BDNF rs962369 Is Associated with Major Depressive Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Genotyping
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Study Groups
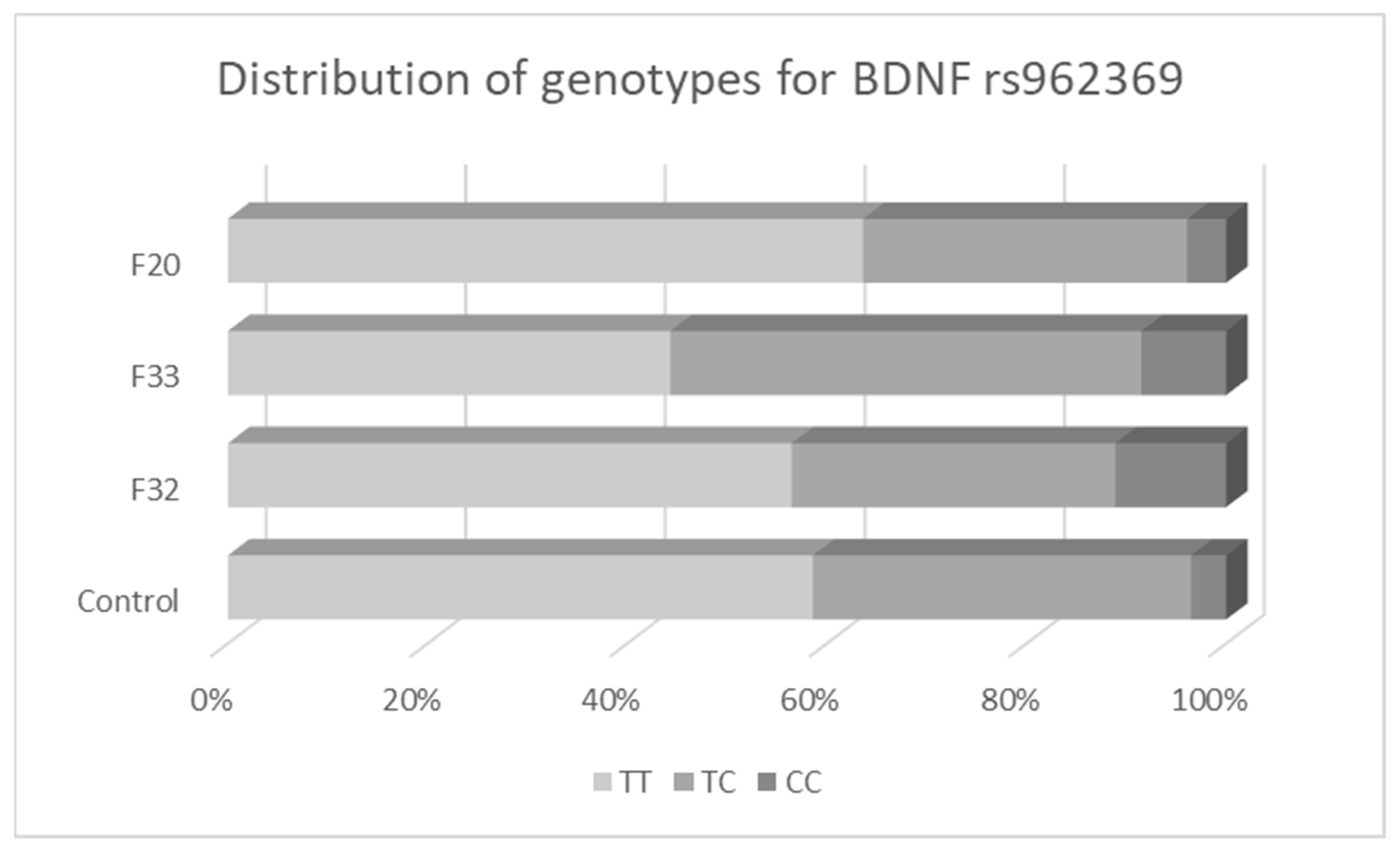
3.2. Associations between BDNF Variants and Different Types of Mental Disorders
3.3. Comparison of Different Types of Mental Disorders in Relation to BDNF Variants
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Mental Health Atlas; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- American Psychiatric Association. Manual Diagnóstico e Estatístico de Transtornos Mentais: DSM-5.5; Artmed: São Paulo, Brazil, 2014. [Google Scholar]
- Ferreira, F.C.; Willatan, S.J.; Rodrigues, G.B.; de Lima, S.M.; de Souza Silva, C.M.; Rodrigues da Silva, I.C. BDNF Genetic Variant and Its Genotypic Fluctuation in Major Depressive Disorder. Behav. Neurol. 2021, 2021, 7117613. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide (accessed on 15 June 2023).
- Gutiérrez-Rojas, L.; Porras-Segovia, A.; Dunne, H.; Andrade-González, N.; Cervilla, J.A. Prevalence and correlates of major depressive disorder: A systematic review. Braz. J. Psychiatry 2020, 42, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Institute of Health Metrics and Evaluation (IHME). Global Health Data Exchange (GHDx). Available online: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/27a7644e8ad28e739382d31e77589dd7 (accessed on 12 June 2023).
- Aleman, A.; Kahn, R.S.; Selten, J.P. Sex differences in the risk of schizophrenia: Evidence from meta-analysis. Arch. Gen. Psychiatry 2003, 60, 565–571. [Google Scholar] [CrossRef]
- Moldin, S.O. Indicators of liability to schizophrenia: Perspectives from genetic epidemiology. Schizophr. Bull. 1994, 20, 169–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harrison, P.J.; Weinberger, D.R. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol. Psychiatry 2005, 10, 40–68. [Google Scholar] [CrossRef] [PubMed]
- Krynicki, C.R.; Upthegrove, R.; Deakin, J.F.W.; Barnes, T.R.E. The relationship between negative symptoms and depression in schizophrenia: A systematic review. Acta Psychiatr. Scand. 2018, 137, 380–390. [Google Scholar] [CrossRef]
- Upthegrove, R.; Marwaha, S.; Birchwood, M. Depression and Schizophrenia: Cause, consequence or trans-diagnostic issue? Schizophr. Bull. 2016, 2, 240–244. [Google Scholar] [CrossRef]
- Craddock, N.; Owen, M.J. The Kraepelinian dichotomy—Going, going but still not gone. Br. J. Psychiatry 2010, 196, 92–95. [Google Scholar] [CrossRef]
- Koutsouleris, N.; Meisenzahl, E.M.; Borgwardt, S. Individualized differential diagnosis of schizophrenia and mood disorders using neuroanatomical biomarkers. Brain 2015, 138, 2059–2073. [Google Scholar] [CrossRef]
- Barnes, T.R.E. Clinical assessment of the negative symptoms of schizophrenia. Eur. Neuropsychopharmacol. 1993, 3, 207–208. [Google Scholar] [CrossRef]
- Malla, A.K. Negative symptoms and affective disturbance in schizophrenia and related disorders. Can. J. Psychiatry 1995, 40 (Suppl. S2), S55–S59. [Google Scholar] [CrossRef]
- van de Leemput, J.; Hess, J.L.; Glatt, S.J.; Tsuang, M.T. Genetics of Schizophrenia: Historical Insights and Prevailing Evidence. Adv. Genet. 2016, 96, 99–141. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Kendler, K.S.; Neale, M.C. Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry 2003, 60, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F.; Neale, M.C.; Kendler, K.S. Genetic epidemiology of major depression: Review and meta-analysis. Am. J. Psychiatry 2000, 157, 1552–1562. [Google Scholar] [CrossRef]
- Polderman, T.J.C.; Benyamin, B.; de Leeuw, C.A.; Sullivan, P.F.; van Bochoven, A.; Visscher, P.M.; Posthuma, D. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat. Genet. 2015, 47, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Postolache, T.T.; Del Bosque-Plata, L.; Jabbour, S.; Vergare, M.; Wu, R.; Gragnoli, C. Co-shared genetics and possible risk gene pathway partially explain the comorbidity of schizophrenia, major depressive disorder, type 2 diabetes, and metabolic syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2019, 180, 186–203. [Google Scholar] [CrossRef]
- Perry, B.I.; Upthegrove, R.; Kappelmann, N.; Jones, P.B.; Burgess, S.; Khandaker, G.M. Associations of immunological proteins/traits with schizophrenia, major depression and bipolar disorder: A bi-directional two-sample mendelian randomization study. Brain Behav. Immun. 2021, 97, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Shnayder, N.A.; Novitsky, M.A.; Neznanov, N.G.; Limankin, O.V.; Asadullin, A.R.; Petrov, A.V.; Dmitrenko, D.V.; Narodova, E.A.; Popenko, N.V.; Nasyrova, R.F. Genetic Predisposition to Schizophrenia and Depressive Disorder Comorbidity. Genes 2022, 13, 457. [Google Scholar] [CrossRef]
- García-Juárez, M.; Camacho-Morales, A. Defining the Role of Anti- and Pro-inflammatory Outcomes of Interleukin-6 in Mental Health. Neuroscience 2022, 492, 32–46. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Egan, M.; Kojima, M.; Callicott, J.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef]
- Bednarova, A.; Habalova, V.; Iannaccone, S.F.; Tkac, I.; Jarcuskova, D.; Krivosova, M.; Marcatili, M.; Hlavacova, N. Association of HTTLPR, BDNF, and FTO Genetic Variants with Completed Suicide in Slovakia. J. Pers. Med. 2023, 10, 501. [Google Scholar] [CrossRef]
- Kishi, T.; Yoshimura, R.; Ikuta, T.; Iwata, N. Brain-derived neurotrophic factor and major depressive disorder: Evidence from meta-analyses. Front. Psychiatry 2018, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Kheirollahi, M.; Kazemi, E.; Ashouri, S. Brain-Derived Neurotrophic Factor Gene Val66Met Polymorphism and Risk of Schizophrenia: A Meta-Analysis of Case-Control Studies. Cell. Mol. Neurobiol. 2016, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- González-Castro, T.B.; Salas-Magaña, M.; Juárez-Rojop, I.E.; López-Narváez, M.L.; Tovilla-Zárate, C.A.; Hernández-Díaz, Y. Exploring the Association between BDNF Val66Met Polymorphism and Suicidal Behavior: Meta-Analysis and Systematic Review. J. Psychiatr. Res. 2017, 94, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Perroud, N.; Aitchison, K.J.; Uher, R.; Smith, R.; Huezo-Diaz, P.; Marusic, A.; Maier, W.; Mors, O.; Placentino, A.; Henigsberg, N.; et al. Genetic Predictors of Increase in Suicidal Ideation During Antidepressant Treatment in the GENDEP Project. Neuropsychopharmacology 2009, 34, 2517–2528. [Google Scholar] [CrossRef]
- Ropret, S.; Zupanc, T.; Komel, R.; Videtič, P.A. Single Nucleotide Polymorphisms in the BDNF Gene and Suicide in the Slovenian Sample. Neurosci. Lett. 2015, 602, 12–16. [Google Scholar] [CrossRef]
- Januar, V.; Ancelin, M.L.; Ritchie, K.; Saffery, R.; Ryan, J. BDNF promoter methylation and genetic variation in late-life depression. Transl. Psychiatry 2015, 18, 619. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Stewart, R.; Kang, H.J.; Kim, S.Y.; Kim, S.W.; Shin, I.S.; Park, M.S.; Kim, H.R.; Shin, M.G.; Cho, K.H.; et al. A longitudinal study of BDNF promoter methylation and genotype with poststroke depression. J. Affect. Disord. 2013, 149, 93–99. [Google Scholar] [CrossRef]
- Kang, H.J.; Kim, J.M.; Bae, K.Y.; Kim, S.W.; Shin, I.S.; Kim, H.R.; Shin, M.G.; Yoon, J.S. Longitudinal associations between BDNF promoter methylation and late-life depression. Neurobiol. Aging 2015, 36, 1764.e1–1764.e7. [Google Scholar] [CrossRef]
- Schröter, K.; Brum, M.; Brunkhorst-Kanaan, N.; Tole, F.; Ziegler, C.; Domschke, K.; Reif, A.; Kittel-Schneider, S. Longitudinal multi-level biomarker analysis of BDNF in major depression and bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Solé, X.; Guinó, E.; Valls, J.; Iniesta, R.; Moreno, V. SNPStats: A Web Tool for the Analysis of Association Studies. Bioinformatics 2006, 22, 1928–1929. [Google Scholar] [CrossRef]
- Surtees, P.G.; Wainwright, N.W.; Willis-Owen, S.A.; Sandhu, M.S.; Luben, R.; Day, N.E.; Flint, J. No association between the BDNF Val66Met polymorphism and mood status in a non-clinical community sample of 7389 older adults. J. Psychiatr. Res. 2007, 41, 404–409. [Google Scholar] [CrossRef]
- Verhagen, M.; van der Meij, A.; van Deurzen, P.A.; Janzing, J.G.; Arias-Vásquez, A.; Buitelaar, J.K.; Franke, B. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: Effects of gender and ethnicity. Mol. Psychiatry 2010, 15, 260–271. [Google Scholar] [CrossRef]
- Herbert, J.; Ban, M.; Brown, G.W.; Harris, T.O.; Ogilvie, A.; Uher, R.; Craig, T.K. Interaction between the BDNF gene Val/66/Met polymorphism and morning cortisol levels as a predictor of depression in adult women. Br. J. Psychiatry 2012, 201, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Aldoghachi, A.F.; Tor, Y.S.; Redzun, S.Z.; Lokman, K.A.B.; Razaq, N.A.A.; Shahbudin, A.F.; Badamasi, I.M.; Cheah, P.S.; Stanslas, J.; Veerakumarasivam, A.; et al. Screening of brain-derived neurotrophic factor (BDNF) single nucleotide polymorphisms and plasma BDNF levels among Malaysian major depressive disorder patients. PLoS ONE 2019, 14, e0211241. [Google Scholar] [CrossRef]
- Akhter, N.; Iqbal, M.U.N.; Maqsood, A.; Sheikh, S.A.; Ali, A.; Khan, T.A. Association of BDNF and SERT gene polymorphisms with depression among Pakistani population. Pak. J. Pharm. Sci. 2021, 34, 103–110. [Google Scholar] [PubMed]
- Rizos, E.N.; Siafakas, N.; Stefanis, N.; Douzenis, A.; Kontaxakis, V.; Laskos, E.; Kastania, A.; Zoumbourlis, V.; Lykouras, L. Association of Serum BDNF and Val66met Polymorphism of the Brain-Derived Neurotrophic Factor in a Sample of First Psychotic Episode Patients. Psychiatriki 2009, 20, 297–304. [Google Scholar] [CrossRef]
- Gratacos, M.; Gonzalez, J.R.; Mercader, J.M.; de Cid, R.; Urretavizcaya, M.; Estivill, X. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: Meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biol. Psychiatry 2007, 61, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zhang, G.; Du, X.; Zhang, Y.; Yin, G.; Dai, J.; He, M.X.; Soares, J.C.; Li, X.; Zhang, X.Y. Suicide attempt, clinical correlates, and BDNF Val66Met polymorphism in chronic patients with schizophrenia. Neuropsychology 2018, 32, 199. [Google Scholar] [CrossRef] [PubMed]
- Terzić, T.; Kastelic, M.; Dolžan, V.; Plesničar, B.K. Genetic Variability Testing of Neurodevelopmental Genes in Schizophrenic Patients. J. Mol. Neurosci. 2015, 56, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Peka-Wysiecka, J.; Wroñski, M.; Jasiewicz, A.; Grzywacz, A.; Tybura, P.; Kucharska-Mazur, J.; Bieñkowski, P.; Samochowiec, J. BDNF Rs 6265 Polymorphism and COMT Rs 4680 Polymorphism in Deficit Schizophrenia in Polish Sample. Pharmacol. Rep. 2013, 65, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huang, Y.; Chen, K.; Li, D.; Han, C.; Kan, Q. The brain-derived neurotrophic factor Val66Met polymorphism is not associated with schizophrenia: An updated meta-analysis of 11,480 schizophrenia cases and 13,490 controls. Psychiatr. Res. 2015, 225, 217–220. [Google Scholar] [CrossRef] [PubMed]

| Gene | Oligonucleotides | Sequences |
|---|---|---|
| BDNF rs6265 | forward-limit | 5′-GCCGAACTTTCTGGTCCTCATCC-3′ |
| reverse-excess | 5′-AAGGCAGGTTCAAGAGGCTTG-3′ | |
| probe | 5′-GCTCTTCTATCACGTGTTCGAAAGTGTC-Phos | |
| BDNF rs962369 | forward-limit | 5′-GACATTTTTATGAGAAGGGTTTACATAAG-3′ |
| reverse-excess | 5′-AAAGAATTGCTCACTGTAATGAC-3′ | |
| probe | 5′-TGCCAAGAGAGTTGAGTCCATGG-Phos |
| Variables | MDD, One Episode F32 | MDD, Recurrent F33 | Schizophrenia F20 | Controls | |
|---|---|---|---|---|---|
| Total | 108 | 106 | 77 | 227 | |
| Sex | Females | 69 (64%) | 80 (75%) | 28 (36%) | 115 (51%) |
| Males | 39 (36%) | 26 (25%) | 49 (63%) | 112 (49%) | |
| Age | Mean ± SD | 48.55 ± 15.72 | 57.04 ± 10.48 | 44.71 ± 13.39 | 54.06 ± 22.55 |
| BDNF rs6265 | BDNF rs962369 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotypes | Alleles Frequency | Genotypes | Alleles Frequency | ||||||||
| CC (%) | CT (%) | TT (%) | C (Val) | T (Met) | TT (%) | TC (%) | CC (%) | T | C | ||
| Controls | |||||||||||
| Total | 227 | 151 (66.5) | 66 (29.1) | 10 (4.4) | 0.81 | 0.19 | 133 (58.6) | 86 (37.9) | 8 (3.5) | 0.78 | 0.22 |
| Females | 115 | 72 (62.6) | 36 (31.3) | 7 (6.1) | 0.78 | 0.22 | 64 (55.6) | 46 (40.0) | 5 (4.3) | 0.76 | 0.24 |
| Males | 112 | 79 (70.5) | 30 (26.8) | 3 (2.7) | 0.84 | 0.16 | 69 (61.6) | 40 (35.7) | 3 (2.7) | 0.79 | 0.21 |
| F32 | |||||||||||
| Total | 108 | 69 (63.9) | 28 (25.9) | 11 (10.2) | 0.77 | 0.23 | 61 (56.5) | 35 (32.4) | 12 (11.1) | 0.73 | 0.27 |
| Females | 69 | 45 (65.2) | 17 (24.6) | 7 (10.2) | 0.78 | 0.22 | 37 (53.6) | 26 (37.7) | 6 (8.7) | 0.72 | 0.28 |
| Males | 39 | 24 (61.5) | 11 (28.2) | 4 (10.3) | 0.76 | 0.24 | 24 (61.5) | 9 (23.1) | 6 (15.4) | 0.73 | 0.27 |
| F33 | |||||||||||
| Total | 106 | 73 (68.9) | 26 (24.5) | 7 (6.6) | 0.81 | 0.19 | 47 (44.3) | 50 (47.2) | 9 (8.5) | 0.68 | 0.32 |
| Females | 80 | 55 (68.8) | 20 (25.0) | 5 (6.3) | 0.81 | 0.19 | 38 (47.5) | 34 (42.5) | 8 (10.0) | 0.69 | 0.31 |
| Males | 26 | 18 (69.2) | 6 (23.1) | 2 (7.7) | 0.81 | 0.19 | 9 (34.6) | 16 (61.5) | 1 (3.8) | 0.65 | 0.35 |
| F20 | |||||||||||
| Total | 77 | 45 (58.4) | 28 (36.4) | 4 (5.2) | 0.77 | 0.23 | 49 (63.6) | 25 (32.5) | 3 (3.9) | 0.80 | 0.20 |
| Females | 28 | 18 (64.3) | 8 (28.6) | 2 (7.1) | 0.79 | 0.21 | 20 (71.4) | 6 (21.4) | 2 (7.1) | 0.82 | 0.18 |
| Males | 49 | 27 (55.1) | 20 (40.8) | 2 (4.1) | 0.76 | 0.24 | 29 (59.2) | 19 (38.8) | 1 (2.0) | 0.79 | 0.21 |
| Disorder | Genetic Model | Crude Analysis | Sex and Age-Adjusted Analysis | |||
|---|---|---|---|---|---|---|
| BDNF rs6265 | OR (95% C.I.) | p (Wald Test) | OR (95% C.I.) | p (Wald Test) | ||
| F32 | recessive (TT vs. CC+CT) | 2.46 (1.01–5.99) | 0.049 | 2.37 (0.96–5.89) | 0.063 | |
| F33 | all tested models | non-significant | >0.05 | non-significant | >0.05 | |
| F20 | all tested models | non-significant | >0.05 | non-significant | >0.05 | |
| BDNF rs962369 | ||||||
| F32 | recessive (CC vs. TT+TC) | 3.42 (1.36–8.64) | 0.009 | 3.47 (1.36–8.85) | 0.009 | |
| F33 | log-additive (per C allele) | 1.71 (1.16–2.51) | 0.006 | 1.65 (1.11–2.45) | 0.013 | |
| F20 | all tested models | non-significant | >0.05 | non-significant | >0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednářová, A.; Habalová, V.; Tkáč, I. BDNF rs962369 Is Associated with Major Depressive Disorder. Biomedicines 2023, 11, 2243. https://doi.org/10.3390/biomedicines11082243
Bednářová A, Habalová V, Tkáč I. BDNF rs962369 Is Associated with Major Depressive Disorder. Biomedicines. 2023; 11(8):2243. https://doi.org/10.3390/biomedicines11082243
Chicago/Turabian StyleBednářová, Aneta, Viera Habalová, and Ivan Tkáč. 2023. "BDNF rs962369 Is Associated with Major Depressive Disorder" Biomedicines 11, no. 8: 2243. https://doi.org/10.3390/biomedicines11082243
APA StyleBednářová, A., Habalová, V., & Tkáč, I. (2023). BDNF rs962369 Is Associated with Major Depressive Disorder. Biomedicines, 11(8), 2243. https://doi.org/10.3390/biomedicines11082243






