Comparative Analysis of Cytokine Profiles in Cerebrospinal Fluid and Blood Serum in Patients with Acute and Subacute Spinal Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Samples Collection and Storage
2.3. Multiplex Analysis
2.4. Statistical Analysis
3. Results
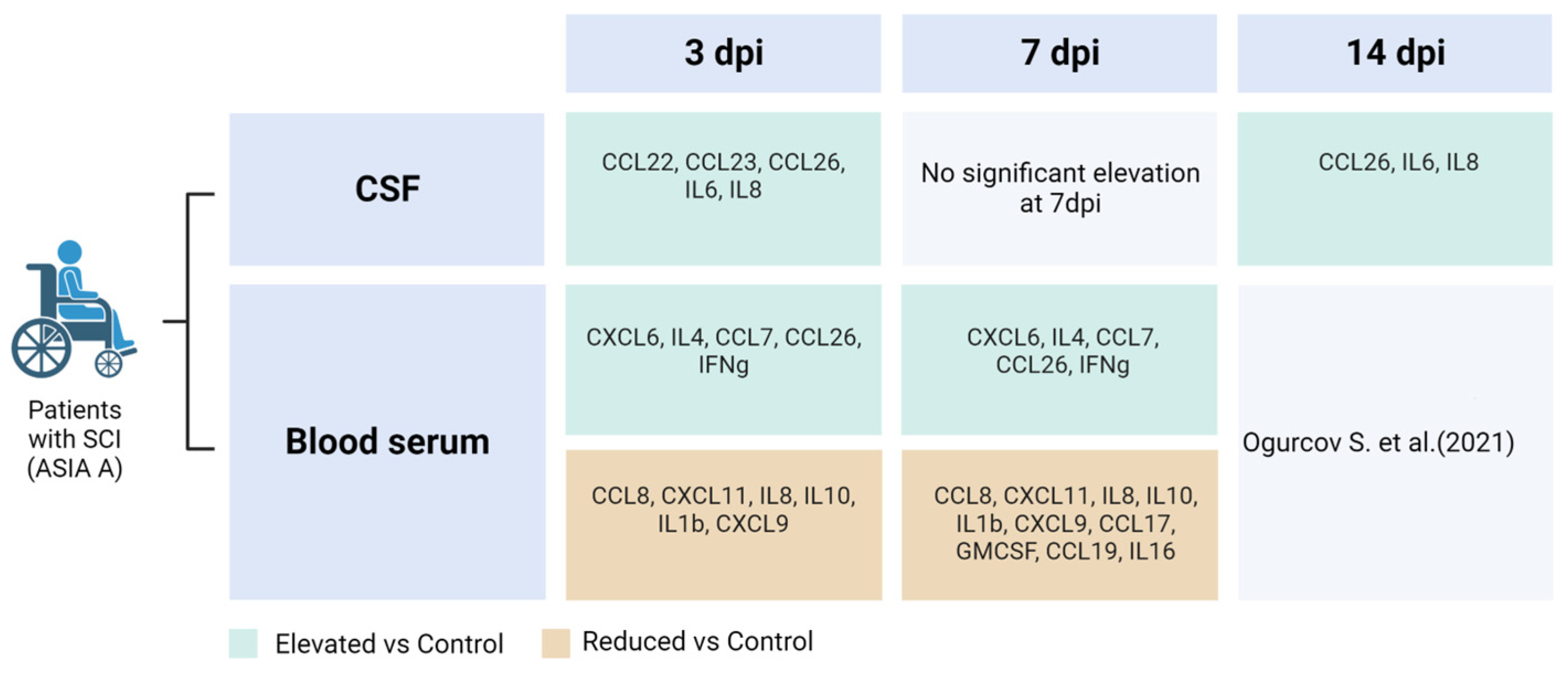
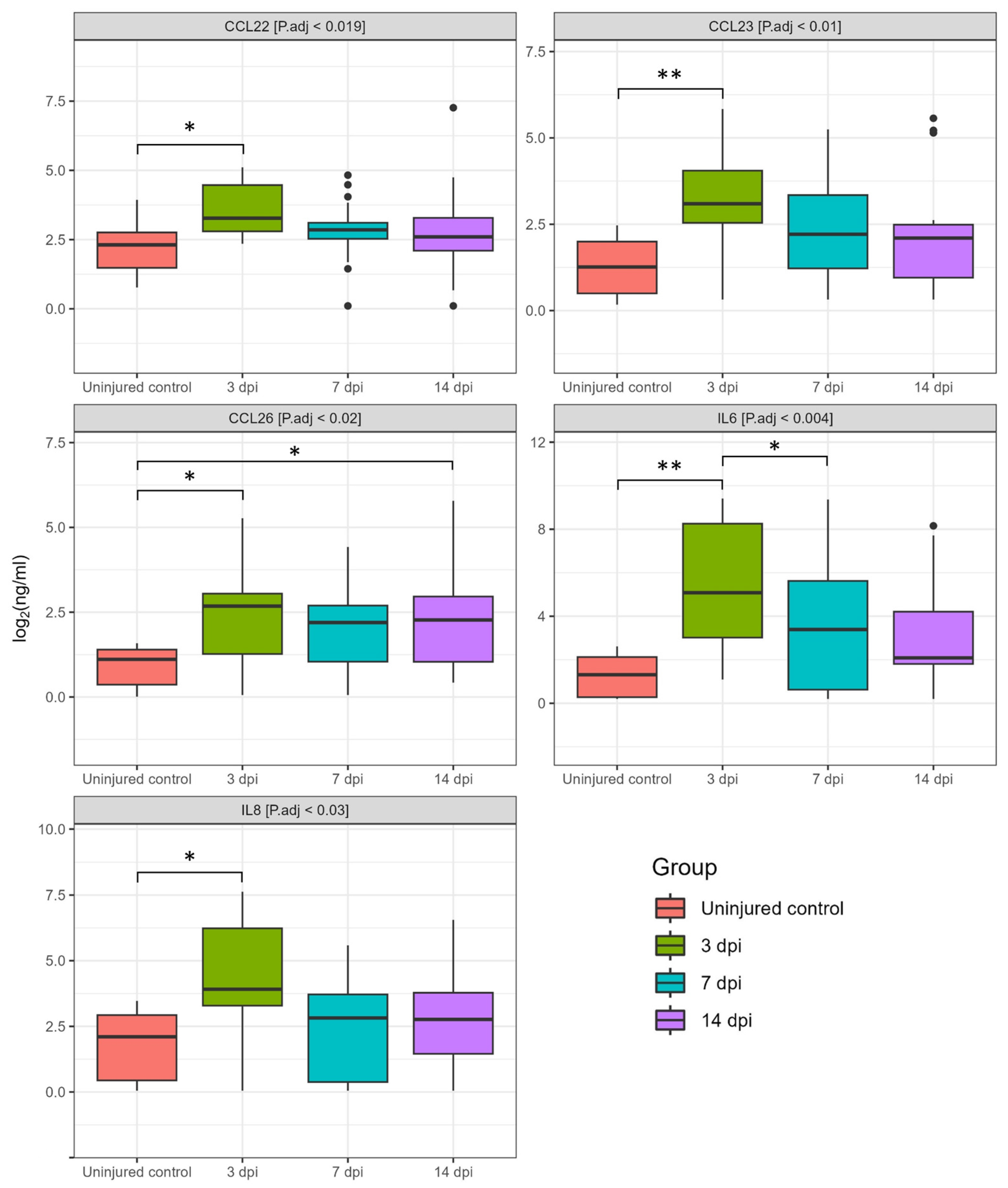
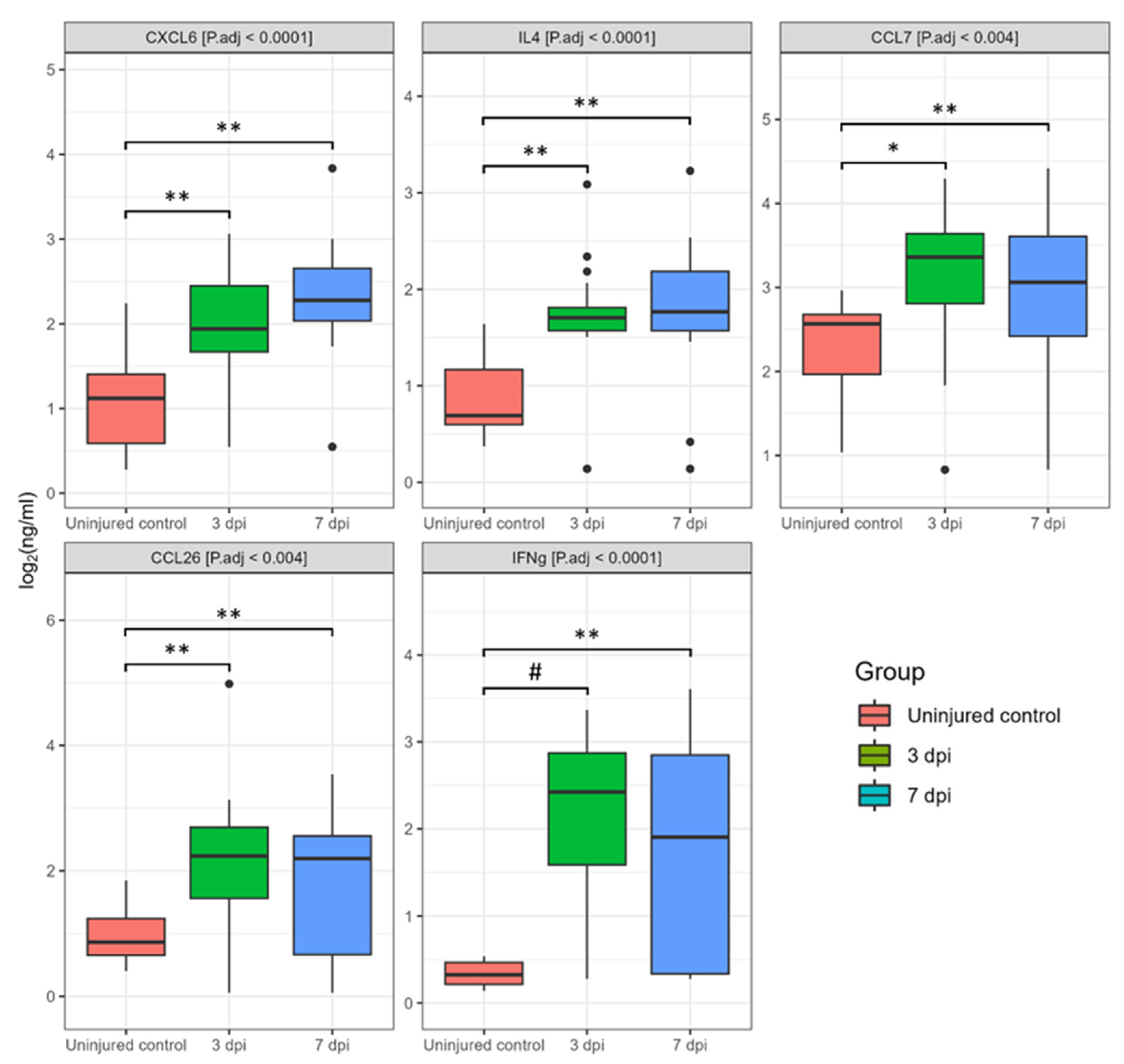
3.1. Dynamics of Cerebrospinal Fluid Cytokine Profile after Spinal Cord Injury
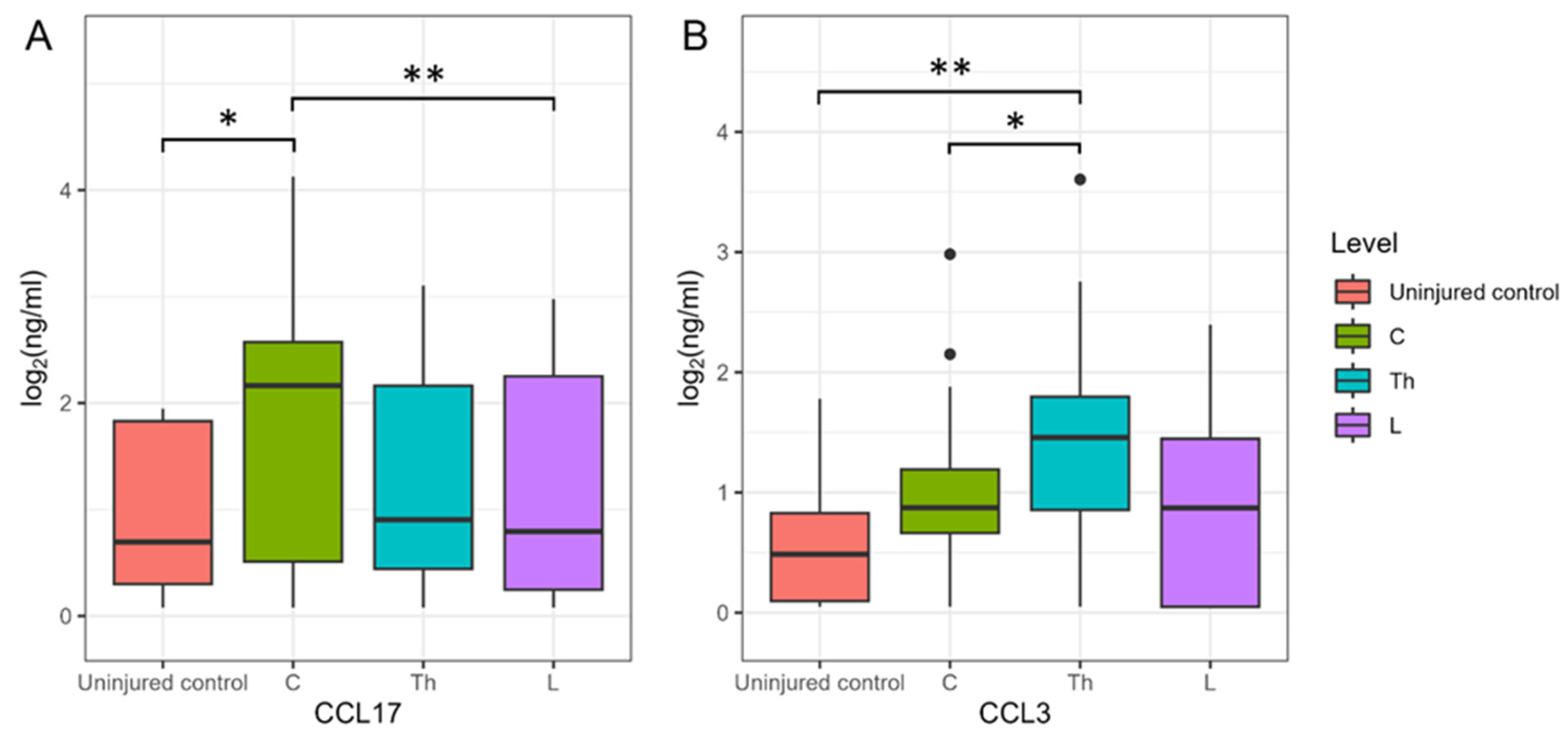
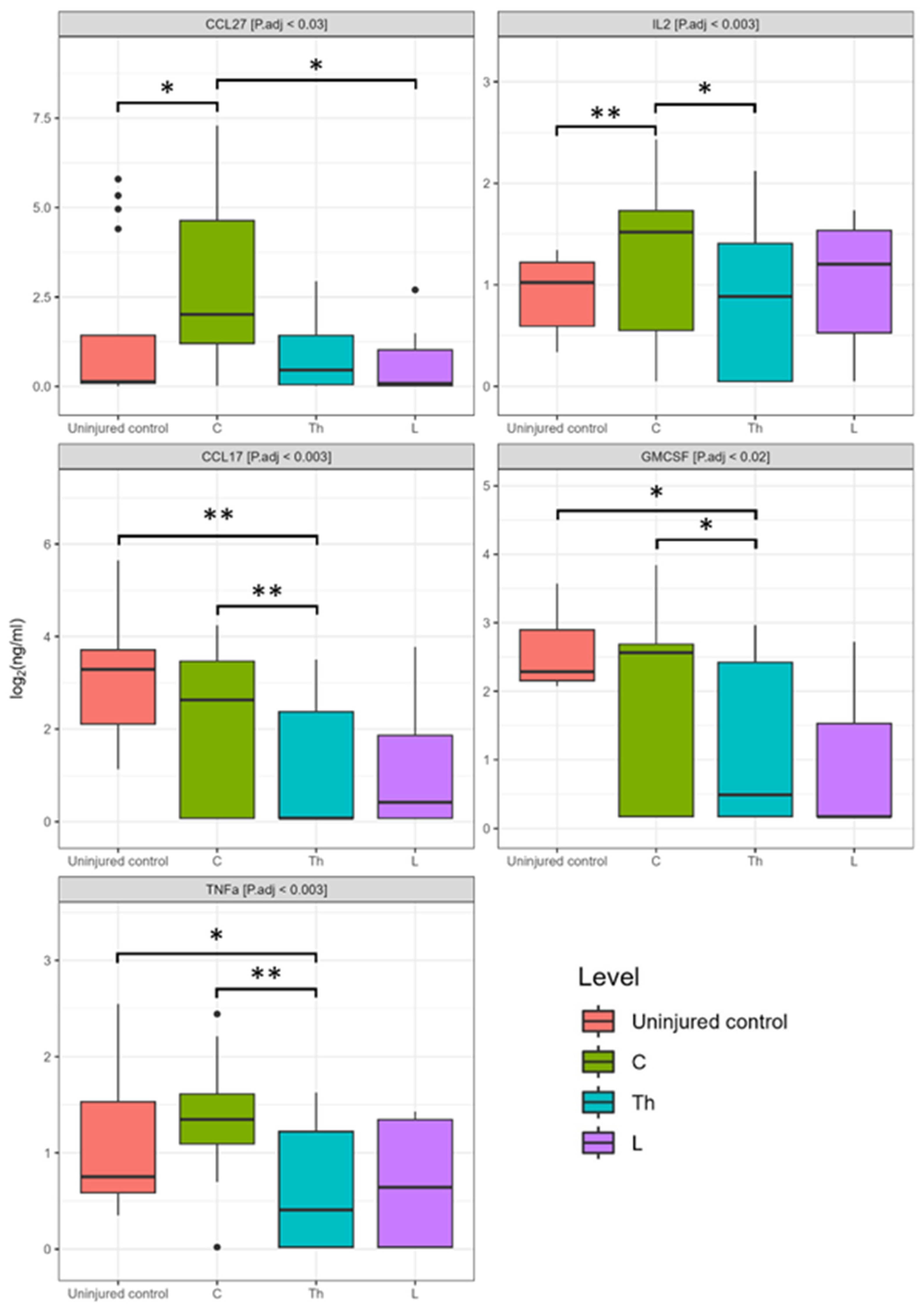
3.2. Dynamics of Blood Serum Cytokine Profile after Spinal Cord Injury
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic Spinal Cord Injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global Prevalence and Incidence of Traumatic Spinal Cord Injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar] [CrossRef] [PubMed]
- Turczyn, P.; Wojdasiewicz, P.; Poniatowski, Ł.A.; Purrahman, D.; Maślińska, M.; Żurek, G.; Romanowska-Próchnicka, K.; Żuk, B.; Kwiatkowska, B.; Piechowski-Jóźwiak, B.; et al. Omega-3 Fatty Acids in the Treatment of Spinal Cord Injury: Untapped Potential for Therapeutic Intervention? Mol. Biol. Rep. 2022, 49, 10797–10809. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Grassner, L.; Belci, M.; Cook, J.; Knight, R.; Davies, L.; Asif, H.; Visagan, R.; Gallagher, M.J.; Thomé, C.; et al. Duroplasty for Injured Cervical Spinal Cord with Uncontrolled Swelling: Protocol of the DISCUS Randomized Controlled Trial. Trials 2023, 24, 497. [Google Scholar] [CrossRef]
- Kimura, A.; Suehiro, K.; Mukai, A.; Fujimoto, Y.; Funao, T.; Yamada, T.; Mori, T. Protective Effects of Hydrogen Gas against Spinal Cord Ischemia–Reperfusion Injury. J. Thorac. Cardiovasc. Surg. 2022, 164, e269–e283. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury—Repair and Regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef]
- Kubaszewski, Ł.; Wojdasiewicz, P.; Rożek, M.; Słowińska, I.E.; Romanowska-Próchnicka, K.; Słowiński, R.; Poniatowski, Ł.A.; Gasik, R. Syndromes with Chronic Non-Bacterial Osteomyelitis in the Spine. Rheumatology 2016, 53, 328–336. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Tetreault, L.A.; Wilson, J.R.; Kwon, B.K.; Burns, A.S.; Martin, A.R.; Hawryluk, G.; Harrop, J.S. A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: Introduction, Rationale, and Scope. Glob. Spine J. 2017, 7, 84S–94S. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International Standards for Neurological Classification of Spinal Cord Injury (Revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef]
- Kirshblum, S.; Snider, B.; Eren, F.; Guest, J. Characterizing Natural Recovery after Traumatic Spinal Cord Injury. J. Neurotrauma 2021, 38, 1267–1284. [Google Scholar] [CrossRef]
- Biglari, B.; Swing, T.; Child, C.; Büchler, A.; Westhauser, F.; Bruckner, T.; Ferbert, T.; Jürgen Gerner, H.; Moghaddam, A. A Pilot Study on Temporal Changes in IL-1β and TNF-α Serum Levels after Spinal Cord Injury: The Serum Level of TNF-α in Acute SCI Patients as a Possible Marker for Neurological Remission. Spinal Cord 2015, 53, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, D.; Cao, S.; Hou, G.; Ma, H.; Shi, B. Association between Serum IL-37 and Spinal Cord Injury: A Prospective Observational Study. Biomed. Res. Int. 2020, 2020, 6664313. [Google Scholar] [CrossRef] [PubMed]
- Ogurcov, S.; Shulman, I.; Garanina, E.; Sabirov, D.; Baichurina, I.; Kuznetcov, M.; Masgutova, G.; Kostennikov, A.; Rizvanov, A.; James, V.; et al. Blood Serum Cytokines in Patients with Subacute Spinal Cord Injury: A Pilot Study to Search for Biomarkers of Injury Severity. Brain Sci. 2021, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Westin, K.; Buchhave, P.; Nielsen, H.; Minthon, L.; Janciauskiene, S.; Hansson, O. CCL2 Is Associated with a Faster Rate of Cognitive Decline during Early Stages of Alzheimer’s Disease. PLoS ONE 2012, 7, e30525. [Google Scholar] [CrossRef]
- Larose, M.-C.; Chakir, J.; Archambault, A.-S.; Joubert, P.; Provost, V.; Laviolette, M.; Flamand, N. Correlation between CCL26 Production by Human Bronchial Epithelial Cells and Airway Eosinophils: Involvement in Patients with Severe Eosinophilic Asthma. J. Allergy Clin. Immunol. 2015, 136, 904–913. [Google Scholar] [CrossRef]
- Zhebrun, D.A.; Totolyan, A.A.; Maslyanskii, A.L.; Titov, A.G.; Patrukhin, A.P.; Kostareva, A.A.; Gol’tseva, I.S. Synthesis of Some CC Chemokines and Their Receptors in the Synovium in Rheumatoid Arthritis. Bull. Exp. Biol. Med. 2014, 158, 192–196. [Google Scholar] [CrossRef]
- Chen, X.; An, Y.; Zhang, Y.; Xu, D.; Chen, T.; Yang, Y.; Chen, W.; Wu, D.; Zhang, X. CCL26 Is Upregulated by Nab-Paclitaxel in Pancreatic Cancer–Associated Fibroblasts and Promotes PDAC Invasiveness through Activation of the PI3K/AKT/MTOR Pathway. Acta Biochim. Biophys. Sin. 2021, 53, 612–619. [Google Scholar] [CrossRef]
- Shou, J.; Peng, J.; Zhao, Z.; Huang, X.; Li, H.; Li, L.; Gao, X.; Xing, Y.; Liu, H. CCL26 and CCR3 Are Associated with the Acute Inflammatory Response in the CNS in Experimental Autoimmune Encephalomyelitis. J. Neuroimmunol. 2019, 333, 576967. [Google Scholar] [CrossRef]
- Kossmann, T.; Stahel, P.F.; Lenzlinger, P.M.; Redl, H.; Dubs, R.W.; Trentz, O.; Schlag, G.; Morganti-Kossmann, M.C. Interleukin-8 Released into the Cerebrospinal Fluid after Brain Injury Is Associated with Blood–Brain Barrier Dysfunction and Nerve Growth Factor Production. J. Cereb. Blood Flow Metab. 1997, 17, 280–289. [Google Scholar] [CrossRef]
- Kwon, B.K.; Stammers, A.M.T.; Belanger, L.M.; Bernardo, A.; Chan, D.; Bishop, C.M.; Slobogean, G.P.; Zhang, H.; Umedaly, H.; Giffin, M.; et al. Cerebrospinal Fluid Inflammatory Cytokines and Biomarkers of Injury Severity in Acute Human Spinal Cord Injury. J. Neurotrauma 2010, 27, 669–682. [Google Scholar] [CrossRef]
- Okada, S.; Nakamura, M.; Mikami, Y.; Shimazaki, T.; Mihara, M.; Ohsugi, Y.; Iwamoto, Y.; Yoshizaki, K.; Kishimoto, T.; Toyama, Y.; et al. Blockade of Interleukin-6 Receptor Suppresses Reactive Astrogliosis and Ameliorates Functional Recovery in Experimental Spinal Cord Injury. J. Neurosci. Res. 2004, 76, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Okada, S.; Toyama, Y.; Okano, H. Role of IL-6 in Spinal Cord Injury in a Mouse Model. Clin. Rev. Allergy Immunol. 2005, 28, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Fertleman, M.; Pereira, C.; Dani, M.; Harris, B.H.L.; Di Giovannantonio, M.; Taylor-Robinson, S.D. Cytokine Changes in Cerebrospinal Fluid and Plasma after Emergency Orthopaedic Surgery. Sci. Rep. 2022, 12, 2221. [Google Scholar] [CrossRef]
- Galimberti, D.; Fenoglio, C.; Comi, C.; Scalabrini, D.; De Riz, M.; Leone, M.; Venturelli, E.; Cortini, F.; Piola, M.; Monaco, F. MDC/CCL22 Intrathecal Levels in Patients with Multiple Sclerosis. Mult. Scler. J. 2008, 14, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.O.; Akhmetzyanova, E.R.; Martynova, E.V.; Khaiboullina, S.F.; Galieva, L.R.; Rizvanov, A.A. Systemic and Local Cytokine Profile Following Spinal Cord Injury in Rats: A Multiplex Analysis. Front. Neurol. 2017, 8, 581. [Google Scholar] [CrossRef]
- Müller, M.; Carter, S.; Hofer, M.J.; Campbell, I.L. The Chemokine Receptor CXCR3 and Its Ligands CXCL9, CXCL10 and CXCL11 in Neuroimmunity—A Tale of Conflict and Conundrum. Neuropathol. Appl. Neurobiol. 2010, 36, 368–387. [Google Scholar] [CrossRef]
- Stein, A.; Panjwani, A.; Sison, C.; Rosen, L.; Chugh, R.; Metz, C.; Bank, M.; Bloom, O. Pilot Study: Elevated Circulating Levels of the Proinflammatory Cytokine Macrophage Migration Inhibitory Factor in Patients with Chronic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2013, 94, 1498–1507. [Google Scholar] [CrossRef]
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after Spinal Cord Injury: A Review of the Critical Timeline of Signaling Cues and Cellular Infiltration. J. Neuroinflammation 2021, 18, 284. [Google Scholar] [CrossRef]
- Shtrichman, R.; Samuel, C.E. The Role of Gamma Interferon in Antimicrobial Immunity. Curr. Opin. Microbiol. 2001, 4, 251–259. [Google Scholar] [CrossRef]
- Kursunel, M.A.; Esendagli, G. The Untold Story of IFN-γ in Cancer Biology. Cytokine Growth Factor. Rev. 2016, 31, 73–81. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabirov, D.; Ogurcov, S.; Shulman, I.; Kabdesh, I.; Garanina, E.; Sufianov, A.; Rizvanov, A.; Mukhamedshina, Y. Comparative Analysis of Cytokine Profiles in Cerebrospinal Fluid and Blood Serum in Patients with Acute and Subacute Spinal Cord Injury. Biomedicines 2023, 11, 2641. https://doi.org/10.3390/biomedicines11102641
Sabirov D, Ogurcov S, Shulman I, Kabdesh I, Garanina E, Sufianov A, Rizvanov A, Mukhamedshina Y. Comparative Analysis of Cytokine Profiles in Cerebrospinal Fluid and Blood Serum in Patients with Acute and Subacute Spinal Cord Injury. Biomedicines. 2023; 11(10):2641. https://doi.org/10.3390/biomedicines11102641
Chicago/Turabian StyleSabirov, Davran, Sergei Ogurcov, Ilya Shulman, Ilyas Kabdesh, Ekaterina Garanina, Albert Sufianov, Albert Rizvanov, and Yana Mukhamedshina. 2023. "Comparative Analysis of Cytokine Profiles in Cerebrospinal Fluid and Blood Serum in Patients with Acute and Subacute Spinal Cord Injury" Biomedicines 11, no. 10: 2641. https://doi.org/10.3390/biomedicines11102641
APA StyleSabirov, D., Ogurcov, S., Shulman, I., Kabdesh, I., Garanina, E., Sufianov, A., Rizvanov, A., & Mukhamedshina, Y. (2023). Comparative Analysis of Cytokine Profiles in Cerebrospinal Fluid and Blood Serum in Patients with Acute and Subacute Spinal Cord Injury. Biomedicines, 11(10), 2641. https://doi.org/10.3390/biomedicines11102641