Targeting Tyrosine Kinases in Ovarian Cancer: Small Molecule Inhibitor and Monoclonal Antibody, Where Are We Now?
Abstract
1. Ovarian Cancer: Current Understanding and Treatment
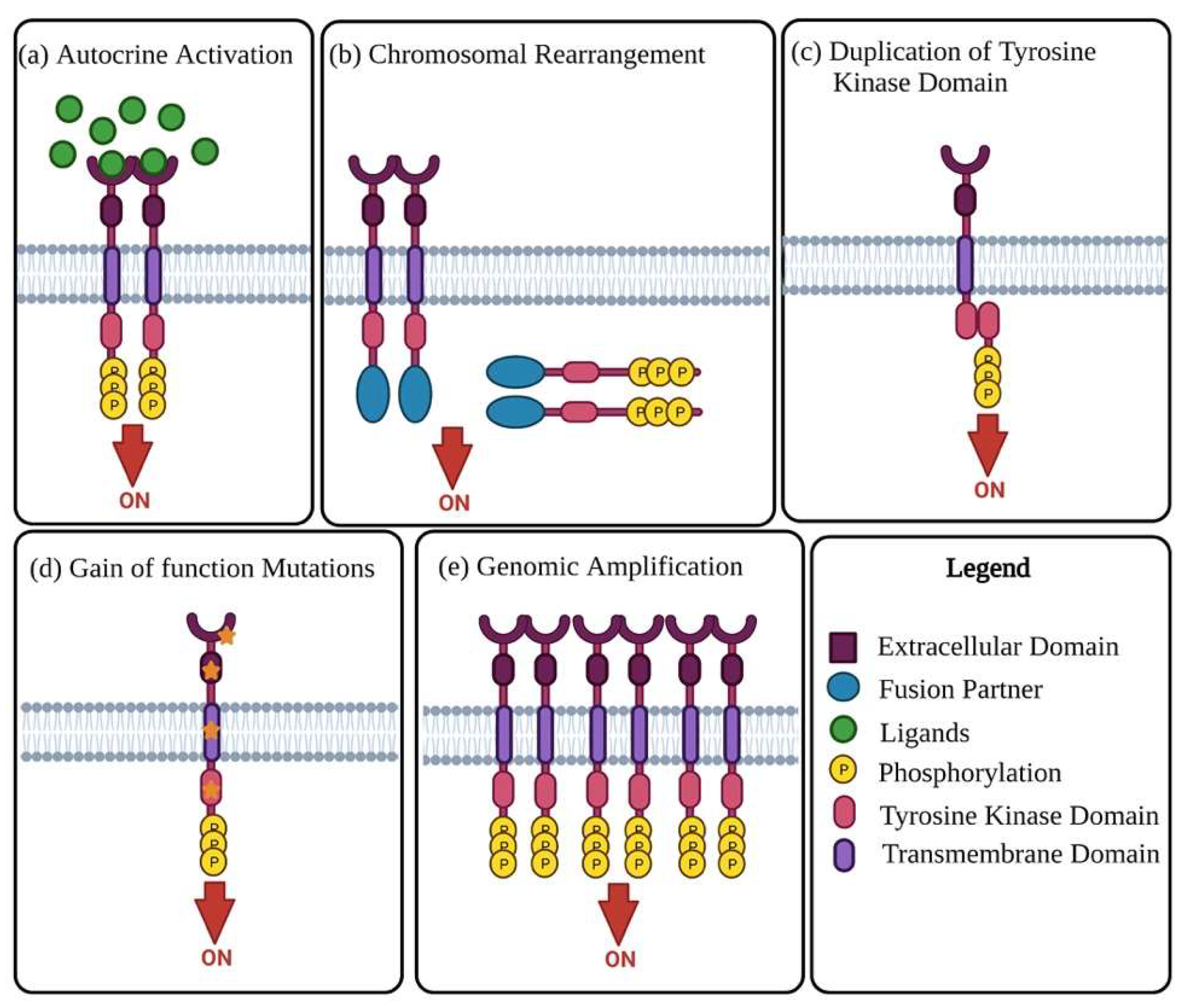
2. Receptor Tyrosine Kinases
3. Epidermal Growth Factor Receptors
4. Vascular Endothelial Growth Factor Receptor and Its Pathways
5. Platelet-Derived Growth Factor Receptors
6. c-MET
7. Other Emerging RTKs
8. Small Molecule Tyrosine Kinase Inhibitors
8.1. Small Molecule ATP-Competitive Tyrosine Kinase Inhibitors
8.1.1. Sorafenib
8.1.2. Sunitinib
8.1.3. Pazopanib
8.1.4. Cediranib
8.1.5. Erlotinib
8.2. Small Molecule Non-ATP-Competitive Tyrosine Kinase Inhibitors
Tivantinib
9. Monoclonal Antibodies Targeting Tyrosine Kinases
9.1. Bevacizumab
9.2. Cetuximab
9.3. Pertuzumab
9.4. Trastuzumab
9.5. Seribantumab
10. Immunotherapeutic Targeting Tyrosine Kinases
11. Future Prospective/Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Prat, J. FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: Abridged republication. J. Gynecol. Oncol. 2015, 26, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Dilley, J.; Burnell, M.; Gentry-Maharaj, A.; Ryan, A.; Neophytou, C.; Apostolidou, S.; Karpinskyj, C.; Kalsi, J.; Mould, T.; Woolas, R.; et al. Ovarian cancer symptoms, routes to diagnosis and survival–Population cohort study in the ‘no screen’arm of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Gynecol. Oncol. 2020, 158, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, K.; Ogden, G.; Mujahid, M.; Razvi, K. Symptoms and risk factors of ovarian cancer: A survey in primary care. Int. Sch. Res. Not. 2012, 2012, 754197. [Google Scholar] [CrossRef]
- Berek, J.S.; Bast, R.C., Jr. Epithelial ovarian cancer. In Holland-Frei Cancer Medicine, 6th ed.; BC Decker: Hamilton, ON, Canada, 2003. [Google Scholar]
- Kobel, M.; Kalloger, S.E.; Boyd, N.; McKinney, S.; Mehl, E.; Palmer, C.; Leung, S.; Bowen, N.J.; Lonescu, D.N.; Rajput, A.; et al. Ovarian carcinoma subtypes are different diseases: Implications for biomarker studies. PLoS Med. 2008, 5, 232. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, S.-G.; Wang, J.; Sun, J.-Y.; He, Z.-Y.; Jin, X.; Zhang, W.-W. The effect of histological subtypes on outcomes of stage IV epithelial ovarian cancer. Front. Oncol. 2018, 8, 577. [Google Scholar] [CrossRef]
- Testa, U.; Petrucci, E.; Pasquini, L.; Castelli, G.; Pelosi, E. Ovarian cancers: Genetic abnormalities, tumor heterogeneity and progression, clonal evolution and cancer stem cells. Medicines 2018, 5, 16. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Etemadmoghadam, D.; Temple, J.; Lynch, A.G.; Riad, M.; Sharma, R.; Stewart, C.; Fereday, S.; Caldas, C.; Defazio, A.; et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J. Pathol. 2010, 221, 49–56. [Google Scholar] [CrossRef]
- Ryland, G.L.; Hunter, S.M.; Doyle, M.A.; Caramia, F.; Li, J.; Rowley, S.M.; Christie, M.; Allan, P.E.; Stephens, A.N.; Bowtell, D.D.L.; et al. Mutational landscape of mucinous ovarian carcinoma and its neoplastic precursors. Genome Med. 2015, 7, 1–12. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Konecny, G.E.; Wang, C.; Hamidi, H.; Winterhoff, B.; Kalli, K.R.; Dering, J.; Ginther, C.; Chen, H.-W.; Dowdy, S.; Cliby, W.; et al. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J. Natl. Cancer Inst. 2014, 106, dju249. [Google Scholar] [CrossRef]
- Tothill, R.W.; Tinker, A.V.; George, J.; Brown, R.; Fox, S.B.; Lade, S.; Johnson, D.S.; Trivett, M.K.; Etemadmoghadam, D.; Locandro, B.; et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin. Cancer Res. 2008, 14, 5198–5208. [Google Scholar] [CrossRef]
- Chen, G.M.; Kannan, L.; Geistlinger, L.; Kofia, V.; Safikhani, Z.; Gendoo, D.M.A.; Parmigiani, G.; Birrer, M.; Haibe-Kains, B.; Waldron, L. Consensus on Molecular Subtypes of High-Grade Serous Ovarian CarcinomaConsensus on Molecular Subtypes of HGSOC. Clin. Cancer Res. 2018, 24, 5037–5047. [Google Scholar] [CrossRef]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef]
- Altman, A.D.; Nelson, G.; Chu, P.; Nation, J.; Ghatage, P. Optimal debulking targets in women with advanced stage ovarian cancer: A retrospective study of immediate versus interval debulking surgery. J. Obstetr. Gynaecol. Can. 2012, 34, 558–566. [Google Scholar] [CrossRef]
- Eisenkop, S.M.; Spirtos, N.M. What are the current surgical objectives, strategies, and technical capabilities of gynecologic oncologists treating advanced epithelial ovarian cancer? Gynecol. Oncol. 2001, 82, 489–497. [Google Scholar] [CrossRef]
- Tewari, D.; Java, J.J.; Salani, R.; Armstrong, D.K.; Markman, M.; Herzog, T.; Monk, B.J.; Chan, J.K. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: A gynecologic oncology group study. J. Clin. Oncol. 2015, 33, 1460–1466. [Google Scholar] [CrossRef]
- Christie, E.L.; Bowtell, D.D.L. Acquired chemotherapy resistance in ovarian cancer. Ann. Oncol. 2017, 28, viii13–viii15. [Google Scholar] [CrossRef]
- Cannistra, S.A. Cancer of the ovary. N. Engl. J. Med. 2004, 351, 2519–2529. [Google Scholar] [CrossRef]
- National Institutes of Health Consensus Development Conference Statement. Ovarian cancer: Screening, treatment, and follow-up. Gynecol. Oncol. 1994, 55, S4–S14. [Google Scholar] [CrossRef]
- Ushijima, K. Treatment for recurrent ovarian cancer—At first relapse. J. Oncol. 2010, 2010, 497429. [Google Scholar] [CrossRef]
- Klempner, S.J.; Myers, A.P.; Mills, G.B.; Westin, S.N. Clinical investigation of receptor and non-receptor tyrosine kinase inhibitors for the treatment of epithelial ovarian cancer. Expert Opin. Pharmacother. 2013, 14, 2171–2182. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Raymond, E.; Faivre, S. Sunitinib: A novel tyrosine kinase inhibitor. A brief review of its therapeutic potential in the treatment of renal carcinoma and gastrointestinal stromal tumors (GIST). Ther. Clin. Risk Manag. 2007, 3, 341–348. [Google Scholar] [CrossRef]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine kinase–role and significance in cancer. Int. J. Med. Sci. 2004, 1, 101–115. [Google Scholar] [CrossRef]
- Mayer, B.J. Perspective: Dynamics of receptor tyrosine kinase signaling complexes. FEBS Lett. 2012, 586, 2575–2579. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Metibemu, D.S.; Akinloye, O.A.; Akamo, A.J.; Ojo, D.A.; Okeowo, O.T.; Omotuyi, I.O. Exploring receptor tyrosine kinases-inhibitors in Cancer treatments. Egypt J. Med. Hum. Genet. 2019, 20, 35. [Google Scholar] [CrossRef]
- Xu, A.M.; Huang, P.H. Receptor Tyrosine Kinase Coactivation Networks in CancerRTK Coactivation Networks. Cancer Res. 2010, 70, 3857–3860. [Google Scholar] [CrossRef]
- Jiao, Y.; Ou, W.; Meng, F.; Zhou, H.; Wang, A. Targeting HSP90 in ovarian cancers with multiple receptor tyrosine kinase coactivation. Mol. Cancer 2011, 10, 125. [Google Scholar] [CrossRef]
- Tvorogov, D.; Carpenter, G. Epidermal Growth Factor Receptor Family. In Encyclopedia of Biological Chemistry; Lennarz, W.J., Lane, M.D., Eds.; Elsevier: New York, NY, USA, 2004; pp. 51–55. [Google Scholar]
- Cao, C.; Lu, S.; Sowa, A.; Kivlin, R.; Amaral, A.; Chu, W.; Yang, H.; Di, W.; Wen, Y. Priming with EGFR tyrosine kinase inhibitor and EGF sensitizes ovarian cancer cells to respond to chemotherapeutical drugs. Cancer Lett. 2008, 266, 249–262. [Google Scholar] [CrossRef]
- Teplinsky, E.; Muggia, F. EGFR and HER2: Is there a role in ovarian cancer? Transl. Cancer Res. 2015, 4, 1. [Google Scholar] [CrossRef]
- Sheng, Q.; Liu, J. The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer. Br. J. Cancer 2011, 104, 1241–1245. [Google Scholar] [CrossRef]
- Mehner, C.; Oberg, A.L.; Goergen, K.M.; Kalli, K.R.; Maurer, M.J.; Nassar, A.; Goode, E.L.; Keeney, G.L.; Jatoi, A.; Radisky, D.C. EGFR as a prognostic biomarker and therapeutic target in ovarian cancer: Evaluation of patient cohort and literature review. Genes Cancer 2017, 8, 589. [Google Scholar] [CrossRef]
- McAlpine, J.N.; Wiegand, K.C.; Vang, R.; Ronnett, B.M.; Adamiak, A.; Köbel, M.; Kalloger, S.E.; Swenerton, K.D.; Huntsman, D.G.; Gilks, C.B.; et al. HER2 overexpression and amplification is present in a subset of ovarian mucinous carcinomas and can be targeted with trastuzumab therapy. BMC Cancer 2009, 9, 433. [Google Scholar] [CrossRef]
- Luo, H.; Xu, X.; Ye, M.; Sheng, B.; Zhu, X. The prognostic value of HER2 in ovarian cancer: A meta-analysis of observational studies. PLoS ONE 2018, 13, 0191972. [Google Scholar] [CrossRef]
- Chung, Y.W.; Kim, S.; Hong, J.H.; Lee, J.K.; Lee, N.W.; Lee, Y.S.; Song, J.Y. Overexpression of HER2/HER3 and clinical feature of ovarian cancer. J. Gynecol. Oncol. 2019, 30, e75. [Google Scholar] [CrossRef]
- Duncan, T.J.; Al-Attar, A.; Rolland, P.; Scott, I.V.; Deen, S.; Liu, D.T.; Spendlove, I.; Durrant, L.G. Vascular endothelial growth factor expression in ovarian cancer: A model for targeted use of novel therapies? Clin. Cancer Res. 2008, 14, 3030–3035. [Google Scholar] [CrossRef]
- Sopo, M.; Anttila, M.; Hämäläinen, K.; Kivelä, A.; Ylä-Herttuala, S.; Kosma, V.-M.; Keski-Nisula, L.; Sallinen, H. Expression profiles of VEGF-A, VEGF-D and VEGFR1 are higher in distant metastases than in matched primary high grade epithelial ovarian cancer. BMC Cancer 2019, 19, 584. [Google Scholar] [CrossRef]
- Henriksen, R.; Funa, K.; Wilander, E.; Bäckström, T.; Ridderheim, M.; Öberg, K. Expression and prognostic significance of platelet-derived growth factor and its receptors in epithelial ovarian neoplasms. Cancer Res. 1993, 53, 4550–4554. [Google Scholar]
- Szubert, S.; Moszynski, R.; Szpurek, D.; Romaniuk, B.; Sajdak, S.; Nowicki, M.; Michalak, S. The expression of Platelet-derived Growth factor receptors (PDGFRs) and their correlation with overall survival of patients with ovarian cancer. Ginekol. Pol. 2019, 90, 242–249. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, H.J.; Kim, H.S.; Kim, B.J.; Park, S.H. Prognostic impact of high c-Met expression in ovarian cancer: A meta-analysis. J. Cancer 2018, 9, 3427–3434. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yoon, A.; Ryu, J.-Y.; Cho, Y.-J.; Choi, J.-J.; Song, S.Y.; Bang, H.; Lee, J.S.; Cho, W.C.; Choi, C.H. c-MET as a potential therapeutic target in ovarian clear cell carcinoma. Sci. Rep. 2016, 6, 38502. [Google Scholar] [CrossRef]
- Quinn, J.M.; Greenwade, M.M.; Palisoul, M.L.; Opara, G.; Massad, K.; Guo, L.; Zhao, P.; Beck-Noia, H.; Hagemann, I.S.; Hangemann, A.R. Therapeutic Inhibition of the Receptor Tyrosine Kinase AXL Improves Sensitivity to Platinum and Taxane in Ovarian CancerAXL Inhibition Improves Chemosensitivity in Ovarian Cancer. Mol. Cancer Ther. 2019, 18, 389–398. [Google Scholar] [CrossRef]
- Vouri, M.; Hafizi, S. TAM receptor tyrosine kinases in cancer drug resistance. Cancer Res. 2017, 77, 2775–2778. [Google Scholar] [CrossRef]
- Rankin, E.B.; Giaccia, A.J. The receptor tyrosine kinase AXL in cancer progression. Cancers 2016, 8, 103. [Google Scholar] [CrossRef]
- Tian, M.; Chen, X.S.; Li, L.Y.; Wu, H.Z.; Zeng, D.; Wang, X.L.; Zhang, Y.; Xioa, S.-S.; Cheng, Y. Inhibition of AXL enhances chemosensitivity of human ovarian cancer cells to cisplatin via decreasing glycolysis. Acta Pharmacol. Sin. 2021, 42, 1180–1189. [Google Scholar] [CrossRef]
- Lee, C. Overexpression of Tyro3 receptor tyrosine kinase leads to the acquisition of taxol resistance in ovarian cancer cells. Mol. Med. Rep. 2015, 12, 1485–1492. [Google Scholar] [CrossRef]
- Suh, Y.A.; Jo, S.Y.; Lee, H.Y.; Lee, C. Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor tyrosine kinases by apigenin circumvent taxol resistance in ovarian cancer cells. Int. J. Oncol. 2015, 46, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Llamosas, E.; Knipprath-Meszaros, A.; Schoetzau, A.; Obermann, E.; Fuenfschilling, M.; Caduff, R.; Fink, D.; Hacker, N.; Ward, R.; et al. Targeting the ROR1 and ROR2 receptors in epithelial ovarian cancer inhibits cell migration and invasion. Oncotarget 2015, 6, 40310–40326. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Llamosas, E.; Djordjevic, A.; Hacker, N.; Ford, C. Migration and invasion is inhibited by silencing ROR1 and ROR2 in chemoresistant ovarian cancer. Oncogenesis 2016, 5, e226. [Google Scholar] [CrossRef] [PubMed]
- Hossein, G.; Arabzadeh, S.; Salehi-Dulabi, Z.; Dehghani-Ghobadi, Z.; Heidarian, Y.; Talebi-Juybari, M. Wnt5A regulates the expression of ROR2 tyrosine kinase receptor in ovarian cancer cells. Biochem. Cell Biol. 2017, 95, 609–615. [Google Scholar] [CrossRef]
- Hojjat-Farsangi, M. Small-molecule inhibitors of the receptor tyrosine kinases: Promising tools for targeted cancer therapies. Int. J. Mol. Sci. 2014, 15, 13768–13801. [Google Scholar] [CrossRef]
- Garuti, L.; Roberti, M.; Bottegoni, G. Non-ATP competitive protein kinase inhibitors. Curr. Med. Chem. 2010, 17, 2804–2821. [Google Scholar] [CrossRef]
- Płużański, A.; Piórek, A. Side effects of tyrosine kinase inhibitors—Management guidelines. Oncol. Clin. Pract. 2016, 12, 113–118. [Google Scholar]
- Matei, D.; Sill, M.W.; Lankes, H.A.; DeGeest, K.; Bristow, R.E.; Mutch, D.; Yamada, S.D.; Cohn, D.; Calvert, V.; Farley, J.; et al. Activity of sorafenib in recurrent ovarian cancer and primary peritoneal carcinomatosis: A gynecologic oncology group trial. J. Clin. Oncol. 2011, 29, 69–75. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Thompson, D.S.; Bismayer, J.A.; Gian, V.G.; Merritt, W.M.; Whorf, R.C.; Finney, L.H.; Dudley, B.S. Paclitaxel/carboplatin with or without sorafenib in the first-line treatment of patients with stage III/IV epithelial ovarian cancer: A randomized phase II study of the Sarah Cannon Research Institute. Cancer Med. 2015, 4, 673–681. [Google Scholar] [CrossRef]
- Pölcher, M.; Eckhardt, M.; Coch, C.; Wolfgarten, M.; Kübler, K.; Hartmann, G.; Kuhn, W.; Rudlowski, C. Sorafenib in combination with carboplatin and paclitaxel as neoadjuvant chemotherapy in patients with advanced ovarian cancer. Cancer Chemother. Pharmacol. 2010, 66, 203–207. [Google Scholar] [CrossRef]
- Ramasubbaiah, R.; Perkins, S.; Schilder, J.; Whalen, C.; Johnson, C.; Callahan, M.; Jones, T.; Sutton, G.; Matei, D. Sorafenib in combination with weekly topotecan in recurrent ovarian cancer, a phase I/II study of the Hoosier Oncology Group. Gynecol. Oncol. 2011, 123, 499–504. [Google Scholar] [CrossRef]
- Welch, S.A.; Hirte, H.W.; Elit, L.; Schilder, R.J.; Wang, L.; MacAlpine, K.; Wright, J.J.; Oza, A.M. Sorafenib in combination with gemcitabine in recurrent epithelial ovarian cancer: A study of the Princess Margaret Hospital Phase II Consortium. Int. J. Gynecol. Cancer 2010, 20. [Google Scholar] [CrossRef]
- Baumann, K.H.; du Bois, A.; Meier, W.; Rau, J.; Wimberger, P.; Sehouli, J.; Kurzeder, C.; Hilpert, F.; Hasenburg, A.; Canzler, U.; et al. A phase II trial (AGO 2.11) in platinum-resistant ovarian cancer: A randomized multicenter trial with sunitinib (SU11248) to evaluate dosage, schedule, tolerability, toxicity and effectiveness of a multitargeted receptor tyrosine kinase inhibitor monotherapy. Ann. Oncol. 2012, 23, 2265–2271. [Google Scholar] [CrossRef]
- Chan, J.K.; Brady, W.; Monk, B.J.; Brown, J.; Shahin, M.S.; Rose, P.G.; Kim, J.-H.; Secord, A.A.; Walker, J.L.; Gershenson, D.M. A phase II evaluation of sunitinib in the treatment of persistent or recurrent clear cell ovarian carcinoma: An NRG oncology/gynecologic oncology group study (GOG-254). Gynecol. Oncol. 2018, 150, 247–252. [Google Scholar] [CrossRef]
- Biagi, J.; Oza, A.; Chalchal, H.; Grimshaw, R.; Ellard, S.; Lee, U.; Hirte, H.; Sederias, J.; Ivy, S.P.; Eisenhauer, E.A. A phase II study of sunitinib in patients with recurrent epithelial ovarian and primary peritoneal carcinoma: An NCIC Clinical Trials Group Study. Ann. Oncol. 2011, 22, 335–340. [Google Scholar] [CrossRef]
- Richardson, D.L.; Sill, M.W.; Coleman, R.L.; Sood, A.K.; Pearl, M.L.; Kehoe, S.M.; Carney, M.E.; Hanjani, P.; Le, L.V.; Zhou, X.C.; et al. Paclitaxel with and without pazopanib for persistent or recurrent ovarian cancer: A randomized clinical trial. JAMA Oncol. 2018, 4, 196–202. [Google Scholar] [CrossRef]
- Friedlander, M.; Hancock, K.C.; Rischin, D.; Messing, M.J.; Stringer, C.A.; Matthys, G.M.; Ma, B.; Hodge, J.P.; Lager, J.J. A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol. Oncol. 2010, 119, 32–37. [Google Scholar] [CrossRef]
- Du Bois, A.; Floquet, A.; Kim, J.-W.; Rau, J.; del Campo, J.M.; Friedlander, M.; Pignata, S.; Fujiwara, K.; Vergote, I.; Colombo, N.; et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J. Clin. Oncol. 2014, 32, 3374–3382. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Berlin, S.; Ivy, P.; Tyburski, K.; Krasner, C.; Zarwan, C.; Berkenblit, A.; Campos, S.; Horowitz, N.; Cannistra, S.A.; et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J. Clin. Oncol. 2009, 27, 5601. [Google Scholar] [CrossRef]
- Liu, J.F.; Barry, W.T.; Birrer, M.; Lee, J.-M.; Buckanovich, R.J.; Fleming, G.F.; Rimel, B.J.; Buss, M.K.; Nattam, S.; Hurteau, J.; et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: A randomised phase 2 study. Lancet Oncol. 2014, 15, 1207–1214. [Google Scholar] [CrossRef]
- Lheureux, S.; Oaknin, A.; Garg, S.; Bruce, J.P.; Madariaga, A.; Dhani, N.C.; Bowering, V.; White, J.; Accardi, S.; Tan, Q.; et al. EVOLVE: A Multicenter Open-Label Single-Arm Clinical and Translational Phase II Trial of Cediranib Plus Olaparib for Ovarian Cancer after PARP Inhibition ProgressionEVOLVE: Post-PARPi Resistance and Treatment of HGSOC. Clin. Cancer Res. 2020, 26, 4206–4215. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.N.; Finkler, N.; Edwards, R.; Garcia, A.; Crozier, M.; Irwin, D.; Barrett, E. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: Results from a phase II multicenter study. Int. J. Gynecol. Cancer 2005, 15, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Hirte, H.; Oza, A.; Swenerton, K.; Ellard, S.; Grimshaw, R.; Fisher, B.; Tsao, M.; Seymour, L. A phase II study of erlotinib (OSI-774) given in combination with carboplatin in patients with recurrent epithelial ovarian cancer (NCIC CTG IND. 149). Gynecol. Oncol. 2010, 118, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.B.; Jimeno, A.; Joly, F.; Katsaros, D.; Coens, C.; Despierre, E.; Marth, C.; Hall, M.; Steer, C.B.; Colombo, N.; et al. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: A European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup study. J. Clin. Oncol. 2014, 32, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Despierre, E.; Vergote, I.; Anderson, R.; Coens, C.; Katsaros, D.; Hirsch, F.R.; Boeckx, B.; Varella-Garcia, M.; Ferrero, A.; Ray-Coquard, I.; et al. Epidermal growth factor receptor (EGFR) pathway biomarkers in the randomized phase III trial of erlotinib versus observation in ovarian cancer patients with no evidence of disease progression after first-line platinum-based chemotherapy. Target. Oncol. 2015, 10, 583–596. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Braden, A.M.; Kolesar, J.M.; Eickhoff, J.C.; Bailey, H.H.; Heideman, J.; Liu, G.; Wisinski, K.B. A phase I study of tivantinib in combination with temsirolimus in patients with advanced solid tumors. Investig. New Drugs 2017, 35, 290–297. [Google Scholar] [CrossRef]
- Kirkland, L.O.; McInnes, C. Non-ATP competitive protein kinase inhibitors as anti-tumor therapeutics. Biochem. Pharmacol. 2009, 77, 1561–1571. [Google Scholar] [CrossRef]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, R.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliverira, C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Kane, R.C.; Farrell, A.T.; Saber, H.; Tang, S.; Williams, G.; Jee, J.M.; Liang, C.; Booth, B.; Nallaperumal, C.; Morse, D.; et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin. Cancer Res. 2006, 12, 7271–7278. [Google Scholar] [CrossRef]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A.; et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 125–134. [Google Scholar] [CrossRef]
- Ji, J.X.; Wang, Y.K.; Cochrane, D.R.; Huntsman, D.G. Clear cell carcinomas of the ovary and kidney: Clarity through genomics. J. Pathol. 2018, 244, 550–564. [Google Scholar] [CrossRef]
- Faivre, S.; Delbaldo, C.; Vera, K.; Robert, C.; Lozahic, S.; Lassau, N.; Bello, C.; Deprimo, S.; Brega, N.; Massimini, G.; et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J. Clin. Oncol. 2006, 24, 25–35. [Google Scholar] [CrossRef]
- Bendell, J.C.; Lim, K.-H.; Burkard, M.E.; Klempner, S.J.; Socinski, M.A.; Gadgeel, S.M.; Reckamp, K.L.; Leland, S.M.; Plessinger, D.; Kunkel, L.A.; et al. CRESTONE: Clinical study of response to seribantumab in tumors with neuregulin-1 (NRG1) fusions—A phase II study of the anti-HER3 mAb for advanced or metastatic solid tumors (NCT04383210). J. Clin. Oncol. 2021, 39. [Google Scholar] [CrossRef]
- Alifrangis, C.; Thornton, A.; Fotopoulou, C.; Krell, J.; Gabra, H. Response to sunitinib (Sutent) in chemotherapy refractory clear cell ovarian cancer. Gynecol. Oncol. Rep. 2016, 18, 42. [Google Scholar] [CrossRef][Green Version]
- Gulia, S.; Ghosh, J.; Bajpai, J.; Rath, S.; Maheshwari, A.; Shylasree, T.S.; Deodhar, K.; Thakur, M.; Gupta, S. Pazopanib and oral cyclophosphamide in women with platinum-resistant or-refractory epithelial ovarian cancer. JCO Glob. Oncol. 2020, 6, 542–547. [Google Scholar] [CrossRef]
- Morgan, R.D.; Banerjee, S.; Hall, M.; Clamp, A.R.; Zhou, C.; Hasan, J.; Orbegoso, C.; Taylor, S.; Tugwood, J.; Lyon, A.R.; et al. Pazopanib and Fosbretabulin in recurrent ovarian cancer (PAZOFOS): A multi-centre, phase 1b and open-label, randomised phase 2 trial. Gynecol. Oncol. 2020, 156, 545–551. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Mack, P.C.; Vokes, E.E.; Longmate, J.; Govindan, R.; Koczywas, M.; Belani, C.P.; Gandara, D.R. Cediranib (AZD2171) for the treatment of recurrent small cell lung cancer (SCLC): A California Consortium phase II study (NCI# 7097). J. Clin. Oncol. 2008, 26, 8078. [Google Scholar] [CrossRef]
- Karakunnel, J.J.; Gulley, J.L.; Arlen, P.M.; Mulquin, M.; Wright, J.J.; Turkbey, I.; Ahlers, C.M.; Figg, W.D.; Dahut, W.L. Phase II trial of cediranib (AZD2171) in docetaxel-resistant, castrate-resistant prostate cancer (CRPC). J. Clin. Oncol. 2008, 26, 5136. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Sorensen, A.G.; di Tomaso, E.; Zhang, W.-T.; Duda, D.G.; Cohen, K.S.; Kozak, K.R.; Cahill, D.P.; Chen, P.-J.; Zhu, M.; et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 2007, 11, 83–95. [Google Scholar] [CrossRef]
- Sridhar, S.S.; Mackenzie, M.J.; Hotte, S.J.; Mukherjee, S.D.; Kollmannsberger, C.; Haider, M.A.; Chen, X.; Wang, L.; Srinivasan, R.; Ivy, S.P.; et al. Activity of cediranib (AZD2171) in patients (pts) with previously untreated metastatic renal cell cancer (RCC). A phase II trial of the PMH Consortium. J. Clin. Oncol. 2008, 26, 5047. [Google Scholar] [CrossRef]
- Drevs, J.; Siegert, P.; Medinger, M.; Mross, K.; Strecker, R.; Zirrgiebel, U.; Harder, J.; Blum, H.; Rpbertson, J.; Jürgensmeier, J.M.; et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2007, 25, 3045–3054. [Google Scholar] [CrossRef]
- Gelmon, K.A.; Tischkowitz, M.; Mackay, H.; Swenerton, K.; Robidoux, A.; Tonkin, K.; Hirte, H.; Huntsam, D.; Clemons, M.; Gilks, B.; et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: A phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011, 12, 852–861. [Google Scholar] [CrossRef]
- Liu, J.; Barry, W.; Birrer, M.; Lee, J.-M.; Buckanovich, R.; Fleming, G.; Rimel, B.J.; Buss, M.K.; Nattam, S.; Hurteau, J.; et al. Overall survival and updated progression-free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer. Ann. Oncol. 2019, 30, 551–557. [Google Scholar] [CrossRef]
- Blank, S.V.; Christos, P.; Curtin, J.P.; Goldman, N.; Runowicz, C.D.; Sparano, J.A.; Liebes, L.; Chen, H.X.; Muggia, F.M. Erlotinib added to carboplatin and paclitaxel as first-line treatment of ovarian cancer: A phase II study based on surgical reassessment. Gynecol. Oncol. 2010, 119, 451–456. [Google Scholar] [CrossRef]
- Munshi, N.; Jeay, S.; Li, Y.; Chen, C.-R.; France, D.S.; Ashwell, M.A.; Hill, J.; Moussa, M.M.; Leggett, D.S.; Li, C.J. ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosine kinase with antitumor activity. Mol. Cancer Ther. 2010, 9, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Rimassa, L.; Assenat, E.; Peck-Radosavljevic, M.; Pracht, M.; Zagonel, V.; Mathurin, P.; Caremoli, E.R.; Porta, C.; Daniele, B.; Bolondi, L.; et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): A final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018, 19, 682–693. [Google Scholar] [CrossRef]
- Schoeberl, B.; Pace, E.A.; Fitzgerald, J.B.; Harms, B.D.; Xu, L.; Nie, L.; Linggi, B.; Kalra, A.; Paragas, V.; Bukhalid, R.; et al. Therapeutically targeting ErbB3: A key node in ligand-induced activation of the ErbB receptor-PI3K axis. Sci. Signal. 2009, 2, ra31. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Schmitz, K.R.; Jeffrey, P.D.; Wiltzius, J.J.; Kussie, P.; Ferguson, K.M. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 2005, 7, 301–311. [Google Scholar] [CrossRef]
- Ghosh, R.; Narasanna, A.; Wang, S.E.; Liu, S.; Chakrabarty, A.; Balko, J.M.; González-Angulo, A.M.; Mills, G.B.; Penuel, E.; Winslow, J.; et al. Trastuzumab has preferential activity against breast cancers driven by HER2 homodimers. Cancer Res. 2011, 71, 1871–1882. [Google Scholar] [CrossRef]
- Carter, P.; Presta, L.; Gorman, C.M.; Ridgway, J.B.; Henner, D.; Wong, W.L.; Rowland, A.M.; Kotts, C.; Carver, M.E.; Shepard, H.M. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl. Acad. Sci. USA 1992, 89, 4285–4289. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use. Avastin: Bevacizumab Assessment Report: European Medicines Agency. 2011. Available online: https://www.ema.europa.eu/en/documents/overview/avastin-epar-summary-public_en.pdf (accessed on 10 January 2022).
- The Therapeutic Goods Administration. Australian Public Assessment for Bevacizumab [Internet]: Australian Government. 2020. Available online: https://www.tga.gov.au/sites/default/files/auspar-bevacizumab-200226.pdf (accessed on 10 January 2022).
- Food and Drugs Administration. Avastin (Bevacizumab) South; Genentech, Inc.: San Francisco, CA, USA, 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125085s323lbl.pdf (accessed on 10 January 2022).
- Wild, R.; Dings, R.P.; Subramanian, I.; Ramakrishnan, S. Carboplatin selectively induces the VEGF stress response in endothelial cells: Potentiation of antitumor activity by combination treatment with antibody to VEGF. Int. J. Cancer 2004, 110, 343–351. [Google Scholar] [CrossRef]
- Chan, J.K.; Brady, M.F.; Penson, R.T.; Huang, H.; Birrer, M.J.; Walker, J.L.; DiSilvestro, P.A.; Rubin, S.C.; Martin, L.P.; Davidson, S.A.; et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N. Engl. J. Med. 2016, 374, 738–748. [Google Scholar] [CrossRef]
- Haunschild, C.E.; Tewari, K.S. Bevacizumab use in the frontline, maintenance and recurrent settings for ovarian cancer. Future Oncol. 2020, 16, 225–246. [Google Scholar] [CrossRef]
- Hinde, S.; Epstein, D.; Cook, A.; Embleton, A.; Perren, T.; Sculpher, M. The cost-effectiveness of bevacizumab in advanced ovarian cancer using evidence from the ICON7 trial. Value Health 2016, 19, 431–439. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J. Clin. Oncol. 2019, 37, 2317. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef]
- Tentori, L.; Lacal, P.M.; Muzi, A.; Dorio, A.S.; Leonetti, C.; Scarsella, M.; Ruffini, F.; Xu, W.; Min, W.; Stoppacciaro, A.; et al. Poly (ADP-ribose) polymerase (PARP) inhibition or PARP-1 gene deletion reduces angiogenesis. Eur. J. Cancer 2007, 43, 2124–2133. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Mirza, M.R.; Lundqvist, E.Å.; Birrer, M.J.; Christensen, R.D.; Nyvang, G.-B.; Malander, S.; Anttila, M.; Werner, T.L.; Lund, B.; Lindahl, G.; et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): A randomised, phase 2, superiority trial. Lancet Oncol. 2019, 20, 1409–1419. [Google Scholar] [CrossRef]
- Lu, J.-F.; Bruno, R.; Eppler, S.; Novotny, W.; Lum, B.; Gaudreault, J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother. Pharmacol. 2008, 62, 779–786. [Google Scholar] [CrossRef]
- Smerdel, M.; Steffensen, K.; Waldstrøm, M.; Brandslund, I.; Jakobsen, A. The predictive value of serum VEGF in multiresistant ovarian cancer patients treated with bevacizumab. Gynecol. Oncol. 2010, 118, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Maltese, G.; Sabatucci, I.; Cresta, S.; Matteo, C.; Ceruti, T.; D’Incalci, M.; Zucchetti, M.; Raspagliesi, F.; Sonetto, C.; et al. Phase I Study of Rucaparib in Combination with Bevacizumab in Ovarian Cancer Patients: Maximum Tolerated Dose and Pharmacokinetic Profile. Target. Oncol. 2021, 16, 59–68. [Google Scholar] [CrossRef]
- Hardesty, M.M.; Krivak, T.C.; Wright, G.S.; Hamilton, E.; Fleming, E.L.; Belotte, J.; Keeton, E.K.; Wang, P.; Gupta, D.; Clements, A.; et al. OVARIO phase II trial of combination niraparib plus bevacizumab maintenance therapy in advanced ovarian cancer following first-line platinum-based chemotherapy with bevacizumab. Gynecol. Oncol. 2022, 166, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Shin, D.M. Monoclonal antibodies to target epidermal growth factor receptor–positive tumors: A new paradigm for cancer therapy. Cancer 2002, 94, 1593–1611. [Google Scholar] [CrossRef] [PubMed]
- Schilder, R.J.; Pathak, H.B.; Lokshin, A.E.; Holloway, R.W.; Alvarez, R.D.; Aghajanian, C.; Min, H.; Devarajan, K.; Ross, E.; Drescher, C.W.; et al. Phase II trial of single agent cetuximab in patients with persistent or recurrent epithelial ovarian or primary peritoneal carcinoma with the potential for dose escalation to rash. Gynecol. Oncol. 2009, 113, 21–27. [Google Scholar] [CrossRef]
- Secord, A.A.; Blessing, J.A.; Armstrong, D.K.; Rodgers, W.H.; Miner, Z.; Barnes, M.N.; Lewandowski, G.; Mannel, R.S.; Gynecologic Oncology Group. Phase II trial of cetuximab and carboplatin in relapsed platinum-sensitive ovarian cancer and evaluation of epidermal growth factor receptor expression: A Gynecologic Oncology Group study. Gynecol. Oncol. 2008, 108, 493–499. [Google Scholar] [CrossRef]
- Franklin, M.C.; Carey, K.D.; Vajdos, F.F.; Leahy, D.J.; de Vos, A.M.; Sliwkowski, M.X. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004, 5, 317–328. [Google Scholar] [CrossRef]
- Takai, N.; Jain, A.; Kawamata, N.; Popoviciu, L.M.; Said, J.W.; Whittaker, S.; Isao Miyakawa, I.; Agus, D.B.; Koeffler, H.P. 2C4, a monoclonal antibody against HER2, disrupts the HER kinase signaling pathway and inhibits ovarian carcinoma cell growth. Cancer 2005, 104, 2701–2708. [Google Scholar] [CrossRef]
- Gordon, M.S.; Matei, D.; Aghajanian, C.; Matulonis, U.A.; Brewer, M.; Fleming, G.F.; Hainsworth, J.D.; Garcia, A.A.; Pegram, M.D.; Schilder, R.J.; et al. Clinical activity of pertuzumab (rhuMAb 2C4), a HER dimerization inhibitor, in advanced ovarian cancer: Potential predictive relationship with tumor HER2 activation status. J. Clin. Oncol. 2006, 24, 4324–4332. [Google Scholar] [CrossRef]
- Makhija, S.; Amler, L.C.; Glenn, D.; Ueland, F.R.; Gold, M.A.; Dizon, D.S.; Paton, V.; Lin, C.Y.; Januario, T.; Ng, K.; et al. Clinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. J. Clin. Oncol. 2010, 28, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Kurzeder, C.; Bover, I.; Marmé, F.; Rau, J.; Pautier, P.; Colombo, N.; Lorusso, D.; Ottevanger, P.; Bjurberg, M.; Marth, C.; et al. Double-Blind, placebo-controlled, randomized phase III trial evaluating pertuzumab combined with chemotherapy for low tumor human epidermal growth factor receptor 3 mRNA–expressing platinum-resistant ovarian cancer (PENELOPE). J. Clin. Oncol. 2016, 34, 2516–2525. [Google Scholar] [CrossRef]
- Tai, W.; Mahato, R.; Cheng, K. The role of HER2 in cancer therapy and targeted drug delivery. J. Control. Release 2010, 146, 264–275. [Google Scholar] [CrossRef]
- Bookman, M.A.; Darcy, K.M.; Clarke-Pearson, D.; Boothby, R.A.; Horowitz, I.R. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: A phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 2003, 21, 283–290. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Guastalla, J.P.; Allouache, D.; Combe, M.; Weber, B.; Cretin, J.; Cure, H.; Nunhuck, S.; Paraiso, D.; Mousseau, M.; et al. HER2 overexpression/amplification and trastuzumab treatment in advanced ovarian cancer: A GINECO phase II study. Clin. Ovarian Cancer 2009, 2, 17–22. [Google Scholar] [CrossRef]
- Sheng, Q.; Liu, X.; Fleming, E.; Yuan, K.; Piao, H.; Chen, J.; Moustafa, Z.; Thomas, R.K.; Greulich, H.; Schinzel, A.; et al. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer Cell 2010, 17, 298–310. [Google Scholar] [CrossRef]
- Jonna, S.; Feldman, R.A.; Swensen, J.; Gatalica, Z.; Korn, W.M.; Borghaei, H.; Ma, P.C.; Nieva, J.J.; Spira, A.I.; Vanderwalde, A.M.; et al. Detection of NRG1 Gene Fusions in Solid TumorsNRG1 Fusions in Solid Tumors. Clin. Cancer Res. 2019, 25, 4966–4972. [Google Scholar] [CrossRef]
- Liu, J.F.; Ray-Coquard, I.; Selle, F.; Poveda, A.M.; Cibula, D.; Hirte, H.; Hilpert, F.; Raspagliesi, F.; Gladieff, L.; Harter, P.; et al. Randomized phase II trial of seribantumab in combination with paclitaxel in patients with advanced platinum-resistant or-refractory ovarian cancer. J. Clin. Oncol. 2016, 34, 4345. [Google Scholar] [CrossRef]
- Odintsov, I.; Lui, A.J.; Sisso, W.J.; Gladstone, E.; Liu, Z.; Delasos, L.; Kurth, R.I.; Sisso, E.M.; Vojnic, M.; Khodos, I.; et al. The anti-HER3 mAb seribantumab effectively inhibits growth of patient-derived and isogenic cell line and xenograft models with oncogenic NRG1 fusions. Clin. Cancer Res. 2021, 27, 3154–3166. [Google Scholar] [CrossRef]
- Reyes, H.D.; Thiel, K.W.; Carlson, M.J.; Meng, X.; Yang, S.; Stephan, J.-M.; Leslie, K.L. Comprehensive profiling of EGFR/HER receptors for personalized treatment of gynecologic cancers. Mol. Diagn. Ther. 2014, 18, 137–151. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.; Wesolowski, R.; Ahn, D.H.; Wu, C.; Mortazavi, A.; Lustberg, M.; Ramaswamy, B.; Fowler, J.; Wei, L.; Overholder, J.; et al. Phase I Immunotherapy Trial with Two Chimeric HER-2 B-Cell Peptide Vaccines Emulsified in Montanide ISA 720VG and Nor-MDP Adjuvant in Patients with Advanced Solid TumorsPhase I Immunotherapy Trial with Two B-cell Vaccines. Clin. Cancer Res. 2019, 25, 3495–3507. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L.; Dang, Y.; Coveler, A.L.; Higgins, D.; Childs, J.; Salazar, L.G. Final report and long-term outcomes: Phase I trial of a HER2 intracellular plasmid-based vaccine in HER2+ advanced stage breast cancer. J. Clin. Oncol. 2021, 39, 2619. [Google Scholar] [CrossRef]
- Urban, N.; McIntosh, M.W.; Andersen, M.; Karlan, B.Y. Ovarian cancer screening. Hematol. Oncol. Clin. N. Am. 2003, 17, 989–1005. [Google Scholar] [CrossRef]
- Buamah, P. Benign conditions associated with raised serum CA-125 concentration. J. Surg. Oncol. 2000, 75, 264–265. [Google Scholar] [CrossRef]
- Scholler, N.; Lowe, K.A.; Bergan, L.A.; Kampani, A.V.; Ng, V.; Forrest, R.M.; Thorpe, J.D.; Gross, J.A.; Garvik, B.M.; Drapkin, R.; et al. Use of yeast-secreted in vivo biotinylated recombinant antibodies (Biobodies) in bead-based ELISA. Clin. Cancer Res. 2008, 14, 2647–2655. [Google Scholar] [CrossRef]
- Huhtinen, K.; Suvitie, P.; Hiissa, J.; Junnila, J.; Huvila, J.; Kujari, H.; Setälä, M.; Härkki, P.; Jalkanen, J.; Fraser, J.; et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br. J. Cancer 2009, 100, 1315–1319. [Google Scholar] [CrossRef]
| Drug | Trial ID | Clinical Trial Phase | Cohort Size | Dose | Side Effects | Outcome | References |
|---|---|---|---|---|---|---|---|
| Sorafenib | NCT00093626 | Phase II | n = 71 | 400 mg twice daily in 4-week cycles until PD or intolerable toxicity | Grades 1–3 dermatologic (76%) and gastrointestinal (79%) AEs most common. | 3.4% PR and 33.9% SD after 6 months | [60] |
| Phase II | n = 85 | Paclitaxel and carboplatin every 21 days for a maximum of 6 cycles, with or without 400 mg sorafenib twice daily. | Increased toxicities with sorafenib. Grade 3/4 non-haematological skin toxicities, hand-foot syndrome, mucositis, and hypertension. | No significant difference in OR, PFS or OS | [61] | ||
| 2006-004644-24 | Phase II | n = 4 | 400 mg twice daily alongside carboplatin/paclitaxel schedule. | Fatigue, anorexia, diarrhoea, rash, and hand-foot skin reaction. | Study terminated on the recommendation of independent safety monitoring board. | [62] | |
| NCT00526799 | Phase I/II | Phase I: n = 16. Phase II: n = 14 | Phase I: 400 mg and 800 mg daily with weekly reducing topotecan dosage. Phase II: 400 mg sorafenib daily with topotecan 3.5 mg/m2 weekly. | Grade 3/4 AEs: leukopenia/neutropenia, thrombocytopenia, anaemia, fatigue, nausea, and vomiting | Phase I: 4 PR. Phase II: 1 PR. OR: 16.7%. 46.7% SD with median OS 14.0 mths | [63] | |
| Phase II | n = 43 | 400 mg twice daily with cyclic dosage of gemcitabine | Hand-foot syndrome, fatigue, diarrhoea, and hypokalaemia. | 4.7% PR; 2.3% SD with median PFS and OS 5.4- and 13.0 mths, respectively. | [64] | ||
| Sunitinib | 2007-003089-16 | Phase II | n = 73 | Either 50 mg once daily for 4 weeks in 6-week cycles or 37.5 mg once daily continuously | Grade 3/4 AEs: 46 in continuous and 60 in non-continuous groups. Includes: haematologic aberrations, and gastro-intestinal syndrome. | OR was 5.4% and 16.7% in continuous and non-continuous dosage groups, respectively. No significant difference in PFS and OS. | [65] |
| NCT00979992 | Phase II | n = 30 | 50 mg once daily for 4 weeks in 6-week cycles. | Grade 3 AEs: fatigue, haematologic aberrations, abdominal pain and hypertension. Grade 4–5 AEs: acute kidney injury, allergic reactions, haematologic aberrations and stroke, | 6.7% had PR or CR. Median PFS and OS was 2.7 and 12.8 mths, respectively. | [66] | |
| NCT00979992 | Phase II | n = 30 | 50 mg once daily for 4 weeks in 6-week cycles. | Grade 3 AEs of fatigue, mucositis, nausea, hand-foot syndrome, diarrhoea, and hypertension. No grade 4 AEs. | Median overall PFS of 4.1 mths 16 with SD and 1 PR | [67] | |
| Pazopanib | NCT01468909 | Phase II | n = 106 | 800 mg every 28-days, with weekly paclitaxel chemotherapy | Grade 3/4 AEs include: neutropenia and hypertension. | No significant change in median PFS or OS. 66% PD in placebo patients compared to 31.5% receiving pazopanib. | [68] |
| NCT0281632 | Phase II | n = 36 | 800 mg once daily | Grade 3 ALT & AST elevation in 8%. Grade 4 peripheral oedema in 2.8% | Decreased CA-125 in 31% patients. Median response time and duration was 29- and 113-days, respectively. OR: 18% | [69] | |
| NCT00866697 | Phase III | n = 940 | 800 mgonce daily, 28-days following first-line chemotherapy | Grade 3/4 AEs of hypertension (30.8%), neutropenia (9.9%), liver-related toxicity (9.4%),and diarrhoea (8.2%). | PFS for pazopanib and placebo was 17.9 and 12.3 mths respectively (p = 0.0021). No signifi-cant difference in OS. | [70] | |
| Cediranib | NCT00275028 | Phase II | n = 46 | 45 mg daily, lowered to 30 mg daily | AEs (all grades) include: diarrhoea, voice changes, hypertension, fatigue, headaches, hypothyroidism, mucositis, and nausea | PR was observed in 17% of patients, with a further 13% with SD. 45% had disease progression. | [71] |
| NCT01116648 | Phase II | n = 44 | Olaparib200 mgandCediranib 30 mgtwice daily | Grade 3 AEs: fatigue, diarrhoea and hypertension. Grade 4 AEs: hypertensive crisis and myelodysplastic syndrome | Median PFSwas 16.5 mthsfor combination therapy vs.8.2 mths in control(p = 0.007). OS at24 mthsfor combination vs.control was 81% and 65% respectively. | [72] | |
| NCT02681237 | Phase II | n = 34 | 200 mg Olaparib combined with 20 mg Cediranib twice daily | Most common all-grade AEs include diarrhoea, nausea, vomiting, and fatigue, mainly all grade 1 or 2. | 2 PR and 4 SD (6 total) in platinum resistant patients, 9 SD but no OR in platinum sensitive patients. 1-year OS for platinum sensitive and resistant was 82% and 69%, respectively. | [73] | |
| Erlotinib | Phase II | n = 28 | 150 mg daily with carboplatin and paclitaxel | Grade 3 diarrhoea, skin and subcutaneous disorders 1 case of grade 4 neutropenia | 42.9% SD and 5.7% PR with PFS and 1-yr survival rate of 8 mths and 35.5%, respectively. 28.6% CR in optimally debulked patients with median PFS and OS of 80.5 and 53.5 mths, respectively. However not clinically significant | [74] | |
| NCT00030446 | Phase II | n = 50 | 150 mg orally with carboplatin and paclitaxel | Grade 3/4 dry skin (1.7%), abdominal pain (2.4%) and increase of gamma-glutamyl transpeptidase (3.4%) | Platinum sensitive patients had 10% CR and 47% PR Platinum resistant patients had 7% PR only No improvement in PFS or OS. | [75] | |
| NCT00263822 | Phase III | n = 835 | 150 mg daily (orally) as maintenance following chemotherapy | Diarrhoea, loss of appetite, nausea/vomiting, and fatigue. | No improvement in PFS or OS | [76,77] | |
| Tivantinib | NCT01625156 | Phase I | n = 6 | 120 mg Tivantinib twice daily with weekly 20 mg IV temsirolimus, increasing exponentially after 28-day cycles. | Grade 2 AEs include: anaemia, fatigue, anorexia, and hypoalbuminemia. Grade 3 AEs: anaemia, hypophosphatemia, hypertension, and hyponatremia. Grade 4 neutropenia in 2 patients | 1 PR 1 SD | [78] |
| Drug | Trial ID | Clinical Trial Phase | Cohort Size | Dose | Side Effects | Outcome | References |
|---|---|---|---|---|---|---|---|
| Bevacizumab | 2005-003929-22 | Phase III | n = 1528 | 7.5 mg/kg of body weight in combination with carboplatin/paclitaxel chemotherapy, every 3 weeks for 12 cycles. | Adverse effects include grade 1–2 muco-cutaneous bleeding (36%), grade 2+ hypertension (18%), grade 3 thrombo-embolic events (7%), and gastrointestinal perforations (1%). | Significant improvement in PFS with 16 mths, compared to control group 10.5 mths (p < 0.001). Improved OS, 39.5 mths | [110] |
| NCT0026287 | Phase III | n = 1873 | 6x 21-day cycles of carboplatin/paclitaxel chemotherapy with the addition of 15 mg/kg body weight bevacizumab or placebo | Most common AEs include pain, hypertension, and neutropenia, all ≥ grade | PFS improved by 3.8 mths with continuous cohort. No significant difference in median OS. Increase OS in grade IV EOC receiving continuous cohort (42.8 mths) vs. grade IV EOC control (32.6 mths) | [111] | |
| NCT00976911 | Phase III | n = 361 | Paclitaxel, PLD andtopotecantreatments +/- bevacizumab10 mg/kg body weight every 2 weeks | Increased incidence of hypertension and proteinuria ≥grade 2 was observed with bevacizumab, along with ≥grade 2 GI perforation and fistulas | Median PFS increased to 6.7 mths for continuous (p < 0.001). Median OS increased to 16.6 mths (p < 0.174). OR of 27.3% with continuous (p = 0.001) | [112] | |
| NCT02477644 | Phase III | n = 806 | Olaparib (300 mg twice daily) with and without bevacizumab (15 mg/kg body weight every 3 weeks) | The most common AEs experienced in the experimental group were fatigue, nausea, and anaemia (all grades) | Increased PFS with combination, 22.1 mths vs. 16.6 mths without (p < 0.001) | [114] | |
| NCT02354131 | Phase II | n = 97 | Niraparib (300 mg) once daily, with bevacizumab (15 mg/kg) once every 3 weeks until disease progression | Combination therapy associated with AEs proteinuria (21%) and hypertension (56%), both of any grade) | Increase PFS 11.9 mths vs. 5.5 mths with and without bevacizumab respectively (p < 0.001) | [115] | |
| Cetuximab | NCT00082212 | Phase II | n = 25 | 21-day cycles of 400 mg/m2 initial dose, followed by weekly 250 mg/m2 doses of cetuximab. | Grade 3 AEs of arthralgia, headache and acneiform rash were recorded. Chills, nausea, stomatitis, diarrhoea, and constipation also were noted, at all grades. | 4% PR with another 36% SD. PFS 2.1 with a 1-year survival rate of 54.8% | [121] |
| NCT00086892 | Phase II | n = 29 | Cyclic 400 mg/m2 and 250 mg/m2, combined with carboplatin | Most common AEs grade 3 dermatologic toxicity (32%), thrombocytopenia (14%) and metabolic toxicity (14%). | CR and PR was observed in 11.5% and 23% of patients respectively. A further 30.8% had SD. 11.5% had PD, and 30.8% not evaluated. | [122] | |
| Pertuzumab | Phase II | n = 123 | Cohort 1- 840 mg loading dose, then 420 mg on day 1 of each 3-week cycle. Cohort 2- 1050 mg on day 1 of each 3-week cycle | Most common AE was diarrhoea, mainly grade 1–2. Other AEs include fatigue, rash/dermatitis, nausea, and abdominal pain across both cohorts. | PR in 3.6% and 4.8% for cohort 1 and 2 respectively. 4 patients in each cohort had SD. Median PFS of 6.6 weeks. Median OS 52.7 weeks | [125] | |
| NCT00096993 | Phase II | n = 130 | Gemcitabine 800 mg/m2 on days 1 and 8 of 21-day cycle with Pertuzumab, loading dose 840 mg followed by 420 mg every 3 weeks. | Higher incidence of AEs in pertuzumab group, including fatigue, nausea, diarrhoea, and backpain. Increased grade 3–4 neutropenia, thrombocytopenia, back pain, and diarrhoea in pertuzumab group. | Median PFS higher in combination group than control (2.9 mths vs. 2.6 mths, p = 0.07). 13.8% PR observed with combination therapy. Median OS similar between groups (13.1 mths placebo vs. 13.0 mths combination, p = 0.65) Increased benefit of combination therapy with HER3 expression (p = 0.0002) | [126,127] | |
| NCT01684878 | Phase III | n = 156 | Chemotherapy delivered (topotecan, paclitaxel or gemcitabine) with pertuzumab 840 mg loading dose followed by 420 mg every 3 weeks. | Common AEs for combination therapy were fatigue, nausea, diarrhoea, neutropenia, and anaemia. | Median PFS 4.3 mths (p = 0.14). PFS benefit for platinum resistant patients as but not in platinum-refractory (p = 0.02) | [118,128] | |
| Trastuzumab | Phase II | n = 41 | Initial dose of 4 mg/kg, then weekly at 2 mg/kg intravenously | Mild toxicities, tolerated well by patients with no treatment related deaths. | OR of 7.3%. 1 CR and 2 PRs recorded. PFS was 2.0 mths | [129] | |
| Phase II | n = 320 | Paclitaxel and carboplatin with trastuzumab in 8 mg/kg initial dose, followed by 6 mg/kg for subsequent cycles, every 3 weeks. | Most common AEs were febrile neutropenia, grade 3 infection, and grade 2 neurotoxicity | 3 patients had CR, with a further 3 observed to have SD. Median PFS and OS were 2.9- (range: 1.5–44.2) and 12.3 mths (range:1.9–44.2), respectively. | [130] | ||
| Seribantumab | NCT01447706 | Phase II | n = 223 | Paclitaxel combined with either Seribantumab (40 mg/kg initial load, followed by 20 mg/kg weekly) or placebo | Increase in AEs observed in combination therapy, including diarrhoea, fatigue, nausea abdominal pain, hypokalaemia, and anaemia. | Median OS 13.75 compared to 10.12 for control group (p = 0.972). Median PFS with seribantumab 3.75 (p = 0.864). Identified potential biomarkers | [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rendell, A.; Thomas-Bland, I.; McCuish, L.; Taylor, C.; Binju, M.; Yu, Y. Targeting Tyrosine Kinases in Ovarian Cancer: Small Molecule Inhibitor and Monoclonal Antibody, Where Are We Now? Biomedicines 2022, 10, 2113. https://doi.org/10.3390/biomedicines10092113
Rendell A, Thomas-Bland I, McCuish L, Taylor C, Binju M, Yu Y. Targeting Tyrosine Kinases in Ovarian Cancer: Small Molecule Inhibitor and Monoclonal Antibody, Where Are We Now? Biomedicines. 2022; 10(9):2113. https://doi.org/10.3390/biomedicines10092113
Chicago/Turabian StyleRendell, Aimee, Isobel Thomas-Bland, Lee McCuish, Christopher Taylor, Mudra Binju, and Yu Yu. 2022. "Targeting Tyrosine Kinases in Ovarian Cancer: Small Molecule Inhibitor and Monoclonal Antibody, Where Are We Now?" Biomedicines 10, no. 9: 2113. https://doi.org/10.3390/biomedicines10092113
APA StyleRendell, A., Thomas-Bland, I., McCuish, L., Taylor, C., Binju, M., & Yu, Y. (2022). Targeting Tyrosine Kinases in Ovarian Cancer: Small Molecule Inhibitor and Monoclonal Antibody, Where Are We Now? Biomedicines, 10(9), 2113. https://doi.org/10.3390/biomedicines10092113






