Transcutaneous Spinal Cord Stimulation Improves Respiratory Muscle Strength and Function in Subjects with Cervical Spinal Cord Injury: Original Research
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical Assessment of Spinal Cord Injury
2.3. Assessment of Respiratory Function
2.4. Neurophysiological Assessment
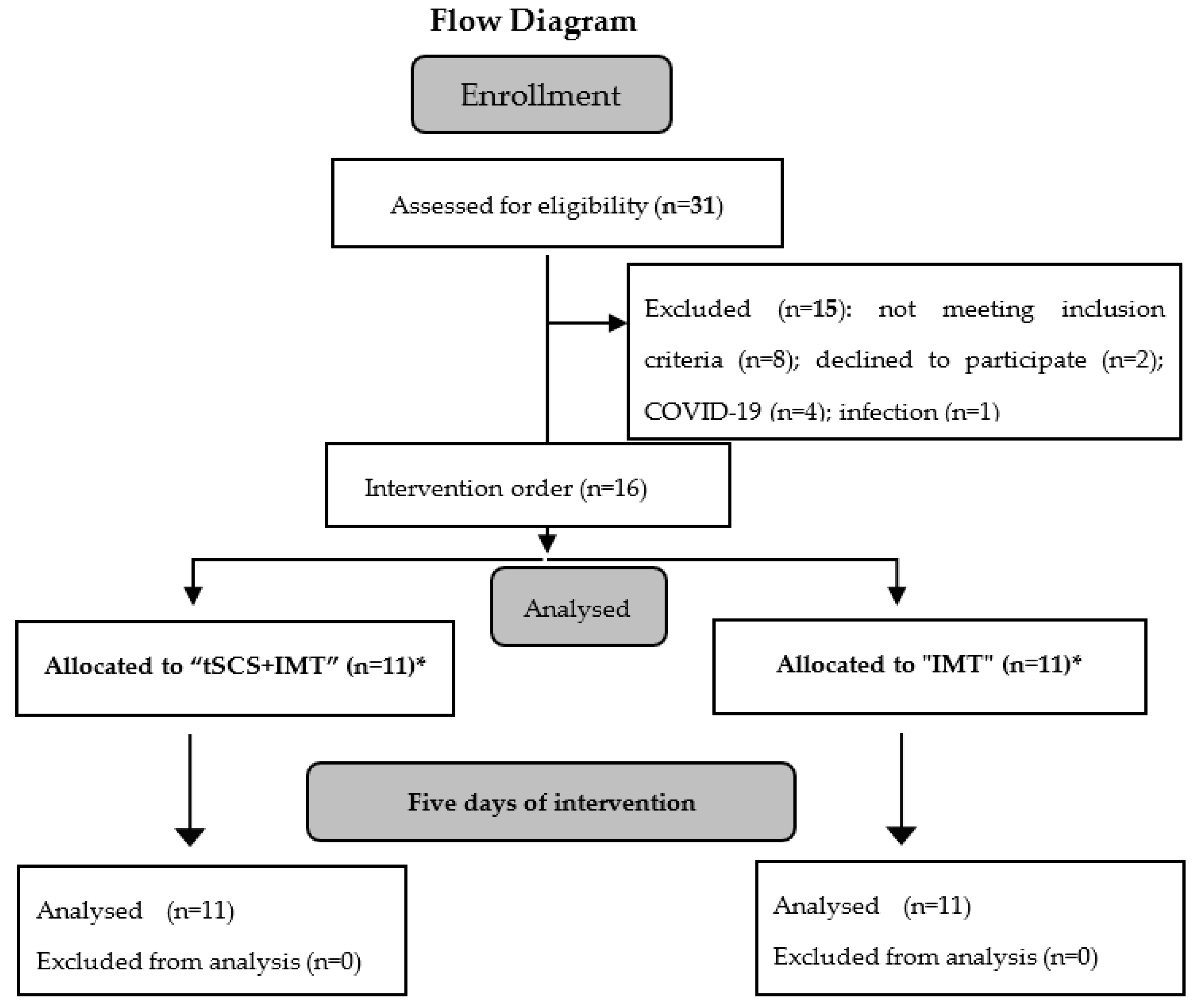
2.5. Experimental Design
2.6. Interventions
2.7. Transcutaneous Spinal Cord Stimulation
2.8. Data and Statistical Analysis
3. Results
3.1. Subjects Clinical and Demographic Characteristics
3.2. Respiratory Assessment
3.2.1. Subjective Evaluation
3.2.2. Objective Evaluation
4. Discussion
Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, A.B.; Groomes, T.E. Incidence of respiratory complications following spinal cord injury. Arch. Phys. Med. Rehabil. 1994, 75, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Berlowitz, D.J.; Tamplin, J. Respiratory muscle training for cervical spinal cord injury. Cochrane Database Syst. Rev. 2013, 7, CD008507. [Google Scholar] [CrossRef]
- Arora, S.; Flower, O.; Murray, N.P.; Lee, B.B. Respiratory care of patients with cervical spinal cord injury: A review. Crit. Care Resusc. 2012, 14, 64–73. [Google Scholar]
- Berlowitz, D.J.; Wadsworth, B.; Ross, J. Respiratory problems and management i people with spinal cord injury. Breathe 2016, 12, 328–340. [Google Scholar] [CrossRef]
- Garshick, E.; Kelley, A.; Cohen, S.A.; Garrison, A.; Tun, C.C.G.; Gagnon, D.; Brown, R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 2005, 43, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Waddimba, A.C.; Jain, N.B.; Stolzmann, K.; Gagnon, D.R.; Burgess, J.F., Jr.; Kazis, L.E.; Garshick, E. Predictors of cardiopulmonary hospitalization in chronic spinal cord injury. Arch. Phys. Med. Rehabil. 2009, 90, 193–200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brûlé, J.F.; Leriche, B.; Normand, J.; Khalife, S.; Torabi, D.; Vallée, D. Phrenic stimulation in respiratory paralysis caused by spinal cord injuries. Neurochirurgie 1991, 37, 127–132. [Google Scholar]
- Onders, R.P.; Elmo, M.; Kaplan, C.; Schilz, R.; Katirji, B.; Tinkoff, G. Long-term experience with diaphragm pacing for traumatic spinal cord injury: Early implantation should be considered. Surgery 2018, 164, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bermejo, J.; LLontop, C.; Similowski, T.; Morélot-Panzini, C. Respiratory neuromodulation in patients with neurological pathologies: For whom and how? Ann. Phys. Rehabil. Med. 2015, 58, 238–244. [Google Scholar] [CrossRef]
- DiMarco, A.F.; Kowalski, K.E.; Geertman, R.T.; Hromyak, D.R.; Frost, F.S.; Creasey, G.H.; Nemunaitis, G.A. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: Results of a National Institutes of Health-Sponsored clinical trial. Part II: Clinical outcomes. Arch. Phys. Med. Rehabil. 2009, 90, 726–732. [Google Scholar] [CrossRef]
- García-Alén, L.; Kumru, H.; Castillo-Escario, Y.; Benito-Penalva, J.; Medina-Casanovas, J.; Gerasimenko, Y.P.; Edgerton, V.R.; García-Alías, G.; Vidal, J. Transcutaneous Cervical Spinal Cord Stimulation Combined with Robotic Exoskeleton Rehabilitation for the Upper Limbs in Subjects with Cervical SCI: Clinical Trial. Biomedicines 2023, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Inanici, F.; Samejima, S.; Gad, P.; Edgerton, V.R.; Hofstetter, C.P.; Moritz, C.T. Transcutaneous electrical spinal stimulation promotes lon-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Inanici, F.; Brighton, L.N.; Samejima, S.; Hofstetter, C.P.; Moritz, C.T. Transcutaneous Spinal Cord Stimulation Restores Hand and Arm Function after Spinal Cord Injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Momeni, K.; Ramanujam, A.; Ravi, M.; Carnahan, J.; Kirshblum, S.; Forrest, G.F. Cervical Spinal Cord Transcutaneous Stimulation Improves Upper Extremity and Hand Function in People with Complete Tetraplegia: A Case Study. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 3167–3174. [Google Scholar] [CrossRef] [PubMed]
- Gad, P.; Lee, S.; Terrafranca, N.; Zhong, H.; Turner, A.; Gerasimenko, Y.P.; Edgerton, V.R. Non-invasive activation of cervical spinal networks after severe paraysis. J. Neurotrauma 2018, 35, 2145–2158. [Google Scholar] [CrossRef]
- Gad, P.; Gerasimenko, Y.; Zdunowski, S.; Turner, A.; Sayenko, D.; Lu, D.C.; Edgerton, V.R. Weight Bearing Over-ground Stepping in an Exoskeleton with Non-invasive Spinal Cord Neuromodulation after Motor Complete Paraplegia. Front. Neurosci. 2017, 11, 333. [Google Scholar] [CrossRef]
- Minassian, K.; Hofstoetter, U.S.; Danner, S.M.; Mayr, W.; Bruce, J.A.; McKay, W.B.; Tansey, K.E. Spinal Rhythm Generation by Step-Induced Feedback and Transcutaneous Posterior Root Stimulation in Complete Spinal Cord–Injured Individuals. Neurorehabil Neural Repair 2016, 30, 233–243. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Krenn, M.; Danner, S.M.; Hofer, C.; Kern, H.; McKay, W.B.; Mayr, W.; Minassian, K. Augmentation of Voluntary Locomotor Activity by Transcutaneous Spinal Cord Stimulation in Motor-Incomplete Spinal Cord-Injured Individuals: Augmentation of Locomotion by tSCS in Incomplete SCI. Artif. Organs 2015, 39, 176–186. [Google Scholar] [CrossRef]
- Rath, M.; Vette, A.H.; Ramasubramaniam, S.; Li, K.; Burdick, J.W.; Edgerton, V.R.; Gerasimenko, Y.P.; Sayenko, D.G. Trunk Stability Enabled by Noninvasive Spinal Electrical Stimulation after Spinal Cord Injury. J. Neurotrauma 2018, 35, 2540–2553. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Rath, M.; Ferguson, A.R.; Burdick, J.W.; Hayton, L.A.; Edgerton, V.R.; Gerasimenko, Y.P. Self-Assisted Standing Enabled by Non-Invasive Spinal Stimulation after Spinal Cord Injury. J. Neurotrauma 2019, 36, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, Y.P.; Lu, D.C.; Modaber, M.; Zdunowski, S.; Gad, P.; Sayenko, D.G.; Morikawa, E.; Haakana, P.; Ferguson, A.R.; Roy, R.R.; et al. Noninvasive Reactivation of Motor Descending Control after Paralysis. J. Neurotrauma 2015, 32, 1968–1980. [Google Scholar] [CrossRef] [PubMed]
- Kumru, H.; Flores, A.; Rodríguez-Cañón, M.; Edgerton, V.R.; García, L.; Benito-Penalva, J.; Navarro, X.; Gerasimenko, Y.; García-Alías, G.; Vidal, J. Cervical Electrical Neuromodulation Effectively Enhances Hand Motor Output in Healthy Subjects by Engaging a Use-Dependent Intervention. J. Clin. Med. 2021, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Kumru, H.; Rodríguez-Cañón, M.; Edgerton, V.R.; García, L.; Soriano, I.; Opisso, E.; Gerasimenko, Y.; Navarro, X.; García-Alías, G.; Vidal, J. Transcutaneous Electrical Neuromodulation of the Cervical Spinal Cord Depends Both on the Stimulation Intensity and the Degree of Voluntary Activity for Training. A Pilot Study. J. Clin. Med. 2021, 10, 3278. [Google Scholar] [CrossRef] [PubMed]
- Gad, P.; Kreydin, E.; Zhong, H.; Edgerton, V.R. Enabling respiratory control after severe chronic tetraplegia: An exploratory case study. J. Neurophysiol. 2020, 124, 774–780. [Google Scholar] [CrossRef]
- Ledsome, J.R.; Sharp, J.M. Pulmonary function in acute cervical cord injury. Am. Rev. Respir. Dis. 1981, 124, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (Revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef]
- Tamplin, J.; Berlowitz, D.J. A systematic review and meta-analysis of the effects of respiratory muscle training on pulmonary function in tetraplegia. Spinal Cord 2014, 52, 175–180. [Google Scholar] [CrossRef]
- Hachmann, J.T.; Grahn, P.J.; Calvert, J.S.; Drubach, D.I.; Lee, K.H.; Lavrov, I.A. Electrical neuromodulation of the respiratory system after spinal cord injury. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2017; Volume 92, pp. 1401–1414. [Google Scholar] [CrossRef]
- Milosevic, M.; Masugi, Y.; Sasaki, A.; Sayenko, D.G.; Nakazawa, K. On the reflex mechanisms of cervical transcutaneous spinal cord stimulation in human subjects. J. Neurophysiol. 2019, 121, 1672–1679. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Gorodnichev, R.; Moshonkina, T.; Sayenko, D.; Gad, P.; Edgerton, V.R. Transcutaneous electrical spinal-cordstimulation in humans HHS Public Access. Ann. Phys. Rehabil. Med. 2015, 58, 225–231. [Google Scholar] [CrossRef]
- Beekhuizen, K.S.; Field-Fote, E.C. Massed practice versus massed practice with stimulation: Effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Neurorehabilit. Neural Repair 2005, 19, 33–45. [Google Scholar] [CrossRef]
- Guiho, T.; Baker, S.N.; Jackson, A. Epidural and transcutaneous spinal cord stimulation facilitates descending inputs to upper-limb motoneurons in monkeys. J. Neural Eng. 2021, 18, 046011. [Google Scholar] [CrossRef] [PubMed]
- DiMarco, A.F.; Kowalski, K.E. Intercostal muscle pacing with high frequency spinal cord stimulation in dogs. Respir. Physiol. Neurobiol. 2010, 171, 218–224. [Google Scholar] [CrossRef] [PubMed][Green Version]



| Age | Sex | SCI Etiology | SCI Level | AIS | Time Since SCI (Month) | Height (cm) | Weight (kg) | tSCS Intensity at C3 (mA) | tSCS Intensity at Th9 (mA) | |
|---|---|---|---|---|---|---|---|---|---|---|
| IMT | 38 * | M | Trauma | C7 | B | 7 | 165 | 54 | - | - |
| IMT | 36 | M | Trauma | C4 | B | 9 | 172 | 57 | - | - |
| IMT | 36 | M | Trauma | C4 | C | 10 | 167 | 61 | - | - |
| IMT | 31 | M | Trauma | C5 | A | 8 | 170 | 76 | - | - |
| IMT | 18 * | M | Trauma | C4 | B | 8 | 174 | 48 | - | - |
| IMT | 45 | M | Trauma | C4 | C | 6 | 175 | 62 | - | - |
| IMT | 21 * | M | Trauma | C5 | B | 9 | 176 | 60 | - | - |
| IMT | 18 * | M | Trauma | C3 | A | 7 | 179 | 58 | - | - |
| IMT | 23 * | F | Trauma | C7 | C | 6 | 170 | 54 | - | - |
| IMT | 47 | M | Trauma | C4 | D | 9 | 172 | 83.2 | - | - |
| IMT | 26 * | M | Trauma | C5 | C | 4 | 178 | 82.5 | - | - |
| tSCS + IMT | 25 | M | Trauma | C4 | A | 9 | 186 | 76 | 67 | 60 |
| tSCS + IMT | 46 | M | Trauma | C4 | C | 8 | 174 | 76 | 80 | 80 |
| tSCS + IMT | 28 | M | Trauma | C6 | A | 7 | 176 | 71 | 77 | 90 |
| tSCS + IMT | 18 * | M | Trauma | C4 | B | 9 | 174 | 48 | 44 | 52 |
| tSCS + IMT | 35 | M | Trauma | C4 | C | 7 | 175 | 62 | 34 | 40 |
| tSCS + IMT | 21 * | M | Trauma | C5 | B | 9 | 176 | 60 | 52 | 66 |
| tSCS + IMT | 18 * | M | Trauma | C3 | B | 10 | 179 | 58.5 | 66 | 70 |
| tSCS + IMT | 23 * | F | Trauma | C7 | C | 9 | 170 | 54 | 62 | 62 |
| tSCS + IMT | 26 * | M | Trauma | C5 | C | 5 | 178 | 82.5 | 57 | 61 |
| tSCS + IMT | 34 | M | Trauma | C4 | D | 4 | 175 | 68.5 | 49 | 59 |
| tSCS + IMT | 38 * | M | Trauma | C7 | B | 8 | 165 | 54 | 78 | 82 |
| p | 0.08 | 1.00 | 0.20 | 0.79 |
| PRE | POST | p ** | ||
|---|---|---|---|---|
| tSCS + IMT | breathlessness | 5.09 ± 1.14 | 3.36 ± 1.36 | F = 8.272, p < 0.009, η2 = 0.293 TimexIntervention F = 19.449, p < 0.001 η2 = 0.493 |
| IMT | 4.91 ± 1.67 | 4.91 ± 1.64 | ||
| p * | 0.336 | |||
| tSCS + IMT | hypophonia | 4.00 ± 2.32 | 2.27 ± 1.35 | F = 18.06, p < 0.001, η2 = 0.475 TimexIntervention F = 9.552, p = 0.006; η2 = 0.323 |
| IMT | 4.18 ± 1.17 | 3.91 ± 0.83 | ||
| p * | 0.819 | |||
| tSCS + IMT | MIP | 61.18 ± 28.01 | 69.64 ± 28.93 | F = 4.452, p < 0.048, η2 = 0.182 TimexIntervention F = 5.813, p = 0.026; η2 = 0.225 |
| IMT | 65.61 ± 26.01 | 65.03 ± 26.69 | ||
| p * | 0.705 | |||
| tSCS + IMT | MEP | 54.48 ± 27.53 | 66.80 ± 32.41 | F = 15.240, p < 0.01, η2 = 0.432 TimexIntervention F = 6.708, p = 0.017; η2 = 0.251 |
| IMT | 45.58 ± 25.15 | 48.09 ± 27.71 | ||
| p * | 0.439 | |||
| spirometric measures | ||||
| PRE | POST | |||
| tSCS + IMT | FVC (L) | 2.28 ± 0.93 | 2.70± 1.37 | p& = 0.013 p & = 0.534 |
| IMT | 2.24 ± 0.79 | 2.18 ± 0.98 | ||
| p@ | 0.898 | |||
| tSCS + IMT | FEV1 (L) | 1.58 ± 0.57 | 1.75 ± 0.70 | F = 0.067, p = 0.799, η2 = 0.003 TimexIntervention F = 6.708, p = 0.018; η2 = 0.251 |
| IMT | 1.78 ± 0.46 | 1.57 ± 0.49 | ||
| p * | 0.406 | |||
| tSCS + IMT | FEV1/FVC (%) | 72.30 ± 22.37 | 70.21 ± 20.92 | F = 2.196, p = 0.154, η2 = 0. 099 TimexIntervention F = 0.062, p = 0.806; η2 = 0.003 |
| IMT | 78.85 ± 12.51 | 75.92 ± 13.95 | ||
| p * | 0.402 | |||
| tSCS + IMT | PEF (L/s) | 2.90 ± 1.39 | 3.04 ± 1.32 | F = 0.004, p = 0.950, η2 = 0.000 TimexIntervention F = 0.840, p = 0.371; η2 = 0.042 |
| IMT | 2.94 ± 0.73 | 2.82 ± 0.85 | ||
| p * | 0.772 | |||
| tSCS + IMT | FEF50% (L/s) | 1.57 ± 0.68 | 1.58 ± 0.70 | F = 0.232, p = 0.636, η2 = 0.012 TimexIntervention F = 0.371, p = 0.550; η2 = 0.019 |
| IMT | 1.78 ± 0.49 | 1.67 ± 0.67 | ||
| p * | 0.482 | |||
| tSCS + IMT | FEF25%/75% (L/s) | 1.62 ± 0.83 | 2.46 ± 3.28 | F = 0.851, p = 0.3670, η2 = 0. 041 TimexIntervention F = 2.388, p = 0.268; η2 = 0.061 |
| IMT | 1.66 ± 0.46 | 1.57 ± 0.61 | ||
| p * | 0.639 | |||
| tSCS + IMT | FEV1/FEV0.5 | 1.44 ± 0.18 | 1.44 ± 0.18 | F = 0.30, p = 0.864; η2 = 0.001 TimexIntervention F = 0.74, p = 0.788, η2 = 0. 004 |
| IMT | 1.45 ± 0.15 | 1.45 ± 0.17 | ||
| p * | 0.880 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumru, H.; García-Alén, L.; Ros-Alsina, A.; Albu, S.; Valles, M.; Vidal, J. Transcutaneous Spinal Cord Stimulation Improves Respiratory Muscle Strength and Function in Subjects with Cervical Spinal Cord Injury: Original Research. Biomedicines 2023, 11, 2121. https://doi.org/10.3390/biomedicines11082121
Kumru H, García-Alén L, Ros-Alsina A, Albu S, Valles M, Vidal J. Transcutaneous Spinal Cord Stimulation Improves Respiratory Muscle Strength and Function in Subjects with Cervical Spinal Cord Injury: Original Research. Biomedicines. 2023; 11(8):2121. https://doi.org/10.3390/biomedicines11082121
Chicago/Turabian StyleKumru, Hatice, Loreto García-Alén, Aina Ros-Alsina, Sergiu Albu, Margarita Valles, and Joan Vidal. 2023. "Transcutaneous Spinal Cord Stimulation Improves Respiratory Muscle Strength and Function in Subjects with Cervical Spinal Cord Injury: Original Research" Biomedicines 11, no. 8: 2121. https://doi.org/10.3390/biomedicines11082121
APA StyleKumru, H., García-Alén, L., Ros-Alsina, A., Albu, S., Valles, M., & Vidal, J. (2023). Transcutaneous Spinal Cord Stimulation Improves Respiratory Muscle Strength and Function in Subjects with Cervical Spinal Cord Injury: Original Research. Biomedicines, 11(8), 2121. https://doi.org/10.3390/biomedicines11082121






