Survival Outcomes in T3 Laryngeal Cancers: Primary Total Laryngectomy vs. Concurrent Chemoradiation or Radiation Therapy—A Meta-Analysis †
Abstract
1. Introduction
2. Methodology
2.1. Reporting and Registration
2.2. Search Strategy
2.3. Search Syntax
2.4. Data Screening and Selection
2.5. Inclusion and Exclusion Criteria
2.6. Data Extraction
2.7. Quality Assessment
2.7.1. Level of Evidence
2.7.2. Evidence Quality
2.7.3. Risk of Bias Assessment
2.7.4. Statistical Analysis
3. Results
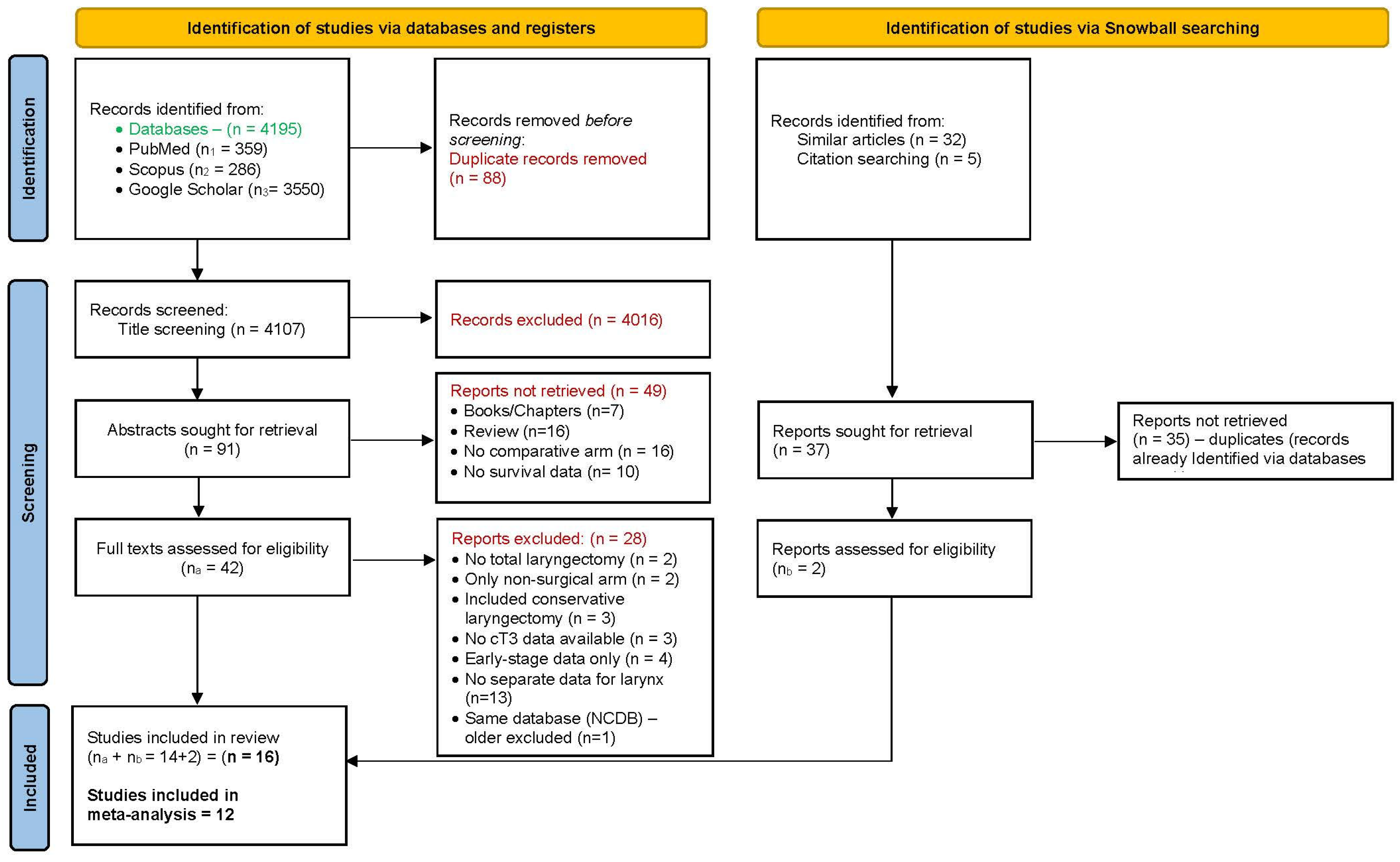
3.1. Literature Retrieval and Data Extraction
3.2. Quality of Included Studies
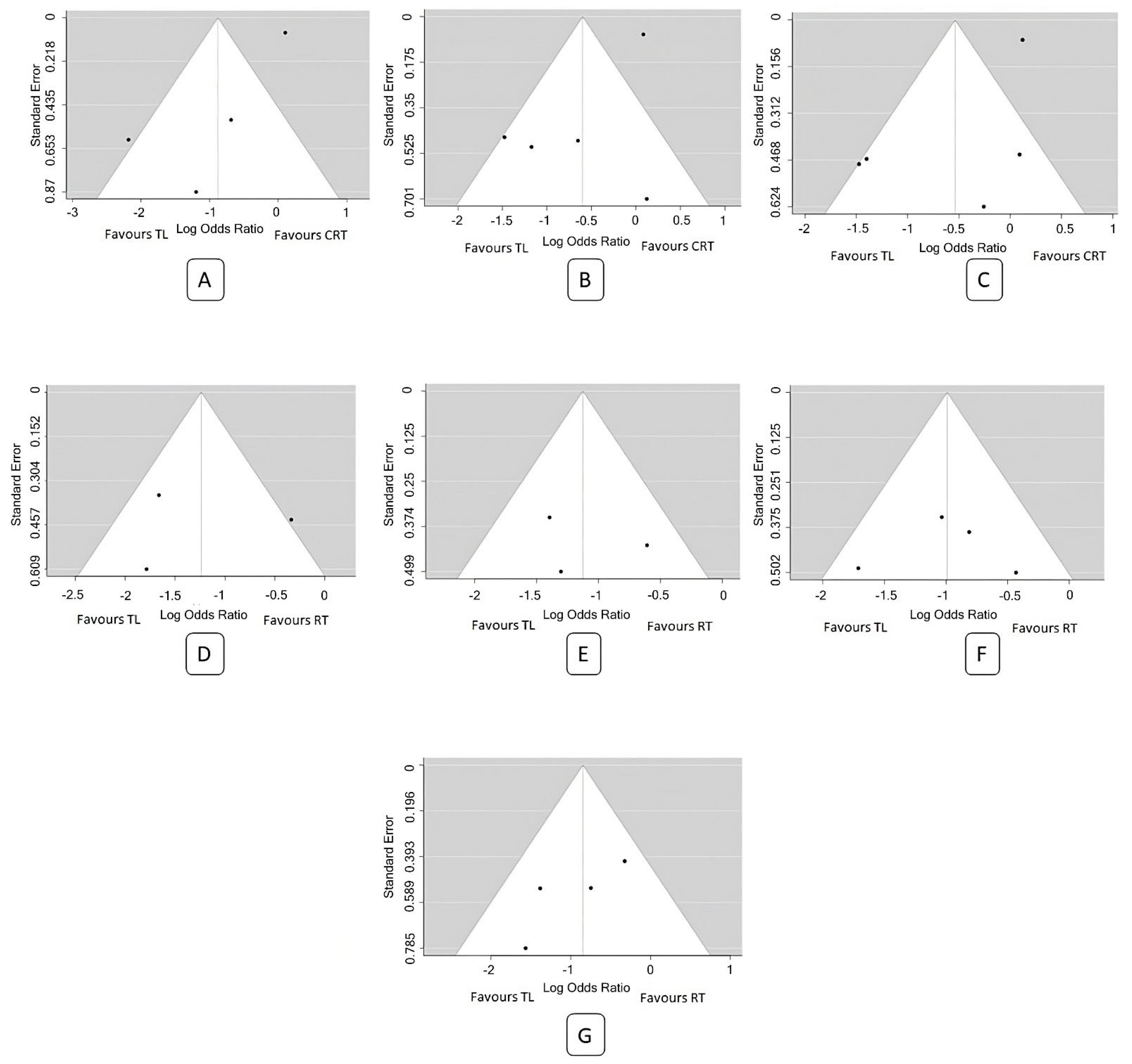
3.3. Analysis of Pooled Data
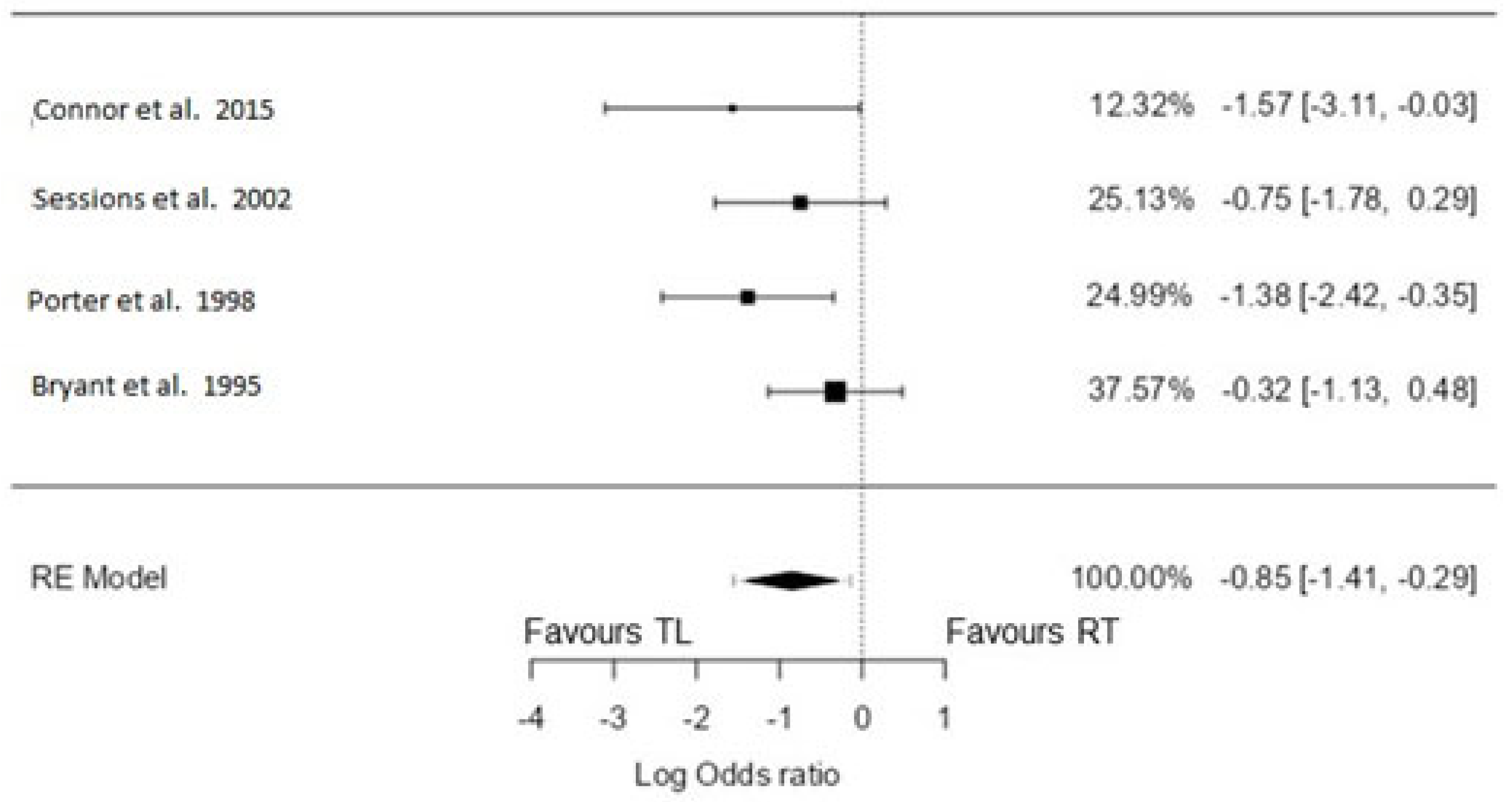
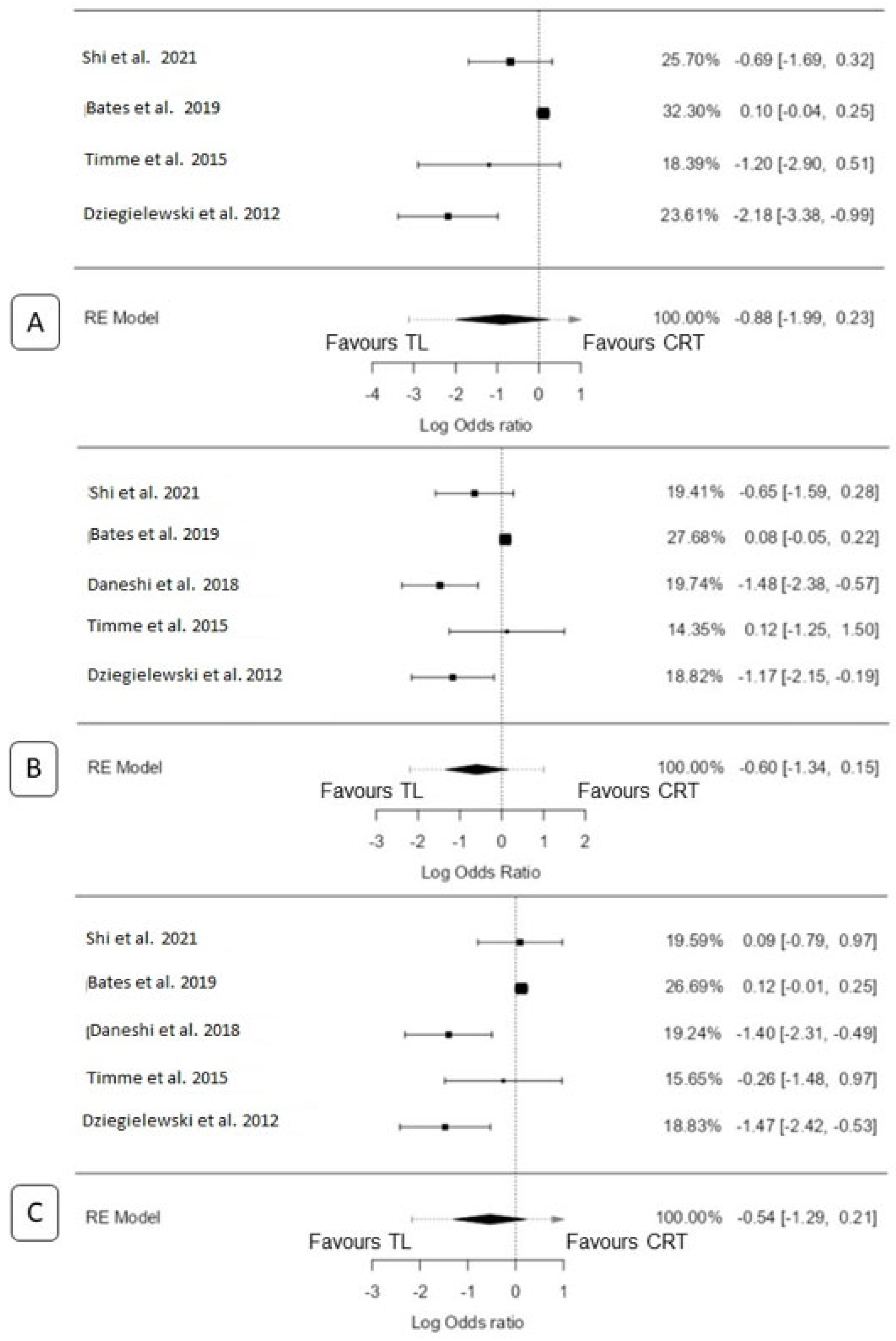
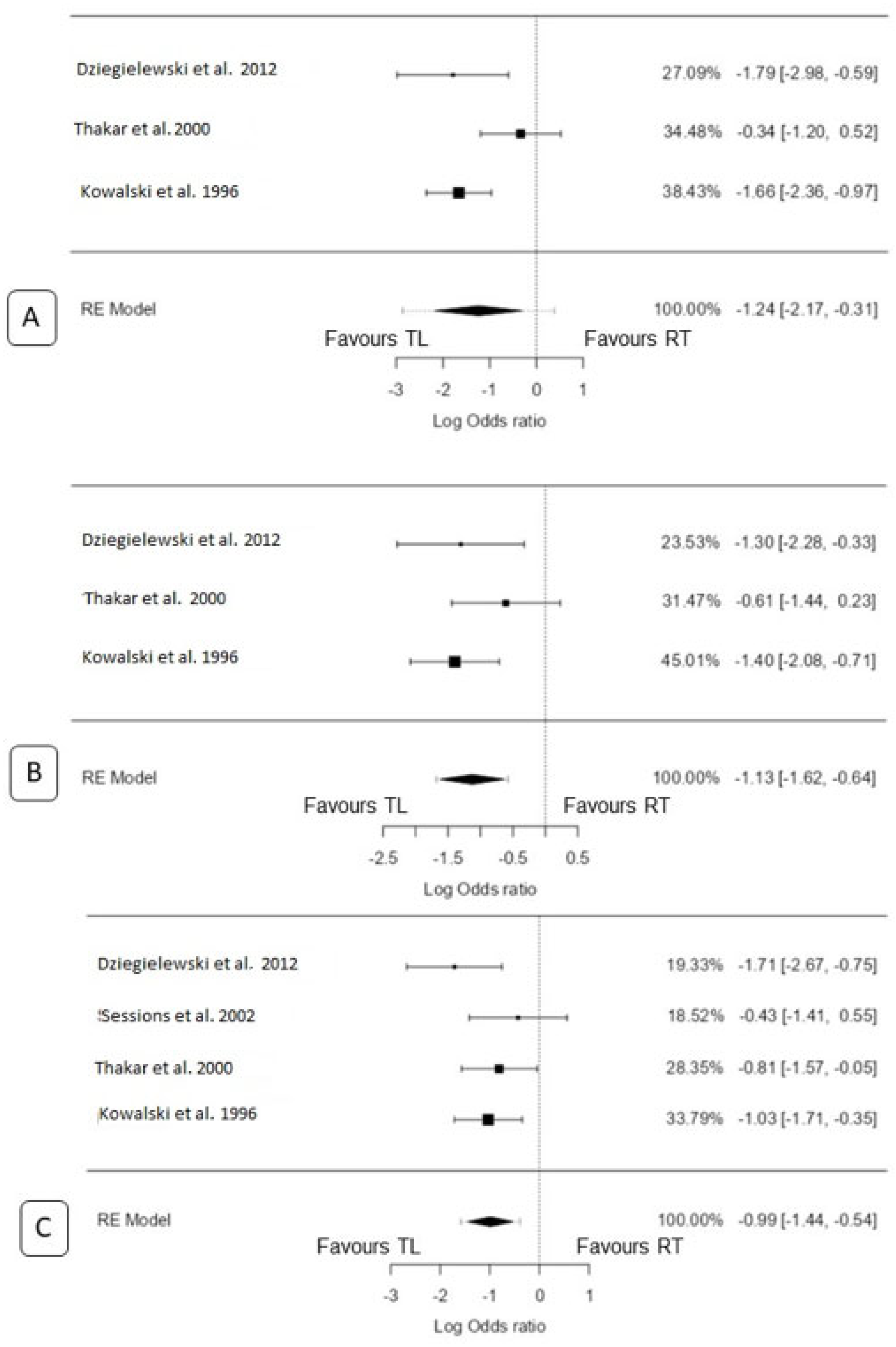
3.4. 2-Year Overall Survival
3.4.1. TL vs. CRT
3.4.2. TL vs. RT only
3.5. 3-Year Overall Survival
3.5.1. TL vs. CRT
3.5.2. TL vs. RT Only
3.6. 5-Year Overall Survival
3.6.1. TL vs. CRT
3.6.2. TL vs. RT Only
3.7. 5-Year Disease-Specific Survival
TL vs. RT Only
4. Discussion
4.1. Survival Outcomes between Total Laryngectomy and Non-Surgical Protocols
4.2. Organ Preservation with Non-Surgical Protocols
4.3. Main Reasons for Failures of Non-Surgical Organ Preservation
4.4. Morbidity following TL
4.5. Morbidity following CRT and RT
4.6. Salvage Laryngectomy
4.7. Pointers to Choose the Appropriate Treatment Modality
4.8. Limitations of Meta-Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- GLOBOCAN 2020: New Global Cancer Data|UICC. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 8 July 2022).
- Department of Veterans Affairs Laryngeal Cancer Study Group; Wolf, G.T.; Fisher, S.G.; Hong, W.K.; Hillman, R.; Spaulding, M.; Laramore, G.E.; Endicott, J.W.; McClatchey, K.; Henderson, W.G. Induction Chemotherapy plus Radiation Compared with Surgery plus Radiation in Patients with Advanced Laryngeal Cancer. N. Engl. J. Med. 1991, 324, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Calais, G.; Pointreau, Y.; Alfonsi, M.; Sire, C.; Tuchais, C.; Tortochaux, J.; Bourhis, J.; Guerrif, S.; Garaud, P. Randomized phase III trial comparing induction chemotherapy using cisplatin (P) fluorouracil (F) with or without docetaxel (T) for organ preservation in hypopharynx and larynx cancer. Preliminary results of GORTEC 2000–01. J. Clin. Oncol. 2006, 24, 5506. [Google Scholar] [CrossRef]
- Sanabria, A.; Chaves, A.L.; Kowalski, L.P.; Wolf, G.T.; Saba, N.F.; Forastiere, A.A.; Beitler, J.J.; Nibu, K.-I.; Bradford, C.R.; Suárez, C.; et al. Organ preservation with chemoradiation in advanced laryngeal cancer: The problem of generalizing results from randomized controlled trials. Auris Nasus Larynx 2017, 44, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.; Nishimoto, I.N.; Califano, J.A.; Kowalski, L.P. Trends in incidence and prognosis for head and neck cancer in the United States: A site-specific analysis of the SEER database. Int. J. Cancer 2005, 114, 806–816. [Google Scholar] [CrossRef]
- Petersen, J.F.; Berlanga, A.; Stuiver, M.M.; Hamming-Vrieze, O.; Hoebers, F.; Lambin, P.; Brekel, M.W.v.D. Improving decision making in larynx cancer by developing a decision aid: A mixed methods approach. Laryngoscope 2019, 129, 2733–2739. [Google Scholar] [CrossRef]
- Grover, S.; Swisher-McClure, S.; Mitra, N.; Li, J.; Cohen, R.B.; Ahn, P.H.; Lukens, J.N.; Chalian, A.A.; Weinstein, G.S.; O’Malley, B.W.; et al. Total Laryngectomy Versus Larynx Preservation for T4a Larynx Cancer: Patterns of Care and Survival Outcomes. Int. J. Radiat. Oncol. Biol Phys. 2015, 92, 594–601. [Google Scholar] [CrossRef]
- Hoffman, H.T.; Porter, K.; Karnell, L.H.; Cooper, J.S.; Weber, R.S.; Langer, C.J.; Ang, K.-K.; Gay, G.; Stewart, A.; Robinson, R.A.; et al. Laryngeal Cancer in the United States: Changes in Demographics, Patterns of Care, and Survival. Laryngoscope 2006, 116, 1–13. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Weber, R.S.; Trotti, A. Organ Preservation for Advanced Larynx Cancer: Issues and Outcomes. J. Clin. Oncol Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3262–3268. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Moher, D.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Bryant, G.P.; Poulsen, M.G.; Tripcony, L.; Dickie, G.J. Treatment decisions in T3N0M0 glottic carcinoma. Int. J. Radiat. Oncol. Biol Phys. 1995, 31, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, L.P.; Batista, M.B.P.; Santos, C.R.; Scopel, A.; Salvajolli, J.V.; Torloni, H. Prognostic Factors in T3,N0-1 Glottic and Transglottic Carcinoma: A Multifactorial Study of 221 Cases Treated by Surgery or Radiotherapy. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.J.; McIvor, N.P.; Morton, R.P.; Hindley, A.C. Audit in the management of T3 fixed-cord laryngeal cancer. Am. J. Otolaryngol. 1998, 19, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Thakar, A.; Bahadur, S.; Mohanti, B.K.; Nivsarkar, S. Clinically staged T3N0M0 laryngeal cancer: How is it best treated? Definitive radiotherapy with salvage surgery v/s combined surgery and radiotherapy. J. Laryngol. Otol. 2000, 114, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Tan, P.F.; Le, Q.T.; Quivey, J.M.; Singer, M.; Terris, D.J.; Goffinet, D.R.; Fu, K.K. Treatment results and prognostic factors of advanced T3--4 laryngeal carcinoma: The University of California, San Francisco (UCSF) and Stanford University Hospital (SUH) experience. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 1172–1180. [Google Scholar] [CrossRef]
- Sessions, D.G.; Lenox, J.; Spector, G.J.; Newland, D.; Simpson, J.; Haughey, B.H.; Chao, K.C. Management of T3N0M0 glottic carcinoma: Therapeutic outcomes. Laryngoscope 2002, 112, 1281–1288. [Google Scholar] [CrossRef]
- Dziegielewski, P.T.; O’Connell, D.A.; Klein, M.; Fung, C.; Singh, P.; Alex Mlynarek, M.; Fung, D.; Harris, J.R.; Seikaly, H. Primary total laryngectomy versus organ preservation for T3/T4a laryngeal cancer: A population-based analysis of survival. J. Otolaryngol.-Head Neck Surg. 2012, 41, S56–S64. [Google Scholar]
- Karlsson, T.R.; Al-Azzawe, M.; Aziz, L.; Hurman, D.; Finizia, C. Survival outcome depending on different treatment strategies in advanced stages III and IV laryngeal cancers: An audit of data from two European centres. Eur. Arch. Oto-Rhino-Laryngol. 2013, 271, 547–554. [Google Scholar] [CrossRef]
- Timme, D.W.; Jonnalagadda, S.; Patel, R.; Rao, K.; Robbins, K.T. Treatment selection for T3/T4a laryngeal cancer: Chemoradiation versus primary surgery. Ann. Otol. Rhinol. Laryngol. 2015, 124, 845–851. [Google Scholar] [CrossRef]
- Connor, K.L.; Pattle, S.; Kerr, G.R.; Junor, E. Treatment, comorbidity and survival in stage III laryngeal cancer. Head Neck 2015, 37, 698–706. [Google Scholar] [CrossRef]
- Timmermans, A.J.; van Dijk, B.A.C.; Overbeek, L.I.H.; van Velthuysen, M.L.F.; van Tinteren, H.; Hilgers, F.J.M.; van den Brekel, M.W. Trends in treatment and survival for advanced laryngeal cancer: A 20-year population-based study in The Netherlands. Head Neck 2016, 38, E1247–E1255. [Google Scholar] [CrossRef]
- Daneshi, N.; Fararouei, M.; Mohammadianpanah, M.; Zare-Bandamiri, M.; Parvin, S.; Dianatinasab, M. Effects of Different Treatment Strategies and Tumor Stage on Survival of Patients with Advanced Laryngeal Carcinoma: A 15-Year Cohort Study. J. Cancer Epidemiol. 2018, 2018, 9678097. [Google Scholar] [CrossRef]
- Čoček, A.; Ambruš, M.; Dohnalová, A.; Chovanec, M.; Kubecová, M.; Licková, K. Locally advanced laryngeal cancer: Total laryngectomy or primary non-surgical treatment? Oncol. Lett. 2018, 15, 6701–6708. [Google Scholar] [CrossRef]
- Bates, J.E.; Amdur, R.J.; Morris, C.M.; Hitchcock, K.E.; Dziegielewski, P.T.; Boyce, B.J.; Mendenhall, W.M.; Silver, N.L.; Shaw, C. curative-dose chemoradiotherapy versus total laryngectomy for stage T3-T4 squamous cell carcinoma of the larynx: An “apples-to-apples” analysis of the national cancer database. Am. J. Clin. Oncol. 2019, 42, 527–533. [Google Scholar] [CrossRef]
- Shi, L.L.; McMullen, C.; Vorwald, K.; Nichols, A.C.; MacNeil, S.D.; Wadsworth, J.T.; Chung, C.H.; Wang, X.; Patel, K.B. Survival outcomes of patients with subglottic squamous cell carcinoma: A study of the national cancer database. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 4923–4932. [Google Scholar] [CrossRef]
- Lee, M.Y.; Belfiglio, M.; Zeng, J.; Fleming, C.W.; Koyfman, S.; Joshi, N.P.; Lamarre, E.; Prendes, B.; Scharpf, J.; Ku, J.A.; et al. Primary total laryngectomy versus organ preservation for locally advanced T3/T4a laryngeal cancer. Laryngoscope 2023, 133, 1122–1131. [Google Scholar] [CrossRef]
- OCEBM Levels of Evidence—Centre for Evidence-Based Medicine (CEBM), University of Oxford. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 2 July 2023).
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment Scale Cohort Studies; University of Ottawa: Ottawa, ON, Canada, 2014. [Google Scholar]
- Chapter 13: Assessing Risk of Bias Due to Missing Results in a Synthesis. Available online: https://training.cochrane.org/handbook/current/chapter-13 (accessed on 22 June 2023).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- West, S.L.; Gartlehner, G.; Mansfield, A.J.; Poole, C.; Tant, E.; Lenfestey, N.; Lux, L.J.; Amoozegar, J.; Morton, S.C.; Carey, T.C.; et al. Table 7, Summary of Common Statistical Approaches to Test for Heterogeneity. Agency for Healthcare Research and Quality (US). 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK53317/table/ch3.t2/ (accessed on 22 June 2023).
- Wilcox, R.R. 12—Multiple comparisons. In Applying Contemporary Statistical Techniques; Wilcox, R.R., Ed.; Academic Press: Burlington, MA, USA, 2003; pp. 407–456. Available online: https://www.sciencedirect.com/science/article/pii/B978012751541050033X (accessed on 22 June 2023).
- Zhu, H.; Ibrahim, J.G.; Cho, H. Perturbation and scaled Cook’s distance. Ann. Stat. 2012, 40, 785–811. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 21 June 2023).
- Jamovi—Open Statistical Software for the Desktop and Cloud. Available online: https://www.jamovi.org/ (accessed on 21 June 2023).
- Fu, X.; Zhou, Q.; Zhang, X. Efficacy Comparison Between Total Laryngectomy and Nonsurgical Organ-Preservation Modalities in Treatment of Advanced Stage Laryngeal Cancer. Medicine 2016, 95, e3142. [Google Scholar] [CrossRef] [PubMed]
- Bozec, A.; Poissonnet, G.; Dassonville, O.; Culié, D. Current Therapeutic Strategies for Patients with Hypopharyngeal Carcinoma: Oncologic and Functional Outcomes. J. Clin. Med. 2023, 12, 1237. [Google Scholar] [CrossRef] [PubMed]
- Terrell, J.E.; Fisher, S.G.; Wolf, G.T.; Veterans Affairs Laryngeal Cancer Study Group. Long-term Quality of Life after Treatment of Laryngeal Cancer. Arch. Otolaryngol. Neck Surg. 1998, 124, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Goepfert, H.; Maor, M.; Pajak, T.F.; Weber, R.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; Chao, C.; et al. Concurrent Chemotherapy and Radiotherapy for Organ Preservation in Advanced Laryngeal Cancer. N. Engl. J. Med. 2003, 349, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Elicin, O.; Giger, R. Comparison of Current Surgical and Non-Surgical Treatment Strategies for Early and Locally Advanced Stage Glottic Laryngeal Cancer and Their Outcome. Cancers 2020, 12, 732. [Google Scholar] [CrossRef]
- Bozec, A.; Culié, D.; Poissonnet, G.; Dassonville, O. Current Role of Total Laryngectomy in the Era of Organ Preservation. Cancers 2020, 12, 584. [Google Scholar] [CrossRef]
- Pignon, J.P.; Bourhis, J.; Domenge, C.; Designé, L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. MACH-NC collaborative group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet 2000, 355, 949–955. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Gong, J.-L.; Wang, Y.-H.; Li, Z.-H.; He, Y.; Liu, Y.X.; Zhou, X.-H. Efficacy comparison between primary total laryngectomy and nonsurgical organ-preservation strategies in treatment of advanced stage laryngeal cancer. Medicine 2018, 97, e10625. [Google Scholar] [CrossRef]
- Janoray, G.; Pointreau, Y.; Garaud, P.; Chapet, S.; Alfonsi, M.; Sire, C.; Jadaud, E.; Calais, G. Long-Term Results of a Multicenter Randomized Phase III Trial of Induction Chemotherapy with Cisplatin, 5-fluorouracil, ± Docetaxel for Larynx Preservation. J. Natl. Cancer Inst. 2015, 108, djv368. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Kainickal, C.T. Current Status of Organ Preservation in Carcinoma Larynx. World J. Oncol. 2018, 9, 39–45. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Ismaila, N.; Lewin, J.; Nathan, C.A.; Adelstein, D.J.; Eisbruch, A.; Fass, G.; Fisher, S.G.; Laurie, S.A.; Le, Q.-T.; et al. Use of Larynx-Preservation Strategies in the Treatment of Laryngeal Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1143–1169. [Google Scholar] [CrossRef]
- Connor, S. Laryngeal cancer: How does the radiologist help? Cancer Imaging 2007, 7, 93–103. [Google Scholar] [CrossRef]
- Malik, N.H.; Fu, R.; Hainc, N.; Noel, C.W.; de Almeida, J.R.; Hosni, A.; Huang, S.H.; Yu, E.; Dzioba, A.; Eskander, A.; et al. Association of primary tumor volume with survival in patients with T3 glottic cancer treated with radiotherapy: A study of the Canadian head & neck collaborative research initiative. JAMA Otolaryngol.-Head Neck Surg. 2023, 149, 103–109. [Google Scholar]
- Sharrett, J.M.; Ward, M.C.; Murray, E.; Scharpf, J.; Lamarre, E.D.; Prendes, B.L.; Lorenz, R.R.; Burkey, B.B.; Koyfman, S.A.; Joshi, N.P.; et al. Tumour volume useful beyond classic criteria in selecting larynx cancers for preservation therapy. Laryngoscope 2020, 130, 2372–2377. [Google Scholar] [CrossRef]
- Hamilton, S.; Venkatesan, V.; Matthews, T.W.; Lewis, C.; Assis, L. Computed Tomographic Volumetric Analysis as a Predictor of Local Control in Laryngeal Cancers Treated with Conventional Radiotherapy. J. Otolaryngol. 2004, 33, 289–294. [Google Scholar] [CrossRef]
- Hanubal, K.S.; Galochkina, Z.; Lee, J.; List, M.A.; Massini, T.C.; Conrad, D.; Hughley, B.; Danan, D.; DeJesus, R.; Hitchcock, K.; et al. Tumor volume as a predictor of survival in advanced laryngeal cancer treated with total laryngectomy. Head Neck 2023, 45, 1376–1388. [Google Scholar] [CrossRef]
- Tangsriwong, K.; Jitreetat, T. Clinical Predictors of Laryngeal Preservation Rate in Stage III-IV Laryngeal Cancer and Hypopharyngeal Cancer Patients Treated with Organ Preservation. Asian Pac. J. Cancer Prev. 2019, 20, 2051–2057. [Google Scholar] [CrossRef]
- Pechacova, Z.; Lohynska, R.; Pala, M.; Drbohlavova, T.; Korinek, T. Impact of comorbidity scores and lifestyle factors in curative radiotherapy in laryngeal cancer. Strahlenther. Onkol. 2023, 1–10. [Google Scholar] [CrossRef]
- Sherman, E.J.; Fisher, S.G.; Kraus, D.H.; Zelefsky, M.J.; Seshan, V.E.; Singh, B.; Shaha, A.R.; Shah, J.P.; Wolf, G.T.; Pfister, D.G. TALK score: Development and validation of a prognostic model for predicting larynx preservation outcome. Laryngoscope 2012, 122, 1043–1050. [Google Scholar] [CrossRef]
- Pantvaidya, G.H.; Raina, S.; Mondal, A.; Deshmukh, A.; Nair, D.; Pai, P.; Chaturvedi, P.; D’Cruz, A. Total laryngectomy: Surgical morbidity and outcomes—A case series. Indian J. Cancer 2017, 54, 621–625. [Google Scholar] [CrossRef]
- Tang, C.G.; Sinclair, C.F. Voice restoration after total laryngectomy. Otolaryngol. Clin. N. Am. 2015, 48, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.C.; Adelstein, D.J.; Bhateja, P.; Nwizu, T.I.; Scharpf, J.; Houston, N.; Lamarre, E.D.; Lorenz, R.; Burkey, B.B.; Koyfman, S.A.; et al. Severe late dysphagia and cause of death after concurrent chemoradiation for larynx cancer in patients eligible for RTOG 91-11. Oral Oncol. 2016, 57, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Staton, J.; Robbins, K.T.; Newman, L.; Samant, S.; Sebelik, M.; Vieira, F. Factors Predictive of Poor Functional Outcome after Chemoradiation for Advanced Laryngeal Cancer. Otolaryngol.-Head Neck Surg. 2002, 127, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Patrik Brodin, N.; Tomé, W.A. Revisiting the dose constraints for head and neck OARs in the current era of IMRT. Oral Oncol. 2018, 86, 8–18. [Google Scholar] [CrossRef]
- Rades, D.; Janssen, S.; Bajrovic, A.; Strojan, P.; Schild, S.E. A Total Radiation Dose of 70 Gy Is Required After Macroscopically Incomplete Resection of Squamous Cell Carcinoma of the Head and Neck. Anticancer Res. 2016, 36, 2989–2992. [Google Scholar]
- Kim, Y.S. Reirradiation of head and neck cancer in the era of intensity-modulated radiotherapy: Patient selection, practical aspects, and current evidence. Radiat. Oncol. J. 2017, 35, 1–15. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.H.; Glisson, B.S.; Trotti, A.; Ridge, J.A.; et al. Long-Term Results of RTOG 91-11: A Comparison of Three Nonsurgical Treatment Strategies to Preserve the Larynx in Patients with Locally Advanced Larynx Cancer. J. Clin. Oncol. 2013, 31, 845–852. [Google Scholar] [CrossRef]
- Shoushtari, S.T.; Gal, J.; Chamorey, E.; Schiappa, R.; Dassonville, O.; Poissonnet, G.; Aloi, D.; Barret, M.; Safta, I.; Saada, E.; et al. Salvage vs. Primary Total Laryngectomy in Patients with Locally Advanced Laryngeal or Hypopharyngeal Carcinoma: Oncologic Outcomes and Their Predictive Factors. J. Clin. Med. 2023, 12, 1305. [Google Scholar] [CrossRef]
- Hasan, Z.; Dwivedi, R.; Gunaratne, D.; Virk, S.; Palme, C.; Riffat, F. Systematic review and meta-analysis of the complications of salvage total laryngectomy. Eur. J. Surg. Oncol. EJSO 2017, 43, 42–51. [Google Scholar] [CrossRef]
- Strojan, P.; Zwitter, M. Mental Disorders after Laryngectomy. Onkologie 2005, 28, 617–618. [Google Scholar] [CrossRef]
- Laccourreye, O.; Gervais, C.; Garcia, D.; Amiri, G.; Mirghani, H.; Giraud, P. Harmful impact of treatment refusal in T3-4M0 endolaryngeal squamous cell carcinoma candidates for total laryngectomy: A STROBE analysis. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2023. [Google Scholar] [CrossRef]
| Author | Country | Year | S | NS | n (cT3) | TL | OP | 2-Year OS | 3-Year OS | 5-Year OS | 5-Year DSS | Type of Non-Surgical Organ Preservation | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TL * | OP * | TL * | OP * | TL * | OP * | TL * | OP * | ||||||||||
| 1 | Bryant et al. [12] | Australia | 1995 | R | 7 | 97 | 42 | 55 | 18 | 28 | RT alone | ||||||
| 2 | Kowalski et al. [13] | Brazil | 1996 | R | 7 | 221 | 176 | 45 | 39 | 27 | 51 | 28 | 69 | 29 | RT alone | ||
| 3 | Porter et al. [14] | NZ | 1998 | R | 7 | 71 | 46 | 25 | 16 | 17 | RT alone | ||||||
| 4 | Thakar et al. [15] | India | 2000 | R | 7 | 119 | 65 | 54 | 13 | 14 | 13 | 17 | 18 | 25 | RT alone | ||
| 5 | Nguyen-Tan et al. [16] | USA | 2001 | R | 7 | 83 | 70 | 13 | CRT | ||||||||
| 6 | Sessions et al. [17] | USA | 2002 | R | 7 | 65 | 36 | 29 | 16 | 16 | 10 | 13 | RT alone | ||||
| 7 | Dziegielewski et al. [18] | Canada | 2012 | R | 7 | 126 | 35 | 91 | 4 | 34 | 8 | 46 | 11 | 63 | Both CRT and RT, separate data available | ||
| 8 | Karlsson et al. [19] | Sweden | 2014 | R | 7 | 71 | 17 | 54 | CRT and RT, separately not specified in data | ||||||||
| 9 | Timme et al. [20] | USA | 2015 | R | 7 | 44 | 19 | 25 | 2 | 7 | 5 | 6 | 11 | 16 | CRT | ||
| 10 | Connor et al. [21] | Scotland | 2015 | R | 7 | 106 | 30 | 76 | 2 | 21 | Both CRT and RT, separate data available | ||||||
| 11 | Timmermans et al. [22] | Netherlands | 2016 | R | 7 | 1922 | 324 | 1598 | 109 | 510 | 131 | 661 | 180 | 973 | CRT and RT, separately not specified in data | ||
| 12 | Daneshi et al. [23] | Iran | 2018 | R | 7 | 151 | 127 | 24 | 27 | 13 | 37 | 15 | CRT | ||||
| 13 | Čoček et al. [24] | Czech Rep. | 2018 | R | 7 | 70 | 45 | 25 | 8 | 6 | 11 | 8 | 17 | 13 | CRT and RT, separately not specified in data | ||
| 14 | Bates et al. [25] | USA | 2019 | R | 7 | 7569 | 1044 | 6525 | 282 | 1631 | 407 | 2414 | 522 | 3067 | CRT | ||
| 15 | Shi et al. [26] | USA | 2021 | R | 7 | 88 | 32 | 56 | 7 | 20 | 9 | 24 | 19 | 32 | CRT | ||
| 16 | Lee et al. [27] | USA | 2023 | R | 7 | 137 | 41 | 96 | 16 | 19 | 20 | 32 | 26 | 51 | CRT and RT, not specified in data | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, K.N.; Pai, P.S.; Dange, P.; Kowalski, L.P.; Strojan, P.; Mäkitie, A.A.; Guntinas-Lichius, O.; Robbins, K.T.; Rodrigo, J.P.; Eisbruch, A.; et al. Survival Outcomes in T3 Laryngeal Cancers: Primary Total Laryngectomy vs. Concurrent Chemoradiation or Radiation Therapy—A Meta-Analysis. Biomedicines 2023, 11, 2128. https://doi.org/10.3390/biomedicines11082128
Rao KN, Pai PS, Dange P, Kowalski LP, Strojan P, Mäkitie AA, Guntinas-Lichius O, Robbins KT, Rodrigo JP, Eisbruch A, et al. Survival Outcomes in T3 Laryngeal Cancers: Primary Total Laryngectomy vs. Concurrent Chemoradiation or Radiation Therapy—A Meta-Analysis. Biomedicines. 2023; 11(8):2128. https://doi.org/10.3390/biomedicines11082128
Chicago/Turabian StyleRao, Karthik Nagaraja, Prathamesh S. Pai, Prajwal Dange, Luiz P. Kowalski, Primož Strojan, Antti A. Mäkitie, Orlando Guntinas-Lichius, K. Thomas Robbins, Juan P. Rodrigo, Avraham Eisbruch, and et al. 2023. "Survival Outcomes in T3 Laryngeal Cancers: Primary Total Laryngectomy vs. Concurrent Chemoradiation or Radiation Therapy—A Meta-Analysis" Biomedicines 11, no. 8: 2128. https://doi.org/10.3390/biomedicines11082128
APA StyleRao, K. N., Pai, P. S., Dange, P., Kowalski, L. P., Strojan, P., Mäkitie, A. A., Guntinas-Lichius, O., Robbins, K. T., Rodrigo, J. P., Eisbruch, A., Takes, R. P., de Bree, R., Coca-Pelaz, A., Piazza, C., Chiesa-Estomba, C., López, F., Saba, N. F., Rinaldo, A., & Ferlito, A. (2023). Survival Outcomes in T3 Laryngeal Cancers: Primary Total Laryngectomy vs. Concurrent Chemoradiation or Radiation Therapy—A Meta-Analysis. Biomedicines, 11(8), 2128. https://doi.org/10.3390/biomedicines11082128