CT-Based Radiomics to Predict KRAS Mutation in CRC Patients Using a Machine Learning Algorithm: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
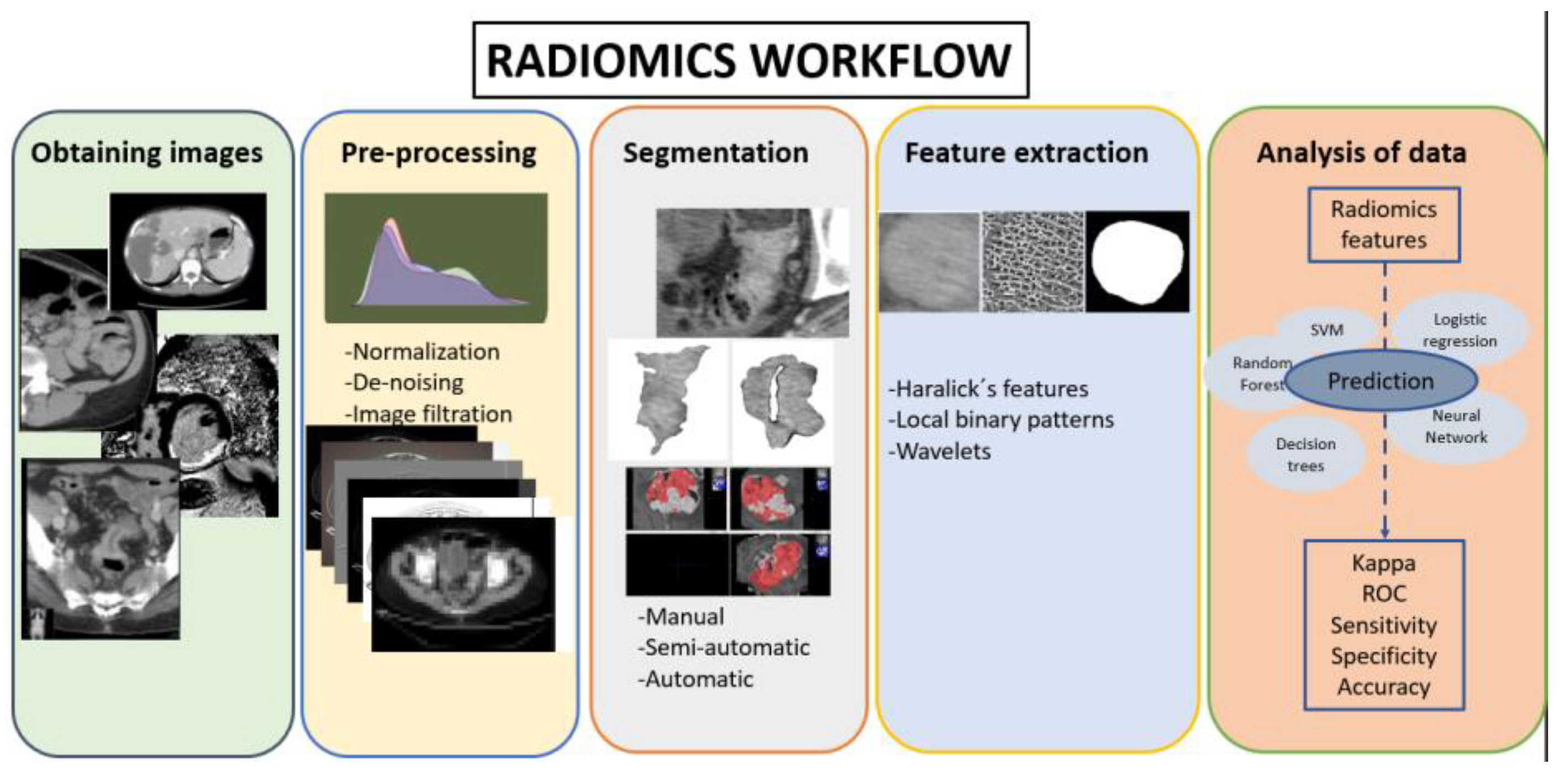
2.1. Radiomics Workflow
2.2. Patient Selection and Obtaining Imaging
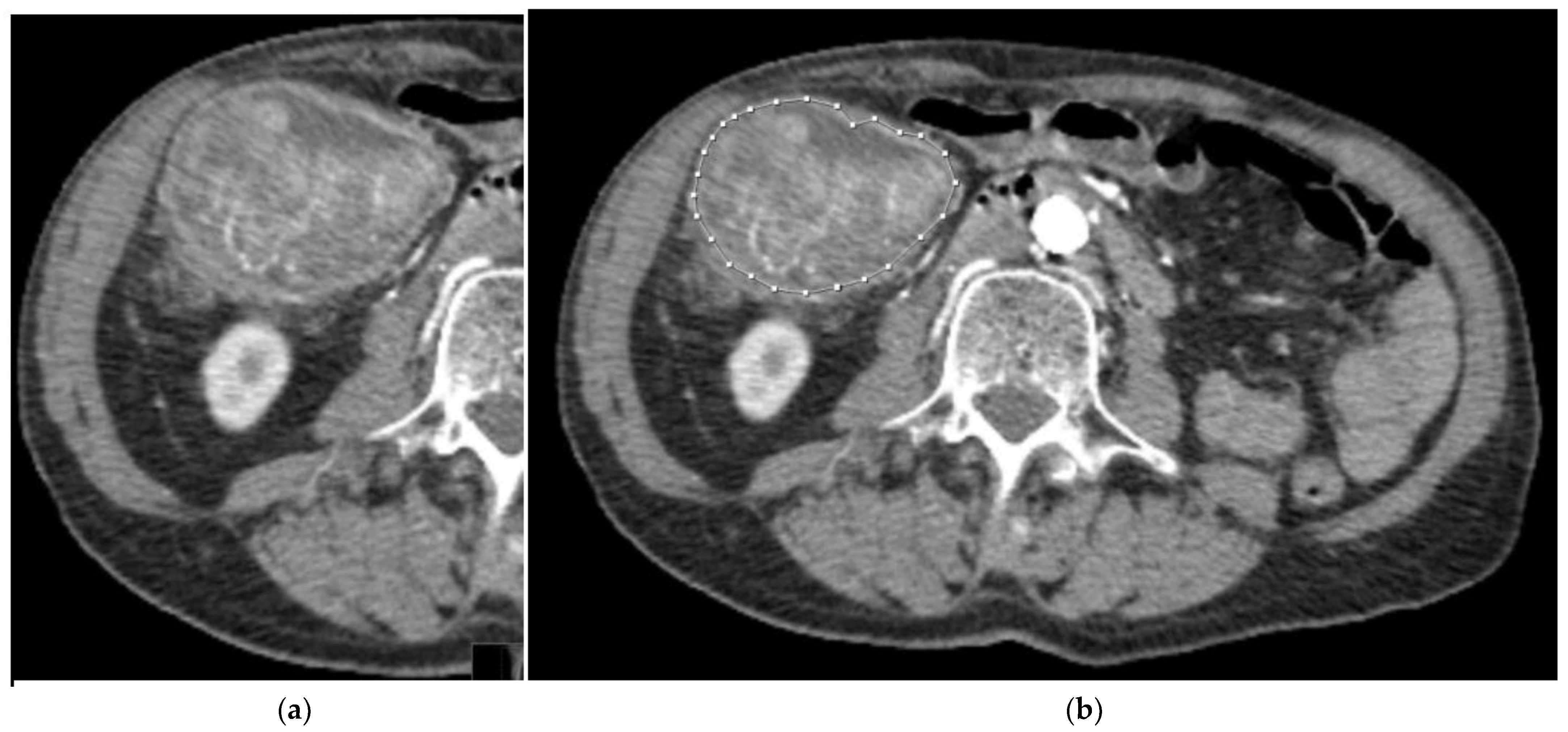
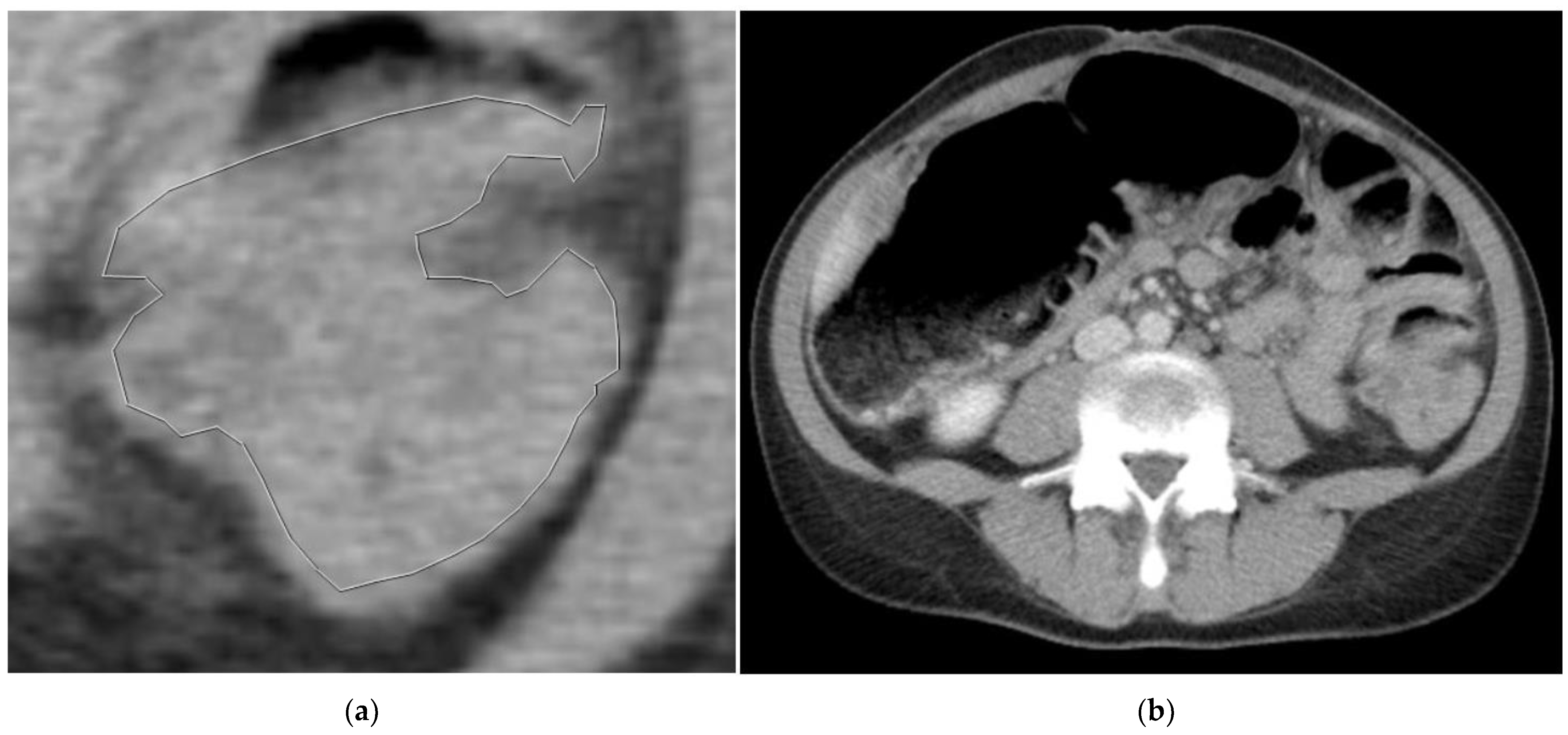
2.3. Segmentation
2.4. Pre-Processing and Feature Extraction
- Applying Haralick coefficients over the different wavelet decomposition levels using a distance d = 1, due to the scale being implicitly included in the downsampling. We computed four vectors, called WDCFfm, varying the number of Haralick coefficients m = {4, 5} and the type of decomposition f = {LL, All} using a distance d = 1: {WDCFfm, f = LL, All, m = 4, 5}.
- Another way to capture multiscale information would be calculating the cooccurrence matrices on the first level of decomposition. We computed the following six vectors (WCFdm) varying the distance d = {1, 2, 3} to calculate the cooccurrences matrices and the number of Haralick’s coefficients m = {4, 5}: {WCFdm, d = 1, 2, 3, m = 4, 5}.
- The multiscalar information can also be captured by calculating the LBP signature using a radius of one pixel over low-pass wavelet decompositions of the original image. Specifically, the four vectors LBPs considering one level of wavelet decomposition s = {1, 2, 3} were computed, developing feature vectors of 10 features. The concatenation of the three previous vectors is called LBP123 with 30 features: {LBPs, s = 1, 2, 3, 123}.
2.5. Machine Learning Models
3. Experimental Setup and Results
3.1. Experimental Setup
- Vector clinical, containing information related to the patient’s life and their histopathology status before any treatment, composed of the following nine values: liver metastasis, pulmonary metastasis, sex (dicotomical variable), age, location of the tumor, T staging (0, 1, 2, 3, or 4), N staging (0, 1, or 2), M staging (0 or 1), and tumor differentiation (stages 1 to 4).
- Texture feature vectors, with features extracted from each slice of the CT as explained in Section 2.4; in our case, three slices of the tumor for each patient (56 patients multiplied by three cuts per patient).
3.2. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, M.; Brunner, V.; Tschurtschenthaler, M. Microbiota and Colorectal Cancer: From Gut to Bedside. Front. Pharmacol. 2021, 12, 760280. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J.D. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef]
- Nosho, K.; Sukawa, Y.; Adachi, Y.; Ito, M.; Mitsuhashi, K.; Kurihara, H.; Kanno, S.; Yamamoto, I.; Ishigami, K.; Igarashi, H.; et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J. Gastroenterol. 2016, 22, 557–566. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Vergara, É.; Alvis, N.; Suárez, A. ¿Existen ventajas clínicas al evaluar el estado de los genes KRAS, NRAS, BRAF, PIK3CA, PTEN y HER2 en pacientes con cáncer colorrectal? Rev. Colomb. Cirugía 2017, 32, 45–55. [Google Scholar] [CrossRef]
- Currais, P.; Rosa, I.; Claro, I. Colorectal cancer carcinogenesis: From bench to bedside. World J. Gastrointest. Oncol. 2022, 14, 654–663. [Google Scholar] [CrossRef]
- Afrăsânie, V.-A.; Marinca, M.V.; Alexa-Stratulat, T.; Gafton, B.; Păduraru, M.; Adavidoaiei, A.M.; Miron, L.; Rusu, C. KRAS, NRAS, BRAF, HER2 and microsatellite instability in metastatic colorectal cancer—Practical implications for the clinician. Radiol. Oncol. 2019, 53, 265–274. [Google Scholar] [CrossRef]
- Garcia-Carbonero, N.; Martinez-Useros, J.; Li, W.; Orta, A.; Perez, N.; Carames, C.; Hernandez, T.; Moreno, I.; Serrano, G.; Garcia-Foncillas, J. KRAS and BRAF Mutations as Prognostic and Predictive Biomarkers for Standard Chemotherapy Response in Metastatic Colorectal Cancer: A Single Institutional Study. Cells 2020, 9, 219. [Google Scholar] [CrossRef]
- Zhu, G.; Pei, L.; Xia, H.; Tang, Q.; Bi, F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer 2021, 20, 143. [Google Scholar] [CrossRef]
- Schirripa, M.; Nappo, F.; Cremolini, C.; Salvatore, L.; Rossini, D.; Bensi, M.; Businello, G.; Pietrantonio, F.; Randon, G.; Fucà, G.; et al. KRAS G12C Metastatic Colorectal Cancer: Specific Features of a New Emerging Target Population. Clin. Color. Cancer 2020, 19, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Limkin, E.J.; Sun, R.; Dercle, L.; Zacharaki, E.I.; Robert, C.; Reuzé, S.; Schernberg, A.; Paragios, N.; Deutsch, E.; Ferté, C. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann. Oncol. 2017, 28, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Porto-Álvarez, J.; Barnes, G.T.; Villanueva, A.; García-Figueiras, R.; Baleato-González, S.; Zapico, E.H.; Souto-Bayarri, M. Digital Medical X-ray Imaging, CAD in Lung Cancer and Radiomics in Colorectal Cancer: Past, Present and Future. Appl. Sci. 2023, 13, 2218. [Google Scholar] [CrossRef]
- Sonka, M.; Hlavac, V.; Boyle, R. Image Processing, Analysis and Machine Vision; Springer: Boston, MA, USA, 2008. [Google Scholar]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I.H. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, 6, 610–621. [Google Scholar] [CrossRef]
- González-Castro, V.; Cernadas, E.; Huelga, E.; Fernández-Delgado, M.; Porto, J.; Antunez, J.R.; Souto-Bayarri, M. CT Radiomics in Colorectal Cancer: Detection of KRAS Mutation Using Texture Analysis and Machine Learning. Appl. Sci. 2020, 10, 6214. [Google Scholar] [CrossRef]
- Ojala, T.; Pietikainen, M.; Maenpaa, T. Multiresolution gray-scale and rotation invariant texture classification with local binary patterns. IEEE Trans. Pattern Anal. Mach. Intell. 2002, 24, 971–987. [Google Scholar] [CrossRef]
- González-Rufino, E.; Carrión, P.; Cernadas, E.; Fernández-Delgado, M.; Domínguez-Petit, R. Exhaustive comparison of colour texture features and classification methods to discriminate cells categories in histological images of fish ovary. Pattern Recognit. 2013, 46, 2391–2407. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, J.; Huang, L.; Zhao, Z.; Zhang, G.; Li, H.; Guo, B.; Xing, Y.; Zhang, Y.; Bao, H.; et al. Development and Validation of Radiomics Signatures to Predict KRAS Mutation Status Based on Triphasic Enhaced Computed Tomography in Patients with Colorectal Cancer. Research Square. 2022. Available online: https://assets.researchsquare.com/files/rs-1261428/v1/155c8784-eb25-4ec9-839c-90d3bb3fab00.pdf?c=1657780161 (accessed on 3 May 2023).
- Jia, L.-L.; Zhao, J.-X.; Zhao, L.-P.; Tian, J.-H.; Huang, G. Current status and quality of radiomic studies for predicting KRAS mutations in colorectal cancer patients: A systematic review and meta-analysis. Eur. J. Radiol. 2022, 158, 110640. [Google Scholar] [CrossRef]
- Taguchi, N.; Oda, S.; Yokota, Y.; Yamamura, S.; Imuta, M.; Tsuchigame, T.; Nagayama, Y.; Kidoh, M.; Nakaura, T.; Shiraishi, S.; et al. CT texture analysis for the prediction of KRAS mutation status in colorectal cancer via a machine learning approach. Eur. J. Radiol. 2019, 118, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dong, D.; Fang, M.; Zhu, Y.; Zang, Y.; Liu, Z.; Zhang, H.; Ying, J.; Zhao, X.; Tian, J. Can CT-based radiomics signature predict KRAS/NRAS/BRAF mutations in colorectal cancer? Eur. Radiol. 2018, 28, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Eresen, A.; Shangguan, J.; Yang, J.; Benson, A.B.; Yaghmai, V.; Zhang, Z. Preoperative prediction of perineural invasion and KRAS mutation in colon cancer using machine learning. J. Cancer Res. Clin. Oncol. 2020, 146, 3165–3174. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; GuoRong, W.; Zhiwei, W.; Zhengyu, J. CT Texture Analysis: A Potential Biomarker for Evaluating KRAS Mutational Status in Colorectal Cancer. Chin. Med. Sci. J. 2020, 35, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Leto, S.M.; Trusolino, L. Primary and acquired resistance to EGFR-targeted therapies in colorectal cancer: Impact on future treatment strategies. J. Mol. Med. 2014, 92, 709–722. [Google Scholar] [CrossRef]
| Family | Classifier | Language | Function (Module): Hyperparameter Tuning (If Any) |
|---|---|---|---|
| Discriminant Analysis | lda: linear discriminant analysis | Octave | Function train_sc with option LD2, package NaN |
| Matlab | Function fitcdiscr | ||
| Python | Function LinearDiscriminantAnalysis, module sklearn.discriminant_analysis | ||
| R | Function lda, package MASS | ||
| dlda: diagonal LDA | Matlab | Function fitcdiscr, option DiscrimType = diaglinear | |
| qda: quadratic discriminant analysis | Matlab | Function fitcdiscr, option DiscrimType = pseudoquadratic | |
| kfd: kernel Fisher discriminant | Python | Function Kfda, package kfda 1 | |
| Neural Network | mlp: multilayer perceptron | Matlab | Function fitcnet, nh = 10, h1 = max(1,⌊N/(I + C)⌋), h0 = max(1,⌊h1/nh⌋),Δ = max(1,(h1-h0)/nh), h (number of hidden neurons) = h0:Δ:h1 N = no. training patterns, I = no. features |
| Python | Function MLPClassifier, module sklearn.neural_network, same h | ||
| nnet: multilayer perceptron | R | Function nnet, package nnet, same h, weight decay = {0, 0.0001, 0.001, 0.01, 0.1} | |
| neuralnet: multilayer perceptron | R | Function neuralnet, package neuralnet, same h | |
| elm: extreme learning machine | Octave | Ad hoc implementation, h (hidden neurons): 20 values in 1.⌊N/(I + C)⌋ | |
| Support Vector Machine | svm | Octave | LibSVM library 2, functions svcmtrain/svmpredict, λ (regularization) = 2−5:2:10, γ (RBF spread) = 2−15:2:10 |
| Python | Function SVC, module sklearn.svm, same tuning | ||
| R | Function ksvm, module kernlab, same tuning | ||
| K-nearest neighbors | knn | Matlab | Function fitcknn, k (no. neighbors) = 1:2:15 |
| R | Function knn, package class, same k | ||
| Ensemble | adaboost | Matlab | Function fitcensemble, option method = AdaBoostM1 T (no. trees) = 10:10:50 |
| Python | Function AdaboostClassifier, same T, learning rate = 0.1:0.1:0.9 | ||
| bagging | R | Function fitcensemble, option method = Bag | |
| rf: random forest | Python | Function RandomForestClassifier, package sklearn.ensemble, T = 5:5:31, F (max. features) = 3:2:I | |
| gbm: gradient boosting machine | Python | Function GradientBoostingClassifier, package sklearn.ensemble, T = {50,100,150,200}, D (max. depth) = {1,3,6,9} | |
| avNNet: committee of neural multilayer perceptrons | R | Function avNNet, package caret, H = 1..9 and as nnet, decay = 0, 0.1, 0.01, 0.001, 0.0001 | |
| Regularized linear regression | lasso | Matlab | Function fitcecoc, option Learners = templateLinear with Learner = svm and Regularization = lasso or ridge, λ (regularization) = 2−3:0.2:3 |
| ridge | Matlab | ||
| sgd: stochastic gradient descent | Python | Function SGDClassifier, module sklearn.linear_model α (regularization) = {10−i}i = 1−5, {5 · 10−i}i = 15 | |
| Logisticregression | logreg | Matlab | Function mnrfit |
| Python | Function LogisticRegression, module sklearn.linear_model | ||
| Decision tree | ctree: classification tree | Matlab | Function fitctree |
| Python | FunctionDecisionTreeClassifier, module sklearn. tree, criterion = {Gini,entropy},splitter = {best,random}, max. features = 3, 4, I, I/4, I/2, , log2(I) | ||
| R | Function ctree, package party, max. depth = 1.5, min. criterion = {0.01, 0.5, 0.745, 0.99} | ||
| rpart: recursive partitioning | R | Function rpart, package rpart | |
| Naive Bayes | nb | Matlab | Function fitcnb |
| R | Function NaiveBayes, package klaR |
| Position | Kappa (%) | Accuracy (%) | Dataset | Classifier | Language |
|---|---|---|---|---|---|
| 1 | 53.7 | 76.8 | clinical | AdaBoost | Python |
| 2 | 46.0 | 73.2 | Hfd123m4 + clinical | mlp | Python |
| 3 | 46.0 | 73.2 | WDCFfm (m = 4, f = All) | rpart | R |
| 4 | 46.0 | 73.2 | WDCFfm (m = 5, f = All) | rpart | R |
| 5 | 45.7 | 73.2 | WCFd3m5 + clinical | ridge | Matlab |
| 6 | 44.9 | 73.2 | Hfd3m4 +clinical | ridge | Matlab |
| 7 | 42.9 | 71.4 | clinical | mlp | Python |
| 8 | 42.6 | 71.4 | clinical | elm | Octave |
| 9 | 42.6 | 71.4 | Hfd2m4 +clinical | lda | Octave |
| 10 | 42.3 | 71.4 | Hfd2m4 +clinical | logreg | Matlab |
| Computer (AdaBoost) | ||||
|---|---|---|---|---|
| Dataset | KRAS+ | KRAS− | ||
| clinical vector kappa = 53.7% acc = 76.8% | Biopsy | KRAS+ | 22 (39.3%) | 8 (14.3%) |
| KRAS- | 5 (8.9%) | 21 (37.5%) | ||
| Computer (rpat) | ||||
| WDCFfm (f = All) kappa = 46% acc = 73.2% | Biopsy | KRAS+ | 23 (41.1%) | 7 (12.5%) |
| KRAS- | 8 (14.3%) | 18 (32.1%) | ||
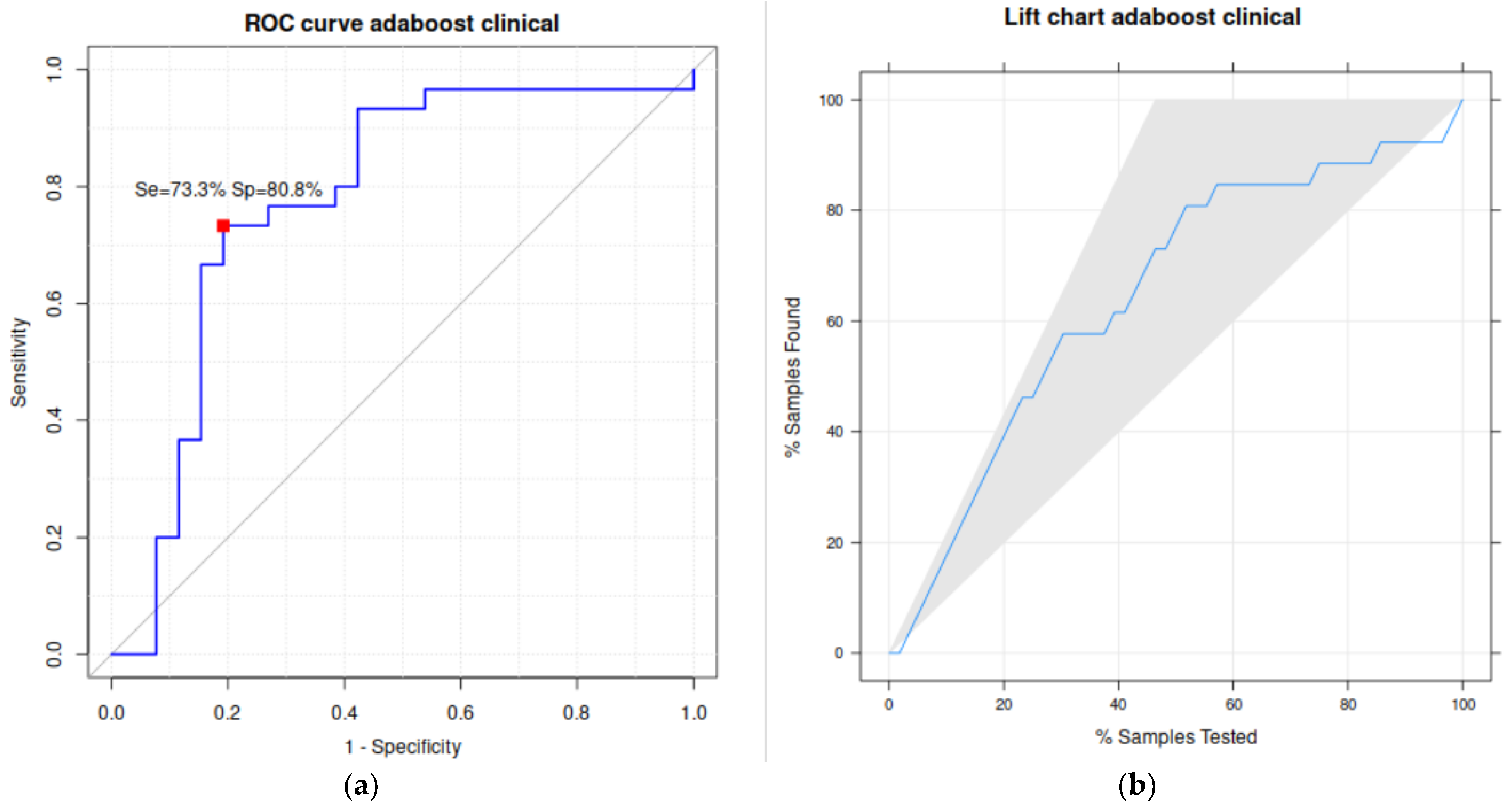
| Kappa | Accuracy | Sensitivity or Recall | Specificity | Precision or Positive Predictivity Value (PPV) | F1 | AUC |
|---|---|---|---|---|---|---|
| 53.7 | 76.8 | 73.3 | 80.8 | 81.5 | 77.2 | 77.8 |
| Texture Descriptor | Kappa(%) | Configuration | Classifier | Language |
|---|---|---|---|---|
| Hfdm | 24.7 | d = 123, m = 5 | ctree | R |
| LPBR | 35.0 | R = 1 | ctree | Python |
| DWT | 12.0 | -- | dlda | Matlab |
| LBPs | 36.9 | s = 2 | ridge | Matlab |
| WCFdm | 28.2 | d = 1 | rpart | R |
| WDCFfm | 46.0 | f = All, m = 4 | rpart | R |
| Texture Descriptor | Kappa(%) | Configuration | Classifier | Language |
|---|---|---|---|---|
| Hfdm | 46.0 | d = 123, m = 4 | mlp | Python |
| LBBR | 42.0 | R = 3 | AdaBoost | Python |
| DWT | 34.7 | -- | gbm | Python |
| LBPs | 42.0 | s = 1 | neuralnet | R |
| 42.0 | s = 3 | AdaBoost | Python | |
| WCFdm | 45.7 | d = 3, m = 5 | mlp | Python |
| WDCFfm | 38.8 | f = LL, m = 4 | sgd | Python |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porto-Álvarez, J.; Cernadas, E.; Aldaz Martínez, R.; Fernández-Delgado, M.; Huelga Zapico, E.; González-Castro, V.; Baleato-González, S.; García-Figueiras, R.; Antúnez-López, J.R.; Souto-Bayarri, M. CT-Based Radiomics to Predict KRAS Mutation in CRC Patients Using a Machine Learning Algorithm: A Retrospective Study. Biomedicines 2023, 11, 2144. https://doi.org/10.3390/biomedicines11082144
Porto-Álvarez J, Cernadas E, Aldaz Martínez R, Fernández-Delgado M, Huelga Zapico E, González-Castro V, Baleato-González S, García-Figueiras R, Antúnez-López JR, Souto-Bayarri M. CT-Based Radiomics to Predict KRAS Mutation in CRC Patients Using a Machine Learning Algorithm: A Retrospective Study. Biomedicines. 2023; 11(8):2144. https://doi.org/10.3390/biomedicines11082144
Chicago/Turabian StylePorto-Álvarez, Jacobo, Eva Cernadas, Rebeca Aldaz Martínez, Manuel Fernández-Delgado, Emilio Huelga Zapico, Víctor González-Castro, Sandra Baleato-González, Roberto García-Figueiras, J Ramon Antúnez-López, and Miguel Souto-Bayarri. 2023. "CT-Based Radiomics to Predict KRAS Mutation in CRC Patients Using a Machine Learning Algorithm: A Retrospective Study" Biomedicines 11, no. 8: 2144. https://doi.org/10.3390/biomedicines11082144
APA StylePorto-Álvarez, J., Cernadas, E., Aldaz Martínez, R., Fernández-Delgado, M., Huelga Zapico, E., González-Castro, V., Baleato-González, S., García-Figueiras, R., Antúnez-López, J. R., & Souto-Bayarri, M. (2023). CT-Based Radiomics to Predict KRAS Mutation in CRC Patients Using a Machine Learning Algorithm: A Retrospective Study. Biomedicines, 11(8), 2144. https://doi.org/10.3390/biomedicines11082144








