Abstract
COVID-19 vaccination was the main measure to overcome the pandemic. As with other drugs and vaccines, mild to moderate adverse events have been reported following vaccination. In addition, several cutaneous reactions have been described. In particular, there are several reports investigating de novo psoriasis or the exacerbation of psoriasis following COVID-19 vaccination. However, data on the possible pathogenetic mechanisms as well as comprehensive manuscripts on the topic are scant. Thus, the aim of our manuscript was to perform a review of the current literature on post-COVID-19 vaccination exacerbations and new-onset psoriasis in order to offer a wide perspective on this area and to point out possible pathogenetic mechanisms. Research on the current literature was performed following PRISMA guidelines. In total, 49 studies involving 134 patients developing new-onset psoriasis (n = 27, 20.1%) or psoriasis exacerbation (n = 107, 79.9%) were collected. Although cases of de novo psoriasis or a worsening of psoriasis have been reported following vaccination, all of the cases have been successfully treated while overall benefit–risk profile of COVID-19 vaccination does not justify vaccine hesitancy due to the risk of psoriasis being developed or worsening. Certainly, further studies are needed to identify possible pathogenetic mechanisms in order to identify “at-risk” patients. Finally, vaccination should not be discouraged.
1. Introduction
Psoriasis is a chronic, inflammatory skin disorder that affects millions of individuals worldwide (with up to a 3% prevalence) [1,2]. Clinically, it is characterized by the presence of thick, red, scaly patches on the skin’s surface [2,3]. Moreover, several comorbidities can be associated with the psoriatic disorder (hypertension, dyslipidemia, obesity, psoriatic arthritis, anxiety/depression, inflammatory bowel disease, diabetes mellitus, etc.), making this disease a burden for patients’ mental and emotional well-being, leading to social isolation and a reduction in quality of life [4,5,6]. Therefore, psoriasis treatment is not limited to skin lesions but also to its comorbidities and the psychosocial aspects of the disease [7,8,9]. Currently, several treatment options for psoriasis are available. These include topical treatments (creams and ointments) which may be used for the mild form of the disease, phototherapy (exposure to ultraviolet light), conventional systemic medications (cyclosporin, methotrexate, acitretin, and fumarates), small molecules, and biologic therapies which are used for moderate-to-severe forms [10,11,12,13]. In particular, the introduction of biologic drugs specifically targeting interleukins (IL) 23 and 17 and tumor necrosis factor-alpha (TNFα), involved in psoriasis pathogenesis, revolutionized the management of the disease, showing promising results in terms of effectiveness and safety [14,15,16]. Globally, treatment plans are tailored to everyone’s specific needs, taking into account the severity of the disease, its impact on a patient’s quality of life, and the comorbidities [17,18].
The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has had a profound impact on global health, economies, and societies worldwide [19,20]. Due to the high transmissibility of the virus, preventive measures such as wearing masks, practicing physical distancing, and frequent hand hygiene played a crucial role in mitigating transmission [21,22]. As regards dermatological clinical practice, teledermatology emerged as a valuable tool in providing remote dermatological care during the pandemic. Indeed, it has played a vital role in maintaining access to dermatological care, reducing viral transmission, improving access to care, and ensuring the continuity of treatment [23,24]. Globally, the introduction of COVID-19 vaccination was the main measure to overcome the pandemic. Indeed, COVID-19 vaccination played a vital role in controlling the spread of the virus and reducing the severity of the disease [25,26,27]. Multiple vaccines have been developed and authorized for emergency use around the world, working by stimulating the immune system to recognize and respond to the SARS-CoV-2 virus, preventing infection or reducing the severity of illness if infection occurs [25,26,27]. In particular, four vaccines have been approved by the European Medicines Agency (EMA), based on two different mechanisms of action: mRNA-based vaccines (Pfizer/BioNTech;BNT162b2 and Moderna; mRNA-1273) and viral vector-based vaccines (AstraZeneca; AZD1222 and Johnson & Johnson; Ad26.COV2.S) [25,26,27].
As with other drugs and vaccines, mild to moderate adverse events (AEs) have been reported following vaccination, including fatigue, diarrhea, headache, fever, muscle aches, pain or redness at the injection site, chills, etc. [28,29]. Fortunately, most of these reactions have been mild and self-limited. In addition, several cutaneous reactions have been described following COVID-19 vaccination [28,29]. In particular, there are several reports investigating de novo psoriasis or an exacerbation of psoriasis following COVID-19 vaccination [30,31]. However, data on the possible pathogenetic mechanisms as well as comprehensive manuscripts on the topic are scant. Thus, the aim of our manuscript was to perform a review of the current literature on post-COVID-19 vaccination exacerbations and new-onset psoriasis in order to offer a wide perspective on COVID-19 vaccination and psoriasis and to point out possible pathogenetic mechanisms.
2. Materials and Methods
Research on the current literature was performed using the following databases: PubMed, Cochrane Skin, Embase, EBSCO, MEDLINE, and Google Scholar (up to 1 June 2023). Studies were identified, screened and extracted for relevant data following PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines [32], using the following keywords: “COVID-19”, “vaccine”, “cutaneous”, “vaccination”, “side effects”, “adverse events”, “safety”, “efficacy”, “skin manifestations”, “mRNA”, “viral vector”, “Pfizer/BioNTech”, “BNT162b2”, “Moderna”, “mRNA-1273”, “AstraZeneca”, “Johnson & Johnson”, “Ad26.COV2.S”, “AZD1222”, and “psoriasis”. The manuscripts analyzed included reviews, meta-analyses, letters to the editor, real-world studies, and case series. Manuscripts that fit the aim of our review were considered. Studies reporting at least one patient who developed new-onset psoriasis or experienced a worsening of psoriasis following at least one dose of COVID-19 vaccine were included. Only the BNT162b2, mRNA-1273, AZD1222 and Ad26.COV2.S vaccines were considered in our review. Studies reporting de novo psoriasis or an exacerbation of psoriasis following other types of vaccines were excluded and studies investigating psoriatic arthritis were not considered. All the clinical phenotypes of psoriasis were included (plaque, guttate, pustular, erythrodermic, inverse, palmoplantar, etc.). The search was then refined by reviewing the texts and abstracts of the collected articles. The bibliography was also revised to include articles that may have been missed. Only English-language manuscripts were considered.
This article is based on previously conducted studies and does not contain studies with human or animal participants conducted by any of the authors.
3. Results
In total, 49 studies involving 134 patients were included in this review (Figure 1) and are summarized in Table 1 (new-onset psoriasis) [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] and Table 2 (psoriasis exacerbation) [32,33,34,35,39,45,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71].
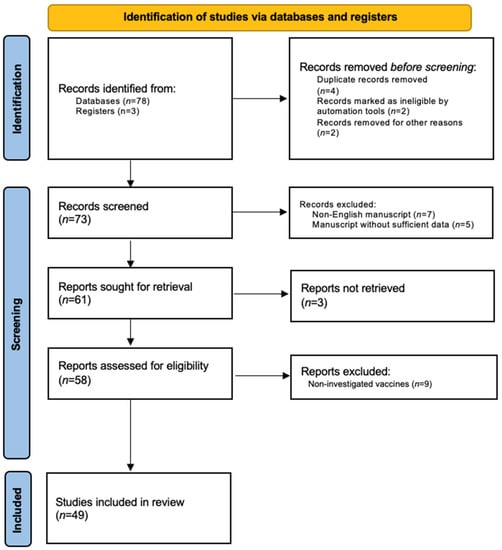
Figure 1.
PRISMA flow-chart.
3.1. New-Onset Psoriasis
New-onset psoriasis refers to the development of psoriasis in individuals who previously did not have the condition. The exact triggers for new-onset psoriasis can vary among individuals, but potential factors include infections, physical trauma to the skin, stress, certain medications, and hormonal changes. As regards cases of new-onset psoriasis following COVID-19 vaccination, a total of 27 (male: 10 (37.0%); female: 13 (48.1%); not reported: 4 (14.8%); mean age: 54.4 ± 20.9 years) cases reported in 20 manuscripts are described and summarized in Table 1 [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. In particular, plaque psoriasis was the commonest clinical phenotype (n = 9 (33.3%)), followed by guttate (7 (25.9%)), pustular (4 (14.8%)), nail (3 (11.1%)) and annular psoriasis (1 (3.7%)), while the clinical phenotype of the remaining 3 (11.1%) subjects has not been reported. Moreover, mRNABNT162b2 was the commonest vaccine associated with psoriasis development (n = 15, 55.5%), followed by AZD1222 (n = 5, 18.5%) and mRNA-1273 (n = 3, 11.1%). Notably, psoriasis development was reported following all of the doses of vaccination, with some patients experiencing psoriasis disease after each single dose. The mean time between vaccine administration and new-onset psoriasis was 10.3 ± 6.4 days (range: 2–30 days) (only cases with reported data were counted). Finally, the majority of cases of de novo psoriasis were mild. Indeed, topical treatments (a topical calcipotriol/betamethasone combination and topical corticosteroids) were successfully used in nine (33.3%) patients, limiting the use of conventional systemic drugs (oral corticosteroids, acitretin and methotrexate) and biologics to three (11.1%) and five (18.5%) cases, respectively. Of note, therapy was not reported in seven (25.9%) patients whereas the remaining subjects were managed with apremilast (n = 2, 7.4%) or without treatment (n = 1, 3.7%).

Table 1.
New-onset psoriasis following COVID-19 vaccination.
Table 1.
New-onset psoriasis following COVID-19 vaccination.
| Authors | Country | Cases | Age/Sex | Type of Psoriasis | Vaccines/Doses | Days * | After |
|---|---|---|---|---|---|---|---|
| Tran et al. [33] | Vietnam | 3 | Pt1: 51/M | Pt1: plaque | Pt1: AZD1222/3 | Pt1: 7 | Calcip/betam + antihistamines |
| Pt2: 68/F | Pt2: plaque | Pt2: BNT162b2/3 | Pt2: 30 | ||||
| Pt3: 73/M | Pt3: guttate | Pt3: BNT162b2/1 | Pt3: 30 | ||||
| Català A et al. [35] | Spain | 3 | NR | NR | NR | NR | NR |
| Nagrani et al. [36] | India | 2 | Pt1: 56/F | Pt1: plaque | Pt1: AZD1222/1-2 | Pt1: 7-2 | Apremilast + antihistamines + emollients |
| Pt2: 65/M | Pt2: plaque | Pt2: AZD1222/2 | Pt2: 10 | ||||
| Ouni et al. [37] | Tunisia | 2 | Pt1: 59/M | Pt1: guttate | Pt1: BNT16B2b2/1-2-3 | Pt1: 7-7-7 | Pt1: TCS |
| Pt2: 23/F | Pt2: guttate | Pt2: Ad26.COV2.S/1 | Pt2: 2 | Pt2: TCS | |||
| Gargiulo et al. [34] | Italy | 2 | Pt1: 82/F | Pt1: plaque | Pt1: BNT162b2/3 | NR | Pt1: secukinumab |
| Pt2: 29/M | Pt2: pustular | Pt1: BNT162b2/2 | Pt2: guselkumab | ||||
| Cortonesi et al. [38] | Italy | 1 | 82/F | Plaque | BNT162b2/1 | 7 | Ixekizumab |
| Wei et al. [39] | USA | 1 | 24/M | Plaque | mRNA-1273/2 | 24 | Ixekizumab + acitretin 25 mg/die |
| Ständer et al. [40] | Germany | 1 | 50/F | Plaque | BNT162b2/2 | 7 | None |
| McMahon et al. [41] | USA | 1 | 67/NR | Plaque | mRNA-1273/NR | NR | NR |
| Song et al. [42] | Korea | 1 | 23/F | Guttate | BNT162b2/1 | 2 | Calcip/betam |
| Magro et al. [43] | USA | 1 | 58/M | Guttate | BNT162b2/2 | 14 | NR |
| Pesqué et al. [44] | Spain | 1 | 72/M | Guttate | mRNA-1273/2 | 6 | Calcip/betam |
| Lehmann et al. [45] | Switzerland | 1 | 79/F | Guttate | BNT162b2/1 | 10 | Calcip/betam + nbUVB |
| Frioui et al. [46] | Tunisia | 1 | 20/M | Pustular | BNT162b2/1 | 4 | Acitretin 25 mg/die + TCS |
| Elamin et al. [47] | United Kingdom | 1 | 66/F | Pustular | AZD1222/1 | 21 | Acitretin 20 mg/die |
| Romagnuolo et al. [48] | Italy | 1 | 64/F | Pustular | BNT162b2/1 | NR | Methotrexate 15 mg per week |
| Ricardo et al. [49] | USA | 1 | 76/F | Nail | BNT162b2/2 | 7 | TCS |
| Ruggiero et al. [50] | Italy | 1 | 47/F | Nail | BNT162b2/2 | 17 | Ixekizumab |
| Lamberti et al. [51] | Italy | 1 | 45/F | Naile | BNT162b2/2 | 3 | NR |
| Chhabra et al. [52] | India | 1 | NR/M | Annular | AZD1222/1 | 14 | NR |
M: male; F: female; AZD1222: AstraZeneca-Oxford AZD1222; mRNA-1273: Moderna mRNA-1273; BNT162b2: Pfizer mRNABNT162b2; Ad26.COV2.S: Johnson & Johnson; calcip/betam: topical calcipotriol/betamethasone combination; nbUVB: narrow-band UVB; TCS: topical corticosteroids; OCS: oral corticosteroids; NR: not reported. * Number of days between new-onset psoriasis and vaccination.
3.2. Psoriasis Exacerbation
Psoriasis exacerbation refers to a worsening or flare-up of symptoms in individuals who already have psoriasis. During an exacerbation, existing psoriasis lesions may become more inflamed, larger, and more widespread. Additionally, new lesions may develop in previously unaffected areas of the body. The severity and duration of an exacerbation can vary from person to person. Several factors can trigger or contribute to a psoriasis exacerbation, including stress, infections, certain medications, changes in weather or climate, hormonal fluctuations, injury or trauma to the skin, and lifestyle factors such as smoking and excessive alcohol consumption. In this scenario, in total, 107 cases (male: 61 (57.0%); female: 46 (43.0%); mean age: 56.5 ± 13.4 years) of a flare-up of psoriasis collected in 29 articles are described and summarized in Table 2 [32,33,34,35,39,45,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71]. Of these, 74 (69.2%), 12 (11.2%), 8 (7.5%), 5 (4.7%), 4 (3.7%) and 4 (3.7%) subjects developed a flare-up of plaque, guttate, pustular, erythrodermic, nail and palmoplantar psoriasis. In particular, mRNABNT162b2 was the commonest vaccine associated with psoriasis development (n = 66, 61.7%), followed by AZD1222 (n = 21, 19.6%) and mRNA-1273 (n = 20, 18.7%). Of note, psoriasis development was reported following all of the doses of vaccination. The mean time between vaccination and psoriasis worsening was 13.7 ± 14.4 days (range: 2–90 days) (only cases with reported data were counted). Differently from new-onset psoriasis, there are 5 case series reporting at least 10 cases of psoriasis exacerbation following vaccination. Of these, Gargiulo et al. reported the largest cohort of cases (n = 16), followed by Huang et al. [53] (n = 15) and Sotiriou et al. (n = 14) [54]. Finally, even though psoriasis therapy before and after vaccination was not reported in 57 (53.3%) and 22 cases (20.6%), biologic treatment was the commonest drug administered for the management of psoriasis exacerbation (n = 39, 36.4%), followed by the addition of topical treatments to current therapies (a topical calcipotriol/betamethasone combination: 12, 11.2%; topical corticosteroids: 11, 10.3%), conventional systemic drugs (cyclosporin, acitretin and methotrexate) (n = 8, 7.5%), and phototherapy (n = 5, 4.7%). As regards biologic treatments, 33 (30.8%) patients started biologics for the first time (anti-IL23: 18 (54.5%); anti-IL17: 9 (27.3%); anti-IL12/23: 2 (6.1%); anti-TNFα; 4 (12.1%)) whereas current biologic treatment was switched to in 6 (5.6%) subjects. Differently from de novo psoriasis, several cases of moderate-to-severe forms of disease were reported following vaccination (as well as three cases of erythrodermic psoriasis), leading to the use of conventional systemic drugs and biologics in 47 (44.0%) patients. Notably, 19 (17.8%) patients who developed psoriasis exacerbation were under biologic treatment for psoriasis at the moment of the flare-up.

Table 2.
Psoriasis exacerbation following COVID-19 vaccination.
Table 2.
Psoriasis exacerbation following COVID-19 vaccination.
| Authors | Cases | Age/Sex | Type of Psoriasis | Vaccines/Doses | Days * | Treatment ** | After *** |
|---|---|---|---|---|---|---|---|
| Gargiulo et al. [34] | 16 | Pt1: 40/F | Pt1: plaque | Pt1: BNT162b2/3 | NR | NR | Pt1: tildrakizumab |
| Pt2: 50/M | Pt2: plaque | Pt2: BNT162b2/2 | Pt2: tildrakizumab | ||||
| Pt3: 25/M | Pt3: plaque | Pt3: BNT162b2/2 | Pt3: ixekizumab | ||||
| Pt4: 40/F | Pt4: plaque | Pt4: mRNA-1273/3 | Pt4: tildrakizumab | ||||
| Pt5: 53/M | Pt5: plaque | Pt5: BNT162b2/2 | Pt5: risankizumab | ||||
| Pt6: 50/M | Pt6: plaque | Pt6: BNT162b2/2 | Pt6: guselkumab | ||||
| Pt7: 38/M | Pt7: plaque | Pt7: BNT162b2/2 | Pt7: risankizumab | ||||
| Pt8: 56/M | Pt8: plaque | Pt8: BNT162b2/1 | Pt8: secukinumab | ||||
| Pt9: 50/M | Pt9: plaque | Pt9: mRNA-1273/2 | Pt9: brodalumab | ||||
| Pt10: 78/M | Pt10: plaque | Pt10: BNT162b2/3 | Pt10: risankizumab | ||||
| Pt11: 67/F | Pt11: plaque | Pt11: BNT162b2/3 | Pt11: risankizumab | ||||
| Pt12: 57/M | Pt12: guttate | Pt12: BNT162b2/2 | Pt12: guselkumab | ||||
| Pt13: 53/M | Pt13: plaque | Pt13: BNT162b2/2 | Pt13: bimekizumab | ||||
| Pt14: 61/M | Pt14: plaque | Pt14: mRNA-1273/2 | Pt14: risankizumab | ||||
| Pt15: 72/F | Pt15: plaque | Pt15: BNT162b2/2 | Pt15: ustekinumab | ||||
| Pt16: 76/M | Pt16: plaque | Pt16: BNT162b2/3 | Pt16: guselkumab | ||||
| Huang et al. [53] | 15 | NR/8M-7F | 8 plaque 7 guttate | AZD1222/1 (3) AZD1222/2 (4) AZD1222/1-2 (1) mRNA-1273/1 (1) mRNA-1273/2 (6) | 9.3 | NR | NR |
| Sotiriou et al. [54] | 14 | Pt1: 69/F | Plaque | Pt1: AZD1222/2 | Pt1: 8 | NR | Pt1: PUVA |
| Pt2: 82/F | Pt2: mRNA-1273/2 | Pt2: 10 | Pt2: calcip/betam | ||||
| Pt3: 62/F | Pt3: BNT162b2/2 | Pt3: 6 | Pt3: calcip/betam | ||||
| Pt4: 73/M | Pt4: BNT162b2/2 | Pt4: 7 | Pt4: calcip/betam | ||||
| Pt5: 66/M | Pt5: AZD1222/1 | Pt5: 22 | Pt5: risankizumab | ||||
| Pt6: 62/F | Pt6: AZD1222/2 | Pt6: 13 | Pt6: apremilast | ||||
| Pt7: 78/F | Pt7: BNT162b2/2 | Pt7: 5 | Pt7: calcip/betam | ||||
| Pt8: 64/F | Pt8: AZD1222/2 | Pt8: 6 | Pt8: PUVA | ||||
| Pt9: 69/M | Pt9: AZD1222/1 | Pt9: 32 | Pt9: nbUVB | ||||
| Pt10: 83/M | Pt10: BNT162b2/2 | Pt10: 9 | Pt10: calcip/betam | ||||
| Pt11: 61/F | Pt11: AZD1222/2 | Pt11: 3 | Pt11: nbUVB | ||||
| Pt12: 49/M | Pt12: BNT162b2/2 | Pt12: 10 | Pt12: ixekizumab | ||||
| Pt13: 55/F | Pt13: BNT162b2/2 | Pt13: 7 | Pt13: cyclosporine | ||||
| Pt14: 64/F | Pt14: AZD1222/2 | Pt14: 7 | Pt14: guselkumab | ||||
| Koumaki et al. [55] | 12 | Pt1: 34/F | Pt1: plaque | Pt1: BNT162b2/2 | Pt1: 10 | Pt1: secukinumab | Pt1: secukinumab + emollients |
| Pt2: 61/M | Pt2: plaque | Pt2: AZD1222/1 | Pt2: 14 | Pt2: TCS | Pt2: apremilast | ||
| Pt3: 45/F | Pt3: plaque | Pt3: BNT162b2/2 | Pt3: 10 | Pt3: calcip/betam | Pt3: TCS + calcip/betam | ||
| Pt4: 56/F | Pt4: plaque | Pt4: BNT162b2/2 | Pt4: 2 | Pt4: adalimumab | Pt4: adalimumab + TCS | ||
| Pt5: 53/F | Pt5: pustular | Pt5: BNT162b2/1 | Pt5: 20 | Pt5: adalimumab | Pt5: TCS+ OCS | ||
| Pt6: 56/F | Pt6: plaque | Pt6: BNT162b2/1-2 | Pt6: 3 | Pt6: secukinumab | Pt6: secukinumab | ||
| Pt7: 34/F | Pt7: plaque | Pt7: BNT162b2/1-2 | Pt7:7 | Pt7: secukinumab | Pt7: secukinumab | ||
| Pt8: 61/F | Pt8: pustular | Pt8: BNT162b2/2 | Pt8: 4 | Pt8: methotrexate | Pt8: methylprednisolone | ||
| Pt9: 66/F | Pt9: plaque | Pt9: BNT162b2/1 | Pt9: 20 | Pt9: ustekinumab | Pt9: ustekinumab + TCS | ||
| Pt10: 67/F | Pt10: plaque | Pt10: BNT162b2/2 | Pt10: 20 | Pt10: OCS | Pt10: TCS + OCS | ||
| Pt11: 56/M | Pt11: plaque | Pt11: BNT162b2/2 | Pt11: 20 | Pt11: calcip/betam | Pt11: calcip/betam | ||
| Pt12: 51/M | Pt12: plaque | Pt12: BNT162b2/2 | Pt12: 25 | Pt12: TCS | Pt12: calcip/betam | ||
| Megna et al. [56] | 11 | Pt1: 55/M | Pt1: plaque | Pt1: BNT162b2/2 | Pt1: 5 | Pt1: none | Pt1: methotrexate |
| Pt2: 49/M | Pt2: plaque | Pt2: BNT162b2/2 | Pt2: 6 | Pt2: none | Pt2: adalimumab | ||
| Pt3: 45/M | Pt3: plaque | Pt3: AZD1222/1 | Pt3: 10 | Pt3: secukinumab | Pt3: secukinumab + calcip/betam | ||
| Pt4: 61/M | Pt4: plaque | Pt4: BNT162b2/2 | Pt4: 12 | Pt4: adalimumab | Pt4: ixekizumab | ||
| Pt5: 62/M | Pt5: plaque | Pt5: mRNA-1273/2 | Pt5: 8 | Pt5: none | Pt5: brodalumab | ||
| Pt6: 47/M | Pt6: guttate | Pt6: BNT162b2/2 | Pt6: 9 | Pt6: ixekizumab | Pt6: ixekizumab + calcip/betam | ||
| Pt7: 70/F | Pt7: plaque | Pt7: BNT162b2/2 | Pt7: 8 | Pt7: calcip/betam | Pt7: adalimumab | ||
| Pt8: 39/F | Pt8: plaque | Pt8: AZD1222/2 | Pt8: 7 | Pt8: guselkumab | Pt8: guselkumab + calcip/betam | ||
| Pt9: 58/M | Pt9: plaque | Pt9: BNT162b2/2 | Pt9: 5 | Pt9: secukinumab | Pt9: secukinumab + calcip/betam | ||
| Pt10: 55/F | Pt10: plaque | Pt10: AZD1222/2 | Pt10: 10 | Pt10: nbUVB | Pt10: risankizumab | ||
| Pt11: 59/M | Pt11: plaque | Pt11: BNT162b2/1 | Pt11: 14 | Pt11: etanercept | Pt11: ixekizumab | ||
| Wei et al. [39] | 6 | Pt1: 76/M | Pt1: plaque | Pt1: mRNA-1273/2 | Pt1: 62 | Pt1: NR | Pt1: apremilast + nbUVB |
| Pt2: 69/M | Pt2: plaque | Pt2: mRNA-1273/2 | Pt2: 21 | Pt2: NR | Pt2: apremilast + tildrakizumab | ||
| Pt3: 68/F | Pt3: plaque | Pt3: mRNA-1273/2 | Pt3: 6 | Pt3: NR | Pt3: risankizumab | ||
| Pt4: 67/M | Pt4: plaque | Pt4: mRNA-1273/2 | Pt4: 60 | Pt4: NR | Pt4: tildrakizumab + TCS | ||
| Pt5: 52/F | Pt5: plaque | Pt5: mRNA-1273/2 | Pt5: 7 | Pt5: NR | Pt5: risankizumab + TCSt | ||
| Pt6: 27/F | Pt6: plaque | Pt6: BNT162b2/2 | Pt6: 90 | Pt6: NR | Pt6: TCS | ||
| Ständer et al. [40] | 4 | Pt1: 35/M | Pt1: guttate | Pt1: BNT162b2/2 | Pt1: 7 | Pt1: NR | Pt1: none |
| Pt2: 62/M | Pt2: guttate | Pt2: mRNA-1273/2 | Pt2: 10 | Pt2: NR | Pt2: ustekinumab | ||
| Pt3: 58/F | Pt3: plaque | Pt3: BNT162b2/2 | Pt3: 5 | Pt3: NR | Pt3: ixekizumab | ||
| Pt4: 54/M | Pt4: plaque | Pt4: BNT162b2/3 | Pt4: 7 | Pt4: NR | Pt4: ixekizumab | ||
| Ruggiero et al. [50] | 4 | Pt1: 55/M | Pt1: nail | Pt1: BNT162b2/2 | Pt1: 19 | Pt1: none | Pt1: methotrexate |
| Pt2: 66/M | Pt2: nail | Pt2: mRNA-1273/1 | Pt2: 21 | Pt2: apremilast | Pt2: ixekizumab | ||
| Pt3: 41/M | Pt3: nail | Pt3: BNT162b2/2 | Pt3: 16 | Pt3: adalimumab | Pt3: brodalumab | ||
| Pt4: 52/F | Pt4: nail | Pt4: BNT162b2/2 | Pt4: 25 | Pt4: secukinumab | Pt4: secukinumab + TCS +TK | ||
| Durmaz et al. [57] | 3 | Pt1:64/M | Pt1: plaque | Pt1: BNT162b2/3 | Pt1: 42 | None | NR |
| Pt2: 64/M | Pt2: palmoplantar | Pt2: BNT162b2/2 | Pt2: 7 | ||||
| Pt3: 25/F | Pt3: pustular | Pt3: BNT162b2/1 | Pt3: 3 | ||||
| Piccolo et al. [58] | 2 | Pt1: 57/M | Pt1: palmoplantar | Pt1: BNT162b2/1 | Pt1: 30 | Pt1: TCS | Pt1: acitretin |
| Pt2: 63/F | Pt2: palmoplantar | Pt2: BNT162b2/1 | Pt2: 30 | Pt2: TCS | Pt2: acitretin | ||
| Tran et al. [59] | 2 | Pt1: 30/F | Pt1: Erythrodermic | Pt1: BNT162b2/2 | Pt1: 7 | Pt1: secukinumab | Pt1: acitretin |
| Pt2: 45/F | Pt2: Erythrodermic | Pt2: BNT162b2/2 | Pt2: 7 | Pt2: calcip/betam | Pt2: NR | ||
| Bostan et al. [60] | 1 | 51/M | Plaque | BNT162b2/2 | 14 | NR | NR |
| Krajewski et al. [61] | 1 | 46/M | Plaque | BNT162b2/2 | 5 | Deucravacitinib | NR |
| Fang et al. [62] | 1 | 34/F | Plaque | AZD1222/1 | 7 | Ustekinumab + cyclosporine | TCS |
| Mieczkowska et al. [63] | 1 | 65/M | Plaque | BNT162b2/1 | 7 | Apremilast + calcip/betam | NR |
| Niebel et al. [64] | 1 | 62/M | Plaque | BNT162b2/2 | 20 | None | Balneophototherapy |
| Kabbani et al. [65] | 1 | 53/M | Plaque | BNT162b2/1 | 7 | None | Cyclosporine |
| Burlando et al. [66] | 1 | 56/M | Plaque | BNT162b2/2 | 16 | NR | NR |
| Pesqué et al. [44] | 1 | 30/F | Plaque | mRNA-1273/1 | 10 | TCS | Calcip/betam |
| Phuan et al. [67] | 1 | 80/F | Guttate | BNT162b2/3 | 7 | Cyclosporine | Cyclosporine + TCS |
| Quattrini et al. [68] | 1 | 83/F | Palmoplantar | BNT162b2/2 | 2 | Methotrexate | OCS + methotrexate |
| Onsun et al. [69] | 1 | 72/M | Pustular | AZD1222/1 | 4 | TCS | Infliximab |
| Perna et al. [70] | 1 | NR/M | Pustular | BNT162b2/1 | 5 | None | Infliximab + cyclosporine |
| Yatsuzuka et al. [71] | 1 | 65/M | Pustular | BNT162b2/2 | 12 | Infliximab | Secukinumab + OCS |
| Pavia et al. [72] | 1 | 47/F | Pustular | BNT162b2/2 | 10 | Ustekinumab | Risankizumab |
| Rouai et al. [73] | 1 | 66/M | Pustular | BNT162b2/1 | 4 | None | TCS |
| Durmus et al. [74] | 1 | 42/M | Erythrodermic | BNT162b2/1 | 28 | Secukinumab | Ixekizumab + OCS |
| Lopez et al. [75] | 1 | 58/M | Erythrodermic | BNT162b2/2 | 4 | None | TCS |
| Nia et al. [76] | 1 | 58/M | Erythrodermic | BNT162b2/1 | 2 | None | Cyclosporine + nbUVB + TCS |
M: male; F: female; AZD1222: AstraZeneca-Oxford AZD1222; mRNA-1273: Moderna mRNA-1273; BNT162b2: Pfizer mRNABNT162b2; Ad26.COV2.S: Johnson & Johnson; calcip/betam: topical calcipotriol/betamethasone combination; nbUVB: narrow-band UVB; PUVA: psoralen-UVA; TCS: topical corticosteroids; OCS: oral corticosteroids; TK: topical keratolytics; NR: not reported. * Number of days between new-onset psoriasis and vaccination. ** Psoriasis treatment before vaccination. *** Psoriasis treatment following COVID-19 vaccination-related psoriasis flare-up.
4. Discussion
Psoriasis is a complex autoimmune disorder characterized by an abnormal immune response that leads to chronic inflammation and the accelerated growth of skin cells [77]. Cytokines play a crucial role in the pathogenesis of psoriasis, serving as key mediators in the inflammatory process [78,79]. In particular, TNFα, IL23, IL17, IL22 and IL6 have been reported to play a central role in the initiation and maintenance of psoriatic inflammation, promoting the recruitment and activation of immune cells, and the production of other cytokines [78,79]. Targeting these cytokines has been a successful approach in the treatment of psoriasis [80,81,82]. Indeed, biologic drugs, such as anti-TNFα, IL17, and IL23, have been developed to specifically block the actions of these cytokines and reduce inflammation in psoriatic skin, revolutionizing the psoriasis treatment scenario [80,81,82].
The COVID-19 pandemic impacted daily clinical practice [83,84]. In particular, several strategies have been adopted to contain the spreading of the infection [85,86]. Among these, vaccination was the main one. However, although the preliminary safety concerns and doubts raised at the beginning of the vaccination campaign related to vaccines’ safety were overcome, several cutaneous adverse events were reported, most of these not being shown in clinical trials [87,88,89]. Fortunately, the majority of these were mild and self-limiting, and did not require medical attention [90,91]. In addition, the vaccination campaign was also limited by several personal burdens (a fear of vaccination and its side effects, stress from needing a vaccination to travel or work, etc.) [84,92,93].
As regards psoriasis, several cases of exacerbation or a new onset of the disease were reported. However, comprehensive manuscripts collecting all these data in order to offer a wide perspective are scant. In this context, we performed a review with the purpose of showing a wide analysis of COVID-19 vaccination and psoriasis development/exacerbation and pointing out possible pathogenetic mechanisms. It is important to note that the information provided is based on the current understanding of these topics and may evolve as further research becomes available. Globally, in total, 49 studies involving 134 patients developing new-onset psoriasis (n = 27, 20.1%) or experiencing a psoriasis exacerbation (n = 107, 79.9%) were collected. In both cases, mRNABNT162b2 was the commonest vaccine associated and plaque psoriasis was the commonest clinical phenotype, while a significant gender predominance was not reported and cutaneous reactions were reported following each dose of a vaccine. In our opinion, mRNABNT162b2 was the commonest vaccine related to cutaneous reactions since it was the most commonly used during the vaccination campaign, and plaque psoriasis is the commonest clinical phenotype according to psoriasis phenotype epidemiology. Moreover, all of the cases have been successfully treated with topical or systemic medications, including biologics. In particular, a difference between patients starting or switching to biologic treatment for psoriasis has been found between new-onset and flare-up groups (11.1% vs. 36.4%). In our opinion, the increased awareness of psoriatic disease in patients already suffering from the disease has reduced the number of consultations for mild exacerbations. Indeed, patients affected by psoriasis are more used to self-medication with topical drugs, reducing the need for medical advice in the case of mild forms of the disease. This may explain the difference between the predominance of moderate-to-severe forms of disease in patients who developed a psoriasis flare-up and those with mild forms in de novo cases where the use of topical treatments for the management was predominant (33.3% vs. 21.5%). Unfortunately, ongoing treatment prior to COVID-19 vaccination was often not reported, which prevented the results from being analyzed to reveal whether or not some psoriatic treatments may increase the risk of disease exacerbation.
Of interest is that 19 (17.8%) patients who developed psoriasis exacerbation were under biologic treatment for psoriasis at the moment of the flare-up. Reviewing the current literature, being on biologic drugs at the moment of vaccination seems to reduce the risk of psoriasis worsening [30,31,94,95], but clinical studies comparing patients undergoing biologics and patients receiving other medications and/or a placebo at the moment of vaccination are absent, not allowing a confirmation of these data. Certainly, biologic drugs were also shown to be safe and effective during the pandemic period.
In addition, the number of days between new-onset psoriasis or the exacerbation of psoriasis and vaccination is not reported in most of the studies. Moreover, the onset or exacerbation of nail psoriasis should also be discussed. Indeed, there are few cases reporting the onset or worsening of this form of disease and the time between vaccination and reporting is too short to limit this condition to the COVID-19 vaccine.
Thus, while there have been anecdotal reports of new-onset psoriasis or psoriasis exacerbation following COVID-19 vaccination, it is essential to evaluate these cases in the context of existing scientific knowledge. Firstly, there is currently no direct evidence linking COVID-19 vaccination to the development of psoriasis. Vaccines, including COVID-19 vaccines, work by stimulating the immune system to produce a protective response against the virus [96,97,98]. The mechanisms involved in vaccine-induced immune responses are different from those implicated in psoriasis pathogenesis [96,97,98]. It is unlikely that COVID-19 vaccination would directly trigger the development of psoriasis in individuals without a pre-existing predisposition [96,97,98]. However, it is important to consider the potential of immune system activation or modulation following vaccination [96,97,98]. In some cases, vaccines can induce immune responses that may lead to transient inflammation or immune system activation [96,97,98]. This immune activation may theoretically contribute to the exacerbation of pre-existing psoriasis in individuals already diagnosed with the condition [96,97,98]. In addition, the induction of neutralizing antibodies and T-cell responses via vaccination may lead to an increasement and production of TNFα and Interferon (IFN) γ [96,97,98]. Similarly, vaccination can activate plasmacytoid and dermal myeloid dendritic cells which may be a trigger for the psoriasis cascade [96,97,98]. Finally, vaccinations might induce the production of IL6, which may be a trigger for Th17 cells to produce IL22, which itself stimulates keratinocyte proliferation [96,97,98].
Notably, cases of de novo psoriasis or an exacerbation of psoriasis have been reported following both mRNA and viral vector-based vaccines, suggesting that the onset or the worsening of the disease is not related to the mechanism of action of the vaccines but to the vaccination itself.
Furthermore, it is crucial to differentiate between coincidence and causation when assessing the relationship between COVID-19 vaccination and new-onset psoriasis. Psoriasis is a relatively common skin condition, and it is possible for new cases to emerge coincidentally after vaccination, without a direct causal relationship. Robust epidemiological studies and careful evaluation of individual cases are needed to determine any potential association between COVID-19 vaccination and the development of psoriasis. Moreover, it is mandatory to emphasize the overall benefit–risk profile of COVID-19 vaccination. Indeed, COVID-19 is a severe and potentially life-threatening illness, and the benefits of vaccination in preventing infection, reducing severe disease, and limiting the spread of the virus far outweigh the potential risks.
5. Strengths and Limitations
The number of investigated studies and the literature review using the PRISMA methods are the main strengths of our manuscript. The absence of clinical studies and consistent data such as data from registries are the main limitations. Moreover, we hypothesize that cases of psoriasis exacerbations or de novo disease developed following vaccination were underestimated, since not all patients seek medical advice due to the limited severity of the disease, tending to self-medicate (particularly in patients affected by psoriasis who are more accustomed to self-medication with topical drugs, reducing the need for medical advice in the case of mild forms of the disease), and many reactions have not been reported. Finally, psoriatic arthritis was not considered in our manuscript.
6. Conclusions
To sum up, the COVID-19 vaccination campaign was a success. Although cases of de novo psoriasis or disease worsening have been reported following vaccination, all of the cases have been successfully treated (mainly with topicals in de novo cases and systemic treatments in psoriasis flare-ups), while the overall benefit–risk profile of COVID-19 vaccination do not justify the vaccine hesitancy due to the risk of psoriasis being developed or worsening. Globally, plaque psoriasis was the most common clinical phenotype both in terms of de novo psoriasis and the exacerbation of psoriasis. Moreover, more severe forms of the disease have been reported in patients with a history of psoriasis compared to the new onset cases where mild forms were predominant. This difference may be explained by psoriatic patients’ ability to self-medicate with topical drugs for mild forms of the disease, reducing the need for medical advice for moderate-to-severe conditions. Certainly, further studies are needed to identify possible pathogenetic mechanisms in order to identify “at-risk” patients. Finally, vaccination should not be discouraged.
Author Contributions
L.P.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. T.B.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. S.C.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. A.R.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. F.M.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. L.F.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. E.C.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. M.M.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are reported in the current study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Langley, R.G.B. Psoriasis: Epidemiology, clinical features, and quality of life. Ann. Rheum. Dis. 2005, 64 (Suppl. S2), ii18–ii23, discussion ii24–ii25. [Google Scholar] [CrossRef]
- Griffiths, C.E.; Barker, J.N.W.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Megna, M.; Ocampo-Garza, S.S.; Potestio, L.; Fontanella, G.; Gallo, L.; Cacciapuoti, S.; Ruggiero, A.; Fabbrocini, G. New-Onset Psoriatic Arthritis under Biologics in Psoriasis Patients: An Increasing Challenge? Biomedicines 2021, 9, 1482. [Google Scholar] [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases: Epidemiology. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases: Implications for management. J. Am. Acad. Dermatol. 2017, 76, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Camela, E.; Potestio, L.; Fabbrocini, G.; Ruggiero, A.; Megna, M. New frontiers in personalized medicine in psoriasis. Expert Opin. Biol. Ther. 2022, 22, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Camela, E.; Potestio, L.; Fabbrocini, G.; Pallotta, S.; Megna, M. The holistic approach to psoriasis patients with comorbidities: The role of investigational drugs. Expert Opin. Investig. Drugs 2023, 32, 537–552. [Google Scholar] [CrossRef]
- Megna, M.; Potestio, L.; Fabbrocini, G.; Cinelli, E. Tildrakizumab: A new therapeutic option for erythrodermic psoriasis? Dermatol. Ther. 2021, 34, e15030. [Google Scholar] [CrossRef]
- Megna, M.; Camela, E.; Battista, T.; Genco, L.; Martora, F.; Noto, M.; Picone, V.; Ruggiero, A.; Monfrecola, G.; Fabbrocini, G.; et al. Efficacy and safety of biologics and small molecules for psoriasis in pediatric and geriatric populations. Part II: Focus on elderly patients. Expert Opin. Drug Saf. 2023, 22, 43–58. [Google Scholar] [CrossRef]
- Bakshi, H.; Nagpal, M.; Singh, M.; Dhingra, G.A.; Aggarwal, G. Treatment of Psoriasis: A Comprehensive Review of Entire Therapies. Curr. Drug Saf. 2020, 15, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, M.; Mabuchi, T. New Treatment Addressing the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 7488. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Ruggiero, A.; Camela, E.; Potestio, L.; Fabbrocini, G.; Megna, M. Drug safety evaluation of tildrakizumab for psoriasis: A review of the current knowledge. Expert Opin. Drug Saf. 2022, 21, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Potestio, L.; Cacciapuoti, S.; Gallo, L.; Battista, T.; Camela, E.; Fabbrocini, G.; Megna, M. Tildrakizumab for the treatment of moderate to severe psoriasis: Results from a single center preliminary real-life study. Dermatol. Ther. 2022, 35, e15941. [Google Scholar] [CrossRef]
- Megna, M.; Battista, T.; Potestio, L.; Ruggiero, A.; Ventura, V.; Fabbrocini, G.; Picone, V. A case of erythrodermic psoriasis rapidly and successfully treated with Bimekizumab. J. Cosmet. Dermatol. 2022, 22, 1146–1148. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Picone, V.; Martora, F.; Fabbrocini, G.; Megna, M. Guselkumab, Risankizumab, and Tildrakizumab in the Management of Psoriasis: A Review of the Real-World Evidence. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1649–1658. [Google Scholar] [CrossRef]
- Marasca, C.; Fornaro, L.; Martora, F.; Picone, V.; Fabbrocini, G.; Megna, M. Onset of vitiligo in a psoriasis patient on ixekizumab. Dermatol. Ther. 2021, 34, e15102. [Google Scholar] [CrossRef]
- Lytras, T.; Tsiodras, S. Lockdowns and the COVID-19 pandemic: What is the endgame? Scand. J. Public Health 2021, 49, 37–40. [Google Scholar] [CrossRef]
- Kaul, V.; de Moraes, A.G.; Khateeb, D.; Greenstein, Y.; Winter, G.; Chae, J.; Stewart, N.H.; Qadir, N.; Dangayach, N.S. Medical Education During the COVID-19 Pandemic. Chest 2021, 159, 1949–1960. [Google Scholar] [CrossRef]
- Ilgen, O.; Saatli, B.; Timur, T.; Kula, H.; Kandemir, S.; Kurt, S.; Cagliyan, E. Measures against COVID-19 pandemic—A single tertiary center experience. J. Obstet. Gynaecol. 2022, 42, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U. Challenges of COVID-19 pandemic for dermatology. Dermatol. Ther. 2020, 33, e13430. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Martora, F.; Fabbrocini, G.; Villani, A.; Marasca, C.; Megna, M.; Fornaro, L.; Comune, R.; Potestio, L. The Role of Teledermatology During the COVID-19 Pandemic: A Narrative Review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2785–2793. [Google Scholar] [CrossRef] [PubMed]
- Marasca, C.; Annunziata, M.C.; Camela, E.; Di Guida, A.; Fornaro, L.; Megna, M.; Napolitano, M.; Patruno, C.; Potestio, L.; Fabbrocini, G. Teledermatology and Inflammatory Skin Conditions during COVID-19 Era: New Perspectives and Applications. J. Clin. Med. 2022, 11, 1511. [Google Scholar] [CrossRef]
- Hassine, I.H. Covid-19 vaccines and variants of concern: A review. Rev. Med. Virol. 2022, 32, e2313. [Google Scholar] [CrossRef]
- Brüssow, H. COVID-19: Vaccination problems. Environ. Microbiol. 2021, 23, 2878–2890. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.-J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Soiza, R.L.; Scicluna, C.; Thomson, E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing 2021, 50, 279–283. [Google Scholar] [CrossRef]
- Kricorian, K.; Civen, R.; Equils, O. COVID-19 vaccine hesitancy: Misinformation and perceptions of vaccine safety. Hum. Vaccines Immunother. 2022, 18, 1950504. [Google Scholar] [CrossRef]
- Megna, M.; Potestio, L.; Battista, T.; Camela, E.; Genco, L.; Noto, M.; Fabbrocini, G.; Martora, F. Immune response to COVID-19 mRNA vaccination in patients with psoriasis undergoing treatment with biologics. Clin. Exp. Dermatol. 2022, 47, 2310–2312. [Google Scholar] [CrossRef]
- Ruggiero, A.; Martora, F.; Picone, V.; Potestio, L.; Camela, E.; Battista, T.; Fabbrocini, G.; Megna, M. The impact of COVID-19 infection on patients with psoriasis treated with biologics: An Italian experience. Clin. Exp. Dermatol. 2022, 47, 2280–2282. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ Clin. Res. Ed. 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.A.; Nguyen, T.T.P.; Pham, N.N.; Pham, N.T.U.; Vu, T.T.P.; Nguyen, H.T. New onset of psoriasis following COVID-19 vaccination. Dermatol. Ther. 2022, 35, e15590. [Google Scholar] [CrossRef]
- Gargiulo, L.; Ibba, L.; Vignoli, C.A.; Piscazzi, F.; Cortese, A.; Fiorillo, G.; Toso, F.; Pavia, G.; Valenti, M.; Avagliano, J.; et al. New-onset and flares of psoriasis after COVID-19 infection or vaccination successfully treated with biologics: A case series. J. Dermatol. Treat. 2023, 34, 2198050. [Google Scholar] [CrossRef]
- Català, A.; Muñoz-Santos, C.; Galván-Casas, C.; Roncero Riesco, M.; Revilla Nebreda, D.; Solá-Truyols, A.; Giavedoni, P.; Llamas-Velasco, M.; González-Cruz, C.; Cubiró, X. Cutaneous reactions after SARS-CoV-2 vaccination: A cross-sectional Spanish nationwide study of 405 cases. Br. J. Derm. 2022, 186, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Nagrani, P.; Jindal, R.; Goyal, D. Onset/flare of psoriasis following the ChAdOx1 nCoV-19 Corona virus vaccine (Oxford-AstraZeneca/Covishield): Report of two cases. Dermatol. Ther. 2021, 34, e15085. [Google Scholar] [CrossRef]
- Ouni, N.; Korbi, M.; Chahed, F.; Ben Fadhel, N.; Bellalah, A.; Belhadjali, H.; Aouam, K.; Zili, J. New-onset guttate psoriasis following coronavirus disease 2019 vaccination: About two cases. Dermatol. Ther. 2022, 35, e15617. [Google Scholar] [CrossRef]
- Cortonesi, G.; Orsini, C.; Rubegni, P.; Trovato, E. New-onset psoriasis after Comirnaty (BNT162b2, BioNTech /Pfizer) vaccine successfully treated with ixekizumab. Dermatol. Ther. 2022, 35, e15606. [Google Scholar] [CrossRef]
- Wei, N.; Kresch, M.; Elbogen, E.; Lebwohl, M. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022, 19, 74–77. [Google Scholar] [CrossRef]
- Ständer, S.; Zirpel, H.; Bujoreanu, F.; Tatu, A.L.; Ludwig, R.J.; Thaçi, D. Case report: Clinical features of COVID-19 vaccine-induced exacerbation of psoriasis—A case series and mini review. Front. Med. 2022, 9, 995150. [Google Scholar] [CrossRef]
- McMahon, D.E.; Kovarik, C.L.; Damsky, W.; Rosenbach, M.; Lipoff, J.B.; Tyagi, A.; Chamberlin, G.; Fathy, R.; Nazarian, R.M.; Desai, S.R.; et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: A registry-based study. J. Am. Acad. Dermatol. 2022, 86, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Lim, Y.; Jo, S.J. De novo guttate psoriasis following coronavirus disease 2019 vaccination. J. Dermatol. 2022, 49, e30–e31. [Google Scholar] [CrossRef]
- Magro, C.; Crowson, A.N.; Franks, L.; Schaffer, P.R.; Whelan, P.; Nuovo, G. The histologic and molecular correlates of COVID-19 vaccine-induced changes in the skin. Clin. Dermatol. 2021, 39, 966–984. [Google Scholar] [CrossRef] [PubMed]
- Pesqué, D.; Lopez-Trujillo, E.; Marcantonio, O.; Giménez-Arnau, A.M.; Pujol, R.M. New-onset and exacerbations of psoriasis after mRNA COVID-19 vaccines: Two sides of the same coin? J. Eur. Acad. Dermatol. Venereol. 2021, 36, e80–e81. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Schorno, P.; Hunger, R.; Heidemeyer, K.; Feldmeyer, L.; Yawalkar, N. New onset of mainly guttate psoriasis after COVID-19 vaccination: A case report. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e752–e755. [Google Scholar] [CrossRef]
- Frioui, R.; Chamli, A.; Zaouak, A.; Hlel, I.; Khanchel, F.; Fenniche, S.; Hammami, H. A case of new-onset acute generalized pustular psoriasis following Pfizer-BioNTech COVID-19 vaccine. Dermatol. Ther. 2022, 35, e15444. [Google Scholar] [CrossRef]
- Elamin, S.; Hinds, F.; Tolland, J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin. Exp. Dermatol. 2022, 47, 153–155. [Google Scholar] [CrossRef]
- Romagnuolo, M.; Pontini, P.; Muratori, S.; Marzano, A.V.; Moltrasio, C. De novo annular pustular psoriasis following mRNA COVID-19 vaccine. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e603–e605. [Google Scholar] [CrossRef]
- Ricardo, J.W.; Lipner, S.R. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021, 17, 18–20. [Google Scholar] [CrossRef]
- Ruggiero, A.; Potestio, L.; Battista, T.; Fabbrocini, G.; Megna, M. Reply to ‘Nail psoriasis: A rare mRNA COVID-19 vaccine reaction’ by Lamberti A et al. J. Eur. Acad. Dermatol. Venereol. 2022, 37, e41–e42. [Google Scholar] [CrossRef]
- Lamberti, A.; Lora, V.; Graceffa, D.; Bonifati, C.; Cota, C. Nail psoriasis: A rare mRNA COVID-19 vaccine reaction. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e745–e746. [Google Scholar] [CrossRef]
- Chhabra, N.; George, A. A case of de novo annular-plaque type psoriasis following Oxford-AstraZeneca COVID-19 vaccination. Curr. Drug Saf. 2023, 18, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-W.; Tsai, T.-F. Exacerbation of Psoriasis Following COVID-19 Vaccination: Report From a Single Center. Front. Med. 2021, 8, 812010. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, E.; Tsentemeidou, A.; Bakirtzi, K.; Lallas, A.; Ioannides, D.; Vakirlis, E. Psoriasis exacerbation after COVID-19 vaccination: A report of 14 cases from a single centre. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e857–e859. [Google Scholar] [CrossRef]
- Koumaki, D.; Krueger-Krasagakis, S.; Papadakis, M.; Katoulis, A.; Gkiaouraki, I.; Zografaki, K.; Mylonakis, D.; Krasagakis, K. Psoriasis flare-up after AZD1222 and BNT162b2 COVID-19 mRNA vaccines: Report of twelve cases from a single centre. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e411–e415. [Google Scholar] [CrossRef]
- Megna, M.; Potestio, L.; Gallo, L.; Caiazzo, G.; Ruggiero, A.; Fabbrocini, G. Reply to “Psoriasis exacerbation after COVID-19 vaccination: Report of 14 cases from a single centre” by Sotiriou E et al. J. Eur. Acad. Dermatol. Venereol. 2021, 36, e11–e13. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, I.; Turkmen, D.; Altunisik, N.; Toplu, S.A. Exacerbations of generalized pustular psoriasis, palmoplantar psoriasis, and psoriasis vulgaris after mRNA COVID-19 vaccine: A report of three cases. Dermatol. Ther. 2022, 35, e15331. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, V.; Russo, T.; Mazzatenta, C.; Bassi, A.; Argenziano, G.; Cutrone, M.; Darlington, M.E.S.D.; Grimalt, R. COVID vaccine-induced pustular psoriasis in patients with previous plaque type psoriasis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e330–e332. [Google Scholar] [CrossRef]
- Tran, T.B.; Pham, N.T.U.; Phan, H.N.; Nguyen, H.T. Generalized erythrodermic psoriasis triggered by vaccination against severe acute respiratory syndrome Coronavirus 2. Dermatol. Ther. 2022, 35, e15464. [Google Scholar] [CrossRef]
- Bostan, E.; Elmas, L.; Yel, B.; Yalici-Armagan, B. Exacerbation of plaque psoriasis after inactivated and BNT162b2 mRNA COVID-19 vaccines: A report of two cases. Dermatol. Ther. 2021, 34, e15110. [Google Scholar] [CrossRef]
- Krajewski, P.K.; Szepietowski, J.C. Psoriasis flare-up associated with second dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e632–e634. [Google Scholar] [CrossRef]
- Fang, W.; Chiu, L.; Hu, S.C. Psoriasis exacerbation after first dose of AstraZeneca coronavirus disease 2019 vaccine. J. Dermatol. 2021, 48, e566–e567. [Google Scholar] [CrossRef] [PubMed]
- Mieczkowska, K.; Kaubisch, A.; McLellan, B.N. Exacerbation of psoriasis following COVID-19 vaccination in a patient previously treated with PD-1 inhibitor. Dermatol. Ther. 2021, 34, e15055. [Google Scholar] [CrossRef] [PubMed]
- Niebel, D.; Wenzel, J.; Wilsmann-Theis, D.; Ziob, J.; Wilhelmi, J.; Braegelmann, C. Single-Center Clinico-Pathological Case Study of 19 Patients with Cutaneous Adverse Reactions Following COVID-19 Vaccines. Dermatopathology 2021, 8, 463–476. [Google Scholar] [CrossRef]
- Kabbani, M.; Poskin, M.; Benhadou, F. Psoriasis exacerbation after COVID-19 vaccination in high-risk group: How to manage it? Dermatol. Ther. 2022, 35, e15368. [Google Scholar] [CrossRef]
- Burlando, M.; Herzum, A.; Micalizzi, C.; Cozzani, E.; Parodi, A. Cutaneous reactions to COVID-19 vaccine at the dermatology primary care. Immun. Inflamm. Dis. 2022, 10, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Phuan, C.Z.Y.; Choi, E.C.-E.; Oon, H.H. Temporary exacerbation of pre-existing psoriasis and eczema in the context of COVID-19 messenger RNA booster vaccination: A case report and review of the literature. JAAD Int. 2022, 6, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Quattrini, L.; Verardi, L.; Caldarola, G.; Peluso, G.; De Simone, C.; D’agostino, M. New onset of remitting seronegative symmetrical synovitis with pitting oedema and palmoplantar psoriasis flare-up after Sars-Cov-2 vaccination. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e727–e729. [Google Scholar] [CrossRef]
- Onsun, N.; Kaya, G.; Işık, B.G.; Güneş, B. A generalized pustular psoriasis flare after CoronaVac COVID-19 vaccination: Case report. Health Promot. Perspect. 2021, 11, 261–262. [Google Scholar] [CrossRef]
- Perna, D.; Jones, J.; Schadt, C.R. Acute generalized pustular psoriasis exacerbated by the COVID-19 vaccine. JAAD Case Rep. 2021, 17, 1–3. [Google Scholar] [CrossRef]
- Yatsuzuka, K.; Murakami, M.; Kuroo, Y.; Fukui, M.; Yoshida, S.; Muto, J.; Shiraishi, K.; Sayama, K. Flare-up of generalized pustular psoriasis combined with systemic capillary leak syndrome after coronavirus disease 2019 mRNA vaccination. J. Dermatol. 2022, 49, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Pavia, G.; Gargiulo, L.; Spinelli, F.; Avagliano, J.; Valenti, M.; Borroni, R.G.; Costanzo, A.; Narcisi, A. Generalized pustular psoriasis flare in a patient affected by plaque psoriasis after BNT162b2 mRNA COVID-19 vaccine, successfully treated with risankizumab. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e502–e505. [Google Scholar] [CrossRef] [PubMed]
- Rouai, M.; Ben Slimane, M.; Sassi, W.; Alaoui, F.; Chelly, I.; Mokni, M. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: A case report. Dermatol. Ther. 2022, 35, e15465. [Google Scholar] [CrossRef] [PubMed]
- Durmus, O.; Akdogan, N.; Karadag, O.; Gokoz, O. Erythroderma related with the first dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine in a patient with psoriasis. Dermatol. Ther. 2022, 35, e15363. [Google Scholar] [CrossRef]
- Lopez, E.D.; Javed, N.; Upadhyay, S.; Shekhar, R.; Sheikh, A.B. Acute exacerbation of psoriasis after COVID-19 Pfizer vaccination. Bayl. Univ. Med. Cent. Proc. 2022, 35, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Nia, A.M.; Silva, M.M.; Spaude, J.; Gonzalez-Fraga, J.D. Erythrodermic psoriasis eruption associated with SARS-CoV-2 vaccination. Dermatol. Ther. 2022, 35, e15380. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- De Alcantara, C.C.; Reiche, E.M.V.; Simão, A.N.C. Cytokines in psoriasis. Adv. Clin. Chem. 2021, 100, 171–204. [Google Scholar] [CrossRef]
- Baliwag, J.; Barnes, D.H.; Johnston, A. Cytokines in psoriasis. Cytokine 2015, 73, 342–350. [Google Scholar] [CrossRef]
- Megna, M.; Potestio, L.; Camela, E.; Fabbrocini, G.; Ruggiero, A. Ixekizumab and brodalumab indirect comparison in the treatment of moderate to severe psoriasis: Results from an Italian single-center retrospective study in a real-life setting. Dermatol. Ther. 2022, 35, e15667. [Google Scholar] [CrossRef]
- Megna, M.; Potestio, L.; Fabbrocini, G.; Camela, E. Treating psoriasis in the elderly: Biologics and small molecules. Expert Opin. Biol. Ther. 2022, 22, 1503–1520. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Ruggiero, A.; Battista, T.; Marano, L.; Cacciapuoti, S.; Potestio, L. Long-Term Efficacy and Safety of Risankizumab for Moderate to Severe Psoriasis: A 2-Year Real-Life Retrospective Study. J. Clin. Med. 2023, 12, 3233. [Google Scholar] [CrossRef]
- De Lucia, M.; Potestio, L.; Costanzo, L.; Fabbrocini, G.; Gallo, L. Scabies outbreak during COVID-19: An Italian experience. Int. J. Dermatol. 2021, 60, 1307–1308. [Google Scholar] [CrossRef]
- Martora, F.; Battista, T.; Marasca, C.; Genco, L.; Fabbrocini, G.; Potestio, L. Cutaneous Reactions Following COVID-19 Vaccination: A Review of the Current Literature. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Marasca, C.; Fabbrocini, G.; Ruggiero, A. Strategies adopted in a southern Italian referral centre to reduce adalimumab discontinuation: Comment on ‘Can we increase the drug survival time of biologic therapies in hidradenitis suppurativa?’. Clin. Exp. Dermatol. 2022, 47, 1864–1865. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Camela, E.; Villani, A.; Tajani, A.; Fabbrocini, G.; Potestio, L. Teledermatology: A useful tool also after COVID-19 era? J. Cosmet. Dermatol. 2022, 21, 2309–2310. [Google Scholar] [CrossRef]
- Huang, Z.; Su, Y.; Zhang, T.; Xia, N. A review of the safety and efficacy of current COVID-19 vaccines. Front. Med. 2022, 16, 39–55. [Google Scholar] [CrossRef]
- Potestio, L.; Fabbrocini, G.; D’Agostino, M.; Piscitelli, I.; Martora, F. Cutaneous reactions following COVID-19 vaccination: The evidence says “less fear”. J. Cosmet. Dermatol. 2023, 22, 28–29. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S.; Firouzabadi, N.; Dehshahri, A.; Vazin, A. A focused review on technologies, mechanisms, safety, and efficacy of available COVID-19 vaccines. Int. Immunopharmacol. 2021, 100, 108162. [Google Scholar] [CrossRef]
- Potestio, L.; Villani, A.; Fabbrocini, G.; Martora, F. Cutaneous reactions following booster dose of COVID-19 mRNA vaccination: What we should know? J. Cosmet. Dermatol. 2022, 21, 5339–5340. [Google Scholar] [CrossRef]
- Martora, F.; Villani, A.; Marasca, C.; Fabbrocini, G.; Potestio, L. Skin reaction after SARS-CoV-2 vaccines Reply to ‘cutaneous adverse reactions following SARS-CoV-2 vaccine booster dose: A real-life multicentre experience’. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e43–e44. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Fabbrocini, G.; Nappa, P.; Megna, M. Reply to ‘Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2’ by Solimani et al. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e750–e751. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Villani, A.; Battista, T.; Fabbrocini, G.; Potestio, L. COVID-19 vaccination and inflammatory skin diseases. J. Cosmet. Dermatol. 2023, 22, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Sayad, B.; Rahimi, Z. COVID-19 and psoriasis: Biologic treatment and challenges. J. Dermatol. Treat. 2022, 33, 699–703. [Google Scholar] [CrossRef]
- Wack, S.; Patton, T.; Ferris, L.K. COVID-19 vaccine safety and efficacy in patients with immune-mediated inflammatory disease: Review of available evidence. J. Am. Acad. Dermatol. 2021, 85, 1274–1284. [Google Scholar] [CrossRef]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef]
- Farkas, A.; Tonel, G.; Nestle, F. Interferon-α and viral triggers promote functional maturation of human monocyte-derived dendritic cells. Br. J. Dermatol. 2008, 158, 921–929. [Google Scholar] [CrossRef]
- Takayama, K.; Satoh, T.; Hayashi, M.; Yokozeki, H. Psoriatic Skin Lesions Induced by BCG Vaccination. Acta Derm. Venereol. 2008, 88, 621–622. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
