Abstract
Epithelial ovarian cancer (EOC) is a significant cause of cancer-related mortality in women. Despite advances in diagnosis and treatment, EOC remains a challenging disease to manage, and the 5-year survival rate is still poor. The role of hormone receptors (HRs) in EOC carcinogenesis and prognosis has been actively explored; however, the role of hormone therapy (HT) in the treatment of these tumors is not well established. Most available data on HT mainly come from retrospective series and small early clinical trials. Several of these studies suggest that HT may have a role in adjuvant, maintenance therapy, or in the case of recurrent disease, especially for some subtypes of EOC (e.g., low-grade serous EOC). Furthermore, HT has recently been combined with targeted therapies, but most studies evaluating these combinations are still ongoing. The main aim of this review is to provide an overview of the progress made in the last decade to characterize the biological and prognostic role of HRs for EOC and the developments in their therapeutic targeting through HT.
1. Introduction
Epithelial ovarian cancer (EOC) is the third most common gynecological malignancy worldwide, with 313,959 new cases reported in 2020. It is also one of the deadliest malignancies, with over 200,000 deaths reported globally in the same year [1]. EOC accounts for about 90% of ovarian tumors and comprises several distinct subgroups with different molecular profiles, biological behaviors, and clinical features. Currently, five subgroups of EOC are described: high-grade serous EOC (70%), endometrioid (10%), clear cell (10%), mucinous (3%), and low-grade serous EOC (<5%) [2,3,4]. High-grade serous EOC is characterized by several molecular aberrations and mutations: TP53 is mutated in almost all cases [5,6,7,8], and somatic or germline mutations of homologous recombination genes such as BRCA1 and BRCA2 are also involved in EOC carcinogenesis [5,6]. Moreover, high-grade serous EOC shows widespread accumulation of copy number alterations [7,8], and other pathways involved in high-grade serous EOC are FXM1, Rb1, PI3K, and Notch 1 [5,9]. Clear cell EOC and endometrial EOC share similar patterns of mutations, including alterations in ARID1A, PIK3CA, PTEN, and KRAS [10,11]. Several mucinous EOCs have KRAS mutations and HER2 amplification [12,13,14], while low-grade serous EOC exhibits activation of the mitogen-activated protein kinase (MAPK) pathway via NRAS, KRAS or BRAF mutations [15,16,17]. EOC has a poor prognosis, with a 5-year relapse rate of 75% for patients diagnosed with advanced disease (International Federation of Gynecology and Obstetrics-FIGO stage III-IV) [15] and a low 5-year overall survival (OS) [12]. To address these poor survival outcomes, better treatment strategies have been developed over the last decade, including optimal debulking surgery to achieve no macroscopic residual tumor and new targeted therapies, particularly for high-grade serous EOC. For instance, bevacizumab, a recombinant humanized monoclonal antibody that blocks angiogenesis by inhibiting vascular endothelial growth factor (VEGF), is approved for maintenance treatment of high-risk EOC [16]. Several trials evaluated the efficacy of inhibitors of the poly ADP ribose polymerase (PARPi) enzyme, and these drugs have been recently approved for treating EOC. Olaparib for BRCA-mutated patients as a maintenance treatment after first-line chemotherapy and at platinum-sensitive relapse; Niraparib and Rucaparib at platinum-sensitive relapse regardless of BRCA status. PARPis improve progression-free survival (PFS), but, to date, no significant impact on OS has been observed [17,18,19,20,21]. Among emerging target therapies for EOC, several studies have investigated the role of immune checkpoint inhibitors, but with little impact on survival [22,23,24]. This scenario highlights the need to explore alternative treatment options capable of improving survival outcomes without decreasing quality of life. Hormone therapy (HT) is an old but important option with promising results in the maintenance treatment of EOC, especially for the low-grade serous histotype [25,26]. However, the evidence about the efficacy of HT is limited to retrospective studies and small phase II trials. The aims of this review are to summarize the developments in the field of HT in the last decade and offer an overview of the biological and prognostic significance of the hormone receptors (HRs) in EOC.
2. The Role of Hormone Receptors in Ovarian Cancer Carcinogenesis
Estrogen Receptor (ER) is a key receptor in the development and progression of EOC. Two different ERs isoforms have been described: ERα and ERβ encoded by ESR1 (6q25.1) and ESR2 (14q23.2), respectively. They share a similar structure with other steroid hormone receptors, with an N-terminal domain (NTD), a C-terminal domain corresponding to the DNA binding domain (DBD), a hinge region, and a ligand binding domain (LBD). When the ligand binds to the receptor, the latter forms a dimer and translocates from the cytoplasm to the nucleus, where it acts as a transcription factor (TF), binding to Estrogen Responsive Elements (EREs) [27].
ERα and ERβ expression levels vary in different tissues, and in the ovary, ERβ is more dominant [28]. In preclinical models, ERβ acts as a tumor suppressor and ERα as a pro-tumorigenic factor in breast, prostate, colon, and ovarian cancer cells [27]. Treeck et al. showed that ERβ inhibits cell proliferation by increasing p21 and triggering apoptosis in SK-OV-3 ovarian cancer cells [29]. Furthermore, Bossard et al. showed that ERβ also reduces pro-tumoral factors such as P-AKT, P-RB1, CycD1, and CycA2 in BG-1 (ERα-positive) and PEO14 (ERα-negative) cell lines transfected with ESR2 adenoviruses. These results were confirmed in mouse models [30].
Additionally, Liu et al. used RNA-seq analysis to show that ERβ can alter the expression of several pro-tumoral genes when activated by an agonist. They also showed that ERβ inhibits NF-kB through a non-canonical interaction with its subunit p65 and that ERβ enhances the sensitivity of chemoresistant EOC cell lines to chemotherapy. Pinton et al. confirmed this effect in naïve EOC cell lines [31,32].
However, not all ERβ isoforms have anti-tumoral effects. Chan et al. transfected EOC cell lines with different ERβ isoforms and found that isoforms -2 and -5 increased the aggressiveness and dissemination of the EOC cells. Specifically, ERβ-5 activated the FAK/Src pathway, which promoted cell migration and proliferation. These effects were reversed by a FAK inhibitor [33]. The loss of ERβ and the imbalance of the ERα/ERβ ratio are crucial for EOCs carcinogenesis, tumor progression, and dissemination, as shown by in vitro experiments [34,35,36]. These findings have been validated in patient-derived samples by immunohistochemical analysis of surgical specimens. A cohort study on 171 EOC patients (mainly represented by the serous histotype: 134/171, 78.36%) at different FIGO stages revealed a higher expression of the inactive cytoplasmic form of ERβ (cERβ) [37], confirming a previous result on a case series of 58 serous EOCs [38]. This finding is supported by a recent study on ERβ performed on TMA samples of EOC, which also reported a correlation between ERβ nuclear/cytoplasmic staining and known clinical risk factors such as the number of pregnancies [39].
ERα activates downstream pathways crucial for carcinogenesis, such as IL6/STAT3, PI3K/AKT, MAPK signaling, and pro-invasive pathways [40]. Additionally, Benhadjeba et al., through in vitro experiments on EOC cell lines, proved a feed-forward mechanism between ERα and the CXCR7/CXCL11 chemokines axis, which activates Erk1/2 and phosphorylates ERα at Ser-118, leading to a more aggressive pro-metastatic tumor phenotype [41]. Moreover, in mice models, Hodgkinson et al. showed the role of GREB1 (Growth Regulation by Estrogen in Breast cancer) in EOC as a promoter of tumor development and growth, being a possible cofactor of ERα in the transcription of ERE genes [42].
Progesterone is another steroid hormone involved in female cancer development by modulating the transcription of several genes. The two main isoforms of progesterone receptor (PR), PRα and PRβ, are encoded by PGR, localized on the long arm of chromosome 11 (11q22). PRα and PRβ have the canonical structure of steroid HRs but differ in a 164 amino acid region absent at the N-terminal of PRα, leading to different binding of Progesterone Responsive Elements (PREs) [43].
Progesterone and PR have an anti-tumoral role in ovarian carcinogenesis, unlike their pro-tumoral role in breast cancer [44]. Therefore, the contrasting interaction between the ER and PR in EOC is expected [45]. Mukherjee et al. compared ovarian (OVCA) and breast (MCF-7) cancer cell lines stimulated with estrogen, demonstrating that EOC cells are characterized by an ER-dependent downregulation of PRG expression that can be reverted through ER antagonists [46]. Progesterone alone or with estrogen also inhibited the ER-dependent activation of the WNT/β-catenin pathway in EOC—initiating lesions in both human serous EOC cells and murine models [47]. This anti-tumoral ability was also linked to the induction of specific cell death programs such as senescence and necroptosis. Diep et al. observed a strong interaction between FOXO1 and PRβ in vitro in PEO4 cell lines (ERα-positive cells). FOXO1 is a direct interactor of specific steroid HRs, including both PR isoforms [48]. In EOC, PRβ appears to recruit FOXO1 and form a transcriptional complex upon progestin stimulation. This complex enhances the expression of the pro-senescent factor p21 or other senescence effectors (p15, p16, and p27) in case of p21 loss [49,50]. Studies on high-grade serous EOC in murine models, which is characterized by TP53 loss [51], revealed the importance of progesterone and PR for activating the necroptosis death program, which depends on the TNFα/RIPK1/RIPK3/MLKL pathway [52,53]. PR’s anti-tumoral effects also seem to be related to the induction of A Disintegrin and Metalloproteinase with ThromboSpondin motifs (ADAMTS) proteases [54]. ADAMTS are involved in fertility-related physiological functions of the ovary as well as anti-tumoral effects [55]. ADAMTS1, an inhibitor of the VEGFR pathway, resulted in being directly induced by PR through C/EBPβ, NF1-like factor, and Sp1/3 co-factors in a murine model [56]. According to a recent in vitro study, activation of PR increases both its and ADAMTS4′s expressions; this mechanism seems to be protective against ovarian carcinogenesis. Conversely, PR and ADAMTS losses occur in metastatic cancers [57,58]. Recently the PR anti-tumoral role in the ovary has been questioned since some authors provided data about its relevance in cancer development and quiescence. Wetendorf et al. used transgenic mice to demonstrate that PR overexpression, especially of PRβ, can promote the formation of hormone-dependent ovarian and endometrial neoplasms through the activation of the PI3K-AKT pathway and a cyclin D1-mediated deregulation of the cell cycle [59]. Moreover, Mauro et al., studying p53-mutant fallopian tube epithelial cells transfected (FTE) with PRα or PRβ constructs, assessed the role of PR and progestins in the progression of Serous Tubal Intraepithelial Carcinoma (STIC) into high-grade serous EOC. Through several in vitro experiments, the authors demonstrated different behaviors depending on the presence of progestins; in the absence of progestins, PR+-p53 mutant-FTE cells proliferated due to the inhibition of dual-specificity dimerization partners DP1/2, Rb-like p130/p107, E2F4/5 plus the core complex MuvB (LIN9, LIN37, LIN52, LIN54, RBBP4 proteins)/tyrosine-regulated protein kinases (DREAM/DYRK1) complex, while, on the contrary, in presence of progestins, the DREAM/DYRK1 complex is activated. The latter causes a tumor-quiescent status and promotes PR+ emboli formation, increasing invasive features mainly responsible for the EOC dissemination in the peritoneal cavity [60].
Androgens also exhibit an important role in female physiological and pathological processes. Notably, these hormones can be converted into estrogens by CYP19A1-mediated in situ conversion, thus promoting ER-related cancer growth [61]. Moreover, androgens can also activate their specific receptor, whose gene (AR) is localized on chromosome X (Xq11-Xq-12), and induce the transcription of Androgen Responsive Elements (AREs). The expression of androgen receptors (AR) varies among the different EOC histotypes, being higher in serous than in non-serous neoplasms, and ARs have also been shown to enhance cell proliferation. In vitro studies showed a higher number of cells in S-phase, inhibition of p21 and p27 (master regulators of the cell cycle), and the up-regulation of telomerase expression/activity after androgen stimulation, ultimately promoting tumor growth [62,63,64].
AR-dependent cancer growth stimulation is also mediated by the suppression of anti-tumoral pathways. Specifically, in ovarian cancer cells, the activated form of the receptor seems to be capable of sequestering SMAD3, thus leading to a downregulation of the transforming growth factor β (TGFβ) pathway and resulting in pro-tumoral activity [65]. This correlation between activated AR and TGFβ has also been confirmed in other studies [66,67]. SMAD3 is also involved in other cross-talks affecting AR regulation: Kollara et al., exploring the AR interactome, demonstrated that a ligand-independent interaction between AR and VEPH1 inhibits SMAD3 and p-Akt, resulting in a tumor boost caused by higher AR levels [68].
AR can also activate specific cross-talks with pro-tumor pathways by promoting epidermal growth factor receptor (EGFR) signaling and the secretion of the pro-tumoral IL 6 and 8 [69,70].
Furthermore, the oncogenic activity of AR is attributable to its activity as a transcription factor and the formation of molecular complexes with other proteins. GLI3, a Hedgehog-activated transcription factor, has been described as an interactor of AR in both ovarian and breast cancer cell lines, promoting malignancy [71]. Another recently identified interactor of AR is Nanog, a known stem cell phenotype inducer; Ling et al. showed the increase of Nanog, SOX2, and OCT4 expressions after androgen stimulation in EOC cell lines. These findings illustrate the importance of AR in the establishment of a cancer stem cell niche that promotes cancer growth, progression, and dissemination [72].
These data show that steroid hormones play a key role in EOC development through multiple synergic mechanisms, which could be investigated as novel therapeutic targets.
A summary of the pathways activated by hormone receptors and involved in the pathogenesis of EOC are reported in Figure 1.
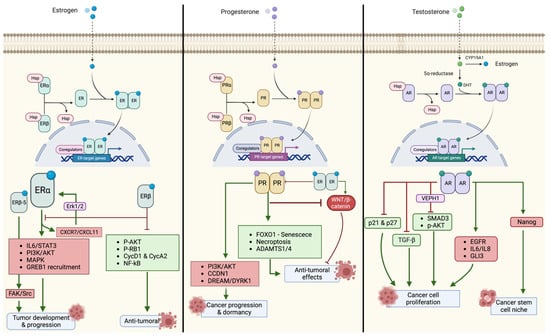
Figure 1.
Main pathways activated by hormone receptors and involved in ovarian cancer tumorigenesis.
3. Prognostic Role of Hormone Receptors in EOC
The expression of HRs in EOC has been widely studied as a prognostic factor. Different histotypes of EOC have different expression patterns of HRs. A large analysis of the Ovarian Tissue Analysis Consortium Study on 2933 EOCs showed that high-grade serous, endometrioid, and low-grade serous EOC had strong ER expressions (defined as ≥50% tumor nuclear staining: 60%, 60%, and 71% respectively). In contrast, clear cell and mucinous EOC had low expression of ER (14% and 16%) [73].
ER and PR expressions were also associated with improved survival even after adjusting for age, tumor site, stage, and histological grade at diagnosis [Hazard Ratio: 0.33, 95% Confidence Interval (CI) 0.21–0.51; p < 0.0001)]. Moreover, two recent meta-analyses confirmed the favorable prognostic role of ER [74] and PR [75] expression in terms of PFS and OS. ER expression was also associated with less aggressive histological features such as lower lymphovascular space invasion [76], and both ER and PR have also been found to be related to platinum sensitivity [77]. However, a recent meta-analysis questioned these findings, as the use of different antibody clones to determine ER immunohistochemical expression may have influenced the results of previous prognostic studies [78]. On the other hand, the role of AR was controversial. Some studies suggested that women with longer AR CAG repeats had a lower risk of developing EOC, but other authors did not confirm this finding [79]. Similarly, the prognostic impact of AR expression was unclear, as some authors reported a favorable prognostic role, while others obtained inconclusive results [79]. Other studies suggest that low AR expression correlated with a higher risk of developing extra-pelvic metastases, in particular brain metastases [80,81,82,83].
The expression of HRs may correlate with the response to HT, but the evidence is mostly based on retrospective studies [84,85,86]. There is no reliable randomized phase III studies addressing the predictive value of HRs, and this lack of data is due to multiple reasons, including the challenges in defining a meaningful cut-off for stratifying HR expression, the low incidence of some EOC subtypes (e.g., low-grade serous EOC), and the differences in the methods used for their assessment. For example, a recent study suggests that ER immunohistochemistry is not an effective predictive marker of response to HT for low-grade serous EOC. Instead, multigene assays should be used to evaluate ER pathway activation, which could help identify patients who are unlikely to benefit from single-agent HT and those who may need combination therapies [87].
4. Aromatase Inhibitors
Aromatase inhibitors (AIs) (e.g., letrozole, exemestane, and anastrozole) are commonly used HTs for postmenopausal women with ER/PR-positive breast cancer in adjuvant, neoadjuvant, or metastatic settings [88]. AIs block the aromatase enzymes that convert testosterone to estradiol (Figure 2), thereby reducing estrogen production in postmenopausal women [89]. In the 2000s, several studies and trials tested the efficacy of AIs for the treatment of EOC, especially using letrozole and anastrozole, with conflicting results [90]. One of the largest pioneering phase II studies evaluated 60 patients with EOC detected by elevated CA125 levels who received letrozole (2.5 mg daily). Unfortunately, no partial or complete responses were observed by computed tomography in any of the patients, although 10 patients showed disease stabilization for more than 12 weeks [91]. Based on these results, at least seven other phase II clinical trials were conducted between 2003 and 2007 to evaluate the role of AIs in recurrent EOC. The response rates varied from 0% to 38% [92,93,94,95,96,97,98]. Moreover, a recent comprehensive review and meta-analysis of 2490 EOC patients treated with HT reported a summary estimate of a 39% (95% CI 0.29–0.50) clinical benefit ratio for AIs [99]. More recently, a new phase II study (PARAGON—ANZGOG-0903) evaluated the role of anastrozole in 49 women with HR-positive platinum-resistant or refractory recurrent EOC. The study reported a clinical benefit in 13 patients (27%; 95% CI 16–40) despite the absence of complete or partial responses (based on the RECIST criteria). The median PFS was of 2.7 months (95% CI 2.0–2.8 months) [100].
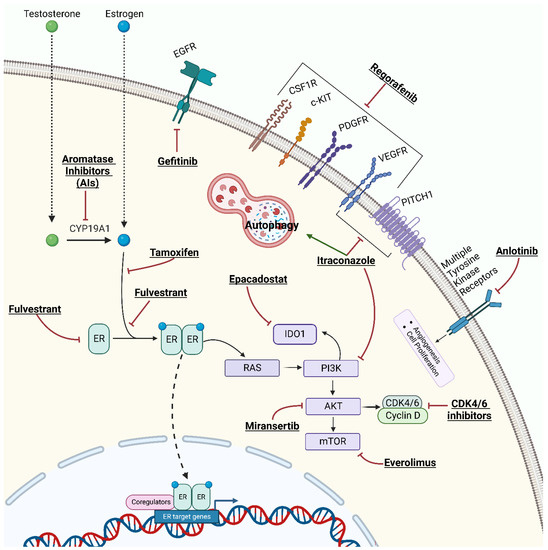
Figure 2.
Mechanism of action of hormone therapies and combined target therapies for epithelial ovarian cancer.
In another phase II study, the PARAGON investigators evaluated the efficacy of anastrozole in 52 patients with asymptomatic HR-positive EOC relapse diagnosed by CA125 elevation. These patients had a low tumor burden and had received only one line of prior chemotherapy. The study reported a 4% complete response and 35% clinical benefit [101]. However, this study has been criticized for its patient selection criteria (e.g., patients with low HR expression levels) [102].
Stanley et al. performed a large retrospective study on 269 patients with relapsed EOC treated with HT, mostly with AIs (77.0% letrozole, 18.6% tamoxifen, 2.2% megestrol acetate, 2.2% other). They investigated the predictive role of HR expression in HT. The CA125 response and clinical benefit rates (response or stable disease) were 8.1% and 40.1%, respectively. The authors also reported that an ER histoscore value > 200 and a time-free interval ≥ 180 days from the last dose of chemotherapy and the initiation of HT were independent predictive factors of response [84].
The role of AIs as first-line maintenance therapy has also been evaluated. In a prospective cohort study on high-grade serous EOC, the addition of letrozole as maintenance was associated with a significantly prolonged recurrence-free survival after 24 months of treatment [60% for letrozole (n = 23) vs. 39% for the control (n = 27); p = 0.035]. A benefit was also observed in patients who received letrozole alongside bevacizumab: 87.5% of patients who received letrozole and bevacizumab had no recurrence after 12 months (p = 0.026) [103].
The studies mentioned above involved patients with advanced and/or relapsed high-grade serous EOC; however, several data are also available on the role of HT in low-grade serous EOC. A retrospective study on 64 patients with recurrent low-grade serous EOC evaluated the response to AIs (letrozole n = 33 and anastrozole n = 21) and tamoxifen (n = 17). Among these patients, six complete responses (6.7%, four with letrozole, one with anastrozole, one with tamoxifen) and two partial responses (2.2%, two with letrozole) were obtained. Disease stabilization was reported in 44 patients (33 with AIs and 11 with tamoxifen). These results may be partly explained by the indolent behavior of low-grade serous EOC, but a potential benefit of HT is suggested [104]. Similar results were reported in a phase II study on 36 women affected by HR-positive low-grade serous OC or serous borderline ovarian tumor treated with anastrozole. The study showed a clinical benefit in 61% of patients after 6 months of therapy, and although no patients achieved a complete response, a partial response was reported in 5 patients (14%) and stable disease in 18 patients (50%) [105].
More intriguing results on low-grade serous EOC were obtained when AIs were used in a maintenance setting. A study compared 70 patients with low-grade serous EOC who received maintenance HT (57.2% AIs, 28.6% tamoxifen, 14.2% others) with 133 who underwent observation after primary surgery followed by platinum-based chemotherapy. Median PFS for patients without maintenance HT was 26.4 months, compared with 64.9 months for those who received HT (p ≤ 0.001). No statistically significant difference in OS was reported between the two groups (102.7 vs. 115.7 months, respectively) [106]. More encouraging results were reported by Fader et al.: out of 27 patients with low-grade serous EOC treated with HT as maintenance (over 90% with AIs), only 6 patients (22.2%) developed a tumor recurrence, and 2 patients died of disease (after a median follow up of 41 months). Recently, maintenance therapy with AIs for low-grade serous EOC has been analyzed from a cost-effectiveness point of view [107]. The study highlighted that maintenance with letrozole is a cost-effective strategy in women with advanced low-grade serous EOC leading to a clinically-relevant improvement in quality-adjusted life years, life years, and a reduction in the number of recurrences [108].
These results suggest an advantage in terms of PFS in patients with low-grade serous EOC receiving HT.
4.1. Combinations Strategies with AIs
Recently, several combinations of target therapy and aromatase inhibitors have been studied to increase the response rate to treatments.
4.1.1. Everolimus and AIs
Oestradiol binds to the ER activating signaling pathways, including PI3K/AKT/mTOR signaling [109]. Small molecules that inhibit the mammalian target of rapamycin (mTOR) kinase activity are being developed to treat various tumors [110,111]. Interestingly, in breast cancer, mTOR inhibitors such as everolimus (Figure 2) can partially overcome AI resistance [112]. A study involving patients with recurrent endometrial cancer found some benefits from treatment with AIs plus everolimus [113]. The PI3K/AKT/mTOR pathway is frequently mutated or activated in EOC and plays a crucial role in tumor progression [114]. In a phase II trial of everolimus and letrozole in relapsed ER-positive high-grade EOC, evaluable patients (n = 19) received both oral everolimus (10 mg) and letrozole (2.5 mg orally) daily until disease progression or intolerable toxicity. Three patients (16%) had a confirmed partial response; however, no patient achieved a complete response. Moreover, seven other patients showed a disease control rate of 53%. After 12 weeks of therapy, the PFS was 47%, with a median PFS time of 3.9 months (95% CI, 2.8–11.0) and a 6-month PFS rate of 32% [115].
4.1.2. CDK 4/6 Inhibitors and AIs
Cyclin-dependent kinases (CDKs) are a family of serine–threonine kinases identified in the 1970s–1980s as gene products involved in cell division control. In particular, CDK4 and CDK6 phosphorylate retinoblastoma protein 1 (RB1) and regulate its activity. The active hypophosphorylated form of RB1 acts as a negative regulator of the cell cycle by forming multiprotein complexes that bind the E2F transcription factors and prevent premature cell division. CDK inhibitors (CDKI) inhibit CDK4/6 and lead to hypophosphorylation of RB1 and arrest of cells in the G1 phase (Figure 2) [116,117]. Several molecules (palbociclib, ribociclib, and abemaciclib) have shown significant survival benefits when combined with AIs or fulvestrant in the treatment of metastatic ER-positive breast cancer, leading to their Food and Drug Administration (FDA) approval [118,119]. Interestingly, a significant fraction of EOC showed aberrant expression of cyclins, CDKs, and/or CDKI supporting the hypothesis that these tumors may also respond to CDK4/6 inhibition. Indeed, cyclin inhibitors have been evaluated in different settings for EOC treatment, either as a single agent or in combination with cytotoxic chemotherapy [120].
Based on this background, the association between AIs and cyclin inhibitors has also been evaluated in the treatment of EOC. In a recent trial involving 40 patients with an ER-positive recurrent cancer (20 affected by EOC and 20 by endometrial cancer) treated with 400 mg of oral ribociclib and 2.5 mg of oral letrozole daily, PFS of 50% and 35% were obtained at 12 and 24 weeks, respectively, in the EOC cohort. Interestingly, the greatest benefit was seen in low-grade serous EOC (3 patients were progression-free after 24 weeks of treatment) [121].
4.1.3. Miransertib and AIs
Miransertib is an AKT1 inhibitor (Figure 2) that has been combined with anastrozole in women with PIK3CA and AKT1-mutant ER-positive endometrial cancer and EOC. Preliminary data showed some efficacy in endometrial cancer but not in EOC (data available for 13 patients) [122].
All studies on AIs performed since 2012 are reported in Table 1.

Table 1.
Main results of studies performed since 2012 exploring the efficacy of hormone therapy for OC.
4.2. Ongoing Trials
Some trials are currently evaluating the use of AIs for EOC therapy. As mentioned previously, data on AIs are based on retrospective/prospective studies or phase II trials (summarized in Table 2). For the first time, a randomized, double-blind placebo-controlled multi-center phase III trial has been proposed to evaluate the role of letrozole (2.5 mg daily) as maintenance therapy in patients with FIGO Stage II-IV low and high-grade serous or endometrioid EOC [127].

Table 2.
Ongoing trials exploring hormone therapy in OC.
Regarding low-grade serous EOC, a multicenter, randomized, open-label phase III trial is evaluating the superiority of letrozole to conventional carboplatin-taxol chemotherapy (LEPRE trial, NCT05601700). The results of this trial are expected in 2029. Another phase III randomized trial is evaluating letrozole with or without paclitaxel and carboplatin chemotherapy (NCT04095364).
A further phase II study (NCT04720807) is evaluating the combination of anlotinib (a multi-target tyrosine kinase inhibitor targeting tumor angiogenesis and proliferative signaling, Figure 2) and letrozole in patients with relapsed EOC and at least two prior lines of chemotherapy. Currently, 13 patients have been enrolled, and preliminary data showed that 2 and 7 patients achieved a partial response and stable disease, respectively, yielding an objective response rate of 16.7% (2/12, 95% CI: 3.3 to 54.3) [128].
Regarding CDK4/6 inhibitors, an open-label phase II study (NCT04469764) is investigating the efficacy and safety of abemaciclib plus anastrozole or letrozole in patients with hormone receptor-positive OC.
Finally, an interesting study (IMPACT NCT03378297) is evaluating the effect on tumor tissue of four different drugs (acetylsalicylic acid, olaparib, metformin, and letrozole). One of these drugs is taken 10–14 days prior to tumor reductive surgery, starting on the day of laparoscopy.
5. Anti-Estrogens
Anti-estrogen agents include a category of drugs that directly interfere with ER signaling and are mainly represented by selective ER modulators (SERMs) and selective ER downregulators (SERDs).
SERMs (e.g., tamoxifen, raloxifene) are anti-estrogen compounds that act as ERα antagonists by competing with estrogen and modulating the transcription of ERα. Tamoxifen is the best-known SERM (Figure 2) which is commonly used for the treatment of premenopausal breast cancer [129]. Currently used SERMs include triphenylethylenes, such as tamoxifen and its analogs; benzothiophenes, such as raloxifene and arzoxifene; phenylindoles, such as bazedoxifene and pipindoxifene; and tetrahydronaphthalenes, such as lasofoxifene. These agents modulate estrogens in breast, bone, and endometrial tissues [130,131]. Differences in the molecular and 3D structures of the co-activators and co-suppressors that modulate the transcriptional activity of the ERs seem to be related to the mechanism of these dual effects that are specific to each tissue [132]. SERDs have antagonistic effects on ERα and ERβ. Fulvestrant is a selective ER degrader that acts by binding, blocking, and degrading the ER, leading to complete inhibition of the estrogen signaling cascade (Figure 2) [133]. Fulvestrant is approved for the treatment of ER-positive metastatic breast cancer alone or in combination with other drugs [133,134].
5.1. Tamoxifen
In the last 30 years, several authors investigated the use of tamoxifen in patients with a diagnosis of recurrent or persistent EOC. One of the pioneering studies of anti-estrogen therapy reported three patients with advanced serous EOC treated with tamoxifen: one patient achieved a complete remission lasting for 18 months, and another obtained a partial response. One of the three cases showed high HR expression suggesting a role of this marker in the response to HT [135]. In 1991, Heatch et al. [136] evaluated the response to tamoxifen (40 mg daily) in 105 patients with recurrent or persistent EOC. They found a completed response rate of 10% and a partial response rate of 8%. Furthermore, higher expression of ERs was found in 89% of patients who achieved a complete response and in 59% of patients who achieved a partial response.
The effectiveness of tamoxifen was later reviewed in a Cochrane Systematic Review that included 32 studies. Data from 623 patients were analyzed, of which 60 achieved a partial or complete response (9.6%), and 31.9% showed stable disease. The response rates varied from 0 to 56%, while the no-disease progression rates varied from 0 to 85%. This review did not support a predictive role for HR expression when patients were treated with tamoxifen. The authors concluded that there is only limited evidence of anti-tumor activity based on phase II studies [137]. Another systematic review reported an overall estimated clinical benefit ratio of 43% (95% CI, 0.30–0.56) for tamoxifen [99].
More recently, a retrospective study evaluated tamoxifen in 92 patients with EOC of different stages (I–IV), histotypes (serous, endometroid, clear cell), and clinical settings (first, second, and third line). The clinical benefit ratio was 56%: 10% of patients achieved a partial or complete response, and 46% had stable disease. Also, ER and PR expressions were analyzed in 47 patients, but no correlation was found between clinical response and HR expression or histotype [85].
Ovaresist is a recent phase III trial that compared the efficacy of single-agent chemotherapy (weekly paclitaxel 80 mg/m2 or four weekly pegylated liposomal doxorubicin 40 mg/m2) and tamoxifen (40 mg daily) in patients with platinum-resistant OC. The primary endpoint was Health-Related Quality of Life (HRQoL), and the secondary endpoints were PFS and OS. Patients (156 and 82) were randomized to chemotherapy and tamoxifen, respectively. Patients treated with tamoxifen had a PFS of 8.3 weeks vs. 12.7 for chemotherapy. OS was not significantly different between the treatment arms. Despite a better PFS in the chemotherapy arm, the patients treated with tamoxifen had fewer side effects and superior HRQoL [123].
Regorafenib is a multi-kinase inhibitor that targets angiogenic (VEGFR1–3, TIE2), stromal (PDGFR-b, FGFR), and oncogenic kinases (KIT, RET, and RAF), as well as tumor immunity (CSF1R) (Figure 2). It is approved for the treatment of refractory metastatic colorectal cancer, unresectable or metastatic gastrointestinal stromal tumors, and hepatocellular carcinoma previously treated with sorafenib [138].
The REGOVAR trial randomized 68 patients to tamoxifen (40 mg daily) or regorafenib (160 or 120 mg daily, 3 weeks on/11 weeks off) until progression or occurrence of toxicity. After a median follow-up of 32 months, there was no difference in PFS and OS between the two groups [124].
Epacadostat is a selective indoleamine 2,3-dioxygenase-1 (IDO1) enzyme inhibitor (Figure 2), currently under investigation in several tumor types [139,140,141]. IDO1 regulates the innate immune response by suppressing T lymphocytes and natural killer cells and by activating regulatory T cells and myeloid-derived suppressor cells [142]. IDO1 also promotes tumor neoangiogenesis through the expression of interferon-γ (IFN-γ) and IL-6 [142,143]. IDO1 is overexpressed in EOC and is associated with advanced stage, chemoresistance, and poor survival [144,145,146].
A randomized, open-label, phase II study [125] compared epacadostat with tamoxifen in biochemically recurrent EOC (CA125 relapse). Forty-two patients were enrolled: the median PFS was 3.75 months for epacadostat (n = 22) versus 5.56 months for tamoxifen (n = 20, p = 0.54). Of evaluable patients, one (5.0%) epacadostat and three (15.8%) tamoxifen patients had confirmed CA125 responses. Despite a supporting preclinical rationale, epacadostat was not superior to tamoxifen in this setting.
The recent studies performed on tamoxifen alone or in combination are summarized in Table 1.
5.1.1. Combination Strategies with Tamoxifen
Tamoxifen has also been investigated in combination with other drugs. AGO-OVAR 2.6 is a phase II trial that investigated the combination of tamoxifen with gefitinib, a signal transduction inhibitor of EGFR tyrosine kinase (Figure 2). However, among 56 patients treated, no survival advantage was observed [126].
5.1.2. Ongoing Trials
Two trials evaluating the use of tamoxifen in combination with other drugs for the treatment of EOC are currently ongoing (reported in Table 2).
The TICTOC study (NCT05156892) is a phase I/II trial investigating the tolerability, toxicity, and efficacy of tamoxifen plus SUBA-itraconazole in platinum-resistant recurrent EOC. Itraconazole is an anti-fungal drug that also has anti-cancer effects by inhibition of angiogenesis, inhibition of the hedgehog pathway, autophagy induction, and reversion of multi-drug resistance (Figure 2) [147,148]. Itraconazole has been tested either as a single agent or with cytotoxic chemotherapy in the treatment of various cancers, including EOC [147]. The TICTOC trial is expected to be completed by 1 January 2025. Another phase II single-arm prospective clinical trial (NCT05669768) is assessing the efficacy and toxicity of the pamiparib + tamoxifen in EOC patients with biochemical recurrence during first-line PARPi maintenance therapy. Pamiparib is a selective PARP1 and PARP2 inhibitor approved in China for the treatment of germline BRCA mutation-associated recurrent advanced ovarian, fallopian tube, or primary peritoneal cancer after two or more lines of chemotherapy [149]. The trial is not currently recruiting patients, and results are expected in 2024.
5.2. Fulvestrant
Fulvestrant was investigated in a single phase II study evaluating 26 recurrent EOC patients. Based on CA125 values, one patient obtained a complete response (4%), one had a partial response (4%), and nine had stable disease (35%) [150]. The response to fulvestrant was related to ER and vimentin expression in EOC tissue [86]. More recently, a heavily pretreated patient with ER-positive, recurrent low-grade serous EOC showed a response to fulvestrant and trametinib (a MEK inhibitor) [151].
Ongoing Trials
Some trials are testing the efficacy of fulvestrant alone or in combination with other drugs. The FUCHSia Study (NCT03926936) is a phase II trial of fulvestrant in women with ER-positive low-grade gynecological cancers, including EOC. Fulvestrant is also being investigated in combination with the multi-targeted kinase inhibitor regorafenib in a phase II single-arm trial for recurrent low-grade serous EOC (NCT05113368). Another phase II study is evaluating the role of PI3K inhibitor copanlisib in combination with fulvestrant in selected ER-positive and/or PgR-positive advanced EOCs and endometrial cancers with PI3K (PIK3CA, PIK3R1) and/or PTEN mutations (NCT05082025). Ongoing studies investigating fulvestrant for EOC treatment are summarized in Table 2.
6. Conclusions
Estrogen, progesterone, and androgens have been studied for their role in EOC carcinogenesis, and literature data suggest that HR-positive EOC have a better prognosis than HR-negative EOC. The currently available studies, mostly retrospective or prospective phase II studies with small sample sizes, have obtained varying and even conflicting results. Nevertheless, HT in EOC could have a similar role to breast cancer, where it is used for adjuvant (first-line), maintenance, and relapse treatment. HT has a relatively low toxicity profile, which makes it suitable for elderly or frail patients who cannot tolerate more aggressive therapies. Moreover, HT seems to prolong the response to chemotherapy and delay disease progression as maintenance therapy. However, more efforts are needed to identify biomarkers that can predict the response to HT and optimize treatment regimens. In this context, some subtypes of EOC, such as low-grade serous EOC, seem to respond better to endocrine therapy. Additional research is needed to ascertain whether combining HT with other drugs, including targeted therapies, can be more effective, and this effort is ongoing. In particular, more multicenter, prospective, well-designed, and randomized clinical trials are warranted to define the role of HT in EOC treatment.
Author Contributions
Conceptualization: F.B., S.F. and L.M.; Methodology: F.B., S.F. and L.M.; Resources: S.C., D.K, P.C. and C.B.; Literature research S.C., J.C., A.R.C. and L.B.; Data curation, S.C., J.C., A.R.C. and L.B.; Interpretation of data: all authors. Writing—original draft preparation: F.B., S.F., and L.M.; Writing—review and editing: all authors; Supervision: P.C., D.K. and C.B.; Project administration, P.C., D.K. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, J.; Chan, W.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.-J.; Elcarte, E.; Withers, M.; et al. Worldwide Burden, Risk Factors, and Temporal Trends of Ovarian Cancer: A Global Study. Cancers 2022, 14, 2230. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.-M. The Dualistic Model of Ovarian Carcinogenesis. Am. J. Clin. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef]
- Meinhold-Heerlein, I.; Fotopoulou, C.; Harter, P.; Kurzeder, C.; Mustea, A.; Wimberger, P.; Hauptmann, S.; Sehouli, J. The New WHO Classification of Ovarian, Fallopian Tube, and Primary Peritoneal Cancer and Its Clinical Implications. Arch. Gynecol. Obstet. 2016, 293, 695–700. [Google Scholar] [CrossRef]
- Duska, L.R.; Kohn, E.C. The New Classifications of Ovarian, Fallopian Tube, and Primary Peritoneal Cancer and Their Clinical Implications. Ann. Oncol. 2017, 28, viii8–viii12. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated Genomic Analyses of Ovarian Carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial Ovarian Cancer: Evolution of Management in the Era of Precision Medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Kroeger, P.T.; Drapkin, R. Pathogenesis and Heterogeneity of Ovarian Cancer. Curr. Opin. Obstet. Gynecol. 2017, 29, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Pesenti, C.; Beltrame, L.; Velle, A.; Fruscio, R.; Jaconi, M.; Borella, F.; Cribiù, F.M.; Calura, E.; Venturini, L.V.; Lenoci, D.; et al. Copy Number Alterations in Stage I Epithelial Ovarian Cancer Highlight Three Genomic Patterns Associated with Prognosis. Eur. J. Cancer 2022, 171, 85–95. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, C.; Erriquez, J.; Arigoni, M.; Capellero, S.; Mittica, G.; Ghisoni, E.; Borella, F.; Katsaros, D.; Privitera, S.; Ribotta, M.; et al. PIK3R1W624R Is an Actionable Mutation in High Grade Serous Ovarian Carcinoma. Cells 2020, 9, 442. [Google Scholar] [CrossRef] [PubMed]
- McConechy, M.K.; Ding, J.; Senz, J.; Yang, W.; Melnyk, N.; Tone, A.A.; Prentice, L.M.; Wiegand, K.C.; McAlpine, J.N.; Shah, S.P.; et al. Ovarian and Endometrial Endometrioid Carcinomas Have Distinct CTNNB1 and PTEN Mutation Profiles. Mod. Patho.l 2014, 27, 128–134. [Google Scholar] [CrossRef]
- Itamochi, H.; Oishi, T.; Oumi, N.; Takeuchi, S.; Yoshihara, K.; Mikami, M.; Yaegashi, N.; Terao, Y.; Takehara, K.; Ushijima, K.; et al. Whole-Genome Sequencing Revealed Novel Prognostic Biomarkers and Promising Targets for Therapy of Ovarian Clear Cell Carcinoma. Br. J. Cancer 2017, 117, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial Ovarian Cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.A.; Luvero, D.; Shafer, A.; O’Connor, D.; Mangili, G.; Friedlander, M.; Pfisterer, J.; Mirza, M.R.; Kim, J.-W.; Alexandre, J.; et al. Gynecologic Cancer InterGroup (GCIG) Consensus Review for Mucinous Ovarian Carcinoma. Int. J. Gynecol. Cancer 2014, 24, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Borella, F.; Mitidieri, M.; Cosma, S.; Benedetto, C.; Bertero, L.; Fucina, S.; Ray-Coquard, I.; Carapezzi, A.; Ferraioli, D. Update on Prognostic and Predictive Markers in Mucinous Ovarian Cancer. Cancers 2023, 15, 1172. [Google Scholar] [CrossRef]
- Yoneoka, Y.; Ishikawa, M.; Uehara, T.; Shimizu, H.; Uno, M.; Murakami, T.; Kato, T. Treatment Strategies for Patients with Advanced Ovarian Cancer Undergoing Neoadjuvant Chemotherapy: Interval Debulking Surgery or Additional Chemotherapy? J. Gynecol. Oncol. 2019, 30, e81. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Raja, F.A.; Fotopoulou, C.; Gonzalez-Martin, A.; Colombo, N.; Sessa, C. Newly Diagnosed and Relapsed Epithelial Ovarian Carcinoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2013, 24, vi24–vi32. [Google Scholar] [CrossRef]
- DiSilvestro, P.; Colombo, N.; Harter, P.; González-Martín, A.; Ray-Coquard, I.; Coleman, R.L. Maintenance Treatment of Newly Diagnosed Advanced Ovarian Cancer: Time for a Paradigm Shift? Cancers 2021, 13, 5756. [Google Scholar] [CrossRef]
- Goh, J.C.H.; Gourley, C.; Tan, D.S.P.; Nogueira-Rodrigues, A.; Elghazaly, H.; Edy Pierre, M.; Giornelli, G.; Kim, B.-G.; Morales–Vasquez, F.; Tyulyandina, A. Optimizing Treatment Selection and Sequencing Decisions for First-Line Maintenance Therapy of Newly Diagnosed Advanced Ovarian Cancer–International Considerations amongst Upper Middle- and High-Income Countries (UMIC and HIC). Gynecol. Oncol. Rep. 2022, 42, 101028. [Google Scholar] [CrossRef]
- Luo, J.; Ou, S.; Wei, H.; Qin, X.; Jiang, Q. Comparative Efficacy and Safety of Poly (ADP-Ribose) Polymerase Inhibitors in Patients With Ovarian Cancer: A Systematic Review and Network Meta-Analysis. Front. Oncol. 2022, 12, 815265. [Google Scholar] [CrossRef]
- Tattersall, A.; Ryan, N.; Wiggans, A.J.; Rogozińska, E.; Morrison, J. Poly(ADP-Ribose) Polymerase (PARP) Inhibitors for the Treatment of Ovarian Cancer. Cochrane Database Syst. Rev. 2022, 2022, CD007929. [Google Scholar] [CrossRef]
- Nag, S.; Aggarwal, S.; Rauthan, A.; Warrier, N. Maintenance Therapy for Newly Diagnosed Epithelial Ovarian Cancer–a Review. J. Ovarian Res. 2022, 15, 88. [Google Scholar] [CrossRef]
- Borella, F.; Ghisoni, E.; Giannone, G.; Cosma, S.; Benedetto, C.; Valabrega, G.; Katsaros, D. Immune Checkpoint Inhibitors in Epithelial Ovarian Cancer: An Overview on Efficacy and Future Perspectives. Diagnostics 2020, 10, 146. [Google Scholar] [CrossRef]
- Dumitru, A.; Dobrica, E.-C.; Croitoru, A.; Cretoiu, S.M.; Gaspar, B.S. Focus on PD-1/PD-L1 as a Therapeutic Target in Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 12067. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; He, X.; Wang, Q. Immune Checkpoint Blockades in Gynecological Cancers: A Review of Clinical Trials. Acta Obstet. Gynecol. Scand. 2022, 101, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Slomovitz, B.; Gourley, C.; Carey, M.S.; Malpica, A.; Shih, I.-M.; Huntsman, D.; Fader, A.N.; Grisham, R.N.; Schlumbrecht, M.; Sun, C.C.; et al. Low-Grade Serous Ovarian Cancer: State of the Science. Gynecol. Oncol. 2020, 156, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Cosio, S. Therapeutic Approach to Low-Grade Serous Ovarian Carcinoma: State of Art and Perspectives of Clinical Research. Cancers 2020, 12, 1336. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, B.; Ou-Yang, L. Role of Estrogen Receptors in Health and Disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef]
- Chan, K.K.L.; Wei, N.; Liu, S.S.; Xiao-Yun, L.; Cheung, A.N.; Ngan, H.Y.S. Estrogen Receptor Subtypes in Ovarian Cancer: A Clinical Correlation. Obstet. Gynecol. 2008, 111, 144–151. [Google Scholar] [CrossRef]
- Treeck, O.; Pfeiler, G.; Mitter, D.; Lattrich, C.; Piendl, G.; Ortmann, O. Estrogen Receptor Β1 Exerts Antitumoral Effects on SK-OV-3 Ovarian Cancer Cells. J. Endocrinol. 2007, 193, 421–433. [Google Scholar] [CrossRef]
- Bossard, C.; Busson, M.; Vindrieux, D.; Gaudin, F.; Machelon, V.; Brigitte, M.; Jacquard, C.; Pillon, A.; Balaguer, P.; Balabanian, K.; et al. Potential Role of Estrogen Receptor Beta as a Tumor Suppressor of Epithelial Ovarian Cancer. PLoS ONE 2012, 7, e44787. [Google Scholar] [CrossRef]
- Liu, J.; Viswanadhapalli, S.; Garcia, L.; Zhou, M.; Nair, B.C.; Kost, E.; Rao Tekmal, R.; Li, R.; Rao, M.K.; Curiel, T.; et al. Therapeutic Utility of Natural Estrogen Receptor Beta Agonists on Ovarian Cancer. Oncotarget 2017, 8, 50002–50014. [Google Scholar] [CrossRef]
- Pinton, G.; Nilsson, S.; Moro, L. Targeting Estrogen Receptor Beta (ERβ) for Treatment of Ovarian Cancer: Importance of KDM6B and SIRT1 for ERβ Expression and Functionality. Oncogenesis 2018, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.L.; Siu, M.K.Y.; Jiang, Y.-X.; Wang, J.-J.; Wang, Y.; Leung, T.H.Y.; Liu, S.S.; Cheung, A.N.Y.; Ngan, H.Y.S. Differential Expression of Estrogen Receptor Subtypes and Variants in Ovarian Cancer: Effects on Cell Invasion, Proliferation and Prognosis. BMC Cancer 2017, 17, 606. [Google Scholar] [CrossRef]
- Brandenberger, A.W.; Tee, M.K.; Jaffe, R.B. Estrogen Receptor Alpha (ER-α) and Beta (ER-β) MRNAs in Normal Ovary, Ovarian Serous Cystadenocarcinoma and Ovarian Cancer Cell Lines: Down-Regulation of ER-β in Neoplastic Tissues. J. Clin. Endocrinol. Metab. 1998, 83, 1025–1028. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pujol, P.; Rey, J.M.; Nirde, P.; Roger, P.; Gastaldi, M.; Laffargue, F.; Rochefort, H.; Maudelonde, T. Differential Expression of Estrogen Receptor-Alpha and -Beta Messenger RNAs as a Potential Marker of Ovarian Carcinogenesis. Cancer. Res. 1998, 58, 5367–5373. [Google Scholar] [PubMed]
- Rutherford, T. Absence of Estrogen Receptor-Î2 Expression in Metastatic Ovarian Cancer. Obstet. Gynecol. 2000, 96, 417–421. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Weber, F.; Skrzypczak, M.; Ortmann, O.; Treeck, O. Estrogen Receptor β Is Associated with Expression of Cancer Associated Genes and Survival in Ovarian Cancer. BMC Cancer 2018, 18, 981. [Google Scholar] [CrossRef]
- De Stefano, I.; Zannoni, G.F.; Prisco, M.G.; Fagotti, A.; Tortorella, L.; Vizzielli, G.; Mencaglia, L.; Scambia, G.; Gallo, D. Cytoplasmic Expression of Estrogen Receptor Beta (ERβ) Predicts Poor Clinical Outcome in Advanced Serous Ovarian Cancer. Gynecol. Oncol. 2011, 122, 573–579. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Babic, A.; Gates Kuliszewski, M.; Rice, M.S.; Townsend, M.K.; Hecht, J.L.; Tworoger, S.S. Estrogen Receptor-β Expression of Ovarian Tumors and Its Association with Ovarian Cancer Risk Factors. Cancer Epidemiol. Biomarkers Prev. 2020, 29, 2211–2219. [Google Scholar] [CrossRef]
- Huang, W.; Chen, L.; Sun, P. ERRα Expression in Ovarian Cancer and Promotes Ovarian Cancer Cells Migration in Vitro. Arch. Gynecol. Obstet. 2022, 305, 1525–1534. [Google Scholar] [CrossRef]
- Benhadjeba, S.; Edjekouane, L.; Sauvé, K.; Carmona, E.; Tremblay, A. Feedback Control of the CXCR7/CXCL11 Chemokine Axis by Estrogen Receptor α in Ovarian Cancer. Mol. Oncol. 2018, 12, 1689–1705. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, K.; Forrest, L.A.; Vuong, N.; Garson, K.; Djordjevic, B.; Vanderhyden, B.C. GREB1 Is an Estrogen Receptor-Regulated Tumour Promoter That Is Frequently Expressed in Ovarian Cancer. Oncogene 2018, 37, 5873–5886. [Google Scholar] [CrossRef]
- Azeez, J.M.; Susmi, T.R.; Remadevi, V.; Ravindran, V.; Sasikumar Sujatha, A.; Ayswarya, R.N.S.; Sreeja, S. New Insights into the Functions of Progesterone Receptor (PR) Isoforms and Progesterone Signaling. Am. J. Cancer. Res. 2021, 11, 5214–5232. [Google Scholar] [PubMed]
- Mote, P.A.; Bartow, S.; Tran, N.; Clarke, C.L. Loss of Co-Ordinate Expression of Progesterone Receptors A and B Is an Early Event in Breast Carcinogenesis. Breast Cancer Res. Treat. 2002, 72, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Langdon, S.P.; Gabra, H.; Bartlett, J.M.; Rabiaz, G.J.; Hawkins, R.A.; Tesdale, A.L.; Ritchie, A.A.; Miller, W.R.; Smyth, J.F. Functionality of the Progesterone Receptor in Ovarian Cancer and Its Regulation by Estrogen. Clin. Cancer Res. 1998, 4, 2245–2251. [Google Scholar]
- Mukherjee, K.; Syed, V.; Ho, S.-M. Estrogen-Induced Loss of Progesterone Receptor Expression in Normal and Malignant Ovarian Surface Epithelial Cells. Oncogene 2005, 24, 4388–4400. [Google Scholar] [CrossRef][Green Version]
- Nagendra, P.B.; Goad, J.; Nielsen, S.; Rassam, L.; Lombard, J.M.; Nahar, P.; Tanwar, P.S. Ovarian Hormones through Wnt Signalling Regulate the Growth of Human and Mouse Ovarian Cancer Initiating Lesions. Oncotarget 2016, 7, 64836–64853. [Google Scholar] [CrossRef]
- Diep, C.H.; Knutson, T.P.; Lange, C.A. Active FOXO1 Is a Key Determinant of Isoform-Specific Progesterone Receptor Transactivation and Senescence Programming. Mol. Cancer Res. 2016, 14, 141–162. [Google Scholar] [CrossRef]
- Diep, C.; Charles, N.; Blake Gilks, C.; Kalloger, S.; Argenta, P.; Lange, C.A. Progesterone Receptors Induce FOXO1-Dependent Senescence in Ovarian Cancer Cells. Cell Cycle 2013, 12, 1433–1449. [Google Scholar] [CrossRef]
- Owen, G.I.; Richer, J.K.; Tung, L.; Takimoto, G.; Horwitz, K.B. Progesterone Regulates Transcription of the P21 Cyclindependent Kinase Inhibitor Gene through Sp1 and CBP/P300. J. Biol. Chem. 1998, 273, 10696–10701. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Etemadmoghadam, D.; Temple, J.; Lynch, A.G.; Riad, M.; Sharma, R.; Stewart, C.; Fereday, S.; Caldas, C.; deFazio, A.; et al. Driver Mutations in TP53 Are Ubiquitous in High Grade Serous Carcinoma of the Ovary: TP53 Mutation in High-Grade Pelvic Serous Carcinoma. J. Pathol. 2010, 221, 49–56. [Google Scholar] [CrossRef]
- Wu, N.-Y.; Huang, H.-S.; Chao, T.H.; Chou, H.M.; Fang, C.; Qin, C.-Z.; Lin, C.-Y.; Chu, T.-Y.; Zhou, H.H. Progesterone Prevents High-Grade Serous Ovarian Cancer by Inducing Necroptosis of P53-Defective Fallopian Tube Epithelial Cells. Cell Rep. 2017, 18, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.G.; Hubbard, N.W.; Messmer, M.N.; Kofman, S.B.; Hagan, C.E.; Orozco, S.L.; Chiang, K.; Daniels, B.P.; Baker, D.; Oberst, A. Intratumoral Activation of the Necroptotic Pathway Components RIPK1 and RIPK3 Potentiates Antitumor Immunity. Sci. Immunol. 2019, 4, eaaw2004. [Google Scholar] [CrossRef]
- Lima, M.A.; da Silva, S.V.; Freitas, V.M. Progesterone Acts via the Progesterone Receptor to Induce Adamts Proteases in Ovarian Cancer Cells. J. Ovarian Res. 2016, 9, 9. [Google Scholar] [CrossRef]
- Mead, T.J.; Apte, S.S. ADAMTS Proteins in Human Disorders. Matrix Biol. 2018, 71–72, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Doyle, K.M.H.; Russell, D.L.; Sriraman, V.; Richards, J.S. Coordinate Transcription of the ADAMTS-1 Gene by Luteinizing Hormone and Progesterone Receptor. Mol. Endocrinol. 2004, 18, 2463–2478. [Google Scholar] [CrossRef]
- Akahira, J.; Suzuki, T.; Ito, K.; Kaneko, C.; Darnel, A.D.; Moriya, T.; Okamura, K.; Yaegashi, N.; Sasano, H. Differential Expression of Progesterone Receptor Isoforms A and B in the Normal Ovary, and in Benign, Borderline, and Malignant Ovarian Tumors. Jpn. J. Cancer Res. 2002, 93, 807–815. [Google Scholar] [CrossRef]
- Freitas, V.M.; do Amaral, J.B.; Silva, T.A.; Santos, E.S.; Mangone, F.R.; de Jesus Pinheiro, J.; Jaeger, R.G.; Nagai, M.A.; Machado-Santelli, G.M. Decreased Expression of ADAMTS-1 in Human Breast Tumors Stimulates Migration and Invasion. Mol. Cancer. 2013, 12, 2. [Google Scholar] [CrossRef]
- Wetendorf, M.; Li, R.; Wu, S.-P.; Liu, J.; Creighton, C.J.; Wang, T.; Janardhan, K.S.; Willson, C.J.; Lanz, R.B.; Murphy, B.D.; et al. Constitutive Expression of Progesterone Receptor Isoforms Promotes the Development of Hormone-Dependent Ovarian Neoplasms. Sci. Signal 2020, 13, eaaz9646. [Google Scholar] [CrossRef] [PubMed]
- Mauro, L.J.; Seibel, M.I.; Diep, C.H.; Spartz, A.; Perez Kerkvliet, C.; Singhal, H.; Swisher, E.M.; Schwartz, L.E.; Drapkin, R.; Saini, S.; et al. Progesterone Receptors Promote Quiescence and Ovarian Cancer Cell Phenotypes via DREAM in P53-Mutant Fallopian Tube Models. J. Clin. Endocrinol. Metab. 2021, 106, 1929–1955. [Google Scholar] [CrossRef]
- Blakemore, J.; Naftolin, F. Aromatase: Contributions to Physiology and Disease in Women and Men. Physiology 2016, 31, 258–269. [Google Scholar] [CrossRef]
- Gruessner, C.; Gruessner, A.; Glaser, K.; AbuShahin, N.; Zhou, Y.; Laughren, C.; Wright, H.; Pinkerton, S.; Yi, X.; Stoffer, J.; et al. Flutamide and Biomarkers in Women at High Risk for Ovarian Cancer: Preclinical and Clinical Evidence. Cancer Prev. Res. 2014, 7, 896–905. [Google Scholar] [CrossRef]
- Elattar, A.; Warburton, K.G.; Mukhopadhyay, A.; Freer, R.M.; Shaheen, F.; Cross, P.; Plummer, E.R.; Robson, C.N.; Edmondson, R.J. Androgen Receptor Expression Is a Biological Marker for Androgen Sensitivity in High Grade Serous Epithelial Ovarian Cancer. Gynecol. Oncol. 2012, 124, 142–147. [Google Scholar] [CrossRef]
- Nourbakhsh, M.; Golestani, A.; Zahrai, M.; Modarressi, M.H.; Malekpour, Z.; Karami-Tehrani, F. Androgens Stimulate Telomerase Expression, Activity and Phosphorylation in Ovarian Adenocarcinoma Cells. Mol. Cell. Endocrinol. 2010, 330, 10–16. [Google Scholar] [CrossRef]
- Kohan-Ivani, K.; Gabler, F.; Selman, A.; Vega, M.; Romero, C. Role of Dihydrotestosterone (DHT) on TGF-Β1 Signaling Pathway in Epithelial Ovarian Cancer Cells. J. Cancer Res. Clin. Oncol. 2016, 142, 47–58. [Google Scholar] [CrossRef]
- Evangelou, A.; Jindal, S.K.; Brown, T.J.; Letarte, M. Down-Regulation of Transforming Growth Factor Beta Receptors by Androgen in Ovarian Cancer Cells. Cancer Res. 2000, 60, 929–935. [Google Scholar] [PubMed]
- Evangelou, A.; Letarte, M.; Jurisica, I.; Sultan, M.; Murphy, K.J.; Rosen, B.; Brown, T.J. Loss of Coordinated Androgen Regulation in Nonmalignant Ovarian Epithelial Cells with BRCA1/2 Mutations and Ovarian Cancer Cells. Cancer Res. 2003, 63, 2416–2424. [Google Scholar] [PubMed]
- Kollara, A.; Shathasivam, P.; Park, S.; Ringuette, M.J.; Brown, T.J. Increased Androgen Receptor Levels and Signaling in Ovarian Cancer Cells by VEPH1 Associated with Suppression of SMAD3 and AKT Activation. J. Steroid Biochem. Mol. Biol. 2020, 196, 105498. [Google Scholar] [CrossRef]
- Ilekis, J.V.; Connor, J.P.; Prins, G.S.; Ferrer, K.; Niederberger, C.; Scoccia, B. Expression of Epidermal Growth Factor and Androgen Receptors in Ovarian Cancer. Gynecol. Oncol. 1997, 66, 250–254. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Gao, Y.; Dong, L.J.; Liu, S.; Yao, Z. Reciprocal Regulation of 5α-Dihydrotestosterone, Interleukin-6 and Interleukin-8 during Proliferation of Epithelial Ovarian Carcinoma. Cancer Biol. Ther. 2007, 6, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zhu, H.; Shen, Q.; Sun, L.-Z.; Zhu, X. GLI3 and Androgen Receptor Are Mutually Dependent for Their Malignancy-Promoting Activity in Ovarian and Breast Cancer Cells. Cell. Signal. 2022, 92, 110278. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Jiang, L.; Liang, S.; Kwong, J.; Yang, L.; Li, Y.; Deng, Q.; Liang, Z. Nanog Interaction with the Androgen Receptor Signaling Axis Induce Ovarian Cancer Stem Cell Regulation: Studies Based on the CRISPR/Cas9 System. J. Ovarian Res. 2018, 11, 36. [Google Scholar] [CrossRef]
- Sieh, W.; Köbel, M.; Longacre, T.A.; Bowtell, D.D.; deFazio, A.; Goodman, M.T.; Høgdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B.; et al. Hormone-Receptor Expression and Ovarian Cancer Survival: An Ovarian Tumor Tissue Analysis Consortium Study. Lancet Oncol. 2013, 14, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Luo, H.; Li, S.; Sheng, B.; Zhao, M.; Zhu, H.; Zhu, X. Correlation between Estrogen Receptor Expression and Prognosis in Epithelial Ovarian Cancer: A Meta-Analysis. Oncotarget 2017, 8, 62400–62413. [Google Scholar] [CrossRef]
- Luo, H.; Li, S.; Zhao, M.; Sheng, B.; Zhu, H.; Zhu, X. Prognostic Value of Progesterone Receptor Expression in Ovarian Cancer: A Meta-Analysis. Oncotarget 2017, 8, 36845–36856. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, J.; Deberti, M.; Body, G.; Carcopino, X.; Touboul, C.; Dabi, Y.; Collinet, P.; Coutant, C.; Akladios, C.; Lavoué, V.; et al. Lymphovascular Space Invasion and Estrogen Receptor Status in High-Grade Serous Ovarian Cancer–A Multicenter Study by the FRANCOGYN Group. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102242. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Song, C.; Wang, D.; Hu, Y.; Liu, D.; Ma, D.; Gao, Q. Expression of Hormone Receptors Predicts Survival and Platinum Sensitivity of High-Grade Serous Ovarian Cancer. Biosci. Rep. 2021, 41, BSR20210478. [Google Scholar] [CrossRef]
- Ng, C.W.; Wong, K.-K. Impact of Estrogen Receptor Expression on Prognosis of Ovarian Cancer According to Antibody Clone Used for Immunohistochemistry: A Meta-Analysis. J. Ovarian. Res. 2022, 15, 63. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, X.; Zheng, L.; Hu, X.; Sun, L.; Zhu, X. The Role of the Androgen Receptor in Ovarian Cancer Carcinogenesis and Its Clinical Implications. Oncotarget 2017, 8, 29395–29405. [Google Scholar] [CrossRef]
- Mittica, G.; Senetta, R.; Scotto, G.; Aglietta, M.; Maggiorotto, F.; Ghisoni, E.; Genta, S.; Boldorini, R.; Manini, C.; Morra, I.; et al. Androgen Receptor Status Predicts Development of Brain Metastases in Ovarian Cancers. Oncotarget 2017, 8, 41143–41153. [Google Scholar] [CrossRef]
- Mittica, G.; Goia, M.; Gambino, A.; Scotto, G.; Fonte, M.; Senetta, R.; Aglietta, M.; Borella, F.; Sapino, A.; Katsaros, D.; et al. Validation of Androgen Receptor Loss as a Risk Factor for the Development of Brain Metastases from Ovarian Cancers. J. Ovarian Res. 2020, 13, 53. [Google Scholar] [CrossRef]
- Borella, F.; Bertero, L.; Morrone, A.; Gambella, A.; Bovetti, M.; Cosma, S.; Carosso, A.; Katsaros, D.; Gemmiti, S.; Preti, M.; et al. Brain Metastases from Ovarian Cancer: Current Evidence in Diagnosis, Treatment, and Prognosis. Cancers 2020, 12, 2156. [Google Scholar] [CrossRef]
- Scotto, G.; Borella, F.; Turinetto, M.; Tuninetti, V.; Valsecchi, A.A.; Giannone, G.; Cosma, S.; Benedetto, C.; Valabrega, G. Biomarkers of Central Nervous System Involvement from Epithelial Ovarian Cancer. Cells 2021, 10, 3408. [Google Scholar] [CrossRef] [PubMed]
- Stanley, B.; Hollis, R.L.; Nunes, H.; Towler, J.D.; Yan, X.; Rye, T.; Dawson, C.; Mackean, M.J.; Nussey, F.; Churchman, M.; et al. Endocrine Treatment of High Grade Serous Ovarian Carcinoma; Quantification of Efficacy and Identification of Response Predictors. Gynecol. Oncol. 2019, 152, 278–285. [Google Scholar] [CrossRef]
- Chan, K.K.L.; Ngu, S.F.; Chu, M.M.Y.; Tse, K.Y.; Ngan, H.Y.S. Tamoxifen Use in Recurrent Ovarian Cancer in a Chinese Population: A 15 -Year Clinical Experience in a Tertiary Referral Center. Asia Pac. J. Clin. Oncol. 2021, 17, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Argenta, P.A.; Um, I.; Kay, C.; Harrison, D.; Faratian, D.; Sueblinvong, T.; Geller, M.A.; Langdon, S.P. Predicting Response to the Anti-Estrogen Fulvestrant in Recurrent Ovarian Cancer. Gynecol. Oncol. 2013, 131, 368–373. [Google Scholar] [CrossRef]
- Grisham, R.N.; Manning-Geist, B.L.; Chui, M.H. Beyond the Estrogen Receptor: In Search of Predictive Biomarkers for Low-grade Serous Ovarian Cancer. Cancer 2023, 129, 1305–1307. [Google Scholar] [CrossRef]
- Hamadeh, I.S.; Patel, J.N.; Rusin, S.; Tan, A.R. Personalizing Aromatase Inhibitor Therapy in Patients with Breast Cancer. Cancer Treat. Rev. 2018, 70, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.X.; Reinert, T.; Chmielewska, I.; Ellis, M.J. Mechanisms of Aromatase Inhibitor Resistance. Nat. Rev. Cancer 2015, 15, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Langdon, S.P.; Gourley, C.; Gabra, H.; Stanley, B. Endocrine Therapy in Epithelial Ovarian Cancer. Expert Rev. Anticancer Ther. 2017, 17, 109–117. [Google Scholar] [CrossRef]
- Bowman, A.; Gabra, H.; Langdon, S.P.; Lessells, A.; Stewart, M.; Young, A.; Smyth, J.F. CA125 Response Is Associated with Estrogen Receptor Expression in a Phase II Trial of Letrozole in Ovarian Cancer: Identification of an Endocrine-Sensitive Subgroup. Clin. Cancer Res. 2002, 8, 2233–2239. [Google Scholar] [PubMed]
- Krasner, C.N.; Debernardo, R.L.; Findley, M.; Penson, R.; Matulonis, U.; Atkinson, T.; Roche, M.; Seiden, M.V. Phase II Trial of Anastrazole in Combination with Gefitinib in Women with Asymptomatic Mullerian Cancer. J. Clin. Oncol. 2005, 23, 5063. [Google Scholar] [CrossRef]
- Papadimitriou, C.A.; Markaki, S.; Siapkaras, J.; Vlachos, G.; Efstathiou, E.; Grimani, I.; Hamilos, G.; Zorzou, M.; Dimopoulos, M.-A. Hormonal Therapy with Letrozole for Relapsed Epithelial Ovarian Cancer. Oncology 2004, 66, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Gourley, C.; Smyth, J.F.; Mackean, M.; Stevenson, A.; Williams, A.; Rye, T.; Reed, N.; Vasey, P.; Gabra, H.; Langdon, S. Phase II Study of Letrozole in Estrogen Receptor (ER) Positive Relapsed Epithelial Ovarian Cancer (EOC). J. Clin. Oncol. 2006, 24, 5025. [Google Scholar] [CrossRef]
- Verma, S.; Alhayki, M.; Le, T.; Baines, K.; Rambout, L.; Hopkins, L.; Fung Kee Fung, M. Phase II Study of Exemestane (E) in Refractory Ovarian Cancer (ROC). J. Clin. Oncol. 2006, 24, 5026. [Google Scholar] [CrossRef]
- Tchekmedyian, N.S.; Liem, A.K.; Quan, E.T.; Burtzo, D.M.; Ucar, K. Aromatase Inhibitor Therapy for Estrogen Receptor Positive Ovarian Cancer. J. Clin. Oncol. 2006, 24, 15038. [Google Scholar] [CrossRef]
- Li, Y.F.; Hu, W.; Fu, S.Q.; Li, J.D.; Liu, J.H.; Kavanagh, J.J. Aromatase Inhibitors in Ovarian Cancer: Is There a Role? Int. J. Gynecol. Cancer 2008, 18, 600–614. [Google Scholar] [CrossRef]
- Kavanagh, J.J.; Hu, W.; Fu, S.; Deavers, M.; Moore, C.; Coleman, R.L.; Levenback, C.F.; Shen, D.; Zheng, H.G.; Yf, L.; et al. Anti-Tumor Activity of Letrozole in Patients with Recurrent Advanced Low Malignant Potential or Low-Grade Serous Ovarian Tumors. J. Clin. Oncol. 2007, 25, 5582. [Google Scholar] [CrossRef]
- Paleari, L.; Gandini, S.; Provinciali, N.; Puntoni, M.; Colombo, N.; DeCensi, A. Clinical Benefit and Risk of Death with Endocrine Therapy in Ovarian Cancer: A Comprehensive Review and Meta-Analysis. Gynecol. Oncol. 2017, 146, 504–513. [Google Scholar] [CrossRef]
- Bonaventura, A.; OʼConnell, R.L.; Mapagu, C.; Beale, P.J.; McNally, O.M.; Mileshkin, L.R.; Grant, P.T.; Hadley, A.M.; Goh, J.C.H.; Sjoquist, K.M.; et al. Paragon (ANZGOG-0903): Phase 2 Study of Anastrozole in Women With Estrogen or Progesterone Receptor-Positive Platinum-Resistant or -Refractory Recurrent Ovarian Cancer. Int. J. Gynecol. Cancer 2017, 27, 900–906. [Google Scholar] [CrossRef]
- Kok, P.-S.; Beale, P.; O’Connell, R.L.; Grant, P.; Bonaventura, T.; Scurry, J.; Antill, Y.; Goh, J.; Sjoquist, K.; DeFazio, A.; et al. PARAGON (ANZGOG-0903): A Phase 2 Study of Anastrozole in Asymptomatic Patients with Estrogen and Progesterone Receptor-Positive Recurrent Ovarian Cancer and CA125 Progression. J. Gynecol. Oncol 2019, 30, e86. [Google Scholar] [CrossRef] [PubMed]
- Gourley, C. Aromatase Inhibition in Ovarian Cancer: Repeated Signals of Efficacy but Tools for Patient Selection Remain Elusive. J. Gynecol. Oncol. 2019, 30, e98. [Google Scholar] [CrossRef]
- Heinzelmann-Schwarz, V.; Knipprath Mészaros, A.; Stadlmann, S.; Jacob, F.; Schoetzau, A.; Russell, K.; Friedlander, M.; Singer, G.; Vetter, M. Letrozole May Be a Valuable Maintenance Treatment in High-Grade Serous Ovarian Cancer Patients. Gynecol. Oncol. 2018, 148, 79–85. [Google Scholar] [CrossRef]
- Gershenson, D.M.; Sun, C.C.; Iyer, R.B.; Malpica, A.L.; Kavanagh, J.J.; Bodurka, D.C.; Schmeler, K.; Deavers, M. Hormonal Therapy for Recurrent Low-Grade Serous Carcinoma of the Ovary or Peritoneum. Gynecol. Oncol. 2012, 125, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; O’Connell, R.L.; Amant, F.; Beale, P.; McNally, O.; Sjoquist, K.M.; Grant, P.; Davis, A.; Sykes, P.; Mileshkin, L.; et al. PARAGON: A Phase II Study of Anastrozole in Patients with Estrogen Receptor-Positive Recurrent/Metastatic Low-Grade Ovarian Cancers and Serous Borderline Ovarian Tumors. Gynecol. Oncol. 2019, 154, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, D.M.; Bodurka, D.C.; Coleman, R.L.; Lu, K.H.; Malpica, A.; Sun, C.C. Hormonal Maintenance Therapy for Women With Low-Grade Serous Cancer of the Ovary or Peritoneum. J. Clin. Oncol. 2017, 35, 1103–1111. [Google Scholar] [CrossRef]
- Fader, A.N.; Bergstrom, J.; Jernigan, A.; Tanner, E.J.; Roche, K.L.; Stone, R.L.; Levinson, K.L.; Ricci, S.; Wethingon, S.; Wang, T.-L.; et al. Primary Cytoreductive Surgery and Adjuvant Hormonal Monotherapy in Women with Advanced Low-Grade Serous Ovarian Carcinoma: Reducing Overtreatment without Compromising Survival? Gynecol. Oncol. 2017, 147, 85–91. [Google Scholar] [CrossRef]
- Nica, A.; Lee, J.Y.J.; Hong, N.L.; May, T. Cost-Effectiveness of Maintenance Hormonal Therapy in Patients with Advanced Low Grade Serous Ovarian Cancer. Gynecol. Oncol. 2021, 160, 206–213. [Google Scholar] [CrossRef]
- Simoncini, T.; Hafezi-Moghadam, A.; Brazil, D.P.; Ley, K.; Chin, W.W.; Liao, J.K. Interaction of Oestrogen Receptor with the Regulatory Subunit of Phosphatidylinositol-3-OH Kinase. Nature 2000, 407, 538–541. [Google Scholar] [CrossRef]
- Pópulo, H.; Lopes, J.M.; Soares, P. The MTOR Signalling Pathway in Human Cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting MTOR for Cancer Therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A.; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Slomovitz, B.M.; Jiang, Y.; Yates, M.S.; Soliman, P.T.; Johnston, T.; Nowakowski, M.; Levenback, C.; Zhang, Q.; Ring, K.; Munsell, M.F.; et al. Phase II Study of Everolimus and Letrozole in Patients with Recurrent Endometrial Carcinoma. J. Clin. Oncol. 2015, 33, 930–936. [Google Scholar] [CrossRef]
- Mabuchi, S.; Kuroda, H.; Takahashi, R.; Sasano, T. The PI3K/AKT/MTOR Pathway as a Therapeutic Target in Ovarian Cancer. Gynecol. Oncol. 2015, 137, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Colon-Otero, G.; Weroha, S.J.; Foster, N.R.; Haluska, P.; Hou, X.; Wahner-Hendrickson, A.E.; Jatoi, A.; Block, M.S.; Dinh, T.A.; Robertson, M.W.; et al. Phase 2 Trial of Everolimus and Letrozole in Relapsed Estrogen Receptor-Positive High-Grade Ovarian Cancers. Gynecol. Oncol. 2017, 146, 64–68. [Google Scholar] [CrossRef]
- Indovina, P.; Pentimalli, F.; Casini, N.; Vocca, I.; Giordano, A. RB1 Dual Role in Proliferation and Apoptosis: Cell Fate Control and Implications for Cancer Therapy. Oncotarget 2015, 6, 17873–17890. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.J.; Bisi, J.E.; Strum, J.C.; Combest, A.J.; Darr, D.B.; Usary, J.E.; Zamboni, W.C.; Wong, K.-K.; Perou, C.M.; Sharpless, N.E. Multiple Roles of Cyclin-Dependent Kinase 4/6 Inhibitors in Cancer Therapy. J. Natl. Cancer Inst. 2012, 104, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Cheng, J.; Bloomquist, E.; Sanchez, J.; Wedam, S.B.; Singh, H.; Amiri-Kordestani, L.; Ibrahim, A.; Sridhara, R.; Goldberg, K.B.; et al. CDK4/6 Inhibitor Treatment for Patients with Hormone Receptor-Positive, HER2-Negative, Advanced or Metastatic Breast Cancer: A US Food and Drug Administration Pooled Analysis. Lancet Oncol. 2020, 21, 250–260. [Google Scholar] [CrossRef]
- Spring, L.M.; Wander, S.A.; Andre, F.; Moy, B.; Turner, N.C.; Bardia, A. Cyclin-Dependent Kinase 4 and 6 Inhibitors for Hormone Receptor-Positive Breast Cancer: Past, Present, and Future. Lancet 2020, 395, 817–827. [Google Scholar] [CrossRef]
- Dall’Acqua, A.; Bartoletti, M.; Masoudi-Khoram, N.; Sorio, R.; Puglisi, F.; Belletti, B.; Baldassarre, G. Inhibition of CDK4/6 as Therapeutic Approach for Ovarian Cancer Patients: Current Evidences and Future Perspectives. Cancers 2021, 13, 3035. [Google Scholar] [CrossRef]
- Colon-Otero, G.; Zanfagnin, V.; Hou, X.; Foster, N.R.; Asmus, E.J.; Wahner Hendrickson, A.; Jatoi, A.; Block, M.S.; Langstraat, C.L.; Glaser, G.E.; et al. Phase II Trial of Ribociclib and Letrozole in Patients with Relapsed Oestrogen Receptor-Positive Ovarian or Endometrial Cancers. ESMO Open 2020, 5, e000926. [Google Scholar] [CrossRef]
- Hyman, D.; Bonafede, M.; O’Cearbhaill, R.; Grisham, R.; Zamarin, D.; Tew, W.; Aghajanian, C.; Cadoo, K.; Friedman, C.; Savage, R.E.; et al. Abstract CT035: A Phase Ib Study of Miransertib (ARQ 092) in Combination with Anastrozole in Patients with PIK3CA or AKT1 -Mutant ER+ Endometrial or Ovarian Cancer. Cancer Res. 2018, 78, CT035. [Google Scholar] [CrossRef]
- Lindemann, K.; Gibbs, E.; Åvall-Lundqvist, E.; dePont Christensen, R.; Woie, K.; Kalling, M.; Auranen, A.; Grenman, S.; Hoegberg, T.; Rosenberg, P.; et al. Chemotherapy vs Tamoxifen in Platinum-Resistant Ovarian Cancer: A Phase III, Randomised, Multicentre Trial (Ovaresist). Br. J. Cancer 2017, 116, 455–463. [Google Scholar] [CrossRef]
- Trédan, O.; Provansal, M.; Abdeddaim, C.; Lardy-Cleaud, A.; Hardy-Bessard, A.-C.; Kalbacher, E.; Floquet, A.; Venat-Bouvet, L.; Lortholary, A.; Pop, O.; et al. Regorafenib or Tamoxifen for Platinum-Sensitive Recurrent Ovarian Cancer with Rising CA125 and No Evidence of Clinical or RECIST Progression: A GINECO Randomized Phase II Trial (REGOVAR). Gynecol. Oncol. 2022, 164, 18–26. [Google Scholar] [CrossRef]
- Kristeleit, R.; Davidenko, I.; Shirinkin, V.; El-Khouly, F.; Bondarenko, I.; Goodheart, M.J.; Gorbunova, V.; Penning, C.A.; Shi, J.G.; Liu, X.; et al. A Randomised, Open-Label, Phase 2 Study of the IDO1 Inhibitor Epacadostat (INCB024360) versus Tamoxifen as Therapy for Biochemically Recurrent (CA-125 Relapse)-Only Epithelial Ovarian Cancer, Primary Peritoneal Carcinoma, or Fallopian Tube Cancer. Gynecol. Oncol. 2017, 146, 484–490. [Google Scholar] [CrossRef]
- Wagner, U.; Dubois, A.; Pfisterer, J.; Huober, J.; Loibl, S.; Luck, H.; Sehouli, J.; Gropp, M.; Stahle, A.; Schmalfeldt, B. Gefitinib in Combination with Tamoxifen in Patients with Ovarian Cancer Refractory or Resistant to Platinum–Taxane Based Therapy—A Phase II Trial of the AGO Ovarian Cancer Study Group (AGO-OVAR 2.6)☆. Gynecol.Oncol. 2007, 105, 132–137. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.M.J.; Klar, M.; Zwimpfer, T.A.; Dutilh, G.; Vetter, M.; Marth, C.; du Bois, A.; Schade-Brittinger, C.; Reuss, A.; Bommer, C.; et al. Maintenance Therapy with Aromatase Inhibitor in Epithelial Ovarian Cancer (MATAO): Study Protocol of a Randomized Double-Blinded Placebo-Controlled Multi-Center Phase III Trial. BMC Cancer 2022, 22, 508. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Zhang, P.; Fang, X.; Wang, Y.; Sun, H. Anlotinib plus Letrozole in Patients with Platinum-Resistant Recurrent Ovarian Cancer: A Prospective, Single-Arm, Open-Label, Phase II Study. J. Clin. Oncol. 2022, 40, e17545. [Google Scholar] [CrossRef]
- Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.; Ciruelos, E.; Burstein, H.J.; et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N. Engl. J. Med. 2018, 379, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.K.; Bihani, T. Selective Estrogen Receptor Modulators (SERMs) and Selective Estrogen Receptor Degraders (SERDs) in Cancer Treatment. Pharmacol. Ther. 2018, 186, 1–24. [Google Scholar] [CrossRef]
- Ozyurt, R.; Ozpolat, B. Molecular Mechanisms of Anti-Estrogen Therapy Resistance and Novel Targeted Therapies. Cancers 2022, 14, 5206. [Google Scholar] [CrossRef]
- Ferreira Almeida, C.; Oliveira, A.; João Ramos, M.; Fernandes, P.A.; Teixeira, N.; Amaral, C. Estrogen Receptor-Positive (ER+) Breast Cancer Treatment: Are Multi-Target Compounds the next Promising Approach? Biochem. Pharmacol. 2020, 177, 113989. [Google Scholar] [CrossRef] [PubMed]
- Nathan, M.R.; Schmid, P. A Review of Fulvestrant in Breast Cancer. Oncol. Ther. 2017, 5, 17–29. [Google Scholar] [CrossRef]
- Mehta, R.S.; Barlow, W.E.; Albain, K.S.; Vandenberg, T.A.; Dakhil, S.R.; Tirumali, N.R.; Lew, D.L.; Hayes, D.F.; Gralow, J.R.; Linden, H.H.; et al. Overall Survival with Fulvestrant plus Anastrozole in Metastatic Breast Cancer. N. Engl. J. Med. 2019, 380, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.M.; Moore, G.E.; Major, F.J. Advanced Ovarian Carcinoma: Response to Antiestrogen Therapy. Cancer 1981, 48, 2368–2370. [Google Scholar] [CrossRef] [PubMed]
- Hatch, K.D.; Beecham, J.B.; Blessing, J.A.; Creasman, W.T. Responsiveness of Patients with Advanced Ovarian Carcinoma to Tamoxifen. A Gynecologic Oncology Group Study of Second-Line Therapy in 105 Patients. Cancer 1991, 68, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Simera, I.; Bryant, A. Tamoxifen for Relapse of Ovarian Cancer. Cochrane Database Syst. Rev. 2010, 2010, CD001034. [Google Scholar] [CrossRef]
- Grothey, A.; Blay, J.-Y.; Pavlakis, N.; Yoshino, T.; Bruix, J. Evolving Role of Regorafenib for the Treatment of Advanced Cancers. Cancer Treat. Rev. 2020, 86, 101993. [Google Scholar] [CrossRef]
- Prendergast, G.C.; Malachowski, W.P.; DuHadaway, J.B.; Muller, A.J. Discovery of IDO1 Inhibitors: From Bench to Bedside. Cancer Res. 2017, 77, 6795–6811. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Hamid, O.; Smith, D.C.; Bauer, T.M.; Wasser, J.S.; Olszanski, A.J.; Luke, J.J.; Balmanoukian, A.S.; Schmidt, E.V.; Zhao, Y.; et al. Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results From a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037). J. Clin. Oncol. 2018, 36, 3223–3230. [Google Scholar] [CrossRef]
- Doi, T.; Fujiwara, Y.; Shitara, K.; Shimizu, T.; Yonemori, K.; Matsubara, N.; Ohno, I.; Kogawa, T.; Naito, Y.; Leopold, L.; et al. The Safety and Tolerability of Epacadostat Alone and in Combination with Pembrolizumab in Patients with Advanced Solid Tumors: Results from a First-in-Japanese Phase I Study (KEYNOTE-434). Investig. New Drugs 2021, 39, 152–162. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 Pathway in Cancer: From Bench to Bedside. J. Hematol. Oncol. 2018, 11, 100. [Google Scholar] [CrossRef]
- Mondal, A.; Smith, C.; DuHadaway, J.B.; Sutanto-Ward, E.; Prendergast, G.C.; Bravo-Nuevo, A.; Muller, A.J. IDO1 Is an Integral Mediator of Inflammatory Neovascularization. EBioMedicine 2016, 14, 74–82. [Google Scholar] [CrossRef]
- Okamoto, A.; Nikaido, T.; Ochiai, K.; Takakura, S.; Saito, M.; Aoki, Y.; Ishii, N.; Yanaihara, N.; Yamada, K.; Takikawa, O.; et al. Indoleamine 2,3-Dioxygenase Serves as a Marker of Poor Prognosis in Gene Expression Profiles of Serous Ovarian Cancer Cells. Clin. Cancer. Res. 2005, 11, 6030–6039. [Google Scholar] [CrossRef]
- Takao, M.; Okamoto, A.; Nikaido, T.; Urashima, M.; Takakura, S.; Saito, M.; Saito, M.; Okamoto, S.; Takikawa, O.; Sasaki, H.; et al. Increased Synthesis of Indoleamine-2,3-Dioxygenase Protein Is Positively Associated with Impaired Survival in Patients with Serous-Type, but Not with Other Types of, Ovarian Cancer. Oncol. Rep. 2007, 17, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Inaba, T.; Ino, K.; Kajiyama, H.; Yamamoto, E.; Shibata, K.; Nawa, A.; Nagasaka, T.; Akimoto, H.; Takikawa, O.; Kikkawa, F. Role of the Immunosuppressive Enzyme Indoleamine 2,3-Dioxygenase in the Progression of Ovarian Carcinoma. Gynecol. Oncol. 2009, 115, 185–192. [Google Scholar] [CrossRef]
- Pantziarka, P. Repurposing Drugs in Oncology (ReDO)—Itraconazole as an Anti-Cancer Agent. Ecancermedicalscience 2015, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Pounds, R.; Leonard, S.; Dawson, C.; Kehoe, S. Repurposing Itraconazole for the Treatment of Cancer. Oncol. Lett. 2017, 14, 2587–2597. [Google Scholar] [CrossRef]
- Markham, A. Pamiparib: First Approval. Drugs 2021, 81, 1343–1348. [Google Scholar] [CrossRef]
- Argenta, P.A.; Thomas, S.G.; Judson, P.L.; Downs, L.S.; Geller, M.A.; Carson, L.F.; Jonson, A.L.; Ghebre, R. A Phase II Study of Fulvestrant in the Treatment of Multiply-Recurrent Epithelial Ovarian Cancer. Gynecol. Oncol. 2009, 113, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Bussies, P.L.; Schlumbrecht, M. Dual Fulvestrant-Trametinib Therapy in Recurrent Low-Grade Serous Ovarian Cancer. Oncologist 2020, 25, e1124–e1126. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).