types, location, and development. Dendritic Cell Protoc. 2010, 595, 21–42. [Google Scholar] [CrossRef]
- Passarelli, A.; Mannavola, F.; Stucci, L.S.; Tucci, M.; Silvestris, F. Immune system and melanoma biology: A balance between immunosurveillance and immune escape. Oncotarget 2017, 8, 106132–106142. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.H. T cell anergy. Annu. Rev. Immunol. 2003, 21, 305–334. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F. Failure at the effector phase: Immune barriers at the level of the melanoma tumor microenvironment. Clin. Cancer Res. 2007, 13, 5256–5261. [Google Scholar] [CrossRef]
- Valdor, R.; Macian, F. Induction and stability of the anergic phenotype in T cells. Semin. Immunol. 2013, 25, 313–320. [Google Scholar] [CrossRef]
- Oliveira, G.; Stromhaug, K.; Klaeger, S.; Kula, T.; Frederick, D.T.; Le, P.M.; Forman, J.; Huang, T.; Li, S.; Zhang, W.; et al. Phenotype, specificity and avidity of antitumour CD8+ T cells in melanoma. Nature 2021, 596, 119–125. [Google Scholar] [CrossRef]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef]
- Giglio, P.; Gagliardi, M.; Bernardini, R.; Mattei, M.; Cotella, D.; Santoro, C.; Piacentini, M.; Corazzari, M. Ecto-Calreticulin is essential for an efficient immunogenic cell death stimulation in mouse melanoma. Genes Immun. 2019, 20, 509–513. [Google Scholar] [CrossRef]
- Venkateswaran, K.; Verma, A.; Bhatt, A.N.; Shrivastava, A.; Manda, K.; Raj, H.G.; Prasad, A.; Len, C.; Parmar, V.S.; Dwarakanath, B.S. Emerging roles of calreticulin in cancer: Implications for therapy. Curr. Protein Pept. Sci. 2018, 19, 344–357. [Google Scholar] [CrossRef]
- Sen Santara, S.; Lee, D.-J.; Crespo, Â.; Hu, J.J.; Walker, C.; Ma, X.; Zhang, Y.; Chowdhury, S.; Meza-Sosa, K.F.; Lewandrowski, M.; et al. The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells. Nature 2023, 616, 348–356. [Google Scholar] [CrossRef]
- Bronietzki, A.W.; Schuster, M.; Schmitz, I. Autophagy in T-cell development, activation and differentiation. Immunol. Cell Biol. 2015, 93, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Dowling, S.D.; Macian, F. Autophagy and T cell metabolism. Cancer Lett. 2018, 419, 20–26. [Google Scholar] [CrossRef]
- DeVorkin, L.; Pavey, N.; Carleton, G.; Comber, A.; Ho, C.; Lim, J.; McNamara, E.; Huang, H.; Kim, P.; Zacharias, L.G.; et al. Autophagy regulation of metabolism is required for CD8+ T cell anti-tumor immunity. Cell Rep. 2019, 27, 502–513.e5. [Google Scholar] [CrossRef] [PubMed]
- Maes, H.; Rubio, N.; Garg, A.D.; Agostinis, P. Autophagy: Shaping the tumor microenvironment and therapeutic response. Trends Mol. Med. 2013, 19, 428–446. [Google Scholar] [CrossRef]
- Messer, J.S. The cellular autophagy/apoptosis checkpoint during inflammation. Cell. Mol. Life Sci. 2017, 74, 1281–1296. [Google Scholar] [CrossRef]
- Li, S.; Song, Y.; Quach, C.; Nemecio, D.; Liang, C. Revisiting the role of autophagy in melanoma. Autophagy 2019, 15, 1843–1844. [Google Scholar] [CrossRef] [PubMed]
- Prieto, K.; Lozano, M.P.; Urueña, C.; Alméciga-Díaz, C.J.; Fiorentino, S.; Barreto, A. The delay in cell death caused by the induction of autophagy by P2Et extract is essential for the generation of immunogenic signals in melanoma cells. Apoptosis 2020, 25, 875–888. [Google Scholar] [CrossRef]
- Martins, I.; Michaud, M.; Sukkurwala, A.Q.; Adjemian, S.; Ma, Y.; Shen, S.; Kepp, O.; Menger, L.; Vacchelli, E.; Galluzzi, L.; et al. Premortem autophagy determines the immunogenicity of chemotherapy-induced cancer cell death. Autophagy 2012, 8, 413–415. [Google Scholar] [CrossRef]
- Yuan, J.; Yuan, X.; Wu, K.; Gao, J.; Li, L. A Local and Low-Dose Chemotherapy/Autophagy-Enhancing Regimen Treatment Markedly Inhibited the Growth of Established Solid Tumors through a Systemic Antitumor Immune Response. Front. Oncol. 2021, 11, 658254. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Jiang, L.; Wang, Z. The progression of HMGB1-induced autophagy in cancer biology. OncoTargets Ther. 2019, 12, 365–377. [Google Scholar] [CrossRef]
- Vacchelli, E.; Sistigu, A.; Yamazaki, T.; Vitale, I.; Zitvogel, L.; Kroemer, G. Autocrine signaling of type 1 interferons in successful anticancer chemotherapy. Oncoimmunology 2015, 4, e988042. [Google Scholar] [PubMed]
- Lamberti, M.J.; Mentucci, F.M.; Roselli, E.; Araya, P.; Rivarola, V.A.; Rumie Vittar, N.B.; Maccioni, M. Photodynamic modulation of type 1 interferon pathway on melanoma cells promotes dendritic cell activation. Front. Immunol. 2019, 10, 2614. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yang, J.; Na, S.; Wang, Y.; Zhang, L.; Wang, J.; Liu, J. Comprehensive characterisation of immunogenic cell death in melanoma revealing the association with prognosis and tumor immune microenvironment. Front. Immunol. 2022, 13, 998653. [Google Scholar] [CrossRef]
- Pfirschke, C.; Engblom, C.; Rickelt, S.; Cortez-Retamozo, V.; Garris, C.; Pucci, F.; Yamazaki, T.; Poirier-Colame, V.; Newton, A.; Redouane, Y.; et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 2016, 44, 343–354. [Google Scholar] [CrossRef]
- Gelmi, M.C.; Houtzagers, L.E.; Strub, T.; Krossa, I.; Jager, M.J. MITF in normal melanocytes, cutaneous and uveal melanoma: A delicate balance. Int. J. Mol. Sci. 2022, 23, 6001. [Google Scholar] [CrossRef]
- Swoboda, A.; Soukup, R.; Eckel, O.; Kinslechner, K.; Wingelhofer, B.; Schörghofer, D.; Sternberg, C.; Pham, H.T.; Vallianou, M.; Horvath, J.; et al. STAT3 promotes melanoma metastasis by CEBP-induced repression of the MITF pathway. Oncogene 2021, 40, 1091–1105. [Google Scholar] [CrossRef]
- Jafari, S.; Lavasanifar, A.; Hejazi, M.S.; Maleki-Dizaji, N.; Mesgari, M.; Molavi, O. STAT3 inhibitory stattic enhances immunogenic cell death induced by chemotherapy in cancer cells. DARU J. Pharm. Sci. 2020, 28, 159–169. [Google Scholar] [CrossRef]
- Coe, E.A.; Tan, J.Y.; Shapiro, M.; Louphrasitthiphol, P.; Bassett, A.R.; Marques, A.C.; Goding, C.R.; Vance, K.W. The MITF-SOX10 regulated long non-coding RNA DIRC3 is a melanoma tumour suppressor. PLoS Genet. 2019, 15, e1008501. [Google Scholar] [CrossRef]
- Beleaua, M.-A.; Jung, I.; Braicu, C.; Milutin, D.; Gurzu, S. SOX11, SOX10 and MITF gene interaction: A possible diagnostic tool in malignant melanoma. Life 2021, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Takahashi, A.; Kikuchi, R.; Nishibu, S.; Lo, J.A.; Hejna, M.; Moon, W.M.; Kato, S.; Zhou, Y.; Hodi, F.S.; et al. SOX10 Regulates Melanoma Immunogenicity through an IRF4–IRF1 AxisSOX10 Regulates Immunogenicity in Melanoma. Cancer Res. 2021, 81, 6131–6141. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhang, Y.-B.; Gui, J.-F.; Lemon, S.M.; Yamane, D. Interferon regulatory factor 1 (IRF1) and anti-pathogen innate immune responses. PLoS Pathog. 2021, 17, e1009220. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Feige, E.; Poling, L.L.; Levy, C.; Widlund, H.R.; Khaled, M.; Kung, A.L.; Fisher, D.E. Pharmacologic suppression of MITF expression via HDAC inhibitors in the melanocyte lineage. Pigment Cell Melanoma Res. 2008, 21, 457–463. [Google Scholar] [CrossRef]
- De Beck, L.; Awad, R.M.; Basso, V.; Casares, N.; De Ridder, K.; De Vlaeminck, Y.; Gnata, A.; Goyvaerts, C.; Lecocq, Q.; José-Enériz, S.; et al. Inhibiting histone and DNA methylation improves cancer vaccination in an experimental model of melanoma. Front. Immunol. 2022, 13, 799636. [Google Scholar] [CrossRef]
- Sheng, W.; LaFleur, M.W.; Nguyen, T.H.; Chen, S.; Chakravarthy, A.; Conway, J.R.; Li, Y.; Chen, H.; Yang, H.; Hsu, P.-H.; et al. LSD1 ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell 2018, 174, 549–563. [Google Scholar] [CrossRef]
- Asadzadeh, Z.; Safarzadeh, E.; Safaei, S.; Baradaran, A.; Mohammadi, A.; Hajiasgharzadeh, K.; Derakhshani, A.; Argentiero, A.; Silvestris, N.; Baradaran, B. Current approaches for combination therapy of cancer: The role of immunogenic cell death. Cancers 2020, 12, 1047. [Google Scholar] [CrossRef]
- Rufo, N.; Garg, A.D.; Agostinis, P. The unfolded protein response in immunogenic cell death and cancer immunotherapy. Trends Cancer 2017, 3, 643–658. [Google Scholar] [CrossRef]
- Zheng, W.; Xie, W.; Yin, D.; Luo, R.; Liu, M.; Guo, F. ATG5 and ATG7 induced autophagy interplays with UPR via PERK signaling. Cell Commun. Signal. 2019, 17, 42. [Google Scholar] [CrossRef]
- Mandula, J.K.; Chang, S.; Mohamed, E.; Jimenez, R.; Sierra-Mondragon, R.A.; Chang, D.C.; Obermayer, A.N.; Moran-Segura, C.M.; Das, S.; Vazquez-Martinez, J.A.; et al. Ablation of the endoplasmic reticulum stress kinase PERK induces paraptosis and type I interferon to promote anti-tumor T cell responses. Cancer Cell 2022, 40, 1145–1160.e9. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wu, L.; Zhang, K.; Wang, H.; Zhang, T.; Gutierrez, L.; O’Connell, D.; Zhang, P.; Li, Y.; Gao, T.; et al. miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. 2018, 25, 1457–1472. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.-l.; Huang, Z.-j.; Lin, Z.-t.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Meng, J.; Deng, S.; Zhang, L.; Wan, C.; Lu, L.; Huang, J.; Hu, Y.; Zhang, Z.; Li, Y.; et al. Targeting CAMKII to reprogram tumor-associated macrophages and inhibit tumor cells for cancer immunotherapy with an injectable hybrid peptide hydrogel. Theranostics 2020, 10, 3049–3063. [Google Scholar] [CrossRef]
- Wang, S.; Yi, X.; Wu, Z.; Guo, S.; Dai, W.; Wang, H.; Shi, Q.; Zeng, K.; Guo, W.; Li, C. CAMKK2 defines ferroptosis sensitivity of melanoma cells by regulating AMPK–NRF2 pathway. J. Investig. Dermatol. 2022, 142, 189–200. [Google Scholar] [CrossRef]
- Tomaszewski, W.H.; Waibl-Polania, J.; Chakraborty, M.; Perera, J.; Ratiu, J.; Miggelbrink, A.; McDonnell, D.P.; Khasraw, M.; Ashley, D.M.; Fecci, P.E.; et al. Neuronal CaMKK2 promotes immunosuppression and checkpoint blockade resistance in glioblastoma. Nat. Commun. 2022, 13, 6483. [Google Scholar] [CrossRef] [PubMed]
- Castiello, L.; Zevini, A.; Vulpis, E.; Muscolini, M.; Ferrari, M.; Palermo, E.; Peruzzi, G.; Krapp, C.; Jakobsen, M.; Olagnier, D.; et al. An optimized retinoic acid-inducible gene I agonist M8 induces immunogenic cell death markers in human cancer cells and dendritic cell activation. Cancer Immunol. Immunother. 2019, 68, 1479–1492. [Google Scholar] [CrossRef]
- Jiang, X.; Muthusamy, V.; Fedorova, O.; Kong, Y.; Kim, D.J.; Bosenberg, M.; Pyle, A.M.; Iwasaki, A. Intratumoral delivery of RIG-I agonist SLR14 induces robust antitumor responses. J. Exp. Med. 2019, 216, 2854–2868. [Google Scholar] [CrossRef]
- Lambing, S.; Tan, Y.P.; Vasileiadou, P.; Holdenrieder, S.; Müller, P.; Hagen, C.; Garbe, S.; Behrendt, R.; Schlee, M.; van den Boorn, J.G.; et al. RIG-I immunotherapy overcomes radioresistance in p53-positive malignant melanoma. J. Mol. Cell Biol. 2023, 15, mjad001. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Giovannetti, E.; Martinez, P.; McCue, S.; Naing, A. Applications and clinical trial landscape using Toll-like receptor agonists to reduce the toll of cancer. NPJ Precis. Oncol. 2023, 7, 26. [Google Scholar] [CrossRef]
- Bourquin, C.; Pommier, A.; Hotz, C. Harnessing the immune system to fight cancer with Toll-like receptor and RIG-I-like receptor agonists. Pharmacol. Res. 2020, 154, 104192. [Google Scholar] [CrossRef]
- Ribas, A.; Medina, T.; Kirkwood, J.M.; Zakharia, Y.; Gonzalez, R.; Davar, D.; Chmielowski, B.; Campbell, K.M.; Bao, R.; Kelley, H.; et al. Overcoming PD-1 blockade resistance with CpG-A toll-like receptor 9 agonist vidutolimod in patients with metastatic melanoma. Cancer Discov. 2021, 11, 2998–3007. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Kim, D.S.; Kim, J.H.; Heo, Y.; Yang, H.; Go, E.-J.; Kim, J.H.; Lee, S.J.; Ahn, B.C.; Yum, J.S.; et al. Intratumoral immunotherapy using a TLR2/3 agonist, L-pampo, induces robust antitumor immune responses and enhances immune checkpoint blockade. J. Immunother. Cancer 2022, 10, e004799. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-W.; Wang, S.-T.; Chang, S.-H.; Chuang, K.-C.; Wang, H.-Y.; Kao, J.-K.; Liang, S.-M.; Wu, C.-Y.; Kao, S.-H.; Chen, Y.-J.; et al. Imiquimod exerts antitumor effects by inducing immunogenic cell death and is enhanced by the glycolytic inhibitor 2-deoxyglucose. J. Investig. Dermatol. 2020, 140, 1771–1783.e6. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, J.; Kong, L.; Yu, Y.; Hu, Q.; Yang, T.; Wang, Y.; Tu, K.; Qiao, Q.; Qin, X.; et al. Immunogenic hybrid nanovesicles of liposomes and tumor-derived nanovesicles for cancer immunochemotherapy. ACS Nano 2021, 15, 3123–3138. [Google Scholar] [CrossRef]
- Qiu, N.; Liu, Y.; Liu, Q.; Chen, Y.; Shen, L.; Hu, M.; Zhou, X.; Shen, Y.; Gao, J.; Huang, L. Celastrol nanoemulsion induces immunogenicity and downregulates PD-L1 to boost abscopal effect in melanoma therapy. Biomaterials 2021, 269, 120604. [Google Scholar] [CrossRef]
- Florêncio, K.G.D.; Edson, E.A.; da Silva Fernandes, K.S.; Luiz, J.P.M.; Pinto, F.d.C.L.; Pessoa, O.D.L.; de Queiroz Cunha, F.; Machado-Neto, J.A.; Wilke, D.V. Chromomycin A5 induces bona fide immunogenic cell death in melanoma. Front. Immunol. 2022, 13, 941757. [Google Scholar] [CrossRef]
- Prieto, K.; Cao, Y.; Mohamed, E.; Trillo-Tinoco, J.; Sierra, R.A.; Urueña, C.; Sandoval, T.A.; Fiorentino, S.; Rodriguez, P.C.; Barreto, A. Polyphenol-rich extract induces apoptosis with immunogenic markers in melanoma cells through the ER stress-associated kinase PERK. Cell Death Discov. 2019, 5, 134. [Google Scholar] [CrossRef]
- Pasquereau-Kotula, E.; Habault, J.; Kroemer, G.; Poyet, J.-L. The anticancer peptide RT53 induces immunogenic cell death. PLoS ONE 2018, 13, e0201220. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Zloza, A.; Rabkin, S.D.; Kaufman, H.L. Oncolytic virus immunotherapy induces immunogenic cell death and overcomes STING deficiency in melanoma. Oncoimmunology 2019, 8, 1591875. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Pala, L.; Conforti, F.; Cocorocchio, E. Talimogene laherparepvec (T-VEC): An intralesional cancer immunotherapy for advanced melanoma. Cancers 2021, 13, 1383. [Google Scholar] [CrossRef]
- Thomas, S.; Kuncheria, L.; Roulstone, V.; Kyula, J.N.; Mansfield, D.; Bommareddy, P.K.; Smith, H.; Kaufman, H.L.; Harrington, K.J.; Coffin, R.S. Development of a new fusion-enhanced oncolytic immunotherapy platform based on herpes simplex virus type 1. J. Immunother. Cancer 2019, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.; Deng, H.; Kong, L.; Wang, Y.; Yang, T.; Hu, Q.; Hu, M.; Yang, C.; Zhang, Z. Reshaping tumor immune microenvironment through acidity-responsive nanoparticles featured with CRISPR/Cas9-mediated programmed death-ligand 1 attenuation and chemotherapeutics-induced immunogenic cell death. ACS Appl. Mater. Interfaces 2020, 12, 16018–16030. [Google Scholar] [CrossRef]
- Liu, M.; Hao, L.; Zhao, D.; Li, J.; Lin, Y. Self-Assembled Immunostimulatory Tetrahedral Framework Nucleic Acid Vehicles for Tumor Chemo-immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 38506–38514. [Google Scholar] [CrossRef]
- Li, Z.; Chu, Z.; Yang, J.; Qian, H.; Xu, J.; Chen, B.; Tian, T.; Chen, H.; Xu, Y.; Wang, F. Immunogenic Cell Death Augmented by Manganese Zinc Sulfide Nanoparticles for Metastatic Melanoma Immunotherapy. ACS Nano 2022, 16, 15471–15483. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Little, N.; Chen, J.; Lambesis, K.T.; Le, K.T.; Han, W.; Scott, A.J.; Lu, J. Immunogenic camptothesome nanovesicles comprising sphingomyelin-derived camptothecin bilayers for safe and synergistic cancer immunochemotherapy. Nat. Nanotechnol. 2021, 16, 1130–1140. [Google Scholar] [CrossRef]
- Dupont, N.; Jiang, S.; Pilli, M.; Ornatowski, W.; Bhattacharya, D.; Deretic, V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. EMBO J. 2011, 30, 4701–4711. [Google Scholar] [CrossRef]
- Michaud, M.; Martins, I.; Sukkurwala, A.Q.; Adjemian, S.; Ma, Y.; Pellegatti, P.; Shen, S.; Kepp, O.; Scoazec, M.; Mignot, G.; et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011, 334, 1573–1577. [Google Scholar] [CrossRef]
- Garg, A.D.; Galluzzi, L.; Apetoh, L.; Baert, T.; Birge, R.B.; Bravo-San Pedro, J.M.; Breckpot, K.; Brough, D.; Chaurio, R.; Cirone, M.; et al. Molecular and translational classifications of DAMPs in immunogenic cell death. Front. Immunol. 2015, 6, 588. [Google Scholar] [CrossRef]
- Huang, C.; Radi, R.H.; Arbiser, J.L. Mitochondrial metabolism in melanoma. Cells 2021, 10, 3197. [Google Scholar] [CrossRef]
- Harel, M.; Ortenberg, R.; Varanasi, S.K.; Mangalhara, K.C.; Mardamshina, M.; Markovits, E.; Baruch, E.N.; Tripple, V.; Arama-Chayoth, M.; Greenberg, E.; et al. Proteomics of melanoma response to immunotherapy reveals mitochondrial dependence. Cell 2019, 179, 236–250.e218. [Google Scholar] [CrossRef]
- Hargadon, K.M. Strategies to improve the efficacy of dendritic cell-based immunotherapy for melanoma. Front. Immunol. 2017, 8, 1594. [Google Scholar] [CrossRef]
- Fallarino, F.; Grohmann, U.; Vacca, C.; Bianchi, R.; Orabona, C.; Spreca, A.; Fioretti, M.; Puccetti, P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002, 9, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Van den Eynde, B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2, 3-dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Puccetti, P.; Grohmann, U. IDO and regulatory T cells: A role for reverse signalling and non-canonical NF-κB activation. Nat. Rev. Immunol. 2007, 7, 817–823. [Google Scholar] [CrossRef]
- Yang, C.; He, B.; Zheng, Q.; Wang, D.; Qin, M.; Zhang, H.; Dai, W.; Zhang, Q.; Meng, X.; Wang, X. Nano-encapsulated tryptanthrin derivative for combined anticancer therapy via inhibiting indoleamine 2, 3-dioxygenase and inducing immunogenic cell death. Nanomedicine 2019, 14, 2423–2440. [Google Scholar] [CrossRef] [PubMed]
- Apolonio, J.S.; de Souza Gonçalves, V.L.; Santos, M.L.C.; Luz, M.S.; Souza, J.V.S.; Pinheiro, S.L.R.; de Souza, W.R.; Loureiro, M.S.; de Melo, F.F. Oncolytic virus therapy in cancer: A current review. World J. Virol. 2021, 10, 229–255. [Google Scholar] [CrossRef]
- Kalus, P.; De Munck, J.; Vanbellingen, S.; Carreer, L.; Laeremans, T.; Broos, K.; Dufait, I.; Schwarze, J.K.; Van Riet, I.; Neyns, B.; et al. Oncolytic Herpes Simplex Virus Type 1 Induces Immunogenic Cell Death Resulting in Maturation of BDCA-1+ Myeloid Dendritic Cells. Int. J. Mol. Sci. 2022, 23, 4865. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.R.; Blomberg, O.S.; Sockolosky, J.T.; Ali, L.; Schmidt, F.I.; Pishesha, N.; Espinosa, C.; Dougan, S.K.; Garcia, K.C.; Ploegh, H.L.; et al. Localized CD47 blockade enhances immunotherapy for murine melanoma. Proc. Natl. Acad. Sci. USA 2017, 114, 10184–10189. [Google Scholar] [CrossRef] [PubMed]
- Jalil, A.R.; Andrechak, J.C.; Hayes, B.H.; Chenoweth, D.M.; Discher, D.E. Human CD47-Derived Cyclic Peptides Enhance Engulfment of mAb-Targeted Melanoma by Primary Macrophages. Bioconjug. Chem. 2022, 33, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shu, Y.; Hu, S.; Qi, Z.; Chen, Y.; Ma, J.; Wang, Y.; Cheng, P. In situ tumor vaccine expressing anti-CD47 antibody enhances antitumor immunity. Front. Oncol. 2022, 12, 897561. [Google Scholar] [CrossRef]
- Garofalo, M.; Pancer, K.W.; Wieczorek, M.; Staniszewska, M.; Salmaso, S.; Caliceti, P.; Kuryk, L. From Immunosuppression to Immunomodulation-Turning Cold Tumours into Hot. J. Cancer 2022, 13, 2884–2892. [Google Scholar] [CrossRef]
- Gleisner, M.A.; Pereda, C.; Tittarelli, A.; Navarrete, M.; Fuentes, C.; Ávalos, I.; Tempio, F.; Araya, J.P.; Becker, M.I.; González, F.E.; et al. A heat-shocked melanoma cell lysate vaccine enhances tumor infiltration by prototypic effector T cells inhibiting tumor growth. J. Immunother. Cancer 2020, 8, e000999. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu-Lieskovan, S.; Chmielowski, B.; Govindan, R.; Naing, A.; Bhardwaj, N.; Margolin, K.; Awad, M.M.; Hellmann, M.D.; Lin, J.J.; et al. A phase Ib trial of personalized neoantigen therapy plus anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell 2020, 183, 347–362.e24. [Google Scholar] [CrossRef]
- Malouff, T.D.; Mahajan, A.; Krishnan, S.; Beltran, C.; Seneviratne, D.S.; Trifiletti, D.M. Carbon ion therapy: A modern review of an emerging technology. Front. Oncol. 2020, 10, 82. [Google Scholar] [CrossRef]
- Particle Therapy Facilities in Clinical Operation. 2023. Available online: https://www.ptcog.site/index.php/facilities-in-operation-public (accessed on 7 March 2023).
- Zhou, H.; Tu, C.; Yang, P.; Li, J.; Kepp, O.; Li, H.; Zhang, L.; Zhang, L.; Zhao, Y.; Zhang, T.; et al. Carbon ion radiotherapy triggers immunogenic cell death and sensitizes melanoma to anti-PD-1 therapy in mice. Oncoimmunology 2022, 11, 2057892. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, P.; Li, H.; Zhang, L.; Li, J.; Zhang, T.; Sheng, C.; Wang, J. Carbon ion radiotherapy boosts anti-tumour immune responses by inhibiting myeloid-derived suppressor cells in melanoma-bearing mice. Cell Death Discov. 2021, 7, 332. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Q.; Li, Z.; Feng, S.; Luo, H.; Liu, R.; Wang, L.; Geng, Y.; Zhao, X.; Yang, Z.; et al. Efficacy and safety of carbon-ion radiotherapy for the malignant melanoma: A systematic review. Cancer Med. 2020, 9, 5293–5305. [Google Scholar] [CrossRef]
- Hou, Y.-J.; Yang, X.-X.; Liu, R.-Q.; Zhao, D.; Guo, C.-X.; Zhu, A.-C.; Wen, M.-N.; Liu, Z.; Qu, G.-F.; Meng, H.-X. Pathological mechanism of photodynamic therapy and photothermal therapy based on nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef]
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Photodynamic therapy for metastatic melanoma treatment: A review. Technol. Cancer Res. Treat. 2018, 17, 1533033818791795. [Google Scholar] [CrossRef] [PubMed]
- Heshmati Aghda, N.; Torres Hurtado, S.; Abdulsahib, S.M.; Lara, E.J.; Tunnell, J.W.; Betancourt, T. Dual photothermal/chemotherapy of melanoma cells with albumin nanoparticles carrying indocyanine green and doxorubicin leads to immunogenic cell death. Macromol. Biosci. 2022, 22, e2100353. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Li, T.; Ye, J.; Sun, F.; Hou, B.; Saeed, M.; Gao, J.; Wang, Y.; Zhu, Q.; Xu, Z.; et al. Acidity-activatable dynamic nanoparticles boosting ferroptotic cell death for immunotherapy of cancer. Adv. Mater. 2021, 33, e2101155. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.A.V.; Almeida, L.R.; Rodrigues, M.C.; Azevedo, R.B.; Muehlmann, L.A. The induction of immunogenic cell death by photodynamic therapy in B16F10 cells in vitro is effected by the concentration of the photosensitizer. Photodiag. Photodyn. Ther. 2021, 35, 102392. [Google Scholar] [CrossRef]
- Konda, P.; Roque III, J.A.; Lifshits, L.M.; Alcos, A.; Azzam, E.; Shi, G.; Cameron, C.G.; McFarland, S.A.; Gujar, S. Photodynamic therapy of melanoma with new, structurally similar, NIR-absorbing ruthenium (II) complexes promotes tumor growth control via distinct hallmarks of immunogenic cell death. Am. J. Cancer Res. 2022, 12, 210–228. [Google Scholar]
- Podolska, M.J.; Shan, X.; Janko, C.; Boukherroub, R.; Gaipl, U.S.; Szunerits, S.; Frey, B.; Muñoz, L.E. Graphene-induced hyperthermia (GIHT) combined with radiotherapy fosters immunogenic cell death. Front. Oncol. 2021, 11, 664615. [Google Scholar] [CrossRef]
- Li, X.; Zhong, S.; Zhang, C.; Li, P.; Ran, H.; Wang, Z. MAGE-targeted gold nanoparticles for ultrasound imaging-guided phototherapy in melanoma. Biomed. Res. Int. 2020, 2020, 6863231. [Google Scholar] [CrossRef]
- Tatsuno, K.; Yamazaki, T.; Hanlon, D.; Han, P.; Robinson, E.; Sobolev, O.; Yurter, A.; Rivera-Molina, F.; Arshad, N.; Edelson, R.L.; et al. Extracorporeal photochemotherapy induces bona fide immunogenic cell death. Cell Death Dis. 2019, 10, 578. [Google Scholar] [CrossRef]
- Curley, C.T.; Sheybani, N.D.; Bullock, T.N.; Price, R.J. Focused ultrasound immunotherapy for central nervous system pathologies: Challenges and opportunities. Theranostics 2017, 7, 3608–3623. [Google Scholar] [CrossRef]
- Dauba, A.; Delalande, A.; Kamimura, H.A.; Conti, A.; Larrat, B.; Tsapis, N.; Novell, A. Recent advances on ultrasound contrast agents for blood-brain barrier opening with focused ultrasound. Pharmaceutics 2020, 12, 1125. [Google Scholar] [CrossRef]
- Kennedy, J.E. High-intensity focused ultrasound in the treatment of solid tumours. Nat. Rev. Cancer 2005, 5, 321–327. [Google Scholar] [CrossRef]
- Yuan, S.-M.; Li, H.; Yang, M.; Zha, H.; Sun, H.; Li, X.-R.; Li, A.-F.; Gu, Y.; Duan, L.; Luo, J.-Y.; et al. High intensity focused ultrasound enhances anti-tumor immunity by inhibiting the negative regulatory effect of miR-134 on CD86 in a murine melanoma model. Oncotarget 2015, 6, 37626–37637. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, L.; Chen, W.R. Host antitumour immune responses to HIFU ablation. Int. J. Hyperth. 2007, 23, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Lu, X.; Pua, E.C.; Zhong, P. The effect of high intensity focused ultrasound treatment on metastases in a murine melanoma model. Biochem. Biophys. Res. Commun. 2008, 375, 645–650. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marra, A.; Ferrone, C.R.; Fusciello, C.; Scognamiglio, G.; Ferrone, S.; Pepe, S.; Perri, F.; Sabbatino, F. Translational research in cutaneous melanoma: New therapeutic perspectives. Anti-Cancer Agents Med. Chem. 2018, 18, 166–181. [Google Scholar] [CrossRef]
- Singh, M.P.; Sethuraman, S.N.; Ritchey, J.; Fiering, S.; Guha, C.; Malayer, J.; Ranjan, A. In-situ vaccination using focused ultrasound heating and anti-CD-40 agonistic antibody enhances T-cell mediated local and abscopal effects in murine melanoma. Int. J. Hyperth. 2019, 36, 64–73. [Google Scholar] [CrossRef]
- Hu, J.; He, J.; Wang, Y.; Zhao, Y.; Fang, K.; Dong, Y.; Chen, Y.; Zhang, Y.; Zhang, C.; Wang, H.; et al. Ultrasound combined with nanobubbles promotes systemic anticancer immunity and augments anti-PD1 efficacy. J. Immunother. Cancer 2022, 10, e003408. [Google Scholar] [CrossRef]
- Prasad, C.; Banerjee, R. Ultrasound-triggered spatiotemporal delivery of topotecan and curcumin as combination therapy for cancer. J. Pharm. Exp. Ther. 2019, 370, 876–893. [Google Scholar] [CrossRef] [PubMed]
- Feril, L.B., Jr.; Yamaguchi, K.; Ikeda-Dantsuji, Y.; Furusawa, Y.; Tabuchi, Y.; Takasaki, I.; Ogawa, R.; Cui, Z.-G.; Tachibana, K. Low-intensity ultrasound inhibits melanoma cell proliferation in vitro and tumor growth in vivo. J. Med. Ultrason. 2021, 48, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.G.; Peric, B.; Mascherini, M.; Spina, R.; Kunte, C.; Kis, E.; Rozsa, P.; Quaglino, P.; Jones, R.P.; Clover, A.J.P.; et al. Combination of pembrolizumab with electrochemotherapy in cutaneous metastases from melanoma: A comparative retrospective study from the InspECT and Slovenian Cancer Registry. Cancers 2021, 13, 4289. [Google Scholar] [CrossRef] [PubMed]
- Gorgizadeh, M.; Azarpira, N.; Lotfi, M.; Daneshvar, F.; Salehi, F.; Sattarahmady, N. Sonodynamic cancer therapy by a nickel ferrite/carbon nanocomposite on melanoma tumor: In vitro and in vivo studies. Photodiag. Photodyn. Ther. 2019, 27, 27–33. [Google Scholar] [CrossRef]
- Sheehan, D.; Sheehan, K.; Sheehan, J. Sonodynamic therapy for metastatic melanoma to the brain. J. Neuro-Oncol. 2021, 153, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Do, A.-V.; Geary, S.M.; Seol, D.; Tobias, P.; Carlsen, D.; Leelakanok, N.; Martin, J.A.; Salem, A.K. Combining ultrasound and intratumoral administration of doxorubicin-loaded microspheres to enhance tumor cell killing. Int. J. Pharm. 2018, 539, 139–146. [Google Scholar] [CrossRef]
- Tamura, Y.; Ito, A.; Wakamatsu, K.; Kamiya, T.; Torigoe, T.; Honda, H.; Yamashita, T.; Uhara, H.; Ito, S.; Jimbow, K. Immunomodulation of melanoma by chemo-thermo-immunotherapy using conjugates of melanogenesis substrate NPrCAP and magnetite nanoparticles: A review. Int. J. Mol. Sci. 2022, 23, 6457. [Google Scholar] [CrossRef]
- Nishikawa, A.; Suzuki, Y.; Kaneko, M.; Ito, A. Combination of magnetic hyperthermia and immunomodulators to drive complete tumor regression of poorly immunogenic melanoma. Cancer Immunol. Immunother. 2022, 72, 1493–1504. [Google Scholar] [CrossRef]
- Pakhomova, O.N.; Gregory, B.W.; Semenov, I.; Pakhomov, A.G. Two modes of cell death caused by exposure to nanosecond pulsed electric field. PLoS ONE 2013, 8, e70278. [Google Scholar] [CrossRef]
- Beebe, S.J.; Fox, P.M.; Rec, L.J.; Willis, L.K.; Schoenbach, K.H. Nanosecond, high-intensity pulsed electric fields induce apoptosis in human cells. FASEB J. 2003, 17, 1493–1495. [Google Scholar] [CrossRef]
- Lin, A.; Gorbanev, Y.; De Backer, J.; Van Loenhout, J.; Van Boxem, W.; Lemière, F.; Cos, P.; Dewilde, S.; Smits, E.; Bogaerts, A. Non-thermal plasma as a unique delivery system of short-lived reactive oxygen and nitrogen species for immunogenic cell death in melanoma cells. Adv. Sci. 2019, 6, 1802062. [Google Scholar] [CrossRef]
- Azzariti, A.; Iacobazzi, R.M.; Di Fonte, R.; Porcelli, L.; Gristina, R.; Favia, P.; Fracassi, F.; Trizio, I.; Silvestris, N.; Guida, G. Plasma-activated medium triggers cell death and the presentation of immune activating danger signals in melanoma and pancreatic cancer cells. Sci. Rep. 2019, 9, 4099. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Pakhomova, O.N.; Pakhomov, A.G.; Weygandt, S.; Bulysheva, A.A.; Murray, L.E.; Mollica, P.A.; Muratori, C. Mechanisms and immunogenicity of nsPEF-induced cell death in B16F10 melanoma tumors. Sci. Rep. 2019, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Sagwal, S.K.; Pasqual-Melo, G.; Bodnar, Y.; Gandhirajan, R.K.; Bekeschus, S. Combination of chemotherapy and physical plasma elicits melanoma cell death via upregulation of SLC22A16. Cell Death Dis. 2018, 9, 1179. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Delgoffe, G.M.; Meyer, C.F.; Chan, W.; Powell, J.D. Anergic T cells are metabolically anergic. J. Immunol. 2009, 183, 6095–6101. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Kiess, A.P. Time to Debunk an Urban Myth? The “Abscopal Effect” with Radiation and Anti-PD-1. J. Clin. Oncol. 2021, 39, 1–3. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Quinn, T.J.; Scandiuzzi, L.; Basu, I.; Partanen, A.; Tomé, W.A.; Macian, F.; Guha, C. Low-intensity focused ultrasound induces reversal of tumor-induced T cell tolerance and prevents immune escape. J. Immunol. 2016, 196, 1964–1976. [Google Scholar] [CrossRef]
- Skalina, K.A.; Singh, S.; Chavez, C.G.; Macian, F.; Guha, C. Low intensity focused ultrasound (LOFU)-mediated acoustic immune priming and ablative radiation therapy for in situ tumor vaccines. Sci. Rep. 2019, 9, 15516. [Google Scholar] [CrossRef]
- Heissmeyer, V.; Macián, F.; Im, S.-H.; Varma, R.; Feske, S.; Venuprasad, K.; Gu, H.; Liu, Y.-C.; Dustin, M.L.; Rao, A. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 2004, 5, 255–265. [Google Scholar] [CrossRef]
| Therapy | Generic Name (Brand Name) | References |
|---|---|---|
| Checkpoint inhibitor therapies | Nivolumab and Relatlimab Combination Therapy (Opdualag) | [3] |
| Ipilimumab (Yervoy) | [4,5] | |
| Pembrolizumab (Keytruda) | [6] | |
| Nivolumab (Opdivo) | [7] | |
| BRAF/MEK inhibitors | Binimetinib (Mektovi) + Encorafenib (Braftovi) | [8] |
| Vemurafenib (Zelboraf) + Cobimetinib (Cotellic) | [9] | |
| Dabrafenib (Tafinlar) + Trametinib (Mekinist) | [10,11] | |
| Cytotoxic chemotherapy | Dacarbazine | [12] |
| Other immunotherapies | Interleukin-2 (Aldesleukin, Proleukin) | [13] |
| Recombinant Interferon Alfa-2b (Intron A) | [14] |
| ICD-Inducer | Examples | Mechanism | References |
|---|---|---|---|
| Chemical/small molecule inducers | Chemotherapeutics (i.e., doxorubicin and paclitaxel) | Increase tumor cell expression of PD-L1 | [87] |
| Celastrol (quinone methide family) | Induces tumor cell autophagy; decreases tumor cell expression of PD-L1 | [88] | |
| Chromomycins A5-8 (antibiotics) | Induces tumor cell apoptosis | [89] | |
| Polyphenols | Induces PERK arm of the UPR | [90] | |
| RT53 (peptide) | Promotes B16-F10 tumor regression | [91] | |
| Oncolytic viruses | Talimogene laherparepvec (T-VEC) | Oncolytic herpes simplex virus HSV-1 that infects tumor cells and encodes granulocyte-macrophage colony stimulating factor; Enhances CD8+ T-cell recruitment via STING-mediated pathway; Modulation to express GALV-GP-R- leads to enhanced ICD and abscopal effect | [92,93,94] |
| FixVac (BNT111) | Liposomal RNA vaccine (Clinical Trial NCT02410733) that enhances anti-tumor immune response when used with other ICD-inducers | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelka, S.; Guha, C. Enhancing Immunogenicity in Metastatic Melanoma: Adjuvant Therapies to Promote the Anti-Tumor Immune Response. Biomedicines 2023, 11, 2245. https://doi.org/10.3390/biomedicines11082245
Pelka S, Guha C. Enhancing Immunogenicity in Metastatic Melanoma: Adjuvant Therapies to Promote the Anti-Tumor Immune Response. Biomedicines. 2023; 11(8):2245. https://doi.org/10.3390/biomedicines11082245
Chicago/Turabian StylePelka, Sandra, and Chandan Guha. 2023. "Enhancing Immunogenicity in Metastatic Melanoma: Adjuvant Therapies to Promote the Anti-Tumor Immune Response" Biomedicines 11, no. 8: 2245. https://doi.org/10.3390/biomedicines11082245
APA StylePelka, S., & Guha, C. (2023). Enhancing Immunogenicity in Metastatic Melanoma: Adjuvant Therapies to Promote the Anti-Tumor Immune Response. Biomedicines, 11(8), 2245. https://doi.org/10.3390/biomedicines11082245





