Drug Discovery and Development of miRNA-Based Nucleotide Drugs for Gastrointestinal Cancer
Abstract
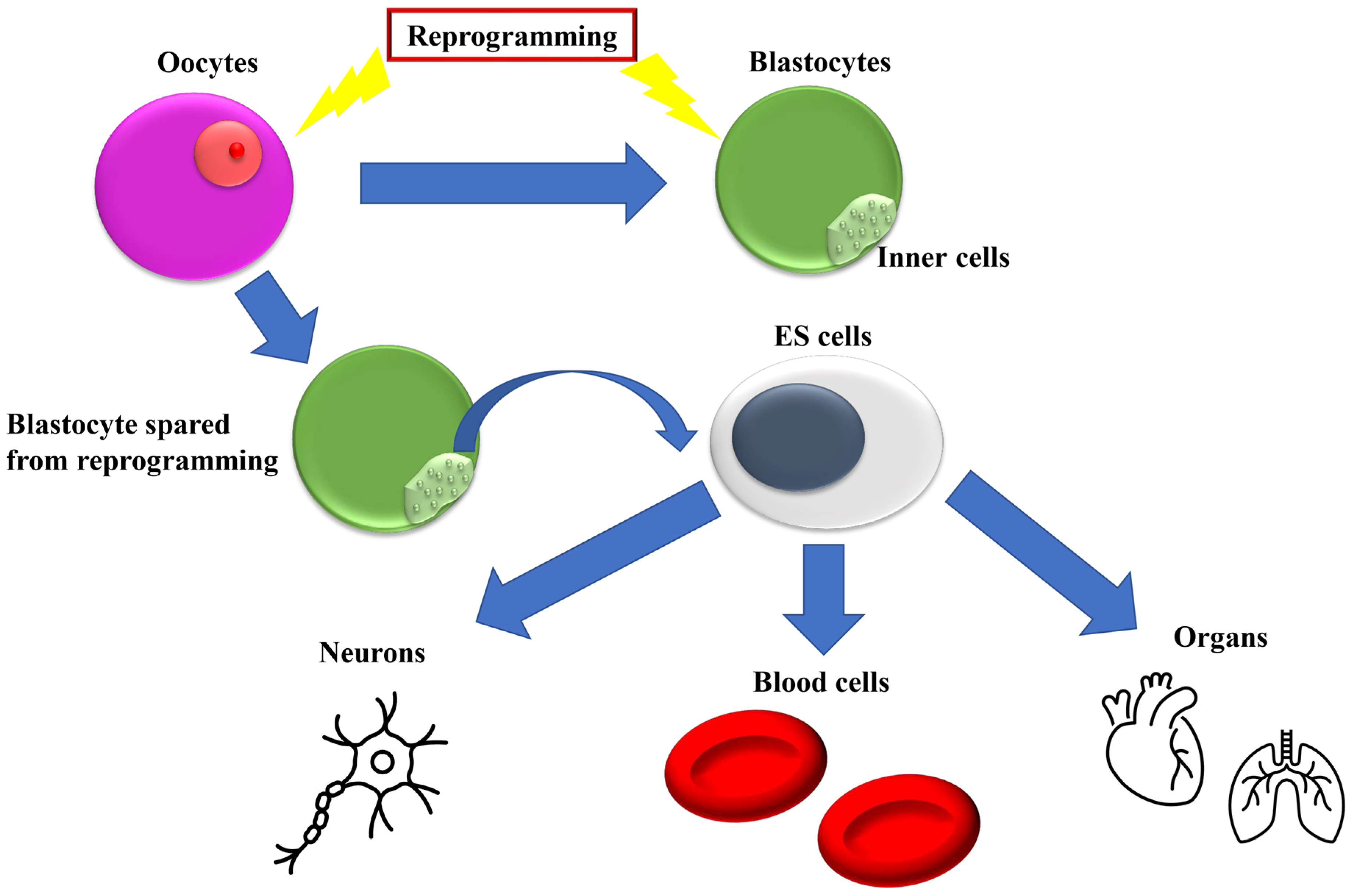
1. Introduction
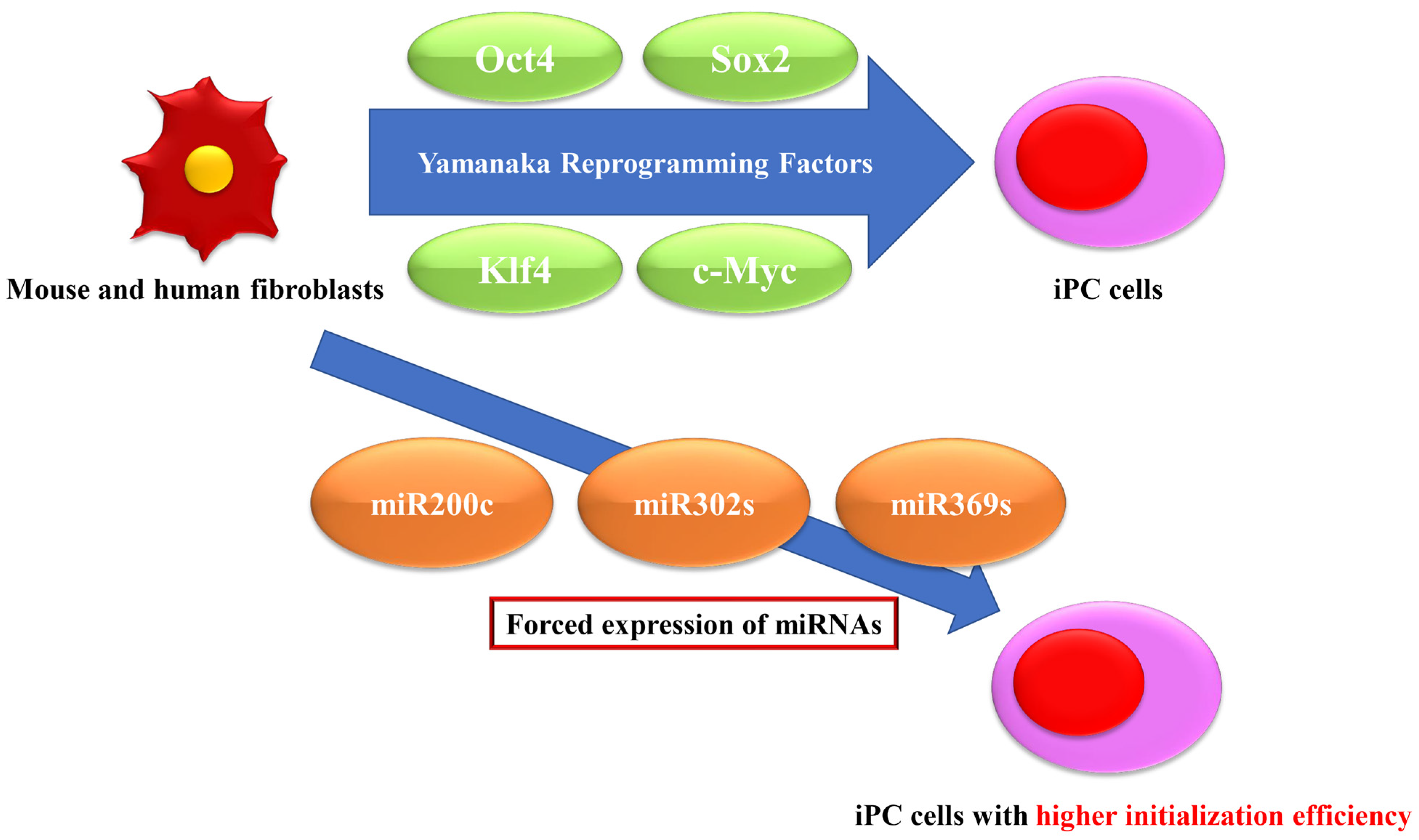
2. Introduction of Transcription Factors for Reprogramming
3. Resolution of Initiation Efficiency by Forced Expression of miRNAs
4. Application to Refractory Gastrointestinal Cancer
4.1. Liver
4.2. Colon and Rectum
4.3. Pancreas
4.4. Esophagus
4.5. Stomach
5. Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reik, W.; Dean, W.; Walter, J. Epigenetic reprogramming in mammalian development. Science 2001, 293, 1089–1093. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Chang, C. Epigenetics in Health and Disease. Adv. Exp. Med. Biol. 2020, 1253, 3–55. [Google Scholar] [CrossRef]
- Reik, W.; Dean, W. DNA methylation and mammalian epigenetics. Electrophoresis 2001, 22, 2838–2843. [Google Scholar] [CrossRef]
- Feng, S.; Jacobsen, S.E.; Reik, W. Epigenetic reprogramming in plant and animal development. Science 2010, 330, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Hochedlinger, K.; Blelloch, R.; Brennan, C.; Yamada, Y.; Kim, M.; Chin, L.; Jaenisch, R. Reprogramming of a melanoma genome by nuclear transplantation. Genes Dev. 2004, 18, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Brambrink, T.; Hochedlinger, K.; Bell, G.; Jaenisch, R. ES cells derived from cloned and fertilized blastocysts are transcriptionally and functionally indistinguishable. Proc. Natl. Acad. Sci. USA 2006, 103, 933–938. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Zaehres, H.; Wu, G.; Gentile, L.; Ko, K.; Sebastiano, V.; Araúzo-Bravo, M.J.; Ruau, D.; Han, D.W.; Zenke, M.; et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 2008, 454, 646–650. [Google Scholar] [CrossRef]
- Yang, J.; Chai, L.; Fowles, T.C.; Alipio, Z.; Xu, D.; Fink, L.M.; Ward, D.C.; Ma, Y. Genome-wide analysis reveals Sall4 to be a major regulator of pluripotency in murine-embryonic stem cells. Proc. Natl. Acad. Sci. USA 2008, 105, 19756–19761. [Google Scholar] [CrossRef]
- Heng, J.-C.D.; Feng, B.; Han, J.; Jiang, J.; Kraus, P.; Ng, J.-H.; Orlov, Y.L.; Huss, M.; Yang, L.; Lufkin, T.; et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell 2010, 6, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.; Markov, G.J.; Brady, J.J.; Palla, A.; Zeng, H.; Sebastiano, V.; Blau, H.M. NKX3-1 is required for induced pluripotent stem cell reprogramming and can replace OCT4 in mouse and human iPSC induction. Nat. Cell Biol. 2018, 20, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Li, W.; Benda, C.; Huang, Z.; Ahmed, T.; Liu, P.; Guo, X.; Ibañez, D.P.; Luo, Z.; Zhang, M.; et al. NCoR/SMRT co-repressors cooperate with c-MYC to create an epigenetic barrier to somatic cell reprogramming. Nat. Cell Biol. 2018, 20, 400–412. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, K.; Ruan, W.; Bo, Z.; Liu, L.; Cao, Z.; Chai, L.; Cao, G. Higher methylation in genomic DNA indicates incomplete reprogramming in induced pluripotent stem cells. Cell. Reprogramming 2013, 15, 92–99. [Google Scholar] [CrossRef]
- Miyoshi, N.; Ishii, H.; Nagano, H.; Haraguchi, N.; Dewi, D.L.; Kano, Y.; Nishikawa, S.; Tanemura, M.; Mimori, K.; Tanaka, F.; et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell 2011, 8, 633–638. [Google Scholar] [CrossRef]
- Hoshino, H.; Nagano, H.; Haraguchi, N.; Nishikawa, S.; Tomokuni, A.; Kano, Y.; Fukusumi, T.; Saito, T.; Ozaki, M.; Sakai, D.; et al. Hypoxia and TP53 deficiency for induced pluripotent stem cell-like properties in gastrointestinal cancer. Int. J. Oncol. 2012, 40, 1423–1430. [Google Scholar] [CrossRef]
- Miyoshi, N.; Ishii, H.; Nagai, K.-I.; Hoshino, H.; Mimori, K.; Tanaka, F.; Nagano, H.; Sekimoto, M.; Doki, Y.; Mori, M. Defined factors induce reprogramming of gastrointestinal cancer cells. Proc. Natl. Acad. Sci. USA 2010, 107, 40–45. [Google Scholar] [CrossRef]
- Nagai, K.-I.; Ishii, H.; Miyoshi, N.; Hoshino, H.; Saito, T.; Sato, T.; Tomimaru, Y.; Kobayashi, S.; Nagano, H.; Sekimoto, M.; et al. Long-term culture following ES-like gene-induced reprogramming elicits an aggressive phenotype in mutated cholangiocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2010, 395, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, D.; Huang, C.; Yin, Y.; Vali, K.; Zhang, M.; Tang, Y. Enhanced human somatic cell reprogramming efficiency by fusion of the MYC transactivation domain and OCT4. Stem Cell Res. 2017, 25, 88–97. [Google Scholar] [CrossRef]
- Miyazaki, S.; Yamamoto, H.; Miyoshi, N.; Takahashi, H.; Suzuki, Y.; Haraguchi, N.; Ishii, H.; Doki, Y.; Mori, M. Emerging methods for preparing iPS cells. Jpn. J. Clin. Oncol. 2012, 42, 773–779. [Google Scholar] [CrossRef]
- Singovski, G.; Bernal, C.; Kuciak, M.; Siegl-Cachedenier, I.; Conod, A.; Ruiz i Altaba, A. In vivo epigenetic reprogramming of primary human colon cancer cells enhances metastases. J. Mol. Cell Biol. 2016, 8, 157–173. [Google Scholar] [CrossRef][Green Version]
- Sandmaier, S.E.; Telugu, B.P. MicroRNA-Mediated Reprogramming of Somatic Cells into Induced Pluripotent Stem Cells. Methods Mol. Biol. 2015, 1330, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wilson, K.D.; Ghosh, Z.; Han, L.; Wang, Y.; Lan, F.; Ransohoff, K.J.; Burridge, P.; Wu, J.C. MicroRNA-302 increases reprogramming efficiency via repression of NR2F2. Stem Cells 2013, 31, 259–268. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Cao, Y.; Wang, L.; Han, Y.; Zhong, X.; Zhou, G.; Cai, Y.; Zhang, H.; Gao, P. Human fibroblast reprogramming to pluripotent stem cells regulated by the miR19a/b-PTEN axis. PLoS ONE 2014, 9, e95213. [Google Scholar] [CrossRef]
- Pfaff, N.; Liebhaber, S.; Möbus, S.; Beh-Pajooh, A.; Fiedler, J.; Pfanne, A.; Schambach, A.; Thum, T.; Cantz, T.; Moritz, T. Inhibition of miRNA-212/132 improves the reprogramming of fibroblasts into induced pluripotent stem cells by de-repressing important epigenetic remodelling factors. Stem Cell Res. 2017, 20, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Mukherjee, B.; Dixit, M. MicroRNA Key to Angiogenesis Regulation: MiRNA Biology and Therapy. Curr. Cancer Drug Targets 2018, 18, 266–277. [Google Scholar] [CrossRef]
- García-Donas, J.; Beuselinck, B.; Inglada-Pérez, L.; Graña, O.; Schöffski, P.; Wozniak, A.; Bechter, O.; Apellániz-Ruiz, M.; Leandro-García, L.J.; Esteban, E.; et al. Deep sequencing reveals microRNAs predictive of antiangiogenic drug response. JCI Insight 2016, 1, e86051. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, G.; Cava, C.; Castiglioni, I. The potential of miRNAs for diagnosis, treatment and monitoring of breast cancer. Scand. J. Clin. Lab Invest. 2016, 245, S34–S39. [Google Scholar] [CrossRef]
- Shu, D.; Li, H.; Shu, Y.; Xiong, G.; Carson, W.E., III; Haque, F.; Xu, R.; Guo, P. Systemic Delivery of Anti-miRNA for Suppression of Triple Negative Breast Cancer Utilizing RNA Nanotechnology. ACS Nano 2015, 9, 9731–9740. [Google Scholar] [CrossRef]
- Sarkar, F.H. Novel Holistic Approaches for Overcoming Therapy Resistance in Pancreatic and Colon Cancers. Med. Princ. Pract. 2016, 25 (Suppl. 2), 3–10. [Google Scholar] [CrossRef]
- Koga, C.; Kobayashi, S.; Nagano, H.; Tomimaru, Y.; Hama, N.; Wada, H.; Kawamoto, K.; Eguchi, H.; Konno, M.; Ishii, H.; et al. Reprogramming using microRNA-302 improves drug sensitivity in hepatocellular carcinoma cells. Ann. Surg. Oncol. 2014, 21 (Suppl. 4), S591–S600. [Google Scholar] [CrossRef]
- Li, H.-P.; Zeng, X.-C.; Zhang, B.; Long, J.-T.; Zhou, B.; Tan, G.-S.; Zeng, W.-X.; Chen, W.; Yang, J.-Y. miR-451 inhibits cell proliferation in human hepatocellular carcinoma through direct suppression of IKK-β. Carcinogenesis 2013, 34, 2443–2451. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Zhang, K.; Chen, D.-Q.; Chen, J.; Feng, B.; Song, H.; Chen, Y.; Zhu, Z.; Lu, L.; De, W.; et al. MicroRNA-451: Epithelial-mesenchymal transition inhibitor and prognostic biomarker of hepatocelluar carcinoma. Oncotarget 2015, 6, 18613–18630. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Li, F.; Lin, Y.; Zhang, X.; Lv, Z.; Jiang, J. MiR-214 inhibits cell growth in hepatocellular carcinoma through suppression of β-catenin. Biochem. Biophys. Res. Commun. 2012, 428, 525–531. [Google Scholar] [CrossRef]
- He, X.-X.; Chang, Y.; Meng, F.-Y.; Wang, M.-Y.; Xie, Q.-H.; Tang, F.; Li, P.-Y.; Song, Y.-H.; Lin, J.-S. MicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene 2012, 31, 3357–3369. [Google Scholar] [CrossRef]
- Shen, G.; Rong, X.; Zhao, J.; Yang, X.; Li, H.; Jiang, H.; Zhou, Q.; Ji, T.; Huang, S.; Zhang, J.; et al. MicroRNA-105 suppresses cell proliferation and inhibits PI3K/AKT signaling in human hepatocellular carcinoma. Carcinogenesis 2014, 35, 2748–2755. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Z.; Li, Y.; Ji, D.; Zhang, P.; Liu, Q.; Yao, Y. [miR-143 inhibits proliferation and invasion of hepatocellular carcinoma cells via down-regulation of TLR2 expression]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2014, 30, 1076–1079. [Google Scholar] [PubMed]
- Yang, Y.; Hou, N.; Wang, X.; Wang, L.; Chang, S.; He, K.; Zhao, Z.; Zhao, X.; Song, T.; Huang, C. miR-15b-5p induces endoplasmic reticulum stress and apoptosis in human hepatocellular carcinoma, both in vitro and in vivo, by suppressing Rab1A. Oncotarget 2015, 6, 16227–16238. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Wang, B.; Xiao, S.; Li, Y.; Chen, Q. miR-125a/b inhibits tumor-associated macrophages mediated in cancer stem cells of hepatocellular carcinoma by targeting CD90. J. Cell. Biochem. 2019, 120, 3046–3055. [Google Scholar] [CrossRef]
- Lin, J.; Huang, S.; Wu, S.; Ding, J.; Zhao, Y.; Liang, L.; Tian, Q.; Zha, R.; Zhan, R.; He, X. MicroRNA-423 promotes cell growth and regulates G1/S transition by targeting p21Cip1/Waf1 in hepatocellular carcinoma. Carcinogenesis 2011, 32, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, W.; Cheng, N.; Wang, K.; Li, B.; Jiang, X.; Sun, S. Hepatitis C virus-induced up-regulation of microRNA-155 promotes hepatocarcinogenesis by activating Wnt signaling. Hepatology 2012, 56, 1631–1640. [Google Scholar] [CrossRef]
- Ni, F.; Zhao, H.; Cui, H.; Wu, Z.; Chen, L.; Hu, Z.; Guo, C.; Liu, Y.; Chen, Z.; Wang, X.; et al. MicroRNA-362-5p promotes tumor growth and metastasis by targeting CYLD in hepatocellular carcinoma. Cancer Lett. 2015, 356, 809–818. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y. miR-342-3p affects hepatocellular carcinoma cell proliferation via regulating NF-κB pathway. Biochem. Biophys. Res. Commun. 2015, 457, 370–377. [Google Scholar] [CrossRef]
- Zan, Y.; Wang, B.; Liang, L.; Deng, Y.; Tian, T.; Dai, Z.; Dong, L. MicroRNA-139 inhibits hepatocellular carcinoma cell growth through down-regulating karyopherin alpha 2. J. Exp. Clin. Cancer Res. 2019, 38, 182. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Yamamoto, H.; Miyoshi, N.; Wu, X.; Ogawa, H.; Uemura, M.; Nishimura, J.; Hata, T.; Takemasa, I.; Mizushima, T.; et al. A Cancer Reprogramming Method Using MicroRNAs as a Novel Therapeutic Approach against Colon Cancer: Research for Reprogramming of Cancer Cells by MicroRNAs. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S1394–S1401. [Google Scholar] [CrossRef]
- Ogawa, H.; Wu, X.; Kawamoto, K.; Nishida, N.; Konno, M.; Koseki, J.; Matsui, H.; Noguchi, K.; Gotoh, N.; Yamamoto, T.; et al. MicroRNAs Induce Epigenetic Reprogramming and Suppress Malignant Phenotypes of Human Colon Cancer Cells. PLoS ONE 2015, 10, e0127119. [Google Scholar] [CrossRef]
- Singh, N.; Liu, G.; Chakrabarty, S. Isolation and characterization of calcium sensing receptor null cells: A highly malignant and drug resistant phenotype of colon cancer. Int. J. Cancer 2013, 132, 1996–2005. [Google Scholar] [CrossRef]
- Wang, J.; Lei, Z.-J.; Guo, Y.; Wang, T.; Qin, Z.-Y.; Xiao, H.-L.; Fan, L.-L.; Chen, D.-F.; Bian, X.-W.; Liu, J.; et al. miRNA-regulated delivery of lincRNA-p21 suppresses β-catenin signaling and tumorigenicity of colorectal cancer stem cells. Oncotarget 2015, 6, 37852–37870. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xi, J.; Xu, X.; Peng, B.; Zhang, B. MiR-148a suppressed cell invasion and migration via targeting WNT10b and modulating β-catenin signaling in cisplatin-resistant colorectal cancer cells. Biomed. Pharmacother. 2019, 109, 902–909. [Google Scholar] [CrossRef]
- Zhao, S.; Mi, Y.; Guan, B.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Cai, S.; Xu, Y.; Li, X.; et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 2020, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Basati, G.; Emami Razavi, A.; Abdi, S.; Mirzaei, A. Elevated level of microRNA-21 in the serum of patients with colorectal cancer. Med. Oncol. 2014, 31, 205. [Google Scholar] [CrossRef]
- Koga, Y.; Yasunaga, M.; Takahashi, A.; Kuroda, J.; Moriya, Y.; Akasu, T.; Fujita, S.; Yamamoto, S.; Baba, H.; Matsumura, Y. MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer Prev. Res. (Phila) 2010, 3, 1435–1442. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Gou, S.; Xiong, J.; Wu, H.; Wang, C.; Yan, H.; Liu, T. MiR-744 increases tumorigenicity of pancreatic cancer by activating Wnt/β-catenin pathway. Oncotarget 2015, 6, 37557–37569. [Google Scholar] [CrossRef]
- Peng, L.; Liu, Z.; Xiao, J.; Tu, Y.; Wan, Z.; Xiong, H.; Li, Y.; Xiao, W. MicroRNA-148a suppresses epithelial-mesenchymal transition and invasion of pancreatic cancer cells by targeting Wnt10b and inhibiting the Wnt/β-catenin signaling pathway. Oncol. Rep. 2017, 38, 301–308. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Z.; Yu, C.; Chen, M.; Tian, S.; Sun, C. MiR-1181 inhibits stem cell-like phenotypes and suppresses SOX2 and STAT3 in human pancreatic cancer. Cancer Lett. 2015, 356, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Ma, T.; Huang, B.; Lin, L.; Zhou, Y.; Yan, J.; Zou, Y.; Chen, S. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-β signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 310. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Wu, S.; Wang, W.; Liu, Z.; Zhang, J.; Wang, Z.; Li, R.; Zhang, Z.; Li, Z.; Dong, S.; et al. miR-942 promotes cancer stem cell-like traits in esophageal squamous cell carcinoma through activation of Wnt/β-catenin signalling pathway. Oncotarget 2015, 6, 10964–10977. [Google Scholar] [CrossRef]
- Kano, M.; Seki, N.; Kikkawa, N.; Fujimura, L.; Hoshino, I.; Akutsu, Y.; Chiyomaru, T.; Enokida, H.; Nakagawa, M.; Matsubara, H. miR-145, miR-133a and miR-133b: Tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int. J. Cancer 2010, 127, 2804–2814. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, J.; Guo, H.; Xu, X.; Xiong, G.; Guan, X.; Liu, B.; Li, J.; Chen, X.; Yang, K.; et al. MiR-196a binding-site SNP regulates RAP1A expression contributing to esophageal squamous cell carcinoma risk and metastasis. Carcinogenesis 2012, 33, 2147–2154. [Google Scholar] [CrossRef]
- Lin, R.-J.; Xiao, D.-W.; Liao, L.-D.; Chen, T.; Xie, Z.-F.; Huang, W.-Z.; Wang, W.-S.; Jiang, T.-F.; Wu, B.-L.; Li, E.-M.; et al. MiR-142-3p as a potential prognostic biomarker for esophageal squamous cell carcinoma. J. Surg. Oncol. 2012, 105, 175–182. [Google Scholar] [CrossRef]
- Gong, H.; Song, L.; Lin, C.; Liu, A.; Lin, X.; Wu, J.; Li, M.; Li, J. Downregulation of miR-138 sustains NF-κB activation and promotes lipid raft formation in esophageal squamous cell carcinoma. Clin. Cancer Res. 2013, 19, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.-H.; Chen, W.-X.; Li, S.; He, X.-Y.; Zhang, Z.-M.; Li, M.; Cao, R.-S.; Hao, B.; Zhang, H.-J.; Qiu, H.-Q.; et al. MicroRNA-183 promotes proliferation and invasion in oesophageal squamous cell carcinoma by targeting programmed cell death 4. Br. J. Cancer 2014, 111, 2003–2013. [Google Scholar] [CrossRef]
- Yang, M.; Liu, R.; Li, X.; Liao, J.; Pu, Y.; Pan, E.; Yin, L.; Wang, Y. miRNA-183 suppresses apoptosis and promotes proliferation in esophageal cancer by targeting PDCD4. Mol. Cells 2014, 37, 873–880. [Google Scholar] [CrossRef]
- Zheng, P.; Chen, L.; Yuan, X.; Luo, Q.; Liu, Y.; Xie, G.; Ma, Y.; Shen, L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 53. [Google Scholar] [CrossRef]
- Cui, H.-Y.; Rong, J.-S.; Chen, J.; Guo, J.; Zhu, J.-Q.; Ruan, M.; Zuo, R.-R.; Zhang, S.-S.; Qi, J.-M.; Zhang, B.-H. Exosomal microRNA-588 from M2 polarized macrophages contributes to cisplatin resistance of gastric cancer cells. World J. Gastroenterol. 2021, 27, 6079–6092. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Bai, M.; Ning, T.; Ge, S.; Deng, T.; Liu, R.; Zhang, L.; Ying, G.; Ba, Y. Exosomes Serve as Nanoparticles to Deliver Anti-miR-214 to Reverse Chemoresistance to Cisplatin in Gastric Cancer. Mol. Ther. 2018, 26, 774–783. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Y.; Guo, L.; Liu, C.; Wang, P.; Ren, W. Exosomal microRNA-107 reverses chemotherapeutic drug resistance of gastric cancer cells through HMGA2/mTOR/P-gp pathway. BMC Cancer 2021, 21, 1290. [Google Scholar] [CrossRef]
- Cheng, C.; Qin, Y.; Zhi, Q.; Wang, J.; Qin, C. Knockdown of long non-coding RNA HOTAIR inhibits cisplatin resistance of gastric cancer cells through inhibiting the PI3K/Akt and Wnt/β-catenin signaling pathways by up-regulating miR-34a. Int. J. Biol. Macromol. 2018, 107, 2620–2629. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, L.; Zhang, C.; Liu, P. Exosomal MiR-500a-3p promotes cisplatin resistance and stemness via negatively regulating FBXW7 in gastric cancer. J. Cell. Mol. Med. 2020, 24, 8930–8941. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, Y.; Xu, Y.; Hou, S.; Huang, J.; Wang, B.; Zhao, J.; Xia, S.; Fan, S.; Yu, X.; et al. Exosomal transfer of miR-501 confers doxorubicin resistance and tumorigenesis via targeting of BLID in gastric cancer. Cancer Lett. 2019, 459, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cai, S.; Shu, Y.; Deng, X.; Zhang, Y.; He, N.; Wan, L.; Chen, X.; Qu, Y.; Yu, S. Exosomal miR-487a derived from m2 macrophage promotes the progression of gastric cancer. Cell Cycle 2021, 20, 434–444. [Google Scholar] [CrossRef]
- Qiu, S.; Xie, L.; Lu, C.; Gu, C.; Xia, Y.; Lv, J.; Xuan, Z.; Fang, L.; Yang, J.; Zhang, L.; et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J. Exp. Clin. Cancer Res. 2022, 41, 296. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.M.; Gao, H.J.; Li, J.M.; Zhang, P.; Li, G. [Effect of exosome-derived miR-223 from macrophages on the metastasis of gastric cancer cells]. Zhonghua Yi Xue Za Zhi 2020, 100, 1750–1755. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, N.; He, S.; Lu, X. Exosomal miR-106a derived from gastric cancer promotes peritoneal metastasis via direct regulation of Smad7. Cell Cycle 2020, 19, 1200–1221. [Google Scholar] [CrossRef]
- Budakoti, M.; Panwar, A.S.; Molpa, D.; Singh, R.K.; Büsselberg, D.; Mishra, A.P.; Coutinho, H.D.M.; Nigam, M. Micro-RNA: The darkhorse of cancer. Cell. Signal. 2021, 83, 109995. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Vázquez, L.A.; Medina-Ríos, I.; Márquez-Gallardo, L.D.; Reyes-Muñoz, J.; Serrano-Cano, F.I.; Pathak, S.; Banerjee, A.; Bandyopadhyay, A.; Duttaroy, A.K.; Paul, S. Functional Implications and Clinical Potential of MicroRNAs in Irritable Bowel Syndrome: A Concise Review. Dig. Dis. Sci. 2023, 68, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-K.; Kafert-Kasting, S.; Thum, T. Preclinical and Clinical Development of Noncoding RNA Therapeutics for Cardiovascular Disease. Circ. Res. 2020, 126, 663–678. [Google Scholar] [CrossRef]
- McGregor, R.A.; Choi, M.S. microRNAs in the regulation of adipogenesis and obesity. Curr. Mol. Med. 2011, 11, 304–316. [Google Scholar] [CrossRef]
- Malczewska, A.; Frampton, A.E.; Mato Prado, M.; Ameri, S.; Dabrowska, A.F.; Zagorac, S.; Clift, A.K.; Kos-Kudła, B.; Faiz, O.; Stebbing, J.; et al. Circulating MicroRNAs in Small-bowel Neuroendocrine Tumors: A Potential Tool for Diagnosis and Assessment of Effectiveness of Surgical Resection. Ann. Surg. 2021, 274, e1–e9. [Google Scholar] [CrossRef]
- Khodaii, Z.; Mehrabani Natanzi, M.; Khalighfard, S.; Ghandian Zanjan, M.; Gharghi, M.; Khori, V.; Amiriani, T.; Rahimkhani, M.; Alizadeh, A.M. Novel targets in rectal cancer by considering lncRNA-miRNA-mRNA network in response to Lactobacillus acidophilus consumption: A randomized clinical trial. Sci. Rep. 2022, 12, 9168. [Google Scholar] [CrossRef]
- Sacar Demirci, M.D.; Yousef, M.; Allmer, J. Computational Prediction of Functional MicroRNA-mRNA Interactions. Methods Mol. Biol. 2019, 1912, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Haraguchi, N.; Mizushima, T.; Ishii, H.; Yamamoto, H.; Mori, M. Targeting cancer stem cells in refractory cancer. Regen. Ther. 2021, 17, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Jafri, M.A.; Zaidi, S.K.; Ansari, S.A.; Al-Qahtani, M.H.; Shay, J.W. MicroRNAs as potential drug targets for therapeutic intervention in colorectal cancer. Expert Opin. Ther. Targets 2015, 19, 1705–1723. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, X.; Wang, Y.; Yu, T.; Huang, L.; Chen, L.; Li, J.; Zhang, C.; Zhang, Y. Multi-Functional Peptide-MicroRNA Nanocomplex for Targeted MicroRNA Delivery and Function Imaging. Chemistry 2018, 24, 2277–2285. [Google Scholar] [CrossRef]
- Esposito, C.L.; Catuogno, S.; de Franciscis, V. Aptamer-MiRNA Conjugates for Cancer Cell-Targeted Delivery. Methods Mol. Biol. 2016, 1364, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
| Cancer Type | miRNA Type | Proliferative or Inhibitory Action against Cancer | Molecular Mechanism of Action Sequence |
|---|---|---|---|
| Liver | miR-302 | Inhibitory | H3K4 methylation and c-Myc suppression through AOF2 downregulation and reprogramming of hepatocellular carcinoma (HCC) cells [31] |
| miR-451 | Inhibitory | Direct suppression of IKK-β and partially targeting c-Myc [32,33] | |
| miR-214 | Inhibitory | Suppression of β-catenin [34] | |
| miR-375 | Inhibitory | Targets AEG-1 and inhibits the growth of HCC [35] | |
| miR-105 | Inhibitory | Inhibits the PI3K/AKT signaling pathway [36] | |
| miR-143 | Inhibitory | Downregulates TLR2, NF-κB, MMP-2, and MMP-9 expression, inhibits HCC growth and invasion, and promotes apoptosis [37] | |
| miR-15b-5p | Inhibitory | Suppresses Rab1A and inhibits HCC cell growth [38] | |
| miR-125a/b | Inhibitory | Inhibits cell proliferation by targeting CD90, a stem cell marker of HCC stem cells [39] | |
| miR-423 | Proliferative | Suppression of expression of the tumor suppressor p21Cip1/Waf1 [40] | |
| miR-155 | Proliferative | Hepatitis C virus infection promotes hepatocyte proliferation and tumorigenesis and simultaneously activates Wnt signaling [41] | |
| miR-362-5p | Proliferative | Activates the NF-κB signaling pathway via cylindromatosis (CYLD) to induce contributions to tumor growth [42] | |
| miR-342 | Proliferative | Regulates the NF-κB pathway and overexpresses Ikk-γ, TAB2, and TAB3 [43] | |
| miR-139 | Inhibitory | Regulates karyopherin alpha 2 [44] | |
| Colon and rectum | miR-302a-d, 369-3p and 5p, and miR-200c | Inhibitory | Suppresses c-Myc expression [45] |
| miR-302s and miR-369s | Inhibitory | Induces cellular initiation and suppresses the malignant phenotype of human colon cancer [46] | |
| miR21, miR135a, and miR135b | Proliferative | Suppresses calcium-sensing receptor (CaSR) in human colonic crypt epithelium, which is associated with cell differentiation [47] | |
| miR145 | Inhibitory | Upregulates CaSR cells [48] | |
| miR-148a | Inhibitory | Suppresses cell invasion and migration by targeting WNT10b and β-catenin signaling activity [49] | |
| miR-934 | Proliferative | Promotes colorectal cancer liver metastasis by regulating cross-talk between colon cancer cells and tumor-associated macrophages [50] | |
| Pancreas | miR-744 | Proliferative | Enhances the Wnt/β-catenin signaling pathway, which is commonly enhanced in pancreatic cancer, and augments the tumorigenic potential [53] |
| miR-148a | Inhibitory | Inhibits epithelial–mesenchymal transition and invasion of pancreatic cancer cells by targeting Wnt10b and inhibiting the Wnt/β-catenin signaling pathway [54] | |
| miR-1181 | Inhibitory | Suppresses sex-determining region Y-box2 (SOX2) and signal transduction and activation of transcription 3 (STAT3) [55] | |
| miR-501-3p | Proliferative | Activates the TGF-β signaling pathway and promotes pancreatic ductal adenocarcinoma development by suppressing the tumor suppressor TGFBR3 gene [56] | |
| Esophagus | miR-942 | Proliferative | Directly binds to c-Myc and upregulates Wnt/β-catenin signaling activity [57] |
| miR-145, miR-133a, and miR-133b | Inhibitory | Directly regulates FSCN1 to suppress cell proliferation and cell invasion in esophageal squamous cell carcinoma (ESCC) cells [58] | |
| miR-196a | Inhibitory | Regulates RAS-related protein (RAP1A) expression Single-nucleotide polymorphism rs6573 inhibits the binding of miR-196a to the RAP1A 3′UTR and increases the constitutive expression of RAP1A, which is overexpressed in the majority of ESCCs [59] | |
| miR-142-3p | Inhibitory | Regulates transcription, cell cycle, and differentiation and is also involved in cell migration and adhesion, transcriptional activation, and apoptosis [60] | |
| miR-138 | Inhibitory | Its suppression promotes K63-bound polyubiquitination of NF-κB signaling intermediates, TRAF2 and RIP1, to sustain NF-κB activation and in lipid rafts, including FLOT1, FLOT2, and caveolin-1, and induces lipid raft formation via upregulation of multiple components [61] | |
| miR-183 | Proliferative | Directly targets PDCD4, and overexpression of miR-183 promotes ESCC cell proliferation and invasion [62,63] | |
| Stomach | miR-21 | Proliferative | Confers resistance to cisplatin (CDDP) [64] |
| miR-588 | Proliferative | Targets CYLD in gastric cancer (GC) cells and promotes the growth of CDDP-resistant GC cells by depleting CYLD [65] | |
| miR-214 | Proliferative | Downregulated miR-214 in tumors, reverses chemotherapy resistance, and inhibits tumor growth in vivo [66] | |
| miR-107 | Proliferative | Enhances sensitivity to CDDP via HMGA2/mTOR/P-gp [67] | |
| miR-34a | Proliferative | HOX transcript antisense RNA (HOTAIR) binds directly to miR-34a, and the knockdown of HOTAIR enhances the inhibitory effect of CDDP on tumor growth in vivo [68] | |
| miR-500a-3p | Proliferative | Increases CDDP resistance and poor progression-free progression by targeting FBXW7 [69] | |
| miR-501 | Proliferative | Induces resistance to doxorubicin by downregulation of cell death inducer (BLID), inactivation of caspase-9/3, and phosphorylation of Akt [70] | |
| miR-487a | Inhibitory | Regulates GC progression and promotes proliferation and tumorigenesis of GC by targeting TIA1 [71] | |
| 519a-3p | Proliferative | Plays an important role in the cross-talk between primary GC tumor cells and intrahepatic macrophages by targeting DUSP2 and activating the MAPK/ERK pathway [72] | |
| miR-223 | Proliferative | Promotes GC cell metastasis via the PTEN-PI3K/AKT pathway [73] | |
| miR-106a | Proliferative | Promotes tumor growth by targeting Smad7 and can contribute to GC peritoneal metastasis [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, H.; Hara, T.; Meng, S.; Tsuji, Y.; Arao, Y.; Sasaki, K.; Miyoshi, N.; Kobayashi, S.; Doki, Y.; Eguchi, H.; et al. Drug Discovery and Development of miRNA-Based Nucleotide Drugs for Gastrointestinal Cancer. Biomedicines 2023, 11, 2235. https://doi.org/10.3390/biomedicines11082235
Sato H, Hara T, Meng S, Tsuji Y, Arao Y, Sasaki K, Miyoshi N, Kobayashi S, Doki Y, Eguchi H, et al. Drug Discovery and Development of miRNA-Based Nucleotide Drugs for Gastrointestinal Cancer. Biomedicines. 2023; 11(8):2235. https://doi.org/10.3390/biomedicines11082235
Chicago/Turabian StyleSato, Hiromichi, Tomoaki Hara, Sikun Meng, Yoshiko Tsuji, Yasuko Arao, Kazuki Sasaki, Norikatsu Miyoshi, Shogo Kobayashi, Yuichiro Doki, Hidetoshi Eguchi, and et al. 2023. "Drug Discovery and Development of miRNA-Based Nucleotide Drugs for Gastrointestinal Cancer" Biomedicines 11, no. 8: 2235. https://doi.org/10.3390/biomedicines11082235
APA StyleSato, H., Hara, T., Meng, S., Tsuji, Y., Arao, Y., Sasaki, K., Miyoshi, N., Kobayashi, S., Doki, Y., Eguchi, H., & Ishii, H. (2023). Drug Discovery and Development of miRNA-Based Nucleotide Drugs for Gastrointestinal Cancer. Biomedicines, 11(8), 2235. https://doi.org/10.3390/biomedicines11082235






