Systolic and Diastolic Blood Pressure Are Independent Risk Factors for Diabetic Retinopathy in Patients with Type 2 Diabetes
Abstract
1. Introduction
2. Methods
2.1. Study Design and Ethics Statement
2.2. Patients
2.3. Demographic Data and Clinical Characteristics
2.4. Markers of Glycemic Control and Lipid Metabolism
2.5. Indicators of Renal Function
2.6. Ophthalmologic Retinal Examination
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Prevalence of Microvascular Complications and Their Correlations
3.3. Predictors of Diabetic Retinopathy and Nephropathy
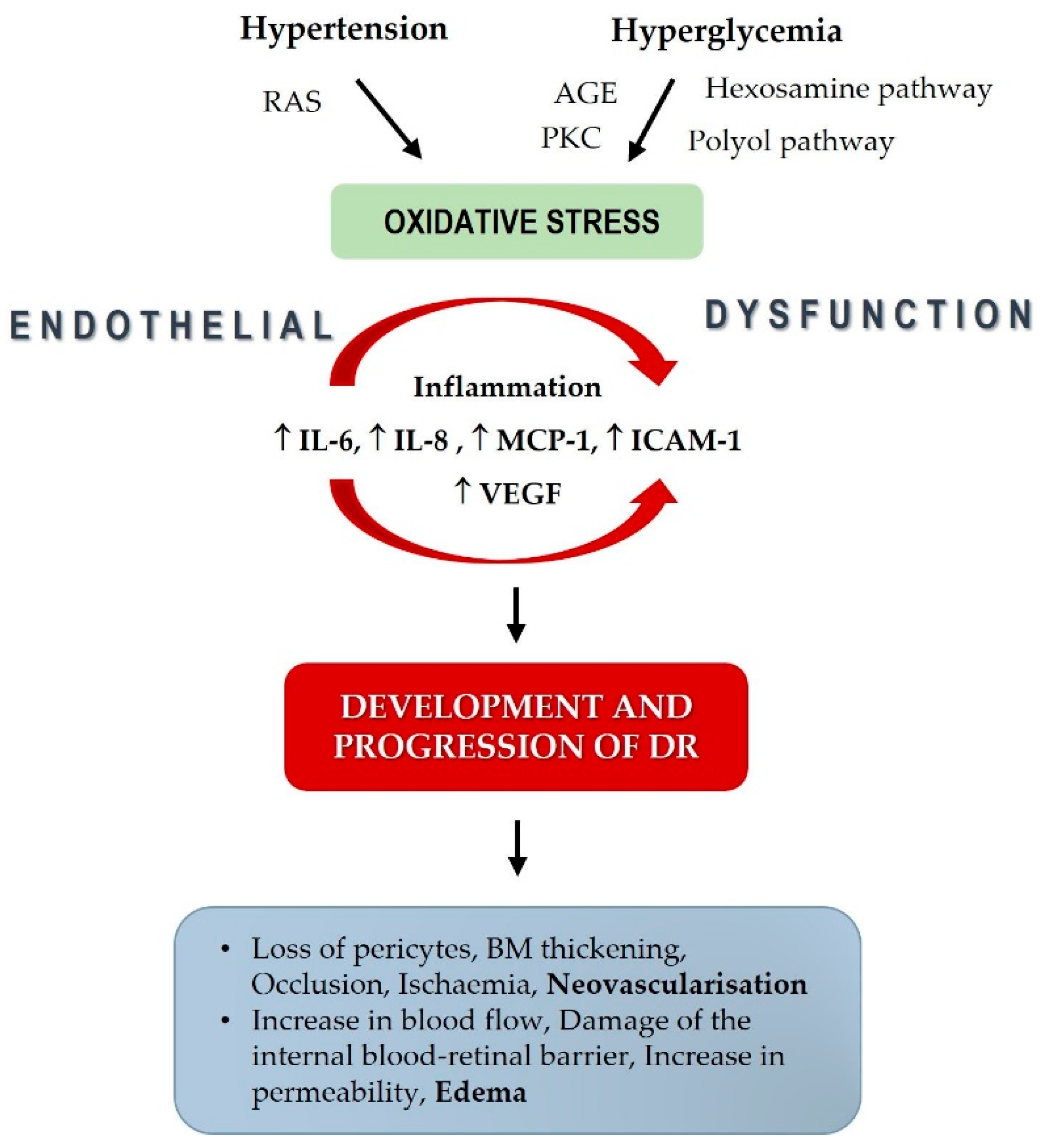
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, M.V.; Dekker, J.M.; Stehouwer, C.D.; Polak, B.C.; Fuller, J.H.; Sjolie, A.K.; Kofinis, A.; Rottiers, R.; Porta, M.; Chaturvedi, N. EURODIAB prospective complications study. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: The EURODIAB Prospective Complications Study. Diabetes Care 2005, 28, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.K. Blood pressure control and diabetic retinopathy. Br. J. Ophthalmol. 2002, 86, 365–367. [Google Scholar] [CrossRef]
- Shaya, F.T.; Aljawadi, M. Diabetic retinopathy. Clin. Ophthalmol. 2007, 1, 259–265. [Google Scholar]
- Poulsen, P.L.; Bek, T.; Ebbehøj, E.; Hansen, K.W.; Mogensen, C.E. 24-h ambulatory blood pressure and retinopathy in normoalbuminuric IDDM patients. Diabetologia 1998, 41, 105–110. [Google Scholar] [CrossRef]
- Mbata, O.; Abo El-Magd, N.F.; El-Remessy, A.B. Obesity, metabolic syndrome and diabetic retinopathy: Beyond hyperglycemia. World. J. Diabetes 2017, 15, 317–329. [Google Scholar] [CrossRef]
- Grunwald, J.E.; Alexander, J.; Ying, G.S.; Maguire, M.; Daniel, E.; Whittock-Martin, R.; Parker, C.; McWilliams, K.; Lo, J.C.; Go, A.; et al. Retinopathy and chronic kidney disease in the Chronic Renal Insufficiency Cohort (CRIC) study. Arch. Ophthalmol. 2012, 130, 1136–1144. [Google Scholar] [CrossRef]
- Arar, N.H.; Freedman, B.I.; Adler, S.G.; Iyengar, S.K.; Chew, E.Y.; Davis, M.D.; Satko, S.G.; Bowden, D.W.; Duggirala, R.; Elston, R.C.; et al. Heritability of the severity of diabetic retinopathy: The FIND-eye study. Inv. Ophthalmol. Vis. Sci. 2008, 9, 3839–3844. [Google Scholar] [CrossRef]
- Wolf, G.; Müller, N.; Mandecka, A.; Müller, U.A. Association of diabetic retinopathy and renal function in patients with types 1 and 2 diabetes mellitus. Clin. Nephrol. 2007, 68, 81–86. [Google Scholar] [CrossRef]
- Tolonen, N.; Hietala, K.; Forsblom, C.; Harjutsalo, V.; Makinen, V.P.; Kyto, J.; Summanen, P.A.; Thorn, L.M.; Wadén, J.; Gordin, D.; et al. Associations and interactions between lipid profiles, retinopathy and nephropathy in patients with type 1 diabetes: The FinnDiane Study. J. Intern. Med. 2013, 274, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, X.; Ye, Q.; Guo, X.; Xu, B.; Guan, T.; Chen, A. The predictive value of diabetic retinopathy on subsequent diabetic nephropathy in patients with type 2 diabetes: A systematic review and meta-analysis of prospective studies. Ren. Fail. 2021, 43, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Kohner, E.M. Diabetic retinopathy. Br. Med. Bull. 1989, 45, 148–173. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Kohner, E.M.; Aldington, S.J.; Turner, R.C.; Holman, R.R.; Manley, S.E.; Matthews, D.R. UKPDS 50: Risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia 2001, 44, 156–163. [Google Scholar] [CrossRef]
- Emdin, C.A.; Rahimi, K.; Neal, B.; Callender, T.; Perkovic, V.; Patel, A. Blood pressure lowering in type 2 diabetes: A systematic review and meta-analysis. JAMA 2015, 313, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Wang, Y.; Hu, X.Y.; Chen, J.H.; Li, Y.Y.; Zhong, Q.Y.; Cheng, H.; Mohammed, B.H.; Liang, X.L.; Hernandez, J.; et al. Association between Systolic Blood Pressure and Diabetic Retinopathy in Both Hypertensive and Normotensive Patients with Type 2 Diabetes: Risk Factors and Healthcare Implications. Healthcare 2021, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Tomić, M.; Vrabec, R.; Ljubić, S.; Bulum, T.; Prkačin, I.; Rahelić, D. Renal function is associated with cataract development in patients with type 2 diabetes. Acta. Clin. Croat. 2021, 60 (Suppl. S1), 18–23. [Google Scholar] [CrossRef]
- Bulum, T.; Blaslov, K.; Duvnjak, L. Risk factors for development and progression of nonproliferative retinopathy in normoalbuminuric patients with type 1 diabetes. Diabetes Res. Clin. Pract. 2014, 106, 555–559. [Google Scholar] [CrossRef][Green Version]
- Levey, A.S.; Stevens, L.A. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am. J. Kidney Dis. 2010, 55, 622–627. [Google Scholar] [CrossRef]
- Aldington, S.J.; Kohner, E.M.; Meuer, S.; Klein, R.; Sjølie, A.K. Methodology for retinal photography and assessment of diabetic retinopathy: The EURODIAB IDDM complications study. Diabetologia 1995, 38, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.P.; Ferris, F.L., 3rd; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Gillow, J.T.; Gibson, J.M.; Dodson, P.M. Hypertension and diabetic retinopathy-what’s the story? Br. J. Ophthalmol. 1999, 83, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Stratton, I.M.; Aldington, S.; Holman, R.R.; Kohner, E.M.; UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus (UKPDS 69). Arch. Ophthalmol. 2004, 122, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Estacio, R.O.; Mehler, P.S.; Hiattet, W.R. Appropriate blood pressure control in hypertensive and normotensive type 2 diabetes mellitus: A summary of the ABCD trial. Nat. Clin. Pract. Nephrol. 2007, 3, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Gaede, P.; Vedel, P.; Parving, H.; Pedersenet, O. Intensified multifactorial intervention in patients with type 2 diabetes and microalbuminuria: The Steno type 2 randomized study. Lancet 1999, 353, 617–622. [Google Scholar] [CrossRef]
- Liu, L.; Quang, N.D.; Banu, R.; Kumar, H.; Tham, Y.-C.; Cheng, C.-Y.; Wong, T.Y.; Sabanayagam, C. Hypertension, blood pressure control and diabetic retinopathy in a large population-based study. PLoS ONE 2020, 15, e0229665. [Google Scholar] [CrossRef]
- Lou, Q.; Chen, X.; Wang, K.; Liu, H.; Zhang, Z.; Lee, Y. The Impact of Systolic Blood Pressure, Pulse Pressure, and Their Variability on Diabetes Retinopathy among Patients with Type 2 Diabetes. J. Diabetes Res. 2022, 2022, 7876786. [Google Scholar] [CrossRef]
- Rajalakshmi, R.; Amutha, A.; Ranjani, H.; Ali, M.K.; Unnikrishnan, R.; Anjana, R.M.; Narayan, K.M.V.; Mohan, V. Prevalence and risk factors for diabetic retinopathy in Asian Indians with young onset type 1 and type 2 diabetes. J. Diabetes Complicat. 2014, 28, 291–297. [Google Scholar] [CrossRef]
- Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Woodward, M.; Billot, L.; Harrap, S.; Poulter, N.; Marre, M.; Cooper, M.; et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): A randomised controlled trial. Lancet 2007, 370, 829–840. [Google Scholar] [CrossRef]
- Kohner, E.M.; Patel, V.; Rassam, S.M.B. Role of blood flow and impaired auto-regulation in the pathogenesis of diabetic retinopathy. Diabetes 1995, 44, 603–607. [Google Scholar] [CrossRef]
- Grunwald, J.E.; Dupont, J.; Riva, C.E. Retinal haemodynamics in patients with early diabetes mellitus. Br. J. Ophthalmol. 1996, 80, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Fuchsjäger-Mayrl, G.; Polak, K.; Luksch, A.; Polska, E.; Dorner, G.T.; Rainer, G.; Eichler, H.G.; Schmetterer, L. Retinal blood flow and systemic blood pressure in healthy young subjects. Graefes. Arch. Clin. Exp. Ophthalmol. 2001, 239, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Pota, C.E.; Apaydın, K.C. Retinal and choroidal microvascular changes during pregnancy detected with OCTA. Can. J. Ophthalmol. 2023, in press. [CrossRef]
- István, L.; Czakó, C.; Benyó, F.; Élő, A.; Mihály, Z.; Sótonyi, P.; Varga, A.; Nagy, Z.Z.; Kovács, I. The effect of systemic factors on retinal blood flow in patients with carotid stenosis: An optical coherence tomography angiography study. Geroscience 2022, 44, 389–401. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Rodriguez-Iturbe, B. Mechanisms of disease: Oxidative stress and inflammation in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2006, 2, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Sjølie, A.K.; Dodsonand, P.; Hobbs, F.R.R. Does renin-angiotensin system blockade have a role in preventing diabetic retinopathy? A clinical review. Int. J. Clin. Pract. 2011, 65, 148–153. [Google Scholar] [CrossRef]
- Cardoso, C.R.L.; Leite, N.C.; Dib, E.; Salles, G.F. Predictors of development and progression of retinopathy in patients with type 2 diabetes: Importance of blood pressure parameters. Sci. Rep. 2017, 7, 4867. [Google Scholar] [CrossRef]
- Do, D.V.; Wang, X.; Vedula, S.S.; Marrone, M.; Sleilati, G.; Hawkins, B.S.; Frank, R.N. Blood pressure control for diabetic retinopathy. Cochrane. Database. Syst. Rev. 2015, 1, CD006127. [Google Scholar] [CrossRef]
- Fraser-Bell, S.; Symes, R.; Vaze, A. Hypertensive eye disease: A review. Clin. Exp. Ophthalmol. 2017, 45, 45–53. [Google Scholar] [CrossRef]
- Schlingerman, R.O.; Hinsbergh, V.W.M. Role of vascular permeability factor/vascular endothelial growth factor in eye disease. Br. J. Ophthalmol. 1997, 81, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Hansson, L.; Zanchetti, A.; Carruthers, S.G. Effects of intensive blood pressure lowering and low dose aspirin in patients with hypertension: Principal results of the hypertension optimal treatment (HOT) randomized trial. Lancet 1998, 351, 1755–1762. [Google Scholar] [CrossRef]
- Pan, C.-W.; Wang, S.; Xu, C.-L.; Song, E. Combined effect of glycemic and blood pressure control on diabetic retinopathy among Chinese with type-2 diabetes mellitus. Diabetol. Metab. Syndr. 2018, 10, 73. [Google Scholar] [CrossRef]
- Wang, B.; Wang, F.; Zhang, Y.; Zhao, S.-H.; Zhao, W.-J.; Yan, S.-L.; Wang, Y.-G. Effects of RAS inhibitors on diabetic retinopathy: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Kotlarsky, P.; Bolotin, A.; Dorfman, K.; Knyazer, B.; Lifshitz, T.; Levy, J. Link between retinopathy and nephropathy caused by complications of diabetes mellitus type 2. Int. Ophthalmol. 2015, 35, 59–66. [Google Scholar] [CrossRef]
- Sun, K.; Lin, D.; Li, F.; Huang, C.; Qi, Y.; Xue, S.; Tang, J.; Yang, C.; Li, Y.; Ren, M.; et al. Discordant associations of lipid parameters with albuminuria and chronic kidney disease: A population-based study. Lipids Health Dis. 2015, 14, 152. [Google Scholar] [CrossRef]
- Minoo, F.; Mahdavi-Mazdeh, M.; Abbasi, M.R.; Sohrabi, S. Impact of the severity of obesity on microalbuminuria in obese normotensive nondiabetic individuals. J. Renal. Inj. Prev. 2015, 4, 34–38. [Google Scholar] [CrossRef]
- Tancredi, M.; Rosengren, A.; Svensson, A.M.; Kosiborod, M.; Pivodic, A.; Gudbjörnsdottir, S.; Wedel, H.; Clements, M.; Dahlqvist, S.; Lind, M. Excess Mortality among Persons with Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 1720–1732. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, K.; Park, J.; Kim, D.H.; Jeon, J.; Jang, H.R.; Hur, K.Y.; Kim, J.H.; Huh, W.; Kim, Y.G.; et al. Prognostic significance of albuminuria in elderly of various ages with diabetes. Sci. Rep. 2023, 13, 7079. [Google Scholar] [CrossRef]
- Gupta, H.; Vidhale, T.; Pustake, M.; Gandhi, C.; Roy, T. Utility of ambulatory blood pressure monitoring in detection of masked hypertension and risk of hypertension mediated organ damage in normotensive patients with type 2 diabetes mellitus. Blood Press. 2022, 31, 50–57. [Google Scholar] [CrossRef] [PubMed]


| All Patients Included in the Study (n = 160) | |
|---|---|
| Age (years) | 64.3 ± 7.6 |
| Gender (m/f) (%) | 60/40 |
| Diabetes duration (years) | 14.0 ± 7.1 |
| BMI (kg/m2) | 29.8 ± 4.7 |
| SBP (mmHg) | 135 (110–170) |
| DBP (mmHg) | 80 (70–110) |
| HbA1c (%) | 7.1 (5.5–12.1) |
| Total cholesterol (mmol/L) | 4.6 (2.7–10.2) |
| HDL cholesterol (mmol/L) | 1.3 (0.8–2.5) |
| LDL cholesterol (mmol/L) | 2.5 (0.9–7.1) |
| Triglycerides (mmol/L) | 1.6 (0.5–7.0) |
| Serum creatinine (μmol/L) | 75.5 (42–163) |
| ACR (mg/mmol) | 1.4 (0.3–49.6) |
| eGFR (mL/min/1.73 m2) | 86.5 (32–108) |
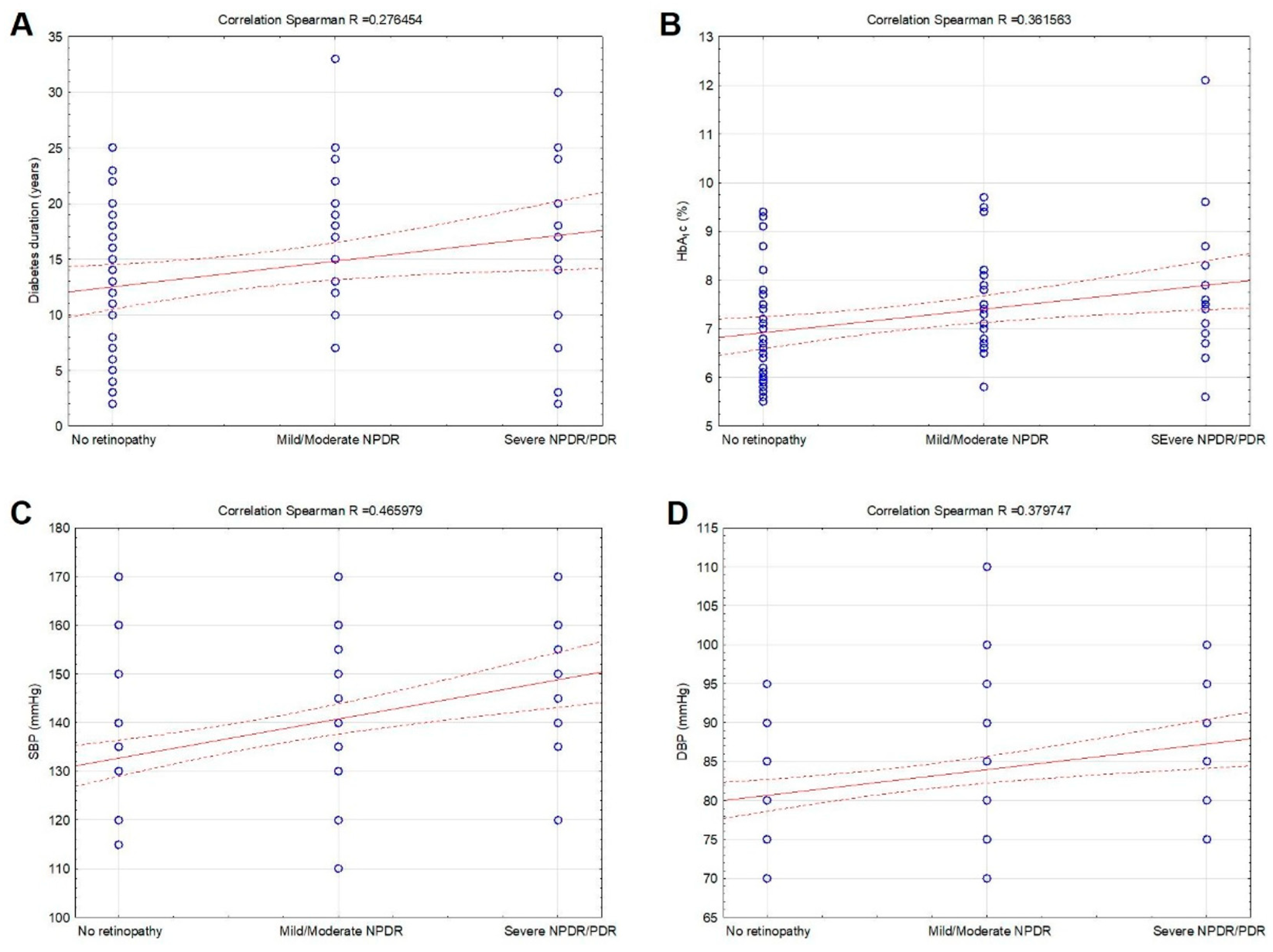
| No DR (n = 86) | Mild/Moderate NPDR (n = 44) | Severe NPDR/PDR (n = 30) | p-Value | |
|---|---|---|---|---|
| Diabetes duration | 11.98 ± 6.76 | 15.60 ± 8.23 | 16.91 ± 5.88 | 0.017 |
| SBP (mmHg) | 130 (115–170) | 137 (110–170) | 150 (120–170) | 0.001 |
| DBP (mmHg) | 80 (70–95) | 85 (70–110) | 85 (75–100) | 0.003 |
| HbA1c (%) | 6.7 (5.5–9.4) | 7.3 (5.8–9.2) | 7.8 (5.6–12.1) | 0.005 |
| Variable | OR (95% CI) | p-Value | AOR (95% CI) * | p-Value * |
|---|---|---|---|---|
| Diabetes duration | 1.10 (1.02–1.18) | 0.008 | / | |
| HbA1c | 1.87 (1.17–2.99) | 0.008 | / | |
| SBP | 1.07 (1.03–1.11) | 0.001 | 1.06 (1.02–1.11) | 0.004 |
| DBP | 1.12 (1.04–1.21) | 0.003 | 1.12 (1.03–1.22) | 0.007 |
| Unstandardized Coefficients | Standardized Coefficients | ||||
|---|---|---|---|---|---|
| Model | B | Std. Error | Beta | t | Sig. |
| (Constant) | 0.138 | 1.839 | 0.075 | 0.941 | |
| Diabetes duration | 0.252 | 0.117 | 0.242 | 2.155 | 0.034 |
| Unstandardized Coefficients | Standardized Coefficients | ||||
|---|---|---|---|---|---|
| Model | B | Std. Error | Beta | t | Sig. |
| (Constant) | 0.138 | 1.839 | 0.075 | 0.941 | |
| Age | −1.413 | 0.247 | −0.554 | −5.728 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulum, T.; Tomić, M.; Vrabec, R.; Brkljačić, N.; Ljubić, S. Systolic and Diastolic Blood Pressure Are Independent Risk Factors for Diabetic Retinopathy in Patients with Type 2 Diabetes. Biomedicines 2023, 11, 2242. https://doi.org/10.3390/biomedicines11082242
Bulum T, Tomić M, Vrabec R, Brkljačić N, Ljubić S. Systolic and Diastolic Blood Pressure Are Independent Risk Factors for Diabetic Retinopathy in Patients with Type 2 Diabetes. Biomedicines. 2023; 11(8):2242. https://doi.org/10.3390/biomedicines11082242
Chicago/Turabian StyleBulum, Tomislav, Martina Tomić, Romano Vrabec, Neva Brkljačić, and Spomenka Ljubić. 2023. "Systolic and Diastolic Blood Pressure Are Independent Risk Factors for Diabetic Retinopathy in Patients with Type 2 Diabetes" Biomedicines 11, no. 8: 2242. https://doi.org/10.3390/biomedicines11082242
APA StyleBulum, T., Tomić, M., Vrabec, R., Brkljačić, N., & Ljubić, S. (2023). Systolic and Diastolic Blood Pressure Are Independent Risk Factors for Diabetic Retinopathy in Patients with Type 2 Diabetes. Biomedicines, 11(8), 2242. https://doi.org/10.3390/biomedicines11082242








