Management of Obesity-Related Inflammatory and Cardiovascular Diseases by Medicinal Plants: From Traditional Uses to Therapeutic Targets
Abstract
1. Introduction
2. Medicinal Plants and Their Anti-Inflammatory Mechanisms
3. Anti-Obesity Medicinal Plants and the Molecular Mechanisms Underlying Their Activities
3.1. Major Basic Anti-Obesity Mechanisms
3.2. Targeting Adipocyte Apoptosis
3.3. Targeting of Adipogenesis
4. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gusev, E.; Zhuravleva, Y. Inflammation: A New Look at an Old Problem. Int. J. Mol. Sci. 2022, 23, 4596. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation–nature’s way to efficiently respond to all types of challenges: Implications for understanding and managing “the epidemic” of chronic diseases. Front. Med. 2018, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Senchenkova, E. Inflammation and the Microcirculation; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Plytycz, B.; Seljelid, R. From inflammation to sickness: Historical perspective. Arch. Immunol. Et Ther. Exp. 2003, 51, 105–109. [Google Scholar]
- Cucu, I. Signaling Pathways in Inflammation and Cardiovascular Diseases: An Update of Therapeutic Strategies. Immuno 2022, 2, 630–650. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef]
- Rana, M.N.; Neeland, I.J. Adipose Tissue Inflammation and Cardiovascular Disease: An Update. Curr. Diabetes Rep. 2022, 22, 27–37. [Google Scholar] [CrossRef]
- Ježek, P.; Jabůrek, M.; Holendová, B.; Plecitá-Hlavatá, L. Fatty Acid-Stimulated Insulin Secretion vs. Lipotoxicity. Molecules 2018, 23, 1483. [Google Scholar] [CrossRef] [PubMed]
- Langlois, A.; Forterre, A.; Pinget, M.; Bouzakri, K. Impact of moderate exercise on fatty acid oxidation in pancreatic β-cells and skeletal muscle. J. Endocrinol. Investig. 2021, 44, 1815–1825. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Farrell, G.C.; Haczeyni, F.; Chitturi, S. Pathogenesis of NASH: How metabolic complications of overnutrition favor lipotoxicity and pro-inflammatory fatty liver disease. Adv. Exp. Med. Biol. 2018, 1061, 19–44. [Google Scholar]
- Wicherska-Pawłowska, K.; Wróbel, T.; Rybka, J. Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases. Int. J. Mol. Sci. 2021, 22, 13397. [Google Scholar] [CrossRef]
- Granzotto, A.; Weiss, J.H.; Sensi, S.L. Editorial: Excitotoxicity turns 50. The death that never dies. Front. Neurosci. 2022, 15, 831809. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Packard, C.J. Remnants, LDL, and the quantification of lipoprotein-associated risk in atherosclerotic cardiovascular disease. Curr. Atheroscler. Rep. 2022, 24, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Moradi, S.Z.; Ash-Rafzadeh, A.; Bishayee, A. Targeting cellular senescence in cancer by plant secondary metabolites: A systematic review. Pharmacol. Res. 2021, 177, 105961. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.K.; Dubey, S.K.; Shah, S.; Kumar, A. A Short Review on Genes Regulating Biosynthesis of Major Secondary. Metabolites 2022, 1, 501–519. [Google Scholar] [CrossRef]
- Ain, Q.-U.; Khan, H.; Mubarak, M.S.; Pervaiz, A. Plant alkaloids as antiplatelet agent: Drugs of the future in the light of recent developments. Front. Pharmacol. 2016, 7, 292. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Püschel, G.P.; Klauder, J.; Henkel, J. Macrophages, Low-Grade Inflammation, Insulin Resistance and Hyperinsulinemia: A Mutual Ambiguous Relationship in the Development of Metabolic Diseases. J. Clin. Med. 2022, 11, 4358. [Google Scholar] [CrossRef]
- Palma, G.; Sorice, G.P.; Genchi, V.A.; Giordano, F.; Caccioppoli, C.; D’oria, R.; Marrano, N.; Biondi, G.; Giorgino, F.; Perrini, S. Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity. Int. J. Mol. Sci. 2022, 23, 7349. [Google Scholar] [CrossRef]
- Zeyda, M.; Stulnig, T.M. Obesity, inflammation, and insulin resistance—A mini-Review. Gerontology 2009, 55, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Chinetti, G.; Fruchart, J.-C.; Staels, B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 2000, 49, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K. Potential roles of the peroxisome proliferatoractivated receptor-γ in macrophage biology and atheroscle-rosis. J. Endocrinol. 2001, 169, 461–464. [Google Scholar] [CrossRef][Green Version]
- Moore, K.J.; Rosen, E.D.; Fitzgerald, M.L.; Randow, F.; Andersson, L.P.; Altshuler, D.; Milstone, D.S.; Mortensen, R.M.; Spiegelman, B.M.; Freeman, M.W. The role of PPAR-γ in macrophage differentiation and cholesterol uptake. Nat. Med. 2001, 7, 41–47. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Murakami, K.; Motojima, K.; Komeda, K.; Ide, T.; Kubota, N.; Terauchi, Y.; Tobe, K.; et al. The mechanisms by which both heterozygous peroxisome prolifera-tor-activated receptor γ (PPARγ) deficiency and PPARγ agonist improve insulin resistance. J. Biol. Chem. 2001, 276, 41245–41254. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Takahashi, N.; Hirai, S.; Kawada, T. Various Terpenoids Derived from Herbal and Dietary Plants Function as PPAR Modulators and Regulate Carbohydrate and Lipid Metabolism. PPAR Res. 2010, 2010, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Saad, B. Prevention and Treatment of Obesity-Related Inflammatory Diseases by Edible and Medicinal Plants and Their Active Compounds. Immuno 2022, 2, 609–629. [Google Scholar] [CrossRef]
- Shehzad, A.; Ha, T.; Subhan, F.; Lee, Y.S. New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur. J. Nutr. 2011, 50, 151–161. [Google Scholar] [CrossRef]
- Shimizu, K.; Funamoto, M.; Sunagawa, Y.; Shimizu, S.; Katanasaka, Y.; Miyazaki, Y.; Wada, H.; Hasegawa, K.; Morimoto, T. Anti-inflammatory Action of Curcumin and Its Use in the Treatment of Lifestyle-related Diseases. Eur. Cardiol. Rev. 2019, 14, 117–122. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Fazelian, S.; Agah, S.; Khazdouz, M.; Rahimlou, M.; Agh, F.; Potter, E.; Heshmati, S.; Heshmati, J. Effect of ginger (Zingiber officinale) on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Cytokine 2020, 135, 155224. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.; Espín, J.C.; González-Sarrías, A. Evidence for health properties of pomegranate juices and extracts beyond nutrition: A critical systematic review of human studies. Trends Food Sci. Technol. 2021, 114, 410–423. [Google Scholar] [CrossRef]
- Jang, D.-I.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2022, 23, 289–303. [Google Scholar] [CrossRef]
- Xu, X.; Lai, Y.; Hua, Z.-C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef]
- Kumar, V.; Rosenzweig, R.; Asalla, S.; Nehra, S.; Prabhu, S.D.; Bansal, S.S. TNFR1 Contributes to Activation-Induced Cell Death of Pathological CD4+ T Lymphocytes During Ischemic Heart Failure. JACC Basic Transl. Sci. 2022, 7, 1038–1049. [Google Scholar] [CrossRef]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef]
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Uciechowski, P.; Dempke, W.C. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology 2020, 98, 131–137. [Google Scholar] [CrossRef]
- Saad, B.; AbouAtta, B.S.; Basha, W.; Hmade, A.; Kmail, A.; Khasib, S.; Said, O. Hypericum triquetrifolium—Derived Factors Downregulate the Production Levels of LPS-Induced Nitric Oxide and Tumor Necrosis Factor-α in THP-1 Cells. Evid.-Based Complement. Altern. Med. 2011, 2011, 586470. [Google Scholar] [CrossRef] [PubMed]
- Saad, B.; Embaslat, W.H.; Abu-Farich, B.; Mahajna, S.; Azab, M. Hypericum triquetrifolium extracts modulate IL-6, IL-10 and TNF-α protein and mRNA expression in LPS-activated human peripheral blood mononuclear cells and THP-1-derived macrophages. Med. Aromat. Plants S 2016, 3, 2167-0412. [Google Scholar]
- Mahajna, S.; Azab, M.; Zaid, H.; Farich, B.A.; Battah, F.; Mashner, S.; Saad, B. In vitro Evaluations of Cytotoxicity and Anti-inflammatory Effects of Peganum harmala Seed Extracts in THP-1-derived Macrophages. Eur. J. Med. Plants 2015, 5, 165–175. [Google Scholar] [CrossRef]
- Mansour, B.; Shaheen, N.; Kmail, A.; Haggag, N.; Saad, B. Rosmarinus officinalis L, Eriobotrya japonica and Olea europaea L attenuate adipogenesis in 3T3-L1-derived adipocytes and inflammatory response in LPS-induced THP-1-derived macro-phages. Biointerface Res. Appl. Chem. 2022, 13, 343–360. [Google Scholar]
- Mansour, B.; Shaheen, N.; Kmail, A.; Haggag, N.; Saad, S.; Sadiq, O.; Zaid, R.; Saad, B. Anti-Inflammatory and Anti-Adipogenesis Effects of Alchemilla vulgaris L., Salvia officinalis L., and Vitis vinifera L. in THP-1-Derived Macrophages and 3T3-L1 Cell Line. Immuno 2023, 3, 148–159. [Google Scholar] [CrossRef]
- Kmail, A.; Jaradat, N.; Mansour, B.; Abu-Labdeh, R.; Zakarneh, S.; Abu-Farha, S.; Hussein, F.; Issa, L.; Saad, B. Phytochemical analysis, cytostatic, cytotoxic, and anti-inflammatory effects of Arum palaestinum, Ocimum basilicum, and Trigonella foenum-graecum in human monocytic cell line (THP-1)-derived macrophages. Eur. J. Integr. Med. 2022, 54, 102159. [Google Scholar] [CrossRef]
- Kmail, A.; Mansour, B.; Hanaisheh, R.; Omar, G.; Jaradat, N.; Said, O.; Saad, B. Modulatory Effects of Leave and Fruit Extracts of Ficus sycomorus on Cytostatic and Inflammatory Mediators in Monocultures and Co-cultures of Human Keratinocyte (HaCat) and Human Monocyte (THP-1) Cell Lines. Eur. J. Med. Plants 2022, 33, 1–14. [Google Scholar] [CrossRef]
- Said, O.; Khamaysi, I.; Kmail, A.; Fulder, S.; AboFarekh, B.; Amin, R.; Daraghmeh, J.; Saad, B. In Vitro and Randomized, Double-Blind, Placebo-Controlled Trial to Determine the Efficacy and Safety of Nine Antiacne Medicinal Plants. Evid. Based Complement. Altern. Med. 2020, 2020, 3231413. [Google Scholar] [CrossRef]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef]
- Daniyal, M.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as antiinflammatory agents. Inflamm. Nat. Prod. 2021, 1, 21–63. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanovac, M.; LeMieuxa, M.; Greerd, B.; Moustaid-Moussa, N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef]
- Hirai, S.; Takahashi, N.; Goto, T.; Lin, S.; Uemura, T.; Yu, R.; Kawada, T. Functional Food Targeting the Regulation of Obesity-Induced Inflammatory Responses and Pathologies. Mediat. Inflamm. 2010, 2010, 1–8. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B. Adipose tissue, inflammation and atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Olefsky, J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021, 35, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; El-Sabbagh, A.S.; Lukas, B.E.; Tanneberger, S.J.; Jiang, Y. Adipose stem cells in obesity: Challenges and opportunities. Biosci. Rep. 2020, 40, BSR20194076. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.S.; Kushwaha, K.; Dubey, R.; Gupta, J. Association between obesity, inflammation and insulin resistance: Insights into signaling pathways and therapeutic interventions. Diabetes Res. Clin. Pract. 2023, 200, 110691. [Google Scholar] [CrossRef]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef]
- Varì, R.; Scazzocchio, B.; Silenzi, A.; Giovannini, C.; Masella, R. Obesity-Associated Inflammation: Does Curcumin Exert a Beneficial Role? Nutrients 2021, 13, 1021. [Google Scholar] [CrossRef]
- Piotrowski, I.; Kulcenty, K.; Suchorska, W. Interplay between inflammation and cancer. Rep. Pract. Oncol. Radiother. 2020, 25, 422–427. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-inflammatory Plant Flavonoids and Cellular Action Mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.-C.; Jee, S.Y.; Lee, S.G.; Park, S.J.; Lee, J.R.; Kim, S.C. Anti-Inflammatory Activity of the Methanol Extract of Moutan Cortex in LPS-Activated Raw264.7 Cells. Evid.-Based Complement. Altern. Med. 2007, 4, 327–333. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Cicero, A.F.G.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2017, 59, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Essafi-Benkhadir, K.; Refai, A.; Riahi, I.; Fattouch, S.; Karoui, H.; Essafi, M. Quince (Cydonia oblonga Miller) peel polyphenols modulate LPS-induced inflammation in human THP-1-derived macrophages through NF-κB, p38MAPK and Akt inhibition. Biochem. Biophys. Res. Commun. 2012, 418, 180–185. [Google Scholar] [CrossRef]
- Buruiana, F.E.; Solà, I.; Alonso-Coello, P. Recombinant human interleukin 10 for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2010, 2012, CD005109. [Google Scholar] [CrossRef]
- Friedrich, M.; Döcke, W.-D.; Klein, A.; Philipp, S.; Volk, H.-D.; Sterry, W.; Asadullah, K. Immunomodulation by Interleukin-10 Therapy Decreases the Incidence of Relapse and Prolongs the Relapse-free Interval in Psoriasis. J. Investig. Dermatol. 2002, 118, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Ally, A.; Powell, I.; Ally, M.M.; Chaitoff, K.; Nauli, S.M. Role of neuronal nitric oxide synthase on cardiovascular functions in physiological and pathophysiological states. Nitric Oxide 2020, 102, 52–73. [Google Scholar] [CrossRef]
- Hasani-Ranjbar, S.; Jouyandeh, Z.; Abdollahi, M. A systematic review of anti-obesity medicinal plants-An update. J. Diabetes Metab. Disord. 2013, 12, 28. [Google Scholar] [CrossRef]
- Said, O.; Saad, B.; Fulder, S.; Khalil, K.; Kassis, E. Weight Loss in Animals and Humans Treated with “Weighlevel”, a Combination of Four Medicinal Plants Used in Traditional Arabic and Islamic Medicine. Evid.-Based Complement. Altern. Med. 2011, 2011, 874538. [Google Scholar] [CrossRef]
- Saad, B.; Zaid, H.; Shanak, S.; Kadan, S. Anti-Diabetes and Anti-Obesity Medicinal Plants and Phytochemicals; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Saad, B.; Ghareeb, B.; Kmail, A. Metabolic and Epigenetics Action Mechanisms of Antiobesity Medicinal Plants and Phytochemicals. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–19. [Google Scholar] [CrossRef]
- Nisar, A. Medicinal plants and phenolic compounds. In Phenolic Compounds: Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; IntechOpen: London, UK, 2022; p. 131. [Google Scholar]
- Chang, E.; Kim, C.Y. Natural Products and Obesity: A Focus on the Regulation of Mitotic Clonal Expansion during Adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef] [PubMed]
- Han, L.K.; Sumiyoshi, M.; Zheng, Y.N.; Okuda, H.; Kimura, Y. Anti-obesity action of Salix matsudana leaves (Part 2). Isolation of anti-obesity effectors from polyphenol fractions of Salix matsudana. Phytother. Res. 2003, 17, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.F.; Shun, C.T.; Chen, T.M.; Chen, Y.H. 3-O-beta-D-glucosyl-(1–N6)-beta-D-glucosyl-kaempferol isolated from Sauropus androgenus reduces body weight gain in Wistar rats. Biol. Pharm. Bull 2006, 29, 2510–2513. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Nelson-Dooley, C.; Della-Fera, M.A.; Yang, J.Y.; Zhang, W.; Duan, J.; Hartzell, D.L.; Hamrick, M.W.; Baile, C.A. Genistein decreases food intake, body weight, and fat pad weight and causes adipose tissue apoptosis in ovariectomized female mice. J. Nutr. 2006, 136, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Naaz, A.; Yellayi, S.; Zakroczymski, M.A.; Bunick, D.; Doerge, D.R.; Lubahn, D.B.; Helferich, W.G.; Cooke, P.S. The Soy Isoflavone Genistein Decreases Adipose Deposition in Mice. Endocrinology 2003, 144, 3315–3320. [Google Scholar] [CrossRef]
- Dang, Z.; Lowik, C.W. The balance between concurrent activation of ERs and PPARs determines diadzein-induced osteogenesis and adipogenesis. J. Bone Miner Res. 2004, 19, 853–861. [Google Scholar] [CrossRef]
- Tsuda, T.; Ueno, Y.; Kojo, H.; Yoshikawa, T.; Osawa, T. Gene expression profile of isolated rat adipocytes treated with anthocyanins. Biochim. Et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2005, 1733, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Wallerstedt, D.; Talpur, N.; Tutuncuoglu, S.O.; Echard, B.; Myers, A.; Bui, M.; Bagchi, D. Effects of niacin-bound chromium and grape seed proanthocyanidin extract on the lipid profile of hypercholesterolemic subjects: A pilot study. J. Med. 2000, 31, 227–246. [Google Scholar]
- Nakagawa, Y.; Iinuma, M.; Matsuura, N.; Yi, K.; Naoi, M.; Nakayama, T.; Nozawa, Y.; Akao, Y. A potent apoptosis-inducing activity of a sesquiterpene lactone, arucanolide, in HL60 cells: A crucial role of apoptosis-inducing factor. J. Pharmacol. Sci. 2005, 97, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, S.; Raederstorff, D.; Preller, M.; Wang, Y.; Teixeira, S.R.; Riegger, C.; Weber, P. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J. Nutr. 2006, 136, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Della-Fera, M.A.; Hartzell, D.L.; Nelson-Dooley, C.; Hausman, D.B.; Baile, C.A. Esculetin Induces Apoptosis and Inhibits Adipogenesis in 3T3-L1 Cells. Obesity 2006, 14, 1691–1699. [Google Scholar] [CrossRef]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; De Oliveira, R.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef]
- Valjevac, A. Neuropeptides and Adipokines in The Control of Food Intake. Meta-Inflamm. Obes. 2020, 29, 44–62. [Google Scholar] [CrossRef]
- Rajan, L.; Palaniswamy, D.; Mohankumar, S.K. Targeting obesity with plant-derived pancreatic lipase inhibitors: A comprehensive review. Pharmacol. Res. 2020, 155, 104681. [Google Scholar] [CrossRef]
- Ahmad, B.; Friar, E.P.; Vohra, M.S.; Garrett, M.D.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Mechanisms of action for the anti-obesogenic activities of phytochemicals. Phytochemistry 2020, 180, 112513. [Google Scholar] [CrossRef]
- Li, H.; Qi, J.; Li, L. Phytochemicals as potential candidates to combat obesity via adipose non-shivering thermogenesis. Pharmacol. Res. 2019, 147, 104393. [Google Scholar] [CrossRef]
- Stuby, J.; Gravestock, I.; Wolfram, E.; Pichierri, G.; Steurer, J.; Burgstaller, J.M. Appetite-Suppressing and Satiety-Increasing Bioactive Phytochemicals: A Systematic Review. Nutrients 2019, 11, 2238. [Google Scholar] [CrossRef]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a potential anti-obesity target: A review of pharmacological treatment and natural products. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Chen, C.-W.; Chen, L.-K.; Chou, H.-Y.; Chang, C.-L.; Juan, C.-C. Curcumin Attenuates Adipogenesis by Inducing Preadipocyte Apoptosis and Inhibiting Adipocyte Differentiation. Nutrients 2019, 11, 2307. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Induction of cell apoptosis in 3T3-L1 preadipocytes by flavonoids is associated with their antioxidant activity. Mol. Nutr. Food Res. 2006, 50, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-L.; Huang, S.-L.; Yen, G.-C. Inhibitory Effect of Phenolic Acids on the Proliferation of 3T3-L1 Preadipocytes in Relation to Their Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 4191–4197. [Google Scholar] [CrossRef]
- Harmon, A.; Harp, J. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am. J. Physiol. Cell Physiol. 2001, 280, C807–C813. [Google Scholar] [CrossRef]
- Kim, H.K.; Della Fera, M.A.; Lin, J.; Baile, C.A. Docosahexaenoic acid inhibits adipocyte differentiation and induces apoptosis in 3T3-L1 preadipocytes. J. Nutr. 2006, 136, 2965–2969. [Google Scholar] [CrossRef]
- Roncari, D.A.; Lau, D.C.; Kindler, S. Exaggerated replication in culture of adipocyte precursors from massively obese persons. Metabolism 1981, 30, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.E.; Florine, D.L.; Wille, J.J., Jr.; Yun, K. Coupling of growth arrest and differentiation at a distinct state in the G1 phase of the cell cycle:GD. Proc. Natl. Acad. Sci. USA 1982, 79, 845–849. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.; Jin, C.; Xiong, Y.; Cheng, Y.Y.; Chen, K. Pomegranate flower extract bidirectionally regulates the proliferation, differentiation and apoptosis of 3T3-L1 cells through regulation of PPARγ expression mediated by PI3K-AKT signaling pathway. Biomed. Pharmacother. 2020, 131, 110769. [Google Scholar] [CrossRef]
- Evans, M.; Geigerman, C.; Cook, J.; Curtis, L.; Kuebler, B.; McIntosh, M. Conjugated linoleic acid suppresses triglyceride accumulation and induces apoptosis in 3T3-L1 preadipocytes. Lipids 2000, 35, 899–910. [Google Scholar] [CrossRef]
- Lin, J.; Della-Fera, M.A.; Baile, C.A. Green Tea Polyphenol Epigallocatechin Gallate Inhibits Adipogenesis and Induces Apoptosis in 3T3-L1 Adipocytes. Obes. Res. 2005, 13, 982–990. [Google Scholar] [CrossRef]
- Kao, Y.H.; Hiipakka, R.A.; Liao, S. Modulation of obesity by a green tea catechin. Am. J. Clin. Nutr. 2000, 72, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 cells. J. Agric. Food Chem. 2007, 55, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Iliakis, G.; Wang, Y.; Guan, J.; Wang, H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene 2003, 22, 5834–5847. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Posovszky, P.; Kukulus, V.; Zulet, M.A.; Debatin, K.M.; Wabitsch, M. Conjugated Linoleic Acids Promote Human Fat Cell Apoptosis. Horm. Metab. Res. 2007, 39, 186–191. [Google Scholar] [CrossRef]
- West, D.B.; DeLany, J.; Camet, P.M.; Blohm, F.; Truett, A.A.; Scimeca, J. Effects of conjugated linoleic acid on body fat and energy metabolism in the mouse. Am. J. Physiol. Integr. Comp. Physiol. 1998, 275, R667–R672. [Google Scholar] [CrossRef]
- Tsuboyama-Kasaoka, N.; Takahashi, M.; Tanemura, K.; Kim, H.J.; Tange, T.; Okuyama, H.; Kasai, M.; Ikemoto, S.; Ezaki, O. Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipodystrophy in mice. Diabetes 2000, 49, 1534–1542. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; He, Y.; Li, Q.; Gao, G.; Tong, G. Regulation of Apelin-13 on Bcl-2 and Caspase-3 and Its Effects on Adipocyte Apoptosis. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Della-Fera, M.A.; Hausman, D.B.; Baile, C.A. Enhancement of ajoene-induced apoptosis by conjugated linoleic acid in 3T3-L1 adipocytes. Apoptosis 2007, 12, 1117–1128. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 protein family: Arbiters of cell survival. Science 1998, 281, 1322–1326. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef]
- Fajas, L. Adipogenesis: A cross-talk between cell proliferation and cell differentiation. Ann. Med. 2003, 35, 79–85. [Google Scholar] [CrossRef]
- Zechner, R.; Bogner-Strauss, J.; Haemmerle, G.; Lass, A.; Zimmermann, R. Lipolysis: Pathway under construction. Curr. Opin. Infect. Dis. 2005, 16, 333–340. [Google Scholar] [CrossRef]
- Yang, A.; Mottillo, E.P. Adipocyte lipolysis: From molecular mechanisms of regulation to disease and therapeutics. Biochem. J. 2020, 477, 985–1008. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.; Cho, E.J. Flavonoids from Acer okamotoanum Inhibit Adipocyte Differentiation and Promote Lipolysis in the 3T3-L1 Cells. Molecules 2020, 25, 1920. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.K.D.S.; Silva, A.M.D.O.E.; Pereira, R.O.; Santos, A.S.; Junior, E.V.B.; Bezerra, M.T.; Barreto, R.S.S.; Quintans-Junior, L.J.; Quintans, J.S.S. Anti-obesity properties and mechanism of action of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 3, 1–22. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Cąkała-Jakimowicz, M.; Puzianowska-Kuźnicka, M. Targeting Abdominal Obesity and Its Complications with Dietary Phytoestrogens. Nutrients 2020, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Szkudelski, T.; Nogowski, L. Daidzein, coumestrol and zearalenone affect lipogenesis and lipolysis in rat adipocytes. Phytomedicine 2002, 9, 338–345. [Google Scholar] [CrossRef]
- Kuppusamy, U.; Das, N. Effects of flavonoids on cyclic AMP phosphodiesterase and lipid mobilization in rat adipocytes. Biochem. Pharmacol. 1992, 44, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Pinent, M.; Bladé, M.C.; Salvadó, M.J.; Arola, L.; Ardévol, A. Intracellular Mediators of Procyanidin-Induced Lipolysis in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2005, 53, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]

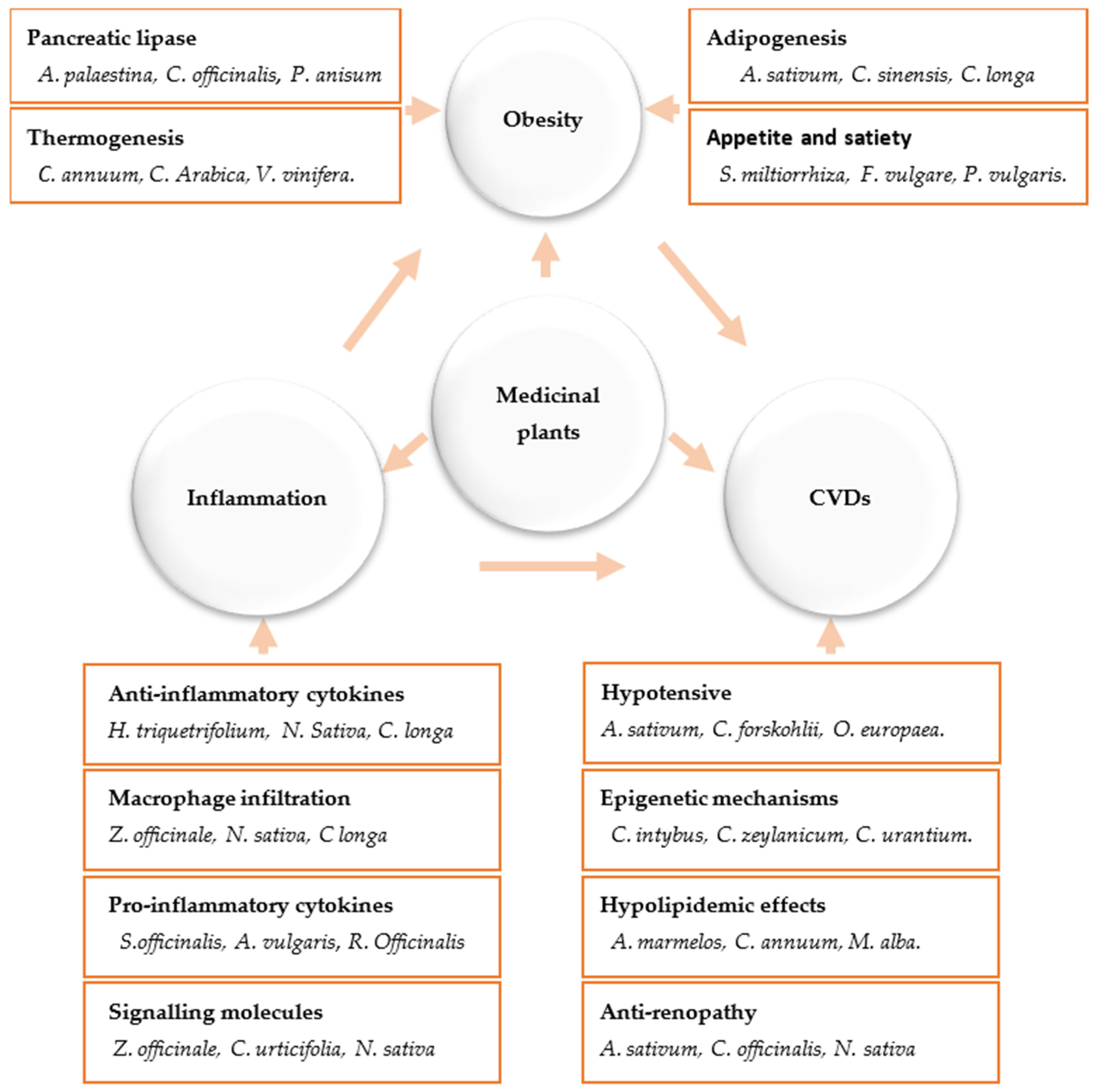
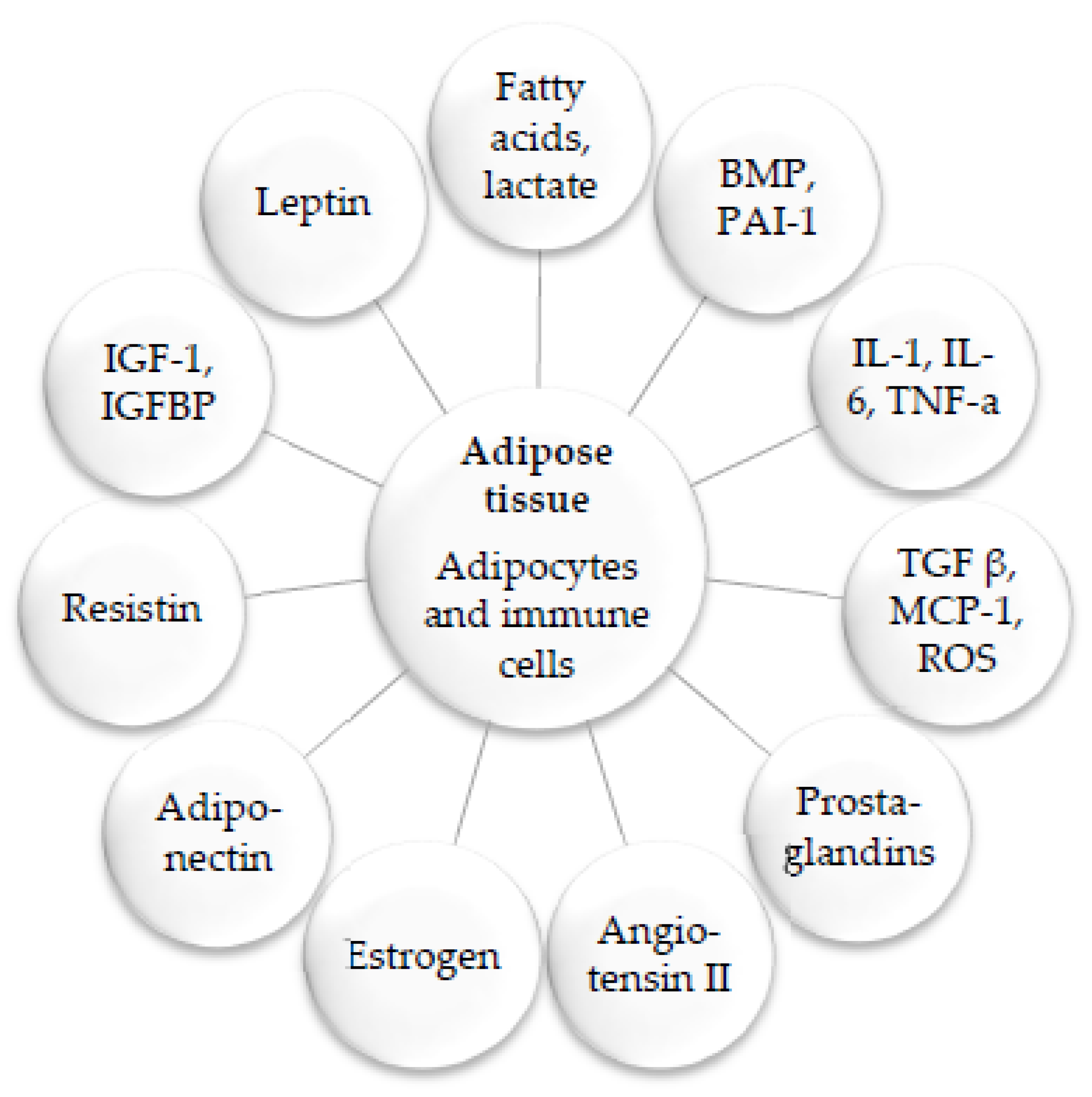
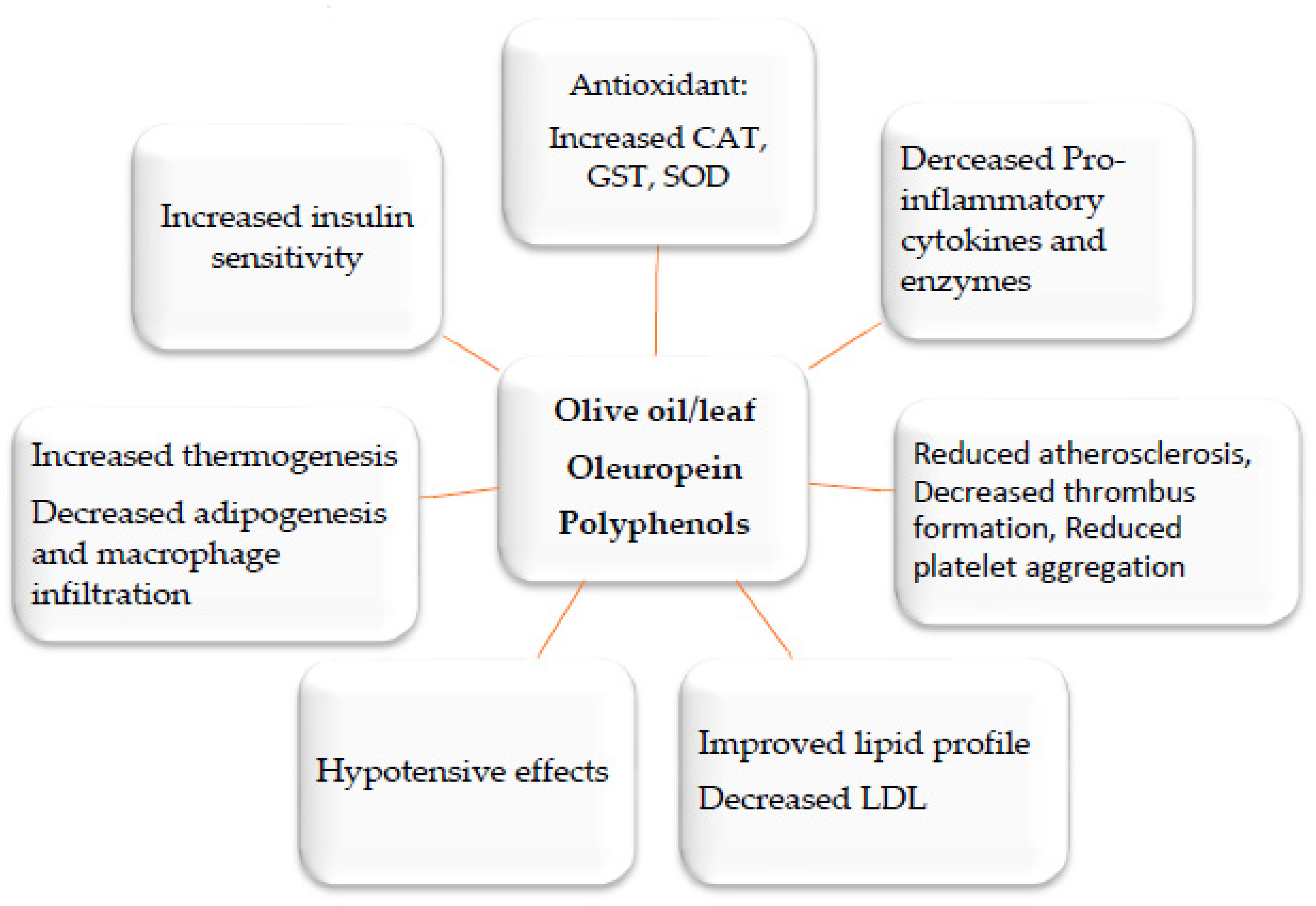
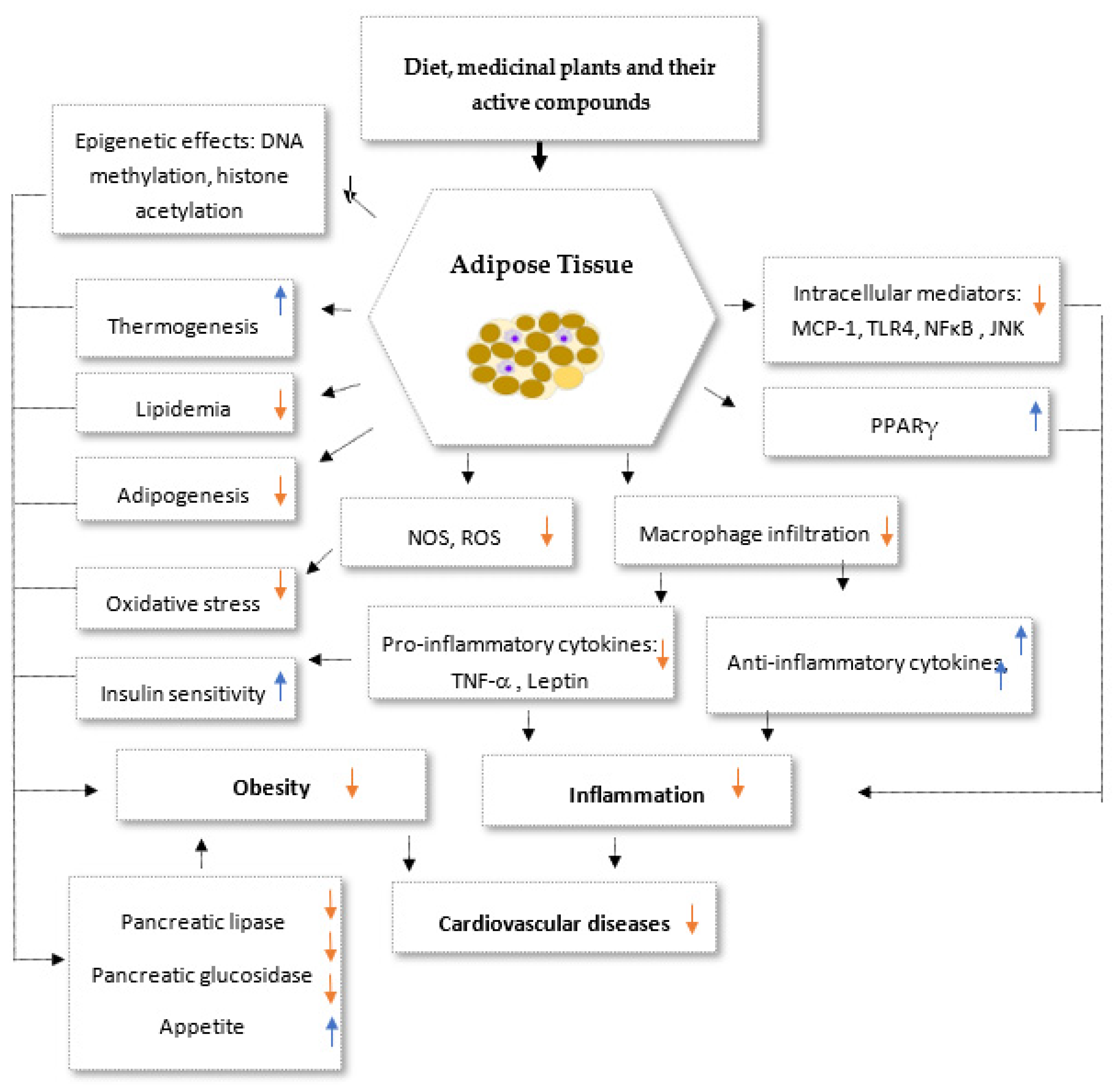
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saad, B. Management of Obesity-Related Inflammatory and Cardiovascular Diseases by Medicinal Plants: From Traditional Uses to Therapeutic Targets. Biomedicines 2023, 11, 2204. https://doi.org/10.3390/biomedicines11082204
Saad B. Management of Obesity-Related Inflammatory and Cardiovascular Diseases by Medicinal Plants: From Traditional Uses to Therapeutic Targets. Biomedicines. 2023; 11(8):2204. https://doi.org/10.3390/biomedicines11082204
Chicago/Turabian StyleSaad, Bashar. 2023. "Management of Obesity-Related Inflammatory and Cardiovascular Diseases by Medicinal Plants: From Traditional Uses to Therapeutic Targets" Biomedicines 11, no. 8: 2204. https://doi.org/10.3390/biomedicines11082204
APA StyleSaad, B. (2023). Management of Obesity-Related Inflammatory and Cardiovascular Diseases by Medicinal Plants: From Traditional Uses to Therapeutic Targets. Biomedicines, 11(8), 2204. https://doi.org/10.3390/biomedicines11082204





