Tuberculosis and COVID-19 Dually Affect Human Th17 Cell Immune Response
Abstract
1. Introduction
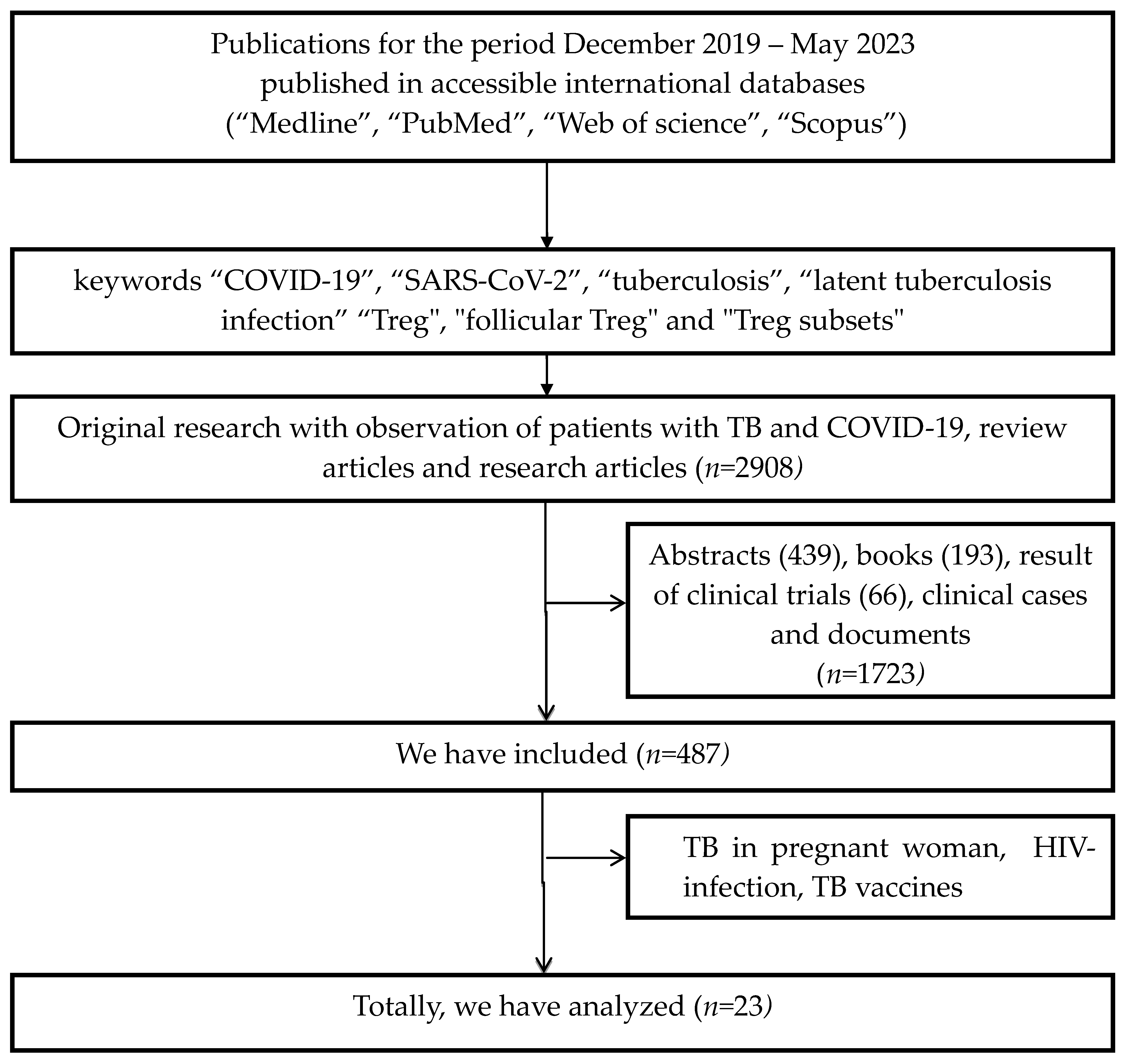
2. Methods
3. Results
3.1. COVID-19 Affecting Immune Response in LTBI and Active Tuberculosis
3.2. Immunological Features in Patients in Tuberculosis
3.3. Immune Response in COVID-19 Patients
3.4. Comparison of M. tuberculosis and SARS-CoV-2-Triggered Immune Response
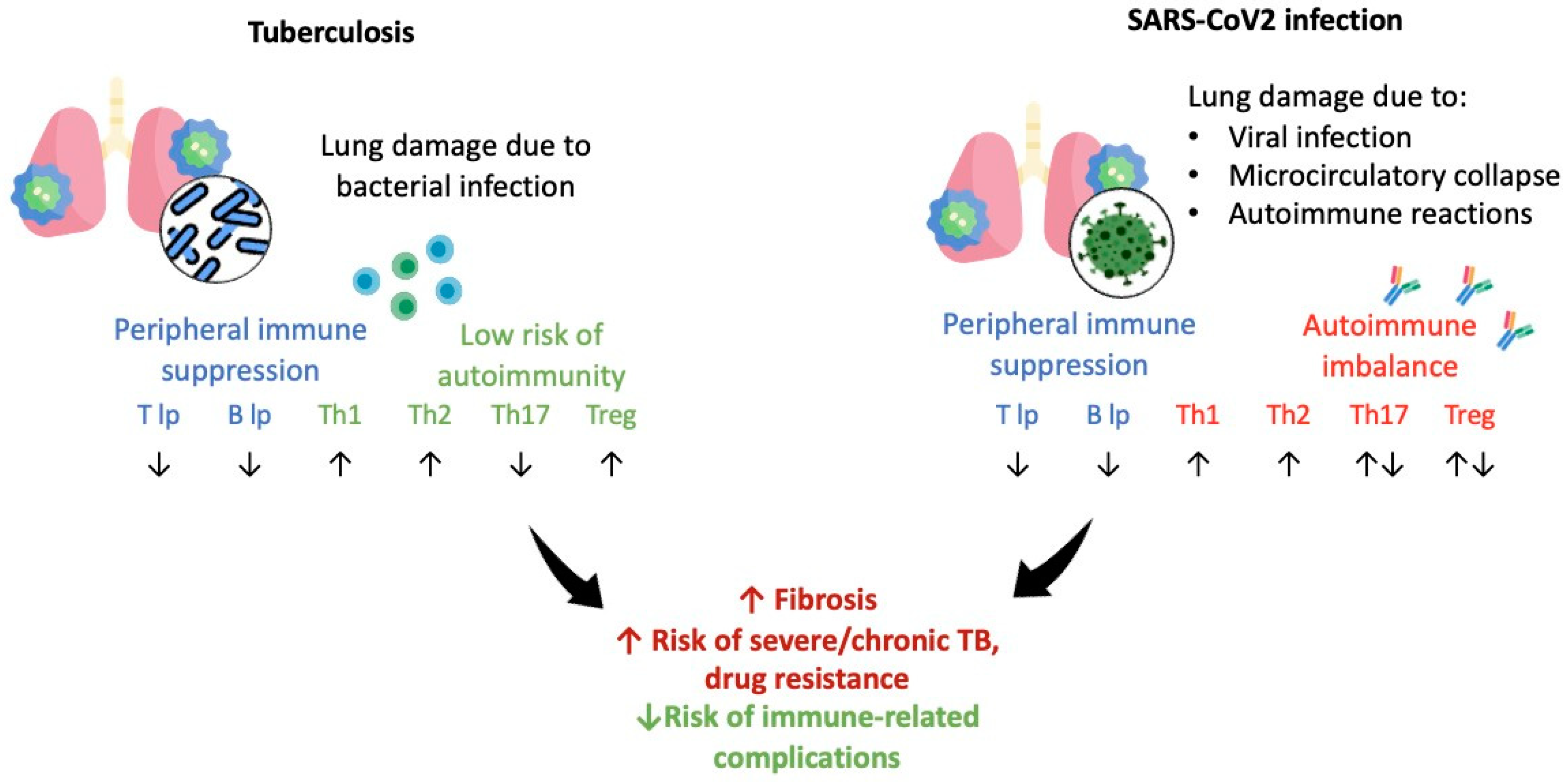
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Coronavirus Disease (COVID-19) Pandemic; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 8 June 2023).
- Sharma, A.; Balda, S.; Apreja, M.; Kataria, K.; Kapalas, N.; Sharma, P. COVID-19 Diagnosis: Current and Future Techniques. Int. J. Biol. Macromol. 2021, 193 (Pt B), 1835–1844. [Google Scholar] [CrossRef]
- Fenollar, F.; Bouam, A.; Ballouche, M.; Fuster, L.; Prudent, E.; Colson, P.; Tissot-Dupont, H.; Million, M.; Drancourt, M.; Raoult, D.; et al. Evaluation of the Panbio COVID-19 Rapid Antigen Detection Test Device for the Screening of Patients with COVID-19. J. Clin. Microbiol. 2021, 59, e02589-20. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; Paul van Schayck, J.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Santa Cruz, A.; Mendes-Frias, A.; Oliveira, A.I.; Dias, L.; Matos, A.R.; Carvalho, A.; Capela, C.; Pedrosa, J.; Castro, A.G.; Silvestre, R. Interleukin-6 Is a Biomarker for the Development of Fatal Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. Front. Immunol. 2021, 12, 613422. [Google Scholar] [CrossRef]
- Guglielmetti, L.; Veziris, N.; Aubry, A.; Brossier, F.; Bernard, C.; Sougakoff, W.; Jarlier, V.; Robert, J. Risk factors for extensive drug resistance in multidrug-resistant tuberculosis cases: A case-case study. Int. J. Tuberc. Lung Dis. 2018, 22, 54–59. [Google Scholar] [CrossRef]
- Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022.
- Bigio, J.; Viscardi, A.; Gore, G.; Matteelli, A.; Sulis, G. A scoping review on the risk of tuberculosis in specific population groups: Can we expand the World Health Organization recommendations? Eur. Respir. Rev. 2023, 32, 220127. [Google Scholar] [CrossRef] [PubMed]
- Mertz, P.; Jeannel, J.; Guffroy, A.; Lescuyer, S.; Korganow, A.S.; Rondeau-Lutz, M.; Weber, J.C. Granulomatous manifestations associated with COVID19 infection: Is there a link between these two diseases? Autoimmun. Rev. 2021, 20, 102824. [Google Scholar] [CrossRef]
- Starshinova, A.; Malkova, A.; Kudryavtsev, I.; Kudlay, D.; Zinchenko, Y.; Yablonskiy, P. Tuberculosis and autoimmunity: Common features. Tuberculosis 2022, 134, 102202. [Google Scholar] [CrossRef]
- Stojanovic, Z.; Gonçalves-Carvalho, F.; Marín, A.; Capa, J.A.; Domínguez, J.; Latorre, I.; Lacoma, A.; Prat-Aymerich, C. Advances in diagnostic tools for respiratory tract infections: From tuberculosis to COVID-19—Changing paradigms? ERJ Open Res. 2022, 8, 00113–2022. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Stamatelopoulos, K.; Terpos, E.; Tsitsilonis, O.E.; Aivalioti, E.; Paraskevis, D.; Kastritis, E.; Pavlakis, G.N.; Dimopoulos, M.A. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J. Biomed. Sci. 2021, 28, 9. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Zhang, M.; Chang, T.L. ACE2-Independent Alternative Receptors for SARS-CoV-2. Viruses 2022, 14, 2535. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Alipoor, S.D.; Mirsaeidi, M. SARS-CoV-2 cell entry beyond the ACE2 receptor. Mol. Biol. Rep. 2022, 49, 10715–10727. [Google Scholar] [CrossRef]
- Watanabe, A.; Yoneda, M.; Ikeda, F.; Terao-Muto, Y.; Sato, H.; Kai, C. CD147/EMMPRIN Acts as a Functional Entry Receptor for Measles Virus on Epithelial Cells. J. Virol. 2010, 84, 4183–4193. [Google Scholar] [CrossRef]
- Muramatsu, T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J. Biochem. 2016, 159, 481–490. [Google Scholar] [CrossRef]
- Wang, L.; Berger, N.A.; Kaelber, D.C.; Pamela BDavis, P.B.; Volkow, N.D.; Xu, R. Comparison of outcomes from COVID infection in pediatric and adult patients before and after the emergence of Omicron. medRxiv 2022. [Google Scholar] [CrossRef]
- Chan, K.W.; Wong, V.T.; Tang, S.C.W. COVID-19: An update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative chinese-western medicine for the management of 2019 novel coronavirus disease. Am. J. Chin. Med. 2020, 48, 737–762. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef]
- Raucci, F.; Mansour, A.A.; Casillo, G.M.; Saviano, A.; Caso, F.; Scarpa, R.; Mascolo, N.; Iqbal, A.J.; Maione, F. Interleukin-17A (IL-17A), a key molecule of innate and adaptive immunity, and its potential involvement in COVID-19-related thrombotic and vascular mechanisms. Autoimmun. Rev. 2020, 19, 102572. [Google Scholar] [CrossRef] [PubMed]
- Elkington, P.; Tebruegge, M.; Mansour, S. Tuberculosis: An Infection-Initiated Autoimmune Disease? Trends Immunol. 2016, 37, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Starshinova, A.; Malkova, A.; Zinchenko, Y.; Kudryavtsev, I.; Serebriakova, M.; Akisheva, T.; Lapin, S.; Mazing, A.; Kudlay, D.; Glushkova, A.; et al. Identification of autoimmune markers in pulmonary tuberculosis. Front. Immunol. 2023, 13, 1059714. [Google Scholar] [CrossRef] [PubMed]
- Khayat, M.; Fan, H.; Vali, Y. COVID-19 promoting the development of active tuberculosis in a patient with latent tuberculosis infection: A case report. Respir. Med. Case Rep. 2021, 32, 101344. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, A.; Kumar, N.P.; Padmapriyadarsini, C.; Nancy, A.; Selvaraj, N.; Karunanithi, K.; Munisankar, S.; Bm, S.; Renji, R.M.; Ambu, T.C.; et al. Latent tuberculosis co-infection is associated with heightened levels of humoral, cytokine and acute phase responses in seropositive SARS-CoV-2 infection. J. Infect. 2021, 83, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Shariq, M.; Sheikh, J.A.; Quadir, N.; Sharma, N.; Hasnain, S.E.; Ehtesham, N.Z. COVID-19 and tuberculosis: The double whammy of respiratory pathogens. Eur. Respir. Rev. 2022, 31, 210264. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Gutiérrez, J.J.; Rodríguez-Guardado, A.; Arias-Guillén, M.; Alonso-Arias, R.; Palacios-Penedo, S.; García-García, J.M.; Balbín, M.; Pérez-Hernández, D.; Sandoval-Torrientes, M.; Torreblanca-Gil, A.; et al. Clinical and Epidemiological Correlates of Low IFN-Gamma Responses in Mitogen Tube of QuantiFERON Assay in Tuberculosis Infection Screening During the COVID-19 Pandemic: A Population-Based Marker of COVID-19 Mortality? Arch. Bronconeumol. 2022, 58, 649–659. [Google Scholar] [CrossRef]
- Petrone, L.; Petruccioli, E.; Vanini, V.; Cuzzi, G.; Gualano, G.; Vittozzi, P.; Nicastri, E.; Maffongelli, G.; Grifoni, A. Coinfection of tuberculosis and COVID-19 limits the ability to in vitro respond to SARS-CoV-2. Int. J. Infect. Dis. 2021, 113 (Suppl. 1), S82–S87. [Google Scholar] [CrossRef] [PubMed]
- Flores-Lovon, K.; Ortiz-Saavedra, B.; Cueva-Chicaña, L.A.; Aperrigue-Lira, S.; Montes-Madariaga, E.S.; Soriano-Moreno, D.R.; Bell, B.; Macedo, R. Immune responses in COVID-19 and tuberculosis coinfection: A scoping review. Front. Immunol. 2022, 13, 992743. [Google Scholar] [CrossRef] [PubMed]
- Bostanghadiri, N.; Jazi, F.M.; Razavi, S.; Fattorini, L.; Darban-Sarokhalil, D. Mycobacterium tuberculosis and SARS-CoV-2 Coinfections: A Review. Front. Microbiol. 2022, 12, 747827. [Google Scholar] [CrossRef]
- De Maio, F.; Bianco, D.M.; Delogu, G. The Dark Side of the COVID-19 Treatments on Mycobacterium Tuberculosis Infection. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022021. [Google Scholar] [CrossRef]
- Wolf, A.J.; Desvignes, L.; Linas, B.; Banaiee, N.; Tamura, T.; Takatsu, K.; Ernst, J.D. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 2008, 205, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.J.; Linas, B.; Trevejo-Nuñez, G.J.; Kincaid, E.; Tamura, T.; Takatsu, K.; Ernst, J.D. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 2007, 179, 2509–2519. [Google Scholar] [CrossRef]
- Corleis, B.; Dorhoi, A. Early dynamics of innate immunity during pulmonary tuberculosis. Immunol. Lett. 2020, 221, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Noguera-Julian, A.; Moroni, R.; Hernández-Bartolomé, A.; Fritschi, N.; Lancella, L.; Cursi, L.; Soler-Garcia, A.; Krüger, R.; Feiterna-Sperling, C.; et al. Performance of QuantiFERON-TB Gold Plus assays in paediatric tuberculosis: A multicentre PTBNET study. Thorax 2022, 78, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management; World Health Organization: Geneva, Switzerland, 2018; Available online: https://apps.who.int/iris/handle/10665/260233 (accessed on 23 February 2022).
- WHO Consolidated Guidelines on Tuberculosis. In Module 3: Diagnosis. Tests for Tuberculosis Infection; World Health Organization: Geneva, Switzerland, 2022.
- Zellweger, J.P.; Sotgiu, G.; Corradi, M.; Durando, P. The diagnosis of latent tuberculosis infection (LTBI): Currently 622 available tests, future developments, and perspectives to eliminate tuberculosis (TB). Med. Lav. 2020, 111, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Starshinova, A.; Zhuravlev, V.; Dovgaluk, I.; Panteleev, A.; Manina, V.; Zinchenko, U.; Istomina, E.; Pavlova, M.; Yablonskiy, P. A Comparison of Intradermal Test with Recombinant Tuberculosis Allergen (Diaskintest) with Other Immunologic Tests in the Diagnosis of Tuberculosis Infection. Int. J. Mycobacteriol. 2018, 1, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Zhou, Y.X.; Wu, S.M.; Pan, Q.; Xia, B.; Zhang, X.L. CFP10 and ESAT6 aptamers as effective 628 Mycobacterial antigen diagnostic reagents. J. Infect. 2014, 69, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Slight, S.R.; Rangel-Moreno, J.; Gopal, R.; Lin, Y.; Fallert Junecko, B.A.; Mehra, S.; Selman, M.; Becerril-Villanueva, E.; Baquera-Heredia, J.; Pavon, L.; et al. CXCR5⁺ T helper cells mediate protective immunity against tuberculosis. J. Clin. Investig. 2013, 123, 712–726. [Google Scholar] [CrossRef]
- Gong, W.; Pan, C.; Cheng, P.; Wang, J.; Zhao, G.; Wu, X. Peptide-Based Vaccines for Tuberculosis. Front. Immunol. 2022, 13, 830497. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Liao, M.; Graner, M.W.; Wu, C.; Yang, Q.; Liu, H.; Zhou, B. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am. J. Respir. Crit. Care Med. 2010, 181, 734–742. [Google Scholar] [CrossRef]
- Li, X.Y.; Wu, Z.B.; Ding, J.; Zheng, Z.H.; Li, X.Y.; Chen, L.N.; Zhu, P. Role of the frequency of blood CD4(+) CXCR5(+) CCR6(+) T cells in autoimmunity in patients with Sjögren’s syndrome. Biochem. Biophys. Res. Commun. 2012, 422, 238–244. [Google Scholar] [CrossRef]
- Kumar, N.P.; Sridhar, R.; Hanna, L.E.; Banurekha, V.V.; Nutman, T.B.; Babu, S. Decreased frequencies of circulating CD4⁺ T follicular helper cells associated with diminished plasma IL-21 in active pulmonary tuberculosis. PLoS ONE 2014, 9, e111098. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, Y.; Lao, S.; Yang, B.; Yu, S.; Zhang, Y.; Wu, C. Mycobacterium tuberculosis-Specific IL-21+IFN-γ+CD4+ T Cells Are Regulated by IL-12. PLoS ONE 2016, 11, e0147356. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Monin, L.; Slight, S.; Uche, U.; Blanchard, E.; Fallert Junecko, B.A.; Ramos-Payan, R.; Stallings, C.L.; Reinhart, T.A.; Kolls, J.K.; et al. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 2014, 10, e1004099. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, G.; Lü, L.; Xu, K.; Chen, Y.; Pan, H.; Burstrom, B.; Burstrom, K.; Wang, J. Genetic polymorphisms of IL-17A, IL-17F, TLR4 and miR-146a in association with the risk of pulmonary tuberculosis. Sci. Rep. 2016, 6, 28586. [Google Scholar] [CrossRef]
- Mourik, B.C.; Lubberts, E.; de Steenwinkel, J.E.M.; Ottenhoff, T.H.M.; Leenen, P.J.M. Interactions between Type 1 Interferons and the Th17 Response in Tuberculosis: Lessons Learned from Autoimmune Diseases. Front. Immunol. 2017, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtsev, I.V.; Serebriakova, M.K.; Starshinova, A.A.; Zinchenko, Y.S.; Basantsova, N.Y.; Belyaeva, E.N.; Pavlova, M.V.; Yablonskiy, P.K. Altered peripheral blood Th17 and follicular T-helper subsets in patients with pulmonary tuberculosis. Russ. J. Infect. Immun. 2019, 9, 304–314. [Google Scholar] [CrossRef]
- Lyadova, I.; Nikitina, I. Cell Differentiation Degree as a Factor Determining the Role for Different T-Helper Populations in Tuberculosis Protection. Front. Immunol. 2019, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Jurado, J.O.; Pasquinelli, V.; Alvarez, I.B.; Peña, D.; Rovetta, A.I.; Tateosian, N.L.; Romeo, H.E.; Musella, R.M.; Palmero, D.; Chuluyán, H.E.; et al. IL-17 and IFN-γ expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J. Leukoc. Biol. 2012, 91, 991–1002. [Google Scholar] [CrossRef]
- Nikitina, I.Y.; Panteleev, A.V.; Kosmiadi, G.A.; Serdyuk, Y.V.; Nenasheva, T.A.; Nikolaev, A.A.; Gorelova, L.A.; Radaeva, T.V.; Kiseleva, Y.Y.; Bozhenko, V.K.; et al. Th1, Th17, and Th1Th17 Lymphocytes during Tuberculosis: Th1 Lymphocytes Predominate and Appear as Low-Differentiated CXCR3+CCR6+ Cells in the Blood and Highly Differentiated CXCR3+/-CCR6- Cells in the Lungs. J. Immunol. 2018, 200, 2090–2103. [Google Scholar] [CrossRef]
- Monin, L.; Griffiths, K.L.; Slight, S.; Lin, Y.; Rangel-Moreno, J.; Khader, S.A. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunol. 2015, 8, 1099–1109. [Google Scholar] [CrossRef]
- Linge, I.; Tsareva, A.; Kondratieva, E.; Dyatlov, A.; Hidalgo, J.; Zvartsev, R.; Apt, A. Pleiotropic Effect of IL-6 Produced by B-Lymphocytes During Early Phases of Adaptive Immune Responses Against TB Infection. Front. Immunol. 2022, 13, 750068. [Google Scholar] [CrossRef] [PubMed]
- Malkova, A.; Starshinova, A.; Zinchenko, Y.; Gavrilova, N.; Kudryavtsev, I.; Lapin, S.; Mazing, A.; Surkova, E.; Pavlova, M.; Belaeva, E.; et al. New laboratory criteria of the autoimmune inflammation in pulmonary sarcoidosis and tuberculosis. Clin. Immunol. 2021, 227, 108724. [Google Scholar] [CrossRef] [PubMed]
- Willem, J.; du Plessis, W.J.; Keyser, A.; Walzl, G.; Loxton, A.G. Phenotypic analysis of peripheral B cell populations during Mycobacterium tuberculosis infection and disease. J. Inflamm. 2016, 13, 23. [Google Scholar] [CrossRef]
- Hinze, C.H.; Colbert, R.A. B-cell depletion in Wegener’s granulomatosis. Clin. Rev. Allergy Immunol. 2008, 34, 327–379. [Google Scholar] [CrossRef] [PubMed]
- Soe, P.T.; Hanthamrongwit, J.; Saelee, C.; Kyaw, S.P.; Khaenam, P.; Warit, S.; Satproedprai, N.; Mahasirimongkol, S.; Yanai, H.; Chootong, P.; et al. Circulating IgA/IgG memory B cells against Mycobacterium tuberculosis dormancy-associated antigens Rv2659c and Rv3128c in active and latent tuberculosis. Int. J. Infect. Dis. 2021, 110, 75–82. [Google Scholar] [CrossRef]
- Semple, P.L.; Binder, A.B.; Davids, M.; Maredza, A.; van Zyl-Smit, R.N.; Dheda, K. Regulatory T cells attenuate mycobacterial stasisin alveolar and blood-derived macrophages from patients with tuberculosis. Am. J. Respir. Crit. Care Med. 2013, 187, 1249–1258. [Google Scholar] [CrossRef]
- Seddiki, N.; Santner-Nanan, B.; Martinson, J.; Zaunders, J.; Sasson, S.; Landay, A.; Solomon, M.; Selby, W.; Alexander, S.I.; Nanan, R.; et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 2006, 203, 1693–1700. [Google Scholar] [CrossRef]
- Dai, Y.C.; Wang, W.D.; Zhang, J.A.; Chen, C.; Luo, H.L.; Xu, H.; Peng, Y.; Luo, H.; Yang, X.R.; Chen, X.; et al. MTB driven B cells producing IL-35 and secreting high level of IL-10 in the patients with active pulmonary tuberculosis. Mol. Immunol. 2019, 112, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Adiga, V.; Nayak, S.; Kumar , J.A.J.U.; Dhar, C.; Sahoo, P.A.; Sundararaj, B.K.; Souza, G.D.; Vyakarnam, A. Circulating HLA-DR+CD4+ effector memory T cells resistant to CCR5 and PD-L1 mediated suppression compromise regulatory T cell function in tuberculosis. PLOS Pathog. 2018, 14, e1007289. [Google Scholar] [CrossRef]
- Liu, W.; Putnam, A.L.; Xu-Yu, Z.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; Fazekas de St Groth, B.; et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef]
- Schultheiß, C.; Paschold, L.; Simnica, D.; Mohme, M.; Willscher, E.; von Wenserski, L.; Scholz, R.; Wieters, I.; Dahlke, C.; Tolosa, E.; et al. Next-Generation Sequencing of T and B Cell Receptor Repertoires from COVID-19 Patients Showed Signatures Associated with Severity of Disease. Immunity 2020, 53, 442–455.e4. [Google Scholar] [CrossRef] [PubMed]
- Chiacchio, T.; Casetti, R.; Butera, O.; Vanini, V.; Carrara, S.; Girardi, E.; Di Mitri, D.; Battistini, L.; Martini, F.; Borsellino, G.; et al. Characterization of regulatory T cells identified as CD4(+)CD25(high)CD39(+) in patients with active tuberculosis. Clin. Exp. Immunol. 2009, 156, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ouyang, J.; Isnard, S.; Mohme, M.; Willscher, E.; von Wenserski, L.; Scholz, R.; Wieters, I.; Dahlke, C.; Tolosa, E.; et al. Sharing CD4+ T Cell Loss: When COVID-19 and HIV Collide on Immune System. Front. Immunol. 2020, 11, 596631. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, F.; Beretta, L.; Scandroglio, A.M.; Colombo, S.; Landoni, G.; Ruggeri, A.; Peccatori, J.; D’Angelo, A.; De Cobelli, F.; Rovere-Querini, P.; et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): An atypical acute respiratory distress syndrome working hypothesis. Crit. Care Resusc. 2020, 22, 95–97. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020, 5, e138999. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Hussaniy, H.A.; Al-Harcan, N.A.H.; Alexiou, A.; Batiha, G.E. Neutrophil Extracellular Traps (NETs) and COVID-19: A new frontiers for therapeutic modality. Int. Immunopharmacol. 2022, 104, 108516. [Google Scholar] [CrossRef]
- Kalinina, O.; Golovkin, A.; Zaikova, E.; Aquino, A.; Bezrukikh, V.; Melnik, O.; Vasilieva, E.; Karonova, T.; Kudryavtsev, I.; Shlyakhto, E. Cytokine Storm Signature in Patients with Moderate and Severe COVID-19. Int. J. Mol. Sci. 2022, 23, 8879. [Google Scholar] [CrossRef]
- Tan, C.W.; Low, J.G.H.; Wong, W.H.; Chua, Y.Y.; Goh, S.L.; Ng, H.J. Critically ill COVID-19 infected patients exhibit increased clot waveform analysis parameters consistent with hypercoagulability. Am. J. Hematol. 2021, 95, E156–E158. Available online: https://pubmed.ncbi.nlm.nih.gov/32267008 (accessed on 22 June 2021). [CrossRef]
- Jovanovic, M.; Sekulic, S.; Jocic, M.; Jurisevic, M.; Gajovic, N.; Jovanovic, M.; Arsenijevic, N.; Jovanovic, M.; Mijailovic, M.; Milosavljevic, M.; et al. Increased Pro Th1 and Th17 Transcriptional Activity in Patients with Severe COVID-19. Int. J. Med. Sci. 2023, 20, 530–541. [Google Scholar] [CrossRef]
- Kudryavtsev, I.V.; Arsentieva, N.A.; Korobova, Z.R.; Isakov, D.V.; Rubinstein, A.A.; Batsunov, O.K.; Khamitova, I.V.; Kuznetsova, R.N.; Savin, T.V.; Akisheva, T.V.; et al. Heterogenous CD8+ T Cell Maturation and ‘Polarization’ in Acute and Convalescent COVID-19 Patients. Viruses 2022, 14, 1906. [Google Scholar] [CrossRef]
- Malkova, A.M.; Kudlay, D.A.; Kudryavtsev, I.V.; Starshinova, A.A.; Yablonsky, P.K.; Shoenfeld, Y. Immunogenetic predictors of severe COVID-19. Vaccines 2021, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, J.N.; Tulyeu, J.; Edahiro, R.; Shirai, Y.; Yamaguchi, Y.; Murakami, T.; Morita, T.; Kato, Y.; Hirata, H.; Takeda, Y.; et al. A sex-biased imbalance between Tfr, Tph, and atypical B cells determines antibody responses in COVID-19 patients. Proc. Natl. Acad. Sci. USA 2023, 120, e2217902120. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Y.; Zhang, M.; Yang, C.X.; Zhang, N.; Wang, X.C.; Yang, X.P.; Dong, X.Q.; Zheng, Y.T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Zahran, A.M.; Abdel-Rahim, M.H.; Nasif, K.A.; Hussein, S.; Hafez, R.; Ahmad, A.B.; Saad, K.; Elhoufey, A.; Hussein, H.A.M.; Thabet, A.A.; et al. Association of follicular helper T and follicular regulatory T cells with severity and hyperglycemia in hospitalized COVID-19 patients. Virulence 2022, 13, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Dai, Y.; Zheng, T.; Cheng, L.; Zhao, D.; Wang, H.; Liu, M.; Pei, H.; Jin, T.; Yu, D.; et al. Peripheral CD4+ T cell subsets and antibody response in COVID-19 convalescent individuals. J. Clin. Investig. 2020, 130, 6588–6599. [Google Scholar] [CrossRef]
- Starshinova, A.A.; Kudryavtsev, I.; Malkova, A.; Zinchenko, U.; Karev, V.; Kudlay, D.; Glushkova, A.; Starshinova, A.Y.; Dominguez, J.; Villar-Hernández, R.; et al. Molecular and Cellular Mechanisms of M. tuberculosis and SARS-CoV-2 Infections—Unexpected Similarities of Pathogenesis and What to Expect from Co-Infection. Int. J. Mol. Sci. 2022, 23, 2235. [Google Scholar] [CrossRef]
- Bonecchi, R.; Bianchi, G.; Bordignon, P.P.; D’Ambrosio, D.; Lang, R.; Borsatti, A.; Sozzani, S.; Allavena, P.; Gray, P.A.; Mantovani, A.; et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998, 187, 129–134. [Google Scholar] [CrossRef]
- Tincati, C.; Cannizzo, E.S.; Giacomelli, M.; Badolato, R.; d’Arminio Monforte, A.; Marchetti, G. Heightened Circulating Interferon-Inducible Chemokines, and Activated Pro-Cytolytic Th1-Cell Phenotype Features COVID-19 Aggravation in the Second Week of Illness. Front. Immunol. 2020, 11, 580987. [Google Scholar] [CrossRef]
- Qi, J.; Liu, C.; Bai, Z.; Li, X.; Yao, G. T follicular helper cells and T follicular regulatory cells in autoimmune diseases. Front. Immunol. 2023, 14, 1178792. [Google Scholar] [CrossRef]
- De Biasi, S.; Lo Tartaro, D.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Mattioli, M.; Paolini, A.; Gozzi, L.; et al. Expansion of plasmablasts and loss of memory B cells in peripheral blood from COVID-19 patients with pneumonia. Eur. J. Immunol. 2020, 50, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kilian, C.; Turner, J.E.; Bosurgi, L.; Roedl, K.; Bartsch, P.; Gnirck, A.C.; Cortesi, F.; Schultheiß, C.; Hellmig, M.; et al. Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients. Sci. Immunol. 2021, 6, eabf6692. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Costa, L.; Ruscitti, P.; Navarini, L.; del Puente, A.; Giacomelli, R.; Scarpa, R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun. Rev. 2020, 19, 102524. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kuo, H.H.; Boucau, J.; Farmer, J.R.; Allard-Chamard, H.; Mahajan, V.S.; Piechocka-Trocha, A.; Lefteri, K.; Osborn, M.; Bals, J.; et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell. 2020, 183, 143–157.e13. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtsev, I.; Rubinstein, A.; Golovkin, A.; Kalinina, O.; Vasilyev, K.; Rudenko, L.; Isakova-Sivak, I. Dysregulated Immune Responses in SARS-CoV-2-Infected Patients: A Comprehensive Overview. Viruses 2022, 14, 1082. [Google Scholar] [CrossRef] [PubMed]
- Haslbauer, J.D.; Matter, M.S.; Stalder, A.K.; Tzankov, A. Reaktionsmuster der lokoregionären Lymphknoten im Abflussgebiet von COVID-19-Lungen [Histomorphological patterns of regional lymph nodes in COVID-19 lungs]. Pathologe 2021, 42, 188–196. [Google Scholar] [CrossRef]
- Duan, Y.Q.; Xia, M.H.; Ren, L.; Zhang, Y.-F.; Ao, Q.-L.; Xu, S.-P.; Kuang, D.; Liu, Q.; Yan, B.; Zhou, Y.-W.; et al. Deficiency of Tfh Cells and Germinal Center in Deceased COVID-19 Patients. Curr. Med. Sci. 2020, 40, 618–624. [Google Scholar] [CrossRef]
- Kalfaoglu, B.; Almeida-Santos, J.; Tye, C.A.; Satou, Y.; Ono, M. T-cell dysregulation in COVID-19. Biochem. Biophys. Res. Commun. 2021, 538, 204–210. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, Y.; Wang, T.; Xie, F. Innate and adaptive immune response in SARS-CoV-2 infection-Current perspectives. Front Immunol. 2022, 13, 1053437. [Google Scholar] [CrossRef]
- Sattler, A.; Angermair, S.; Stockmann, H.; Heim, K.M.; Khadzhynov, D.; Treskatsch, S.; Halleck, F.; Kreis, M.E.; Kotsch, K. SARS-CoV-2-specific T cell responses and correlations with COVID-19 patient predisposition. J. Clin. Investig. 2020, 130, 6477–6489. [Google Scholar] [CrossRef]
- San Segundo, D.; Arnaiz de Las Revillas, F.; Lamadrid-Perojo, P.; Comins-Boo, A.; Gonzalez-Rico, C.; Alonso-Pena, M.; Irure-Ventura, J.; Olmos, J.M.; Farinas, M.C.; Lopez-Hoyos, M. Innate and Adaptive Immune Assessment at Admission to Predict Clinical Outcome in COVID-19 Patients. Biomedicines 2021, 9, 917. [Google Scholar] [CrossRef]
- Lo, C.-Y.; Huang, Y.-C.; Huang, H.-Y.; Chung, F.-T.; Lin, C.-W.; Chung, K.F.; Wang, C.-H. Increased Th1 Cells with Disease Resolution of Active Pulmonary Tuberculosis in Non-Atopic Patients. Biomedicines 2021, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.C.; Wu, M.F.; Wang, J.Y.; Lai, H.C.; Lee, L.N.; Chiang, B.L.; Yu, C.J. Decreased T helper 17 cells in tuberculosis is associated with increased percentages of programmed death ligand 1, T helper 2 and regulatory T cells. Respir. Res. 2017, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Varnaitė, R.; García, M.; Glans, H.; Maleki, K.T.; Sandberg, J.T.; Tynell, J.; Christ, W.; Lagerqvist, N.; Asgeirsson, H.; Ljunggren, H.G.; et al. Expansion of SARS-CoV-2-Specific Antibody-Secreting Cells and Generation of Neutralizing Antibodies in Hospitalized COVID-19 Patients. J Immunol. 2020, 205, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, J.; Huang, Y.; Chen, J.; Jiang, X.; Shi, Y. Characteristics of immune cells and cytokines in patients with coronavirus disease 2019 in Guangzhou, China. Hum Immunol. 2020, 81, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Bautista, J.F.; Rodriguez-Nicolas, A.; Rosales-Castillo, A.; Jiménez, P.; Garrido, F.; Anderson, P.; Ruiz-Cabello, F.; López-Ruz, M. Negative Clinical Evolution in COVID-19 Patients Is Frequently Accompanied with an Increased Proportion of Undifferentiated Th Cells and a Strong Underrepresentation of the Th1 Subset. Front. Immunol. 2020, 11, 596553. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, J.; Feng, R.; Wang, C.; Li, Y.; Wang, X. Follicular Helper T Cells in Pulmonary Tuberculosis: A Retrospective Study. Iran J Immunol. 2022, 19, 2. [Google Scholar] [CrossRef]
- Kozlov, V.A.; Savchenko, A.A.; Kudryavtsev, I.V.; Kozlov, I.G.; Kudlay, D.A.; Prodeus, A.P.; Borisov, A.G. Clinical Immunology. In Krasnoyarsk; Polycor: Krasnoyarsk, Russia, 2020; 386p, ISBN 978-5-6044565-6-9. [Google Scholar]
- Wu, D.; Yang, X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020, 53, 368–370. [Google Scholar] [CrossRef]
- Guyot-Revol, V.; Innes, J.A.; Hackforth, S.; Hinks, T.; Lalvani, A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am. J. Respir. Crit. Care Med. 2006, 173, 803–810. [Google Scholar] [CrossRef]
- Fathi, F.; Sami, R.; Mozafarpoor, S.; Hafezi, H.; Motedayyen, H.; Arefnezhad, R.; Eskandari, N. Immune system changes during COVID-19 recovery play key role in determining disease severity. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420966497. [Google Scholar] [CrossRef]
- Kudryavtsev, I.V.; Arsentieva, N.A.; Batsunov, O.K.; Korobova, Z.R.; Khamitova, I.V.; Isakov, D.V.; Kuznetsova, R.N.; Rubinstein, A.A.; Stanevich, O.V.; Lebedeva, A.A.; et al. Alterations in B Cell and Follicular T-Helper Cell Subsets in Patients with Acute COVID-19 and COVID-19 Convalescents. Curr. Issues Mol. Biol. 2022, 44, 14. [Google Scholar] [CrossRef]
- Tan, M.; Liu, Y.; Zhou, R.; Deng, X.; Li, F.; Liang, K.; Shi, Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology 2020, 160, 261–268. [Google Scholar] [CrossRef]
- Mohebbi, S.R.; Baghaei, K.; Rostami-Nejad, M.; Mojarad, E.N.; Mirjalali, H.; Yadegar, A.; Asri, N.; Abdoulahi, S.; Assadzadeh-Aghdaei, H. Significant changes of CD4, FOXP3, CD25, and IL6 expression level in Iranian COVID-19 patients. Gastroenterol. Hepatol. Bed Bench. 2020, 13, 388. [Google Scholar] [PubMed]
- Almatrafi, M.A.; Awad, K.; Alsahaf, N.; Tayeb, S.; Alharthi, A.; Rabie, N.; Fadag, R.; Alwafi, H.; Salawati, R.; Alhindi, A.K.; et al. Disseminated Tuberculosis Post COVID-19 Infection: A Case Report. Cureus 2022, 14, e31489. [Google Scholar] [CrossRef]
- Dhawan, M.; Rabaan, A.A.; Alwarthan, S.; Alhajri, M.; Halwani, M.A.; Alshengeti, A.; Najim, M.A.; Alwashmi, A.S.S.; Alshehri, A.A.; Alshamrani, S.A.; et al. Regulatory T Cells (Tregs) and COVID-19: Unveiling the Mechanisms, and Therapeutic Potentialities with a Special Focus on Long COVID. Vaccines 2023, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, B.; Trapin, D.; Ettel, P.; Körmöczi, U.; Rottal, A.; Tuppy, F.; Feichter, M.; Gattinger, P.; Borochova, K.; Dorofeeva, Y.; et al. Immunological imprint of COVID-19 on human peripheral blood leukocyte populations. Allergy 2021, 76, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Al-kayali Rawaa, S.; Kashkash Mohamad, F.; Alhussein Alhajji Azzam, H.; Khouri, A. Activation of tuberculosis in recovered COVID-19 patients: A case report. Ann. Med. Surg. 2023, 85, 280–283. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chin, C.H.; Liu, S.F.; Wu, C.C.; Tsen, C.C.; Wang, Y.H.; Chao, T.Y.; Lie, C.H.; Chen, C.J.; Wang, C.C.; et al. Prognostic values of serum IP-10 and IL-17 in patients with pulmonary tuberculosis. Dis. Markers 2011, 31, 101–110. [Google Scholar] [CrossRef]
- Alemu, A.; Bitew, Z.W.; Seid, G.; Diriba, G.; Gashu, E.; Berhe, N.; Mariam, S.H.; Gumi, B. Tuberculosis in individuals who recovered from COVID-19: A systematic review of case reports. PLoS ONE 2022, 17, e0277807. [Google Scholar] [CrossRef]
- Cardona, P.; Cardona, P.-J. Regulatory T Cells in Mycobacterium tuberculosis Infection. Front. Immunol. 2019, 10, 2139. [Google Scholar] [CrossRef]
- Kozlov, V.A.; Tikhonova, E.P.; Savchenko, A.A.; Kudryavtsev, I.V.; Andronova, N.V.; Anisimova, E.N.; Golovkin, A.S.; Demina, D.V.; Zdzitovetsky, D.E.; Kalinina, Y.S.; et al. Clinical Immunology. A Practical Guide for Infectious Disease Specialists; Krasnoyarsk: Polikor, Russia, 2021; 563p. (In Russian) [Google Scholar] [CrossRef]
- Lyadova, I.V.; Panteleev, A.V. Th1 and Th17 Cells in Tuberculosis: Protection, Pathology, and Biomarkers. Mediat. Inflamm. 2015, 2015, 854507. [Google Scholar] [CrossRef]
- Renavikar, P.S.; Crawford, M.P.; Sinha, S.; Karandikar, N.J. Human Tc17 cells harbor potent immune suppressive potential, whereas Tc1 cells lack suppressive ability. J. Immunol. 2019, 202 (Suppl. 1), 57.13. [Google Scholar] [CrossRef]
| TB Infection and COVID-19 | Immune Response | First Author, Year of Publication |
|---|---|---|
| LTBI + COVID-19 (clinical case) | decline in CD4+ T cell count along with latent-to-active TB progression | Khayat M, 2021 [24] |
| LTBI + COVID-19 (study data) | LTBI with COVID-19 showed significantly higher plasma levels of IFN γ, IL-2, TNF α, IL- 1 α, IL-1 β, IL-6, IL-12, IL-15, IL-17, IL-3, GM-CSF, IL-10, IL-25, IL-33, CCL3 and CXCL10 | Rajamanickam A, 2021 [25] |
| LTBI + COVID-19 (review) | decreased cytokine levels shown for IL-2, IL-4, IL-5 and IL-13 | Shariq M, 2022 [26] |
| LTBI + COVID-19 (study data) | LTBI in COVID subjects had lowered CD8+ T cell count and IFN-γ level | Palacios-Gutiérrez JJ, 2022 [27] |
| TB +COVID-19 (study data) | low level of IFN-γ in COVID-19 and TB patients | Petrone L, 2021 [28] |
| TB +COVID-19 (study data) | decreased SARS-CoV-2-specific CD4 T cell level in COVID-19 and TB patients | Flores-Lovon K, 2022 [29] |
| Cells | COVID-19 | Tuberculosis |
|---|---|---|
| Th1 | ↑ [65,91,92]; ↓ [93,94] | ↑ [95,96] |
| Th2 | ↑ [97,98,99] | ↑ [100,101]; not significant [95] |
| Th17 | ↑ [99,102]; ↓ [93,96,101] | ↓ [100,103] |
| Tfh | ↑ [98,104]; ↓ [87,91,99,105] | ↑ [100]; ↓ [101] not significant [95] |
| Treg | ↑ [65,106,107]; ↓ [66,108,109] | ↑ [43,60,110,111,112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starshinova, A.; Kudryavtsev, I.; Rubinstein, A.; Malkova, A.; Dovgaluk, I.; Kudlay, D. Tuberculosis and COVID-19 Dually Affect Human Th17 Cell Immune Response. Biomedicines 2023, 11, 2123. https://doi.org/10.3390/biomedicines11082123
Starshinova A, Kudryavtsev I, Rubinstein A, Malkova A, Dovgaluk I, Kudlay D. Tuberculosis and COVID-19 Dually Affect Human Th17 Cell Immune Response. Biomedicines. 2023; 11(8):2123. https://doi.org/10.3390/biomedicines11082123
Chicago/Turabian StyleStarshinova, Anna, Igor Kudryavtsev, Artem Rubinstein, Anna Malkova, Irina Dovgaluk, and Dmitry Kudlay. 2023. "Tuberculosis and COVID-19 Dually Affect Human Th17 Cell Immune Response" Biomedicines 11, no. 8: 2123. https://doi.org/10.3390/biomedicines11082123
APA StyleStarshinova, A., Kudryavtsev, I., Rubinstein, A., Malkova, A., Dovgaluk, I., & Kudlay, D. (2023). Tuberculosis and COVID-19 Dually Affect Human Th17 Cell Immune Response. Biomedicines, 11(8), 2123. https://doi.org/10.3390/biomedicines11082123








