Co-Targeting Nucleus Accumbens Associate 1 and NF-κB Signaling Synergistically Inhibits Melanoma Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Antibodies
2.4. Western Blotting
2.5. Depletion of NAC1
2.6. Immunofluorescence Staining
2.7. RNA Isolation and Quantitative Real-Time PCR
2.8. Colony Formation Assay
2.9. Mouse Xenograft Model
2.10. Statistical Analysis
3. Results
3.1. Inhibition of NAC1 Activates NF-κB Signaling in Melanoma
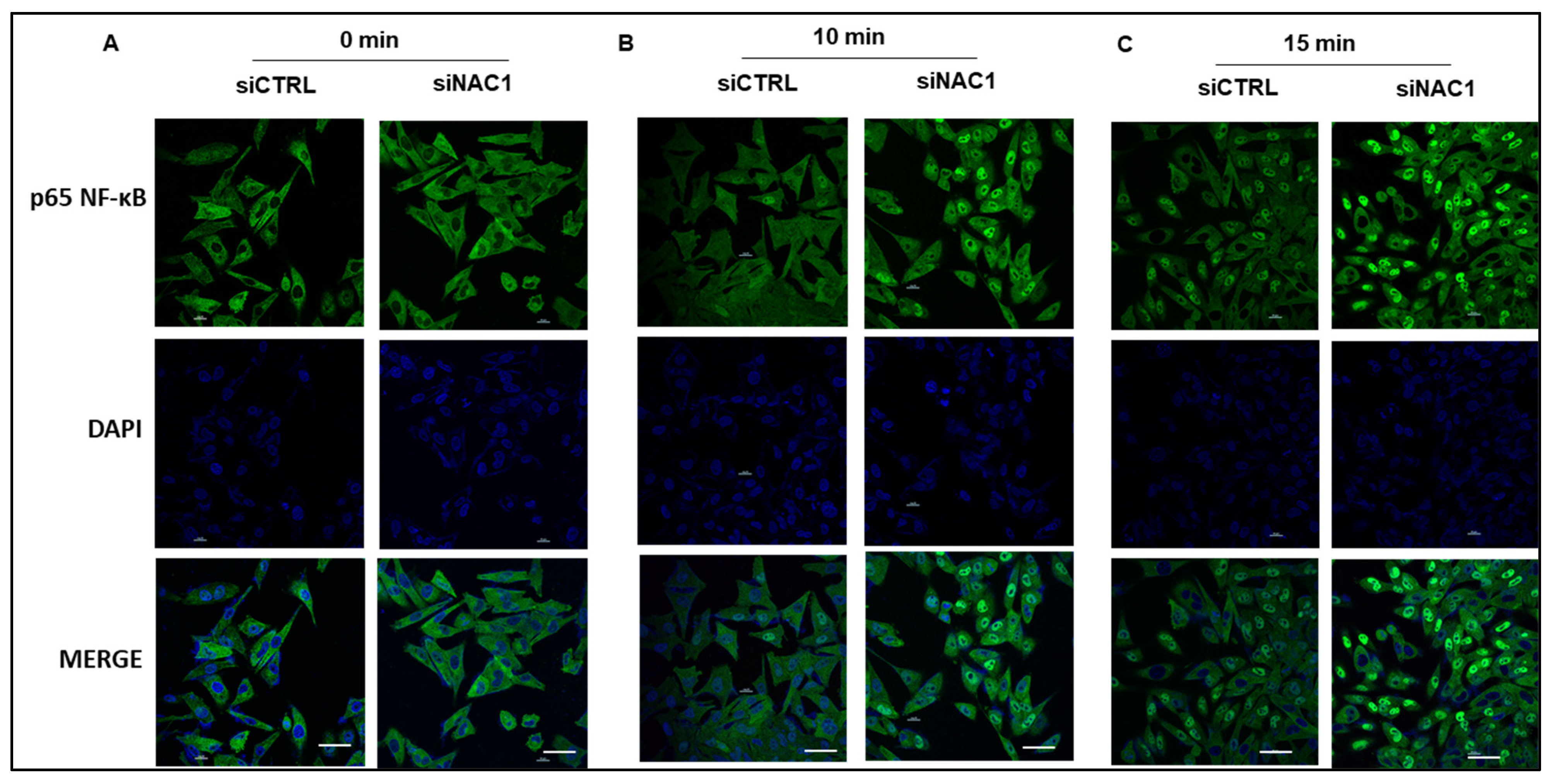
3.2. Knockdown of NAC1 Leads to NF-κB Nuclear Translocation
3.3. Knockdown of NAC1 and Inhibition of NF-κB Act Synergistically in a Melanoma Xenograft Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Khushalani, N.I.; Angeles, C.; Petrella, T.M. Current state of adjuvant therapy for melanoma: Less is more, or more is less? Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Fisher, D.E. Treatment of advanced melanoma in 2020 and beyond. J. Investig. Dermatol. 2021, 141, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.J.; Kumar, A.; Das, J.K.; Peng, H.Y.; Wang, L.Q.; Balllard, D.; Xiong, X.F.; Ren, X.C.; Zhang, Y.; Yang, J.M.; et al. Tumorous expression of NAC1 restrains antitumor immunity through the LDHA-mediated immune evasion. J. Immunother. Cancer 2022, 10, e004856. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Jiang, S.; Wang, H.; Wang, H.; Li, Y.C.; Zhang, W. Upregulation of LINC00963 facilitates melanoma progression through miR-608/NACC1 pathway and predicts poor prognosis. Biochem. Biophys. Res. Commun. 2018, 504, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Fujii, T.; Itami, H.; Itami, H.; Uchiyama, T.; Nakai, T.; Hatakeyama, K.; Sugimoto, A.; Myake, M.; Nakai, Y.; et al. NACC1, as a target of MicroRNA-331- 3p, regulates cell proliferation in urothelial carcinoma cells. Cancers 2018, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.M.; Nakayama, K.; Wu, G.; Nakayama, N.; Zhang, J.H.; Wang, T.L. Amplification of the ch19p13.2 NACC1 locus in ovarian high-grade serous carcinoma. Mod. Pathol. 2011, 24, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z. Circular RNA hsa circ 0001588 promotes the malignant progression of lung adenocarcinoma by modulating miR-524-3p/ NACC1 signaling. Life Sci. 2020, 259, 118157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.W.; Ren, X.; Yang, J.M. NAC1 and HMGB1 enter a partnership for manipulating autophagy. Autophagy 2011, 7, 1557–1558. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.L.; Wang, X.H.; Yang, S.M.; Guo, F.F.; Zhang, J.J.; Ji, C.; Shi, L.R.; Cheng, Y.; Hu, Y.W.; Li, Z.Y.; et al. Mechanistic insights of NAC1 nuclear export and its role in ovarian cancer resistance to docetaxel. Biochem. Pharmcol. 2023, 211, 115533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Y.; Ren, X.; Hori, T.; Huber-Keener, K.J.; Zhang, L.; Yap, K.L.; Liu, D.; Shantz, L.; Qin, Z.H.; et al. Dysfunction of nucleus accumbens-1 activates cellular senescence and inhibits tumor cell proliferation and oncogenesis. Cancer Res. 2012, 72, 4262–4275. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.J.; Wang, X.H.; Ji, C.; Guan, Y.D.; Lu, X.J.; Liu, X.R.; Zhang, H.H.; Guo, L.C.; Xu, Q.H.; Zhu, W.D.; et al. Silencing of Nac1 expression induces cancer cells oxidative stress in hypoxia and potentiates the therapeutic activity of Elesclomol. Front. Pharmacol. 2017, 8, 804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, Y.J.; Guo, L.C.; Hu, J.; Zhang, H.H.; Xu, Q.H.; Zhu, W.D.; Ming, Z.J.; Yuan, Y.S.; Ren, X.; et al. Nucleus accumbens-associated protein-1 promotes glycolysis and survival of hypoxic tumor cells via the HDAC4-HIF-1α axis. Oncogene 2017, 36, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, K.; Oikawa, H.; Tada, H.; Tatemichi, Y.; Muraoka, S.; Miura, S.; Shibazaki, M.; Maeda, F.; Takahashi, K.; Akasaka, T.; et al. Nucleus accumbens-associated 1 contributes to cortactin deacetylation and augments the migration of melanoma cells. J. Investig. Dermatol. 2011, 131, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Madonna, C.; Ullman, C.D.; Gentilcore, G.; Palmieri, G.; Ascierto, P.A. NF-κB as potential target in the treatment of melanoma. J. Transl. Med. 2012, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Bertinato, L.; Staniszewska, M.; Pancer, K.; Wieczorek, M.; Salmaso, S.; Caliceti, P.; Garofalo, M. From Conventional Therapies to Immunotherapy: Melanoma Treatment in Review. Cancers 2020, 12, 3057. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, P.; Singh, A.B.; Ellis, D.L.; Richmond, A. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-kappaB and tumor progression. Cancer Res. 2002, 62, 7335–7342. [Google Scholar] [PubMed]
- Wang, J.; Liao, X.; Jiang, X.; Liu, H.W. Global Trends in Research of NF-κ B in Melanoma from 2000 to 2021: A Study of Bibliometric Analysis. J. Oncol. 2022, 10, 3684228. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Hi, C.; Zhang, H.H.; Shan, Y.; Ren, Y.J.; Hu, Y.W.; Shi, L.R.; Guo, L.C.; Zhu, W.D.; Xia, Y.J.; et al. Identification of a small-molecular compound that inhibits homodimerization of oncogenic NAC1 protein and sensitizes cancer cells to anticancer agents. J. Biol. Chem. 2019, 294, 10009–10017. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Ren, X.; Ngule, C.; Xiong, X.; Song, J.; Li, Z.; Yang, J.-M. Co-Targeting Nucleus Accumbens Associate 1 and NF-κB Signaling Synergistically Inhibits Melanoma Growth. Biomedicines 2023, 11, 2221. https://doi.org/10.3390/biomedicines11082221
Gu L, Ren X, Ngule C, Xiong X, Song J, Li Z, Yang J-M. Co-Targeting Nucleus Accumbens Associate 1 and NF-κB Signaling Synergistically Inhibits Melanoma Growth. Biomedicines. 2023; 11(8):2221. https://doi.org/10.3390/biomedicines11082221
Chicago/Turabian StyleGu, Lixiang, Xingcong Ren, Chrispus Ngule, Xiaofang Xiong, Jianxun Song, Zhiguo Li, and Jin-Ming Yang. 2023. "Co-Targeting Nucleus Accumbens Associate 1 and NF-κB Signaling Synergistically Inhibits Melanoma Growth" Biomedicines 11, no. 8: 2221. https://doi.org/10.3390/biomedicines11082221
APA StyleGu, L., Ren, X., Ngule, C., Xiong, X., Song, J., Li, Z., & Yang, J.-M. (2023). Co-Targeting Nucleus Accumbens Associate 1 and NF-κB Signaling Synergistically Inhibits Melanoma Growth. Biomedicines, 11(8), 2221. https://doi.org/10.3390/biomedicines11082221





