Abstract
The use of periprocedural dual antiplatelet therapy (DAPT) has significantly evolved along with innovations in the endovascular management of intracranial aneurysms. Historically, aspirin and clopidogrel have been the most commonly employed regimen due to its safety and efficacy. However, recent studies highlight the importance of tailoring DAPT regimens to individual patient characteristics which may affect clopidogrel metabolism, such as genetic polymorphisms. In the present report, a systematic review of the literature was performed to determine optimal antiplatelet use with flow diverting stents, intracranial stents, intrasaccular devices, and stent-assisted coiling. Studies were analyzed for the number of aneurysms treated, DAPT regimen, and any thromboembolic complications. Based on inclusion criteria, 368 studies were selected, which revealed the increasing popularity of alternative DAPT regimens with the aforementioned devices. Thromboembolic or hemorrhagic complications associated with antiplatelet medications were similar across all medications. DAPT with ticagrelor, tirofiban, or prasugrel are effective and safe alternatives to clopidogrel and do not require enzymatic activation. Further clinical trials are needed to evaluate different antiplatelet regimens with various devices to establish highest-level evidence-based guidelines and recommendations.
1. Introduction
Interventional cardiology laid the foundation for current developments in endovascular neurosurgery. The early utilization of intra-arterial catheters for interventional purposes were seen in cardiac vasculature targets [1]. There has been a dramatic rise in neuroendovascular treatment modalities for various intracranial pathologies over the last few decades, including intrasaccular coil embolization, stent-assisted coil (SAC) embolization, and flow diverting stents (FDS) [2]. Coil embolization, which involves placing a coil into an aneurysm to promote thrombus formation, has significantly improved the outcomes of patients with cerebral aneurysms [3]. Parent lumen flow diversion devices, such as the Pipeline Embolization Device (PED), are used to treat complex brain aneurysms that are wide-necked, giant, or not amenable for coiling [4]. FDS work by diverting blood flow away from the aneurysm, which promotes occlusion of the aneurysm dome over successive months [3]. The implementation of these neurointerventional devices has dramatically changed the course of treatment for intracranial aneurysms.
Modern neurointerventional procedures require pharmacological intervention to minimize thromboembolic complications. Dual antiplatelet therapy (DAPT), which includes aspirin with another antiplatelet agent, was initially studied in multi-center randomized trials in cardiac patients [5,6,7,8]. While the number of studies specific to neurointervention are increasing, it remains common practice for neurointerventionalists to follow a similar DAPT regimen to cardiac literature [9]. The optimal dosing and duration of DAPT in neurointervention is still debated [9,10,11].
Common antiplatelet agents for DAPT include ticlopidine, clopidogrel, prasugrel, and ticagrelor. Ticlopidine was first used in the late 1990s, but was quickly replaced by clopidogrel due to the occurrence of thrombocytopenia and neutropenia [12]. Clopidogrel has variable responsiveness [13] due to polymorphisms of liver enzymes in the multi-step activation pathway of clopidogrel [14,15]. Due to variations in the liver enzymes CYP2C9 and CYP2C19, it has previously been described that up to 30% of patients fail to respond as intended to clopidogrel [16]. A promising alternative to clopidogrel, prasugrel, has less frequently mutated enzymes [17] and is better at reducing ischemic events [7,15]. Another antiplatelet medication, ticagrelor, is often utilized for patients with clopidogrel-resistance [18]. Although several studies have compared ticagrelor and prasugrel against clopidogrel, the safety results remain unclear. While some studies have found no difference between the three medications [19,20], others found prasugrel and ticagrelor to have higher postoperative bleeding rates [7,21]. Tirofiban is an intravenous glycoprotein IIb/IIIa inhibitor with a short half-life [22,23]. The literature is conflicted when comparing its efficacy to other glycoprotein IIb-IIIa inhibitors and with traditional DAPT [24,25]. A meta-analysis discussing the use of tirofiban versus oral DAPT following SAC in ruptured aneurysms found tirofiban to have lower rates of post-procedural bleeding and thromboembolic complications [26]. However, additional studies have found that tirofiban in combination with DAPT did not increase post-procedural complications [27,28]. The choice of DAPT regimen also varies with the presence of ruptured versus unruptured aneurysms. Use of single versus dual antiplatelet therapy for ruptured aneurysms with subarachnoid hemorrhage remains debated [10]. Further studies are required to delineate optimal antiplatelet use for neurointerventional procedures.
The aforementioned antiplatelet medications are crucial to reduce thromboembolic complications following cerebrovascular procedures. Platelets tend to aggregate around foreign metals, which are the main composition of stents and coils [29]. While there is an abundance of literature on minimizing thrombosis and intracranial hemorrhage following neurointerventional procedures, there are no articles that summarize the use of DAPT therapy with intracranial aneurysm treatment. Here, we collected and analyzed data on the safety of various DAPT combinations with FDS, intrasaccular devices, and SAC procedures.
2. Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The references for this systematic review were conducted using the Embase, Scopus, and PubMed databases on 10 May 2023 using a list of search phrases (Table 1). Information obtained includes: number of aneurysms treated, antiplatelet or anticoagulants used, length of medication use, in-stent stenosis, ischemic events, and hemorrhagic events (Figure 1). Studies were included if they reported on the use of DAPT with the treatment of intracranial aneurysms. Aneurysm management devices include flow diverting stents, stent-assisted coiling, and intrasaccular devices.

Table 1.
Search phrases with their numerical results.
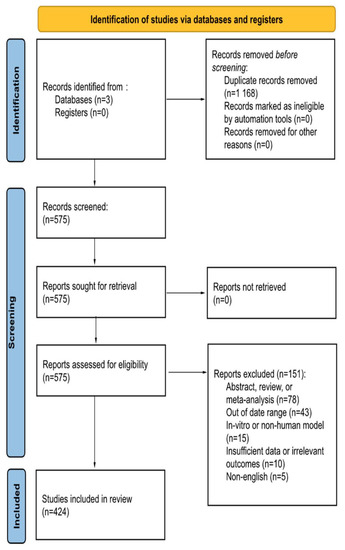
Figure 1.
PRISMA Flow Diagram.
Exclusion criteria includes articles published in a non-English language, use of in-vitro or non-human models, and articles published outside of the date range. Manuscripts were excluded if they failed to report at least one of the following postprocedural complications: ischemic events, in-stent thrombosis or stenosis, and/or hemorrhage. Abstracts, including conference abstracts, literature reviews, systematic reviews, and meta-analyses were excluded. To conduct the review, each of the four independent reviewers screened each article using the title and abstract. Then, BMM, JCCdB, IC, and GA independently reviewed the full text for its content. Discrepancies were addressed by discussion with the authors. The articles identified were published between 1 January 2015 and 1 May 2023. Searches in PubMed returned 250+ articles, Embase returned 800+ articles, and Scopus returned 680+ articles. After applying the aforementioned exclusion criteria, a total of 368 unique articles were reviewed.
The objective was to determine the safety of DAPT use across various endovascular devices utilized for the endovascular treatment of intracranial aneurysms. Safety was defined by the rate of hemorrhagic, ischemic, and in-stent stenosis complications. Hemorrhagic complications included postoperative intracranial or extracranial hemorrhage. Ischemic, or thromboembolic complications, and in-stent stenosis were defined when present on imaging.
Percentages, odds ratios (OR), 95% confidence intervals (CI), and statistical significance were calculated using R software (R Foundation, Indianapolis, IN, USA). To compare the percentages and calculated p-Values, a t-test was used. The p-Value was considered statistically significant if less than 0.05. The rates of hemorrhagic, thromboembolic, and all complications were calculated per 100 cases within each device category and for each medication studied. For analysis, ischemic and in-stent stenosis were grouped into a single category. Studies that placed patients on more than one DAPT regimen were not included in the analysis to prevent confounding of the results. The use of various DAPT regimens were compared to one another for within each complication category using OR.
Review bias was minimized by having each of the four reviewers independently inspect each article using the inclusion and exclusion criteria as mentioned above. All excluded articles were reviewed by JKC to ensure there were none excluded due to bias. No automation tools or artificial intelligence software were used in this process.
3. Results
From 1 January 2015 to 1 May 2023, over 39,000 aneurysms across 368 studies and three types of procedures were included in this review.
3.1. Total Complications
A total of 1551 complications were reported for 16,783 aneurysms treated through three different methods: flow diversion, intrasaccular devices, and stent assisted coiling. For each of these methods, the number of baseline cases were 108 aneurysms (11 complications), 666 aneurysms (12 baseline), and five aneurysms (two complications). Figure 2 provides a breakdown of the total number of cases and complication events per drug type. In flow diverting procedures, we are 95.34% and 91.31% confident that tirofiban and clopidogrel, respectively, had significantly lower complication rates than baseline treatment. Tirofiban and clopidogrel have similar complication rates between each other (Figure 3). The use of clopidogrel and ticagrelor in intrasaccular device procedures showed comparable complication rates (Figure 3).
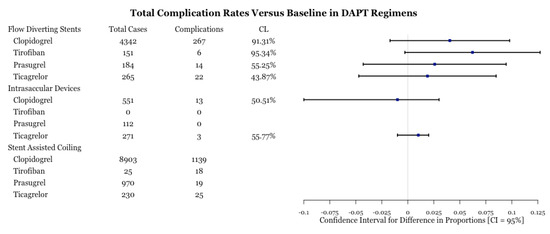
Figure 2.
Test for difference of complication rates by drug type for three procedures. CL is the confidence level at which one can reject the null hypothesis of equality of a complication rate versus that of baseline. A higher CL is indicative that one of the drugs is more effective. Despite CL being high for tirofiban, there is a large margin of error due to a low number of complications.
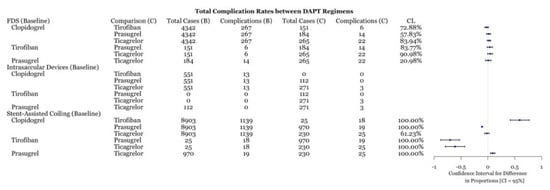
Figure 3.
Difference of complication rates test between DAPT drugs for three procedures with associated confidence level.
3.2. Hemorrhagic Complications
Reported postoperative hemorrhagic complications were compared between the four drug types for each procedure. FDS and intrasaccular devices did not have enough cases for any statistical analysis (n < 5). For stent-assisted coiling, hemorrhages were reported in 413 out of 8903 aneurysms using clopidogrel, 11 out of 25 for tirofiban, 17 out of 970 with prasugrel, and 14 out of 230 for ticagrelor. In terms of hemorrhagic complication rates, clopidogrel had statistically fewer complications than tirofiban, similar complication rates to ticagrelor, and more complications than prasugrel (Figure 4). Both prasugrel and ticagrelor had statistically lower complication rates than tirofiban (Figure 4). Prasugrel and ticagrelor exhibited similar total complication rates (Figure 2).
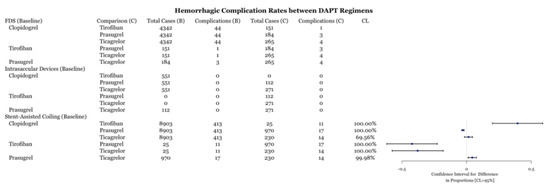
Figure 4.
Difference of hemorrhagic complication rates between DAPT drugs for three procedures with associated confidence levels.
3.3. Ischemic and Thrombotic Complications
Within each medication category, the number of ischemic and thrombotic complications were compared between medication types. All intrasaccular device procedures and stent-assisted coiling procedures with prasugrel did not have enough cases (n < 5) for statistical analysis (Figure 5). All medications performed similarly within flow diversion cases based on the value of the point estimate; however, the confidence level is low for most comparisons (Figure 5). Within stent-assisted coiling cases, ticagrelor had significantly fewer complications than both prasugrel and clopidogrel (Figure 6). Despite the point-estimate being high, there is statistically no difference in complication rates between clopidogrel and tirofiban since the confidence interval includes 0 (Figure 6). Ticagrelor had significantly lower complication rates than tirofiban (Figure 6).
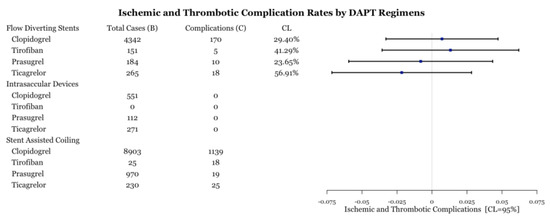
Figure 5.
Test for difference of complication rates by drug type for three procedures. CL is the confidence level at which one can reject the null hypothesis of equality of a complication rate versus that of baseline.

Figure 6.
Difference of ischemic and thrombotic complication rates between DAPT drugs for three procedures with associated confidence levels.
4. Discussion
4.1. General DAPT Use for Aneurysm Treatment
DAPT use in endovascular aneurysm treatment has witnessed significant advancements and refinements since the popularization of intracranial stenting in the early 1990s [30]. Antiplatelet medications have been employed in combination to optimize the prevention of thrombotic events after neurointerventional procedures [31]. Aspirin, a widely used antiplatelet agent, acts by irreversibly inhibiting cyclooxygenase-1, thereby suppressing platelet aggregation [32]. Clopidogrel, another common antiplatelet medication, is a thienopyridine derivative that selectively inhibits adenosine diphosphate-induced platelet activation by binding to the P2Y12 receptor [33]. The combination of aspirin and clopidogrel has historically been the most commonly employed DAPT regimen in unruptured aneurysm treatment due to its safety and efficacy [34]. However, a fixed-dose combination was frequently used without consideration of individual patient variability [35]. Emerging evidence has highlighted the importance of tailoring DAPT regimens based on patient characteristics, such as genetic polymorphisms, that greatly affect clopidogrel metabolism and response [36]. A variety of assessments of antiplatelet responsiveness are available, including aggregometry and platelet reactivity assays, which have enabled clinicians to optimize the antiplatelet effect [37]. This personalized approach has led to the development of other antiplatelet agents, including tirofiban, prasugrel, and ticagrelor, which may exhibit more predictable and potent platelet inhibition [38]. Ongoing research efforts aim to elucidate the optimal duration of DAPT, evaluate the efficacy of new agents, and identify predictors of patient response to guide individualized management plans.
This meta-analysis demonstrates several important points. First, the comparison of individual DAPT regimens to each other for use with FDS demonstrated no difference in preventing ischemic or thrombotic complications (Figure 6). Thus, it may suggest that clopidogrel alternatives, including tirofiban, prasugrel, and ticagrelor, are as effective as clopidogrel at preventing thromboembolic complications. The SAC results demonstrate that ticagrelor was more effective at an 89% confidence level (Figure 6) at preventing ischemic and thrombotic complications than clopidogrel. Clopidogrel was more effective at reducing these complications at an 99.66% confidence level (Figure 6). However, the inclusion of zero in the confidence level indicates a low but statistically significant sample size, which necessitates further data collection. These results support the placement of patients that are hypo-responders to clopidogrel on ticagrelor or using intravenous tirofiban. Likewise, prasugrel was demonstrated to be more effective at preventing all complications in SAC compared to clopidogrel (Figure 3). Examination of the hemorrhagic complications demonstrates that prasugrel is more effective, and ticagrelor as effective, at preventing intracranial hemorrhagic complications with SAC than clopidogrel (Figure 4). Alternatively, clopidogrel was shown to be more effective at preventing intracranial hemorrhagic complications in comparison to tirofiban when used with SAC (Figure 4). While the risk and extent of intracranial bleeding in the management of aneurysms increases with DAPT, more data are required to compare the effectiveness of various DAPT regimens in preventing intracranial hemorrhagic complications in FDS and intrasaccular devices. Randomized clinical trials are required to compare the effectiveness of various DAPT regimens against each other.
4.2. DAPT Use in Stent-Assisted Coiling
Although the combination of aspirin and clopidogrel is historically popular in aneurysm treatment, other agents are increasing in use for SAC. A novel and increasingly favored medication, ticagrelor, has gained popularity in DAPT for SAC due to its more predictable and potent platelet inhibition as compared to clopidogrel [39]. Similar to tirofiban, it has a rapid onset of action and does not require metabolic activation [40]. This decreases the risk of unresponsiveness, which may be seen with clopidogrel use [41]. Across 130 unruptured aneurysms treated with SAC and managed with aspirin and ticagrelor, Ma et al. reported thromboembolic complications in 3.3% of patients, and 1.3% of patients developed extracranial hemorrhage [39]. Narata et al. reported three (1.9%) ischemic and six (3.9%) intracranial hemorrhagic complications following treatment of 154 unruptured aneurysms with FDS and SAC that were on DAPT with ticagrelor [42]. While some have proposed that ticagrelor has an increased risk of intracranial hemorrhagic complications, Yi et al. demonstrated similar thromboembolic complications between ticagrelor and clopidogrel in patients managed with SAC for unruptured aneurysms [43]. A potential disadvantage of ticagrelor is its short half-life, which necessitates twice-daily dosing [44]. This necessity may make the medication dangerous for patients with a history of noncompliance or nonadherence [44].
An increasingly popular oral antiplatelet agent is prasugrel, which has been demonstrated to have lower ischemic events than clopidogrel in acute coronary syndrome [45]. A recent comparative study of DAPT with clopidogrel versus prasugrel in SAC for unruptured aneurysms revealed less thromboembolic complications with similar intracranial hemorrhagic events in the prasugrel group [46]. Likewise, the current study demonstrated lower rates of all complications with prasugrel versus clopidogrel use (Figure 3).
Although rare, the use of aspirin as monotherapy has been shown to be effective in SAC. A multicenter study on antiplatelet use in SAC demonstrated nonsignificant differences in the rates of ischemia and intracranial hemorrhage between aspirin monotherapy and DAPT in ruptured aneurysms [47]. While this supports the use of single antiplatelet therapy (SAPT) in SAC, this study was small and there has been another report of thromboembolic complications with this regimen in ruptured aneurysms [48]. Further studies are required to determine the safety and efficacy of SAPT in SAC.
Lastly, tirofiban is an intravenous GIIb/IIIa antagonist with a rapid onset of action, which makes it particularly useful in SAC procedures that require immediate platelet inhibition [27]. This medication may also be used in the setting of acute stent implantation, ineffective DAPT, or in combination with oral agents to enhance treatment [27,49]. Its efficacy in preventing thromboembolic events is well documented in the literature and may even be useful as inpatient monotherapy [49]. For ruptured aneurysms managed with FDS or SAC, a DELPHI consensus study determined that DAPT with tirofiban or eptifibatide was used as a standard approach amongst the responders [50]. In a case series of 105 patients with ruptured aneurysms who underwent SAC with prophylactic tirofiban, 2.8% of patients had thromboembolic complications without new intracranial hemorrhage [27,51]. This is further supported by a study on SAC and FDS by Samaniego et al., which found that tirofiban with DAPT did not increase the rate of thromboembolic or intracranial hemorrhagic complications in both unruptured and ruptured aneurysms [27]. A recent study found there to be thromboembolic complications in 13.89% and 8.33% of patients on DAPT for treated ruptured aneurysms with clopidogrel versus tirofiban, respectively [52]. Furthermore, intracranial hemorrhage was only found in 1.39% of patients in the tirofiban group. A recent meta-analysis and case series found there to be significantly lower rates of thromboembolic complications without an increase in intracranial hemorrhage when prophylactic therapy with tirofiban versus DAPT was used in SAC for ruptured aneurysms [26,53]. A recent meta-analysis demonstrated lower intracranial hemorrhagic and thromboembolic complications in patients managed with SAPT with tirofiban or eptifibatide over clopidogrel/aspirin in ruptured and unruptured aneurysms [54]. The current study of 114 articles supports these results, demonstrating that ticagrelor and prasugrel were more effective and tirofiban as effective at preventing thromboembolic complications compared to clopidogrel (Figure 6). However, a disadvantage of tirofiban is its short half-life, thus careful monitoring to maintain optimal antiplatelet effect is necessary [55]. Additionally, it can only be given intravenously, making it restricted to inpatient use [56]. Overall, tirofiban is the preferred antiplatelet medication for ruptured aneurysms, while various combinations of DAPT may be used for unruptured aneurysms.
When considering the prevention of thromboembolic events in SAC for aneurysm treatment, an individualized patient approach should be taken. A recent study demonstrated the efficacy of thromboelastography-platelet mapping in stent-assisted coiling for ruptured aneurysms, which would allow for an individualized antiplatelet regimen [57]. This would help prevent thromboembolic complications from occurring in those with antiplatelet resistance [58]. The choice for DAPT in SAC should consider the patient’s risk of bleeding, history of noncompliance, and individual drug metabolism.
4.3. DAPT with FDS and Aneurysm Treatment
While the use of clopidogrel for DAPT in flow diversion is well established, there has been a movement towards the use of various other antiplatelet regimens. The current study recognized the use of antiplatelet therapies across 14,099 cases over 206 studies. There was no statistically significant difference between the various DAPT regimens and the rate of thromboembolic and/or intracranial hemorrhagic complications (Figure 3 and Figure 4). These results suggest that clopidogrel alternatives are safe antiplatelet medications for use in FDS. Several of the original PED, a type of flow diverter, trials used aspirin and clopidogrel to prevent thrombotic complications after device placement in ruptured aneurysms [59]. For example, the PUFS trial placed patients with unruptured aneurysms on aspirin 325 mg and clopidogrel 75 mg once daily [60]. However, as discussed previously, clopidogrel is a prodrug that must first be converted to its active metabolite by a CYP liver enzyme [14]. This creates the possibility of hyper- and hypo-responsiveness due to genetic variations and concurrent medication use that inhibits or activates CYP [61]. As such, there has been a shift towards tirofiban, ticagrelor, or prasugrel with aspirin. Previous studies comparing aspirin and clopidogrel versus aspirin and aspirin and ticagrelor found no significant difference in intracranial hemorrhagic or thromboembolic complications in the management of unruptured aneurysms [62]. A recent meta-analysis reported not only the safety of ticagrelor or prasugrel with DAPT, but it found ticagrelor to be associated with reduced mortality in comparison to clopidogrel in unruptured and ruptured aneurysms [63]. Furthermore, the use of prophylactic tirofiban with conventional DAPT for unruptured aneurysms, being clopidogrel with aspirin, was found to have lower rates of thromboembolic complications as compared to only DAPT [64]. Although these studies are not without their limitations, they suggest that more effective medications exist for commonly treated conditions.
Within the past few years, there have been reports of using triple therapy (TT), which is DAPT with oral anticoagulation. In a case series, Siddiqui et al. compared the use of DAPT and TT in patients with unruptured vertebrobasilar fusiform aneurysms [65]. With a similar number of FDS with adjunctive coiling placed between the two treatment groups, they reported the TT group to have fewer strokes and better overall outcome [65]. However, there was a recent case report of an unruptured aneurysmal recanalization after TT, with subsequent aneurysm re-occlusion after stopping the oral anticoagulant [66]. On a similar topic, Wu et al. reported the periprocedural use of tirofiban with conventional DAPT and found it to be significantly more effective at preventing thromboembolic events in unruptured aneurysms without an increase in intracranial hemorrhages [64]. Further studies are warranted to determine the use, efficacy, and safety of TT in flow diversion.
To minimize the requirements for DAPT, a new FDS with a hydrophilic polymer coating (p48-MW-HPC) has been developed. This has a similar goal to the novel PED-Shield: a FDS with a phosphorylcholine coating designed to reduce thrombogenicity [67]. Either of these devices may be preferred in cases of ruptured aneurysms due to the reduced need for antiplatelet agents [67]. Lobsien et al. demonstrated the effectiveness of the p48-MW-HPC FDS under SAPT across 13 ruptured aneurysms and reported a single thromboembolic complication [68]. A small cohort study demonstrated the limitations of the device with monotherapy with ruptured aneurysms, reporting intraprocedural thrombus formation in 50% of patients, postprocedural thrombus in 12.5% of patients, and vasospasm in 25% of patients [69]. Preliminary animal model studies found there to be significantly more thrombus formation in the PED-Shield group on aspirin versus the DAPT group [70]. A recent meta-analysis on ruptured and unruptured aneurysms demonstrated SAPT with aspirin resulted in significantly more thromboembolic and intracranial hemorrhagic complications than SAPT with prasugrel or ticagrelor [71]. However, there have been successful reports of aspirin monotherapy in flow diversion for unruptured aneurysms in patients with bleeding disorders [72]. Further studies and large clinical trials are required to determine the effectiveness of coated FDS when used with monotherapy or without any antiplatelet agent.
4.4. DAPT with Intrasaccular Devices and Aneurysms
The development of intrasaccular flow disruptors has limited the need for concurrent need for DAPT. These devices are metal mesh spheres that work by volumetrically filling the aneurysm, thus achieving roles as both a flow diverter and coil [73]. One such device, the Woven EndoBridge (WEB) (Microvention, Aliso Viejo, CA, USA), has been demonstrated to be effective in ruptured aneurysms and may only require SAPT with aspirin [74]. WEB is currently the only intrasaccular device approved in the USA and is indicated for use in wide-necked bifurcation aneurysms [73]. If the entire WEB device is within the aneurysm, antiplatelet therapy may not be required at all [73]. If the mesh components protrude into the parent vessel, this poses a risk for thrombosis and antiplatelet medications are recommended [73]. A recent systematic review about WEB use in ruptured and unruptured aneurysms by Xie et al. found 1/7 articles reported any antiplatelet use [75]. This review found thromboembolic complications to occur in 7% of the collected cases [75]. The current study found 48 studies with 3583 cases that used an intrasaccular device for the management of intracranial aneurysms. Although, there was not enough data to suggest an advantage of one DAPT regimen over another for preventing thromboembolic or intracranial hemorrhagic complications (Figure 5 and Figure 6). The lack of data may be attributed to the device’s novelty or the diminished requirement for DAPT therapy and thus additional comparative studies are warranted. The Nautilus Intrasaccular System (EndoStream Medical, Or Akiva, Israel) is a novel intrasaccular device unavailable in the USA that has demonstrated low complication rates without the need for antiplatelet therapy in ruptured aneurysms [76]. The novel ARTISSE (Medtronic, Irvine, CA, USA) intrasaccular device, which is also not available in the USA, demonstrated thromboembolic complications in two out of the nine unruptured cases performed [77]. These patients were placed on antiplatelet therapy to prevent further strokes. While current intrasaccular devices have demonstrated efficacy, further studies are required to determine their safety in the absence of any antiplatelet therapy.
4.5. Limitations
The nature of this article creates inherent limitations. For one, there is still bias present when choosing articles to include even with proper checks and balances. Furthermore, the use of three databases limits the scope of articles that may be reviewed. The inclusion of case reports and series may have skewed the data because these articles are written in the setting of unique pathology or technique which may not be applicable to the general population. The search phrases “intrasaccular device” and “stent-assisted coiling” are limited because not all published works include these phrases in their title or abstract, thus limiting the amount of articles included. Similarly, “dual antiplatelet therapy” may not have been mentioned in the title or abstract if it was not the focus of the study. The use of this review for DAPT use in practice should be approached with caution. Randomized controlled trials of all medications across each device use should be performed to provide a more clear view of recommended DAPT regimens. Ultimately, it remains the physician’s choice and experiences to determine the best DAPT regimen for the individual patients.
4.6. Future Directions
Comparison studies and clinical trials that compare the efficacy and safety of the various antiplatelet regimens employed for the different aneurysm treatment strategies are warranted. Furthermore, studies that examine the optimal duration of antiplatelet use, which balance the risk of thrombus versus intracranial hemorrhagic complications, are justified.
5. Conclusions
While the use of DAPT in the treatment of intracranial pathology is standard, the optimal regimen remains unclear. Aspirin with clopidogrel has become a mainstay for DAPT, although varied clopidogrel responses have led to the popularization of prasugrel, tirofiban, ticagrelor, and cilostazol. If platelet function resting reveals an effective response to clopidogrel, trends in the literature and practice patterns suggest an antiplatelet therapy with clopidogrel or prasugrel given their safety profiles and low cost. In the event of an ineffective response to clopidogrel, oral prasugrel or ticagrelor with or without intraoperative tirofiban has been a developing practice pattern in modern neurointervention. The out-of-pocket cost to patients should be considered when prescribing the aforementioned second generation antiplatelets, as medication compliance is paramount to preventing post-embolization thrombotic complications. The use of devices with antiplatelet coatings is a promising advancement towards monotherapy; however, initial results suggest that DAPT may still be required to reduce thromboembolic complications. Randomized clinical trials of SAPT, DAPT, and TT use during the management of intracranial pathologies is necessary to delineate the optimal treatment regimen.
Author Contributions
B.M.M. and J.K.C. contributed equally to this work. Conceptualization, B.M.M. and J.K.C.; methodology, B.M.M., J.K.C., J.C.C.d.B., I.C., D.A.Z. and B.V.L.; software, J.C.C.d.B.; validation, B.M.M. and J.K.C.; formal analysis, B.M.M., J.C.C.d.B. and I.C.; investigation, B.M.M., J.K.C. and M.W.K.; resources, B.M.M. and J.K.C.; data curation, B.M.M., J.C.C.d.B., G.A. and I.C.; writing—original draft preparation, B.M.M., J.K.C., J.C.C.d.B., M.W.K., I.C. and G.A.; writing—review and editing, B.M.M. and J.K.C.; visualization, B.M.M., J.K.C. and M.W.K.; supervision J.K.C. and A.L.C.; project administration, A.L.C.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not provided.
Conflicts of Interest
Alexander L. Coon is a consultant for Medtronic Neurovascular, MicroVention-Terumo, Stryker Neurovascular, Rapid Medical; and a consultant for Avail MedSystems, Imperative Care, InNeuroCo, Medtronic Neurovascular, MicroVention-Terumo, Q’apel, Rapid Medical, Stryker Neurovascular, and Sequent Medical; a proctor for MicroVention-Termo, Stryker Neurovascular, and Medtronic Neurovascular. All other authors have no conflict of interest. No author received financial support in conjunction with the generation of this submission.
References
- Kamal, H.; Fine, E.J.; Shakibajahromi, B.; Mowla, A. A history of the path towards imaging of the brain: From skull radiography through cerebral angiography. Curr. J. Neurol. 2021, 19, 131–137. [Google Scholar] [CrossRef]
- Phan, K.; Huo, Y.R.; Jia, F.; Phan, S.; Rao, P.J.; Mobbs, R.J.; Mortimer, A.M. Meta-analysis of stent-assisted coiling versus coiling-only for the treatment of intracranial aneurysms. J. Clin. Neurosci. 2016, 31, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.K.; Lien, B.; Wang, A.S.; Lin, L.-M. Advances in endovascular aneurysm management: Coiling and adjunctive devices. Stroke Vasc. Neurol. 2020, 5, 14–21. [Google Scholar] [CrossRef]
- Campos, J.K.; Ii, B.C.; Lien, B.V.; Zarrin, D.A.; Vo, C.D.; Colby, G.P.; Lin, L.-M.; Coon, A.L. Advances in endovascular aneurysm management: Flow modulation techniques with braided mesh devices. Stroke Vasc. Neurol. 2020, 5, 1–13. [Google Scholar] [CrossRef]
- Steinhubl, S.R.; Berger, P.B.; Iii, J.T.M.; Fry, E.T.A.; Delago, A.; Wilmer, C.; Topol, E.; CREDO Investigators. Early and Sustained Dual Oral Antiplatelet Therapy Following Percutaneous Coronary Intervention: A Randomized Controlled Trial. JAMA 2002, 288, 2411–2420, Erratum in JAMA 2003, 289, 987. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Zhao, F.; Mehta, S.R.; Chrolavicius, S.; Tognoni, G.; Fox, K.K. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of Clopidogrel in Addition to Aspirin in Patients with Acute Coronary Syndromes without ST-Segment Elevation. N. Engl. J. Med. 2001, 345, 494–502, Erratum in N. Engl. J. Med. 2001, 345, 1506; Erratum in N. Engl. J. Med. 2001, 345, 1716. [Google Scholar]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Åkerblom, A.; Cannon, C.P.; Emanuelsson, H.; Husted, S.; Katus, H.; Skene, A.; Steg, P.G.; Storey, R.F.; Harrington, R.; et al. Comparison of ticagrelor, the first reversible oral P2Y12 receptor antagonist, with clopidogrel in patients with acute coronary syndromes: Rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am. Heart J. 2009, 157, 599–605. [Google Scholar] [CrossRef]
- Mauri, L.; Kereiakes, D.J.; Normand, S.-L.T.; Wiviott, S.D.; Cohen, D.J.; Holmes, D.R.; Bangalore, S.; Cutlip, D.E.; Pencina, M.; Massaro, J.M. Rationale and design of the dual antiplatelet therapy study, a prospective, multicenter, randomized, double-blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug-eluting stent or bare metal stent placement for the treatment of coronary artery lesions. Am. Heart J. 2010, 160, 1035–1041.e1. [Google Scholar] [CrossRef]
- Nagahama, Y.; Allan, L.; Nakagawa, D.; Zanaty, M.; Starke, R.M.; Chalouhi, N.; Jabbour, P.; Brown, R.D.; Derdeyn, C.P.; Leira, E.C.; et al. Dual antiplatelet therapy in aneurysmal subarachnoid hemorrhage: Association with reduced risk of clinical vasospasm and delayed cerebral ischemia. J. Neurosurg. 2018, 129, 702–710. [Google Scholar] [CrossRef]
- Mauri, L.; Kereiakes, D.J.; Yeh, R.W.; Driscoll-Shempp, P.; Cutlip, D.E.; Steg, P.G.; Normand, S.-L.T.; Braunwald, E.; Wiviott, S.D.; Cohen, D.J.; et al. Twelve or 30 Months of Dual Antiplatelet Therapy after Drug-Eluting Stents. N. Engl. J. Med. 2014, 371, 2155–2166. [Google Scholar] [CrossRef]
- Quinn, M.J.; Fitzgerald, D.J. Ticlopidine and Clopidogrel. Circulation 1999, 100, 1667–1672. [Google Scholar] [CrossRef]
- Brandt, J.T.; Close, S.L.; Iturria, S.J.; Payne, C.D.; Farid, N.A.; Ernest, C.S.; Lachno, D.R.; Salazar, D.; Winters, K.J. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J. Thromb. Haemost. 2007, 5, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Sangkuhl, K.; Klein, T.E.; Altman, R.B. Clopidogrel pathway. Pharmacogenet. Genomics 2010, 20, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Norgard, N.B.; Abu-Fadel, M. Comparison of prasugrel and clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Vasc. Health Risk Manag. 2009, 5, 873–882. [Google Scholar] [CrossRef]
- Mobley, J.E.; Bresee, S.J.; Wortham, D.C.; Craft, R.M.; Snider, C.C.; Carroll, R.C. Frequency of nonresponse antiplatelet activity of clopidogrel during pretreatment for cardiac catheterization. Am. J. Cardiol. 2004, 93, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Dean, L. Prasugrel Therapy and CYP Genotype. In Medical Genetics Summaries; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kattman, B.L., Malheiro, A.J., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2017. [Google Scholar]
- Xia, P.; Huang, Y.; Chen, G. The Effect of Ticagrelor for Endovascular Intervention of Intracranial Aneurysm Patients with or without Clopidogrel Resistant: A Meta-Analysis. Brain Sci. 2022, 12, 1077. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.R.; Fowkes, F.G.R.; Heizer, G.; Berger, J.S.; Baumgartner, I.; Held, P.; Katona, B.G.; Mahaffey, K.W.; Norgren, L.; Jones, W.S.; et al. Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease. N. Engl. J. Med. 2017, 376, 32–40. [Google Scholar] [CrossRef]
- Vranckx, P.; Valgimigli, M.; Jüni, P.; Hamm, C.; Steg, P.G.; Heg, D.; van Es, G.A.; McFadden, E.P.; Onuma, Y.; van Meijeren, C.; et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: A multicentre, open-label, randomised superiority trial. Lancet 2018, 392, 940–949. [Google Scholar] [CrossRef]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Yang, M.; Huo, X.; Miao, Z.; Wang, Y. Platelet Glycoprotein IIb/IIIa Receptor Inhibitor Tirofiban in Acute Ischemic Stroke. Drugs 2019, 79, 515–529. [Google Scholar] [CrossRef]
- Kondo, K.; Umemura, K. Clinical Pharmacokinetics of Tirofiban, a Nonpeptide Glycoprotein IIb/IIIa Receptor Antagonist: Comparison with the monoclonal antibody abciximab. Clin. Pharmacokinet. 2002, 41, 187–195. [Google Scholar] [CrossRef]
- Topol, E.J.; Moliterno, D.J.; Herrmann, H.C.; Powers, E.R.; Grines, C.L.; Cohen, D.J.; Cohen, E.A.; Bertrand, M.; Neumann, F.-J.; Stone, G.W.; et al. Comparison of Two Platelet Glycoprotein IIb/IIIa Inhibitors, Tirofiban and Abciximab, for the Prevention of Ischemic Events with Percutaneous Coronary Revascularization. N. Engl. J. Med. 2001, 344, 1888–1894. [Google Scholar] [CrossRef]
- The RESTORE Investigators. Effects of Platelet Glycoprotein IIb/IIIa Blockade with Tirofiban on Adverse Cardiac Events in Patients with Unstable Angina or Acute Myocardial Infarction Undergoing Coronary Angioplasty. Circulation 1997, 96, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhao, H.; Ding, C.; Chen, H.; Wang, D.; Liu, A. The Prophylactic Use of Tirofiban versus Oral Antiplatelet Medications in Stent-Assisted Coiling of Intracranial Aneurysms: A Meta-analysis. Am. J. Neuroradiol. 2021, 42, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, E.A.; Gibson, E.; Nakagawa, D.; Ortega-Gutierrez, S.; Zanaty, M.; Roa, J.A.; Jabbour, P.; Hasan, D.M. Safety of tirofiban and dual antiplatelet therapy in treating intracranial aneurysms. Stroke Vasc. Neurol. 2019, 4, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Pergolini, P.; Barbieri, L.; Rolla, R.; Nardin, M.; Negro, F.; Suryapranata, H.; De Luca, G. Impact of pre-procedural dual antiplatelet therapy on periprocedural myocardial infarction in patients undergoing percutaneous coronary interventions with adjunctive tirofiban. Thromb. Res. 2018, 164, 17–23. [Google Scholar] [CrossRef]
- Brack, M.J.; Hubner, P.J.; Gershlick, A.H. Anticoagulation after intracoronary stent insertion. Br. Heart J. 1994, 72, 294–296. [Google Scholar] [CrossRef][Green Version]
- Horowitz, M.B.; Pride, G.L.; Graybeal, D.F.; Purdy, P.D. Percutaneous Transluminal Angioplasty and Stenting of Midbasilar Stenoses: Three Technical Case Reports and Literature Review. Neurosurgery 1999, 45, 925–931. [Google Scholar] [CrossRef]
- Schirmer, C.M.; Bulsara, K.R.; Al-Mufti, F.; Haranhalli, N.; Thibault, L.; Hetts, S.W. Antiplatelets and antithrombotics in neurointerventional procedures: Guideline update. J. NeuroInterventional Surg. 2023. [Google Scholar] [CrossRef]
- Maree, A.O.; Curtin, R.J.; Dooley, M.; Conroy, R.M.; Crean, P.; Cox, D.; Fitzgerald, D.J. Platelet Response to Low-Dose Enteric-Coated Aspirin in Patients with Stable Cardiovascular Disease. J. Am. Coll. Cardiol. 2005, 46, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Wijeyeratne, Y.D.; Heptinstall, S. Anti-platelet therapy: ADP receptor antagonists. Br. J. Clin. Pharmacol. 2011, 72, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Kondo, R.; Matsumori, Y.; Shimizu, H.; Takahashi, A.; Tominaga, T. Antiplatelet Therapy for Prevention of Thromboembolic Complications Associated with Coil Embolization of Unruptured Cerebral Aneurysms. Drugs R&D 2012, 12, 1–7. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Fernandez-Ortiz, A.; Bernardo, E.; Alfonso, F.; Macaya, C.; Bass, T.A.; Costa, M.A. Variability in Individual Responsiveness to Clopidogrel: Clinical Implications, Management, and Future Perspectives. J. Am. Coll. Cardiol. 2007, 49, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Rosengart, A.; Collins, M.K.; Hendrix, P.; Uber, R.; Sartori, M.; Jain, A.; Mao, J.; Goren, O.; Schirmer, C.M.; Griessenauer, C.J. P2Y12inhibitors in neuroendovascular surgery: An opportunity for precision medicine. Interv. Neuroradiol. 2021, 27, 682–694. [Google Scholar] [CrossRef]
- Bender, M.T.; Zarrin, D.A.; Campos, J.K.; Jiang, B.; Chandra, A.; Vo, C.D.; Caplan, J.M.; Huang, J.; Tamargo, R.J.; Lin, L.-M.; et al. Precision of VerifyNow P2Y12 Assessment of Clopidogrel Response in Patients Undergoing Cerebral Aneurysm Flow Diversion. Neurosurgery 2018, 85, 543–549. [Google Scholar] [CrossRef]
- Kim, K.S.; Fraser, J.F.; Grupke, S.; Cook, A.M. Management of antiplatelet therapy in patients undergoing neuroendovascular procedures. J. Neurosurg. 2018, 129, 890–905. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Zhang, T.; Feng, Y.; Zhao, W.; Chen, X. Safety and efficacy of dual antiplatelet therapy combining aspirin and ticagrelor in patients with undergoing intracranial stenting procedures. J. Neurosurg. Sci. 2022. [Google Scholar] [CrossRef]
- Teng, R. Ticagrelor: Pharmacokinetic, Pharmacodynamic and Pharmacogenetic Profile: An Update. Clin. Pharmacokinet. 2015, 54, 1125–1138. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; Butler, K.; Antonino, M.J.; Wei, C.; Teng, R.; Rasmussen, L.; Storey, R.F.; Nielsen, T.; Eikelboom, J.W.; et al. Response to Ticagrelor in Clopidogrel Nonresponders and Responders and Effect of Switching Therapies. Circulation 2010, 121, 1188–1199. [Google Scholar] [CrossRef]
- Narata, A.P.; Amelot, A.; Bibi, R.; Herbreteau, D.; Angoulvant, D.; Gruel, Y.; Janot, K. Dual Antiplatelet Therapy Combining Aspirin and Ticagrelor for Intracranial Stenting Procedures: A Retrospective Single Center Study of 154 Consecutive Patients with Unruptured Aneurysms. Neurosurgery 2018, 84, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.-M.; Do, H.P.; Li, Y.-C.; Wang, R.; Zhuang, Z.; Xu, M.-M.; Liu, T.; Shao, T.-F.; Ding, L.-P.; Ge, W.-H. Ticagrelor versus Clopidogrel in the Dual Antiplatelet Regimen for Unruptured Intracranial Aneurysm Treated with Stent-Assisted Coil Embolization: A Single-Center Cohort Study. World Neurosurg. 2023, 170, e755–e765. [Google Scholar] [CrossRef] [PubMed]
- Vrijens, B.; Claeys, M.J.; Legrand, V.; Vandendriessche, E.; Van de Werf, F. Projected inhibition of platelet aggregation with ticagrelor twice daily vs. clopidogrel once daily based on patient adherence data (the TWICE project). Br. J. Clin. Pharmacol. 2014, 77, 746–755. [Google Scholar] [CrossRef][Green Version]
- Roffman, D.S. Developments in Oral Antiplatelet Agents for the Treatment of Acute Coronary Syndromes: Clopidogrel, Prasugrel, and Ticagrelor. J. Pharm. Pract. 2015, 29, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Sedat, J.; Chau, Y.; Gaudart, J.; Sachet, M.; Beuil, S.; Lonjon, M. Prasugrel versus clopidogrel in stent-assisted coil embolization of unruptured intracranial aneurysms. Interv. Neuroradiol. 2016, 23, 52–59. [Google Scholar] [CrossRef]
- Russo, R.; Bradac, G.B.; Castellan, L.; Gallesio, I.; Garbossa, D.; Iannucci, G.; Mardighian, D.; Menozzi, R.; Pitrone, A.; Romano, G.; et al. Neuroform Atlas stent-assisted coiling of ruptured intracranial aneurysms: A multicenter study. J. Neuroradiol. 2020, 48, 479–485. [Google Scholar] [CrossRef]
- Finneran, M.M.; Young, M.; Farhat, H. Antiplatelet Therapy for Stent-Assisted Coil of Ruptured Middle Cerebral Artery Bifurcation Aneurysm: Is There a Right Answer? Cureus 2020, 12, e11612. [Google Scholar] [CrossRef]
- Zi-Liang, W.; Xiao-Dong, L.; Tian-Xiao, L.; Liang-Fu, Z.; Jiang-Yu, X.; Wei-Xing, B.; Ying-Kun, H.; Gang-Qin, X.; Qiu-Ji, S.; Li, L.; et al. Intravenous administration of tirofiban versus loading dose of oral clopidogrel for preventing thromboembolism in stent-assisted coiling of intracranial aneurysms. Int. J. Stroke 2016, 12, 553–559. [Google Scholar] [CrossRef]
- Ospel, J.; Brouwer, P.; Dorn, F.; Arthur, A.; Jensen, M.; Nogueira, R.; Chapot, R.; Albuquerque, F.; Majoie, C.; Jayaraman, M.; et al. Antiplatelet Management for Stent-Assisted Coiling and Flow Diversion of Ruptured Intracranial Aneurysms: A DELPHI Consensus Statement. Am. J. Neuroradiol. 2020, 41, 1856–1862, Erratum in Am. J. Neuroradiol. 2021, 42, E76. [Google Scholar] [CrossRef]
- Ma, Y.; Jia, C.; Zhang, T.; Feng, Y.; Chen, X.; Zhao, W. Safety and efficacy of intravenous tirofiban for stent-assisted coiling in acutely ruptured intracranial aneurysms: A single center experience. Interv. Neuroradiol. 2021, 28, 476–481. [Google Scholar] [CrossRef]
- Shen, G.; Jia, Z.; Zhao, L.; Lu, G.; Liu, S.; Shi, H. The safety and efficacy of a low dose of tirofiban for early complications during and after stent-assisted coiling of ruptured intracranial aneurysms: A propensity matching study. Clin. Neurol. Neurosurg. 2022, 214, 107132. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.-H.; Kang, M.; Cha, J.-K.; Huh, J.-T. Safety and Efficacy of Intravenous Tirofiban as Antiplatelet Premedication for Stent-Assisted Coiling in Acutely Ruptured Intracranial Aneurysms. Am. J. Neuroradiol. 2015, 37, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, C.; Ghozy, S.; Shehata, M.; Ibrahim, M.; Jabal, M.S.; Kobeissi, H.; Gerberi, D.J.; Kadirvel, R.; Kallmes, D.F. The Prophylactic Use of Glycoprotein 2b/3a Inhibitors in the Endovascular Treatment of Intracranial Aneurysms: A Systematic Review and Meta-Analysis. World Neurosurg. 2022, 168, e50–e66. [Google Scholar] [CrossRef] [PubMed]
- Siebler, M.; Hennerici, M.G.; Schneider, D.; von Reutern, G.M.; Seitz, R.J.; Röther, J.; Witte, O.W.; Hamann, G.; Junghans, U.; Villringer, A.; et al. Safety of Tirofiban in Acute Ischemic Stroke. Stroke 2011, 42, 2388–2392. [Google Scholar] [CrossRef] [PubMed]
- McClellan, K.J.; Goa, K.L. Tirofiban. A review of its use in acute coronary syndromes. Drugs 1998, 56, 1067–1080. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Guo, Z.; Zhu, J.; Xu, R.; He, Z.; Sun, X. Standard vs. Modified Antiplatelet Therapy Based on Thromboelastography With Platelet Mapping for Preventing Bleeding Events in Patients Undergoing Stent-Assisted Coil for a Ruptured Intracranial Aneurysm. Front. Neurol. 2021, 11, 615829. [Google Scholar] [CrossRef] [PubMed]
- Xian, E.; Morrison, T.; Wong, J. Hyperacute in-Stent Thrombosis Causing Large Vessel Occlusion after Stent-Assisted Aneurysm Coiling Secondary to Complete Clopidogrel and Prasugrel Resistance: A Case Report. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2022. [Google Scholar] [CrossRef]
- Tonetti, D.A.; Jankowitz, B.T.; Gross, B.A. Antiplatelet Therapy in Flow Diversion. Neurosurgery 2019, 86, S47–S52. [Google Scholar] [CrossRef] [PubMed]
- Becske, T.; Kallmes, D.F.; Saatci, I.; McDougall, C.G.; Szikora, I.; Lanzino, G.; Moran, C.J.; Woo, H.H.; Lopes, D.K.; Berez, A.L.; et al. Pipeline for Uncoilable or Failed Aneurysms: Results from a Multicenter Clinical Trial. Radiology 2013, 267, 858–868. [Google Scholar] [CrossRef]
- Brown, S.-A.; Pereira, N. Pharmacogenomic Impact of CYP2C19 Variation on Clopidogrel Therapy in Precision Cardiovascular Medicine. J. Pers. Med. 2018, 8, 8. [Google Scholar] [CrossRef]
- Park, K.; Ozaki, T.; Kostynskyy, A.; Kortman, H.; Hilario, A.; Nicholson, P.; Agid, R.; Krings, T.; Pereira, V. Ticagrelor versus Clopidogrel in the Dual Antiplatelet Regimen for Intracranial Stenting or Flow-Diverter Treatment for Unruptured Cerebral Aneurysms: A Single-Center Cohort Study. Am. J. Neuroradiol. 2021, 42, 1638–1644. [Google Scholar] [CrossRef]
- Podlasek, A.; Al Sultan, A.A.; Assis, Z.; Kashani, N.; Goyal, M.; Almekhlafi, M.A. Outcome of intracranial flow diversion according to the antiplatelet regimen used: A systematic review and meta-analysis. J. NeuroInterventional Surg. 2019, 12, 148–155. [Google Scholar] [CrossRef]
- Wu, Q.; Shao, Q.; Li, L.; Liang, X.; Chang, K.; Li, T.; He, Y. Prophylactic administration of tirofiban for preventing thromboembolic events in flow diversion treatment of intracranial aneurysms. J. NeuroInterventional Surg. 2020, 13, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.H.; Monteiro, A.; Hanel, R.A.; Kan, P.; Mohanty, A.; Cortez, G.M.; Rabinovich, M.; Matouk, C.; Sujijantarat, N.; Romero, C.; et al. Triple therapy versus dual-antiplatelet therapy for dolichoectatic vertebrobasilar fusiform aneurysms treated with flow diverters. J. NeuroInterventional Surg. 2022, 15, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Geisbush, T.R.; Pulli, B.; Wolman, D.N.; Pendharkar, A.V.; Telischak, N.A. A case of recurrent aneurysm resulting from dual antiplatelet plus anticoagulation after confirmed aneurysm closure following coil-assisted flow diversion. Radiol. Case Rep. 2022, 17, 4075–4078. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Jin, L.; Dong, J.; Fu, Z.; Liu, E.; Yin, S.; Jian, L.; Luo, P.; Liu, B.; Huang, W.; et al. Clinical outcomes of pipeline embolization devices with shield technology for treating intracranial aneurysms. Front. Neurol. 2022, 13, 971664. [Google Scholar] [CrossRef] [PubMed]
- Lobsien, D.; Clajus, C.; Behme, D.; Ernst, M.; Riedel, C.; Abu-Fares, O.; Götz, F.; Fiorella, D.; Klisch, J. Aneurysm Treatment in Acute SAH with Hydrophilic-Coated Flow Diverters under Single-Antiplatelet Therapy: A 3-Center Experience. Am. J. Neuroradiol. 2021, 42, 508–515. [Google Scholar] [CrossRef]
- Aguilar-Perez, M.; Hellstern, V.; AlMatter, M.; Wendl, C.; Bäzner, H.; Ganslandt, O.; Henkes, H. The p48 Flow Modulation Device with Hydrophilic Polymer Coating (HPC) for the Treatment of Acutely Ruptured Aneurysms: Early Clinical Experience Using Single Antiplatelet Therapy. Cardiovasc. Interv. Radiol. 2020, 43, 740–748. [Google Scholar] [CrossRef]
- Matsuda, Y.; Jang, D.-K.; Chung, J.; Wainwright, J.M.; Lopes, D. Preliminary outcomes of single antiplatelet therapy for surface-modified flow diverters in an animal model: Analysis of neointimal development and thrombus formation using OCT. J. NeuroInterventional Surg. 2018, 11, 74–79. [Google Scholar] [CrossRef]
- Senol, Y.C.; Orscelik, A.; Ghozy, S.; Kobeissi, H.; Arul, S.; Bilgin, C.; Kadirvel, R.; Kallmes, D.F. The safety profile of single antiplatelet therapy with flow diverters: Systematic review and meta-analysis. Interv. Neuroradiol. 2023, 15910199231168669. [Google Scholar] [CrossRef]
- Bender, M.T.; Zarrin, D.A.; Jiang, B.; Campos, J.K.; Lin, L.-M.; Young, R.W.; Colby, G.P.; Coon, A.L. Aspirin Monotherapy in Flow Diversion of Selected Internal Carotid Artery Aneurysms. World Neurosurg. 2019, 134, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Hoit, D.; DiNitto, J.; Elijovich, L.; Fiorella, D.; Pierot, L.; Lamin, S.; Spelle, L.; Saatci, I.; Cekirge, S.; et al. How to WEB: A practical review of methodology for the use of the Woven EndoBridge. J. NeuroInterventional Surg. 2020, 12, 512–520. [Google Scholar] [CrossRef]
- Narsinh, K.H.; Caton, M.T.; Mahmood, N.F.; Higashida, R.T.; Halbach, V.V.; Hetts, S.W.; Amans, M.R.; Dowd, C.F.; Cooke, D.L. Intrasaccular flow disruption (WEB) of a large wide-necked basilar apex aneurysm using PulseRider-assistance. Interdiscip. Neurosurg. 2020, 24, 101072. [Google Scholar] [CrossRef] [PubMed]
- Sirakov, A.; Bhogal, P.; Sirakova, K.; Penkov, M.; Minkin, K.; Ninov, K.; Hristov, H.; Hadzhiyanev, A.; Karakostov, V.; Sirakov, S. Endovascular treatment of wide-necked intracranial aneurysms using the Nautilus Intrasaccular System: Initial case series of 41 patients at a single center. J. NeuroInterventional Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tian, H.; Xiang, B.; Liu, J.; Xiang, H. Woven EndoBridge device for the treatment of ruptured intracranial aneurysms: A systematic review of clinical and angiographic results. Interv. Neuroradiol. 2021, 28, 240–249. [Google Scholar] [CrossRef]
- Piotin, M.; Fahed, R.; Redjem, H.; Smajda, S.; Desilles, J.P.; Escalard, S.; Maïer, B.; Hebert, S.; Delvoye, F.; Mazighi, M.; et al. The ARTISSE intrasaccular device for intracranial aneurysm treatment: Short-term, mid-term and long-term clinical and angiographic results. J. NeuroInterventional Surg. 2021, 14, 957–961. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).