Super-Resolution Imaging Reveals the Nanoscale Distributions of Dystroglycan and Integrin Itga7 in Zebrafish Muscle Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Transposase and Clone Generation
2.1.2. Zebrafish Husbandry and Injections
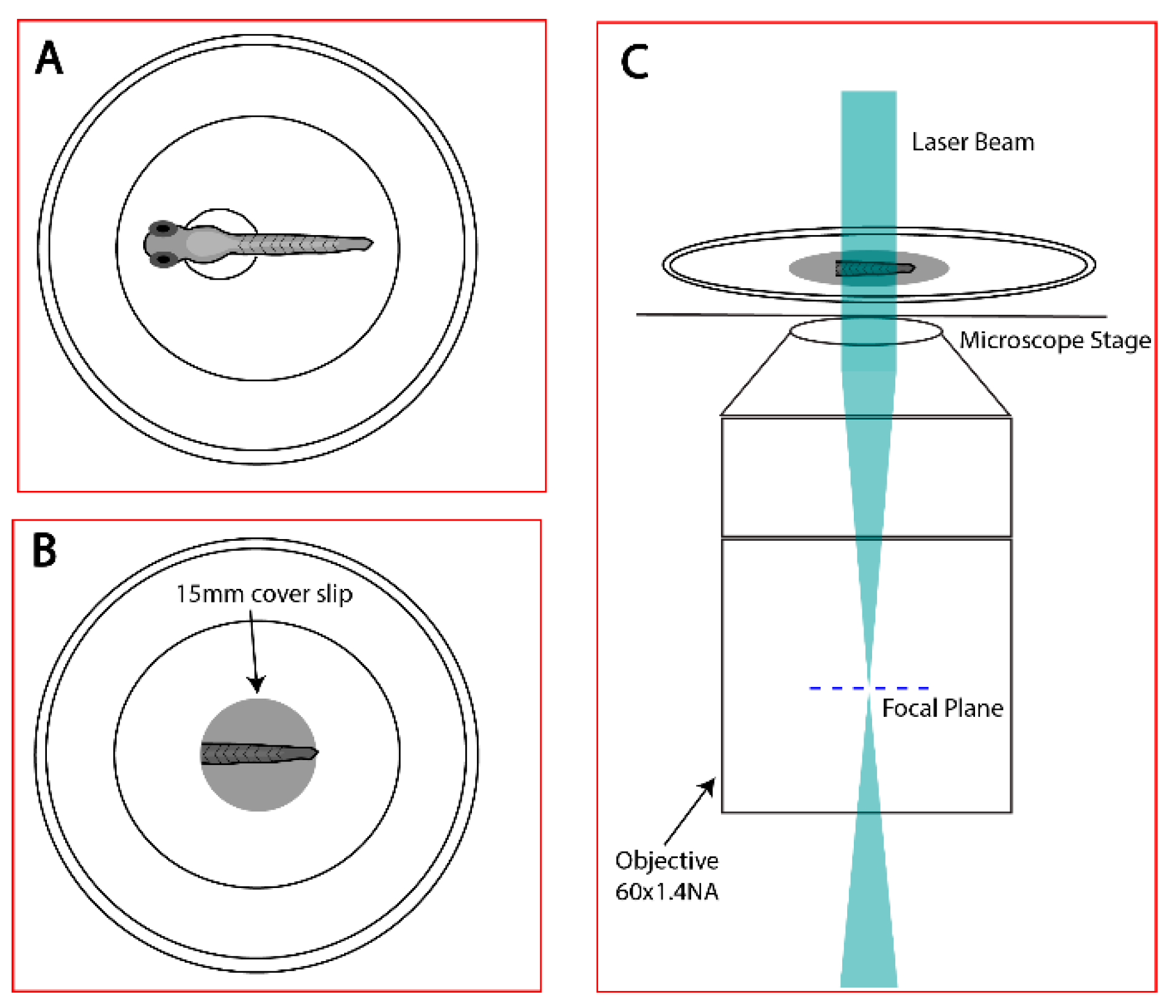
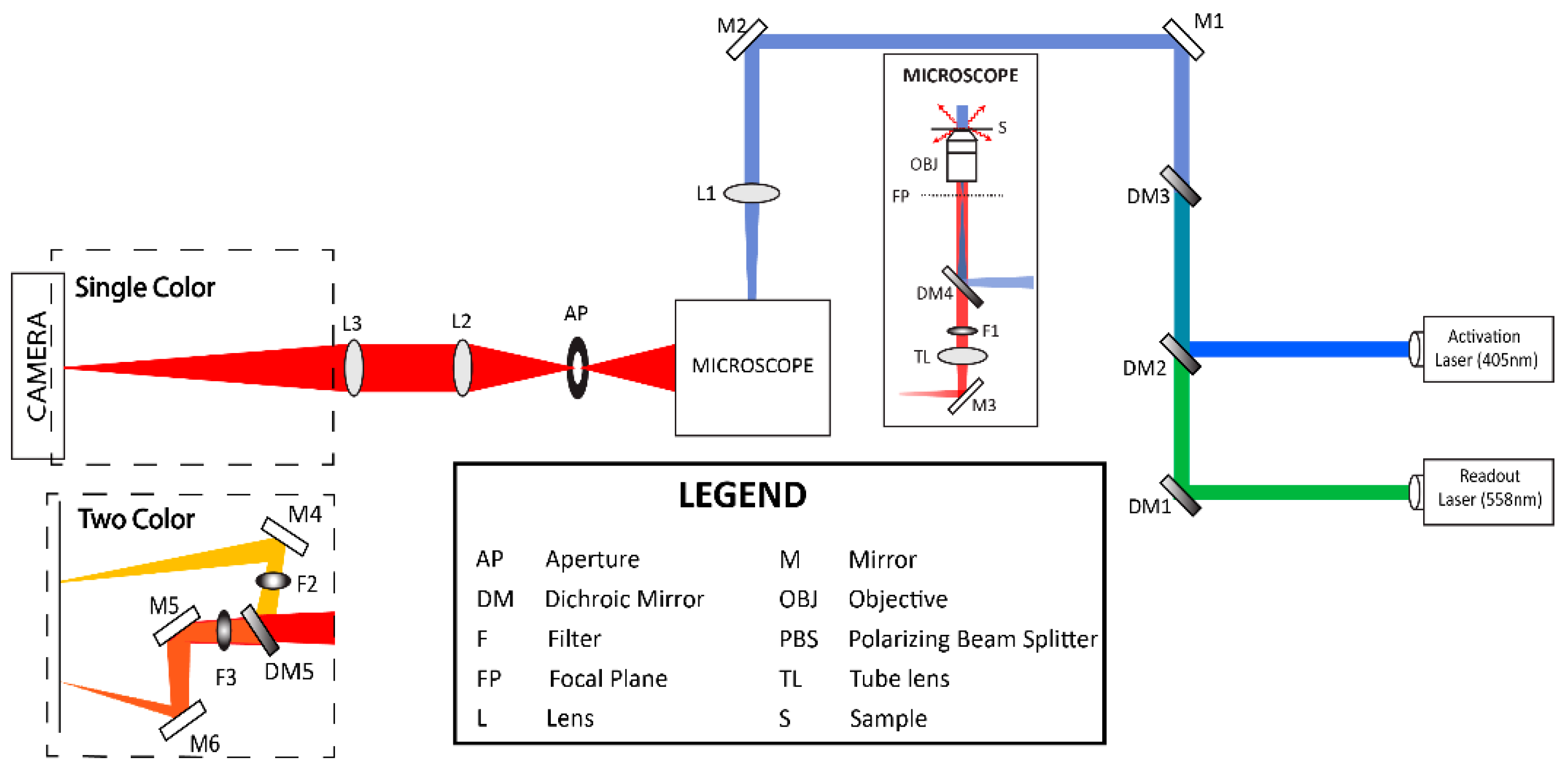
2.2. Localization-Based Super-Resolution Microscopy
Fluorescence Photoactivation Localization Microscopy (FPALM)
2.3. Acquisition Parameters
2.4. Identification of Regions of Interest
2.5. Identification and Localization of Single Molecules
2.6. Cluster Analysis
3. Results
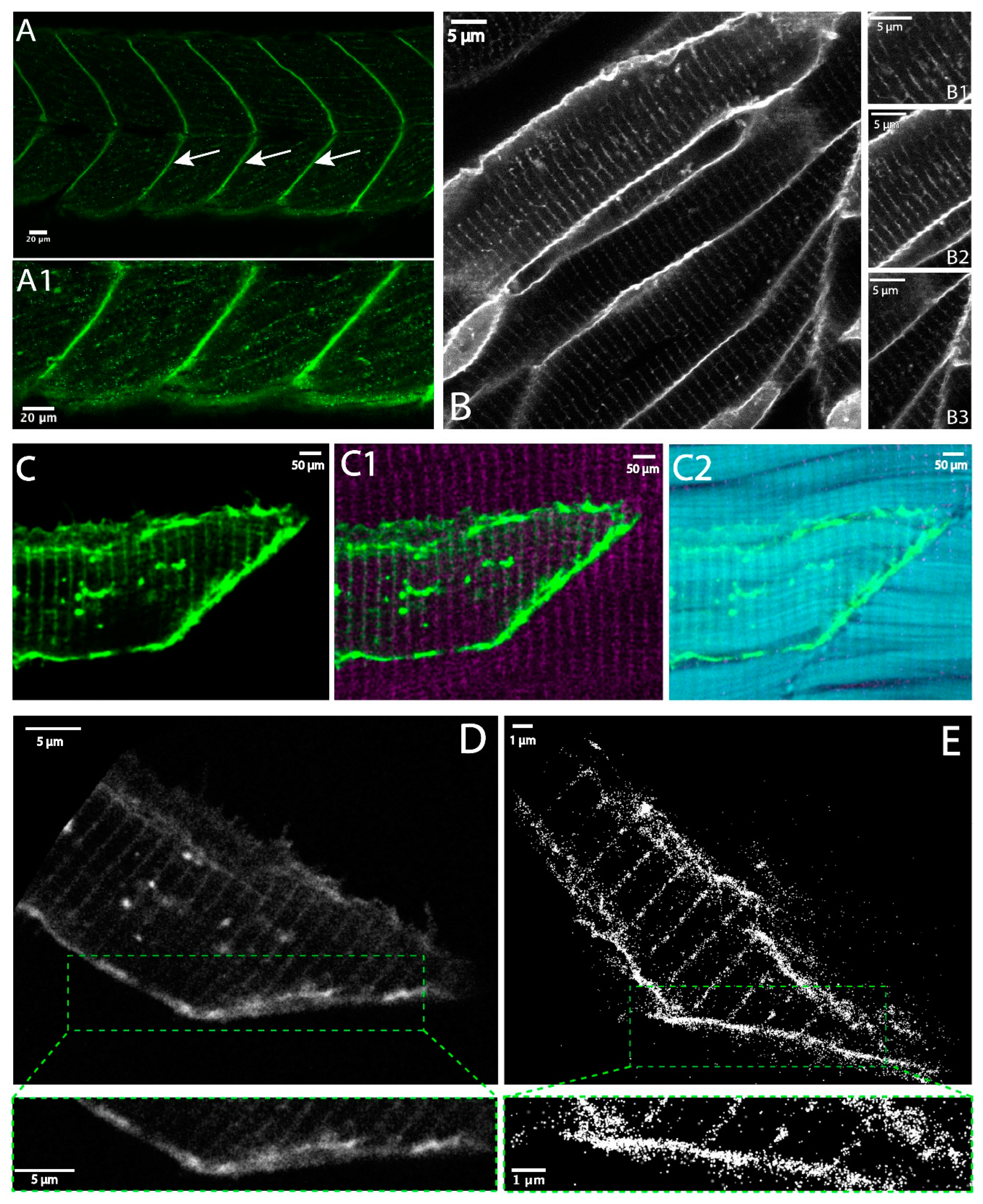
3.1. Dystroglycan in Skeletal Muscle Fibers
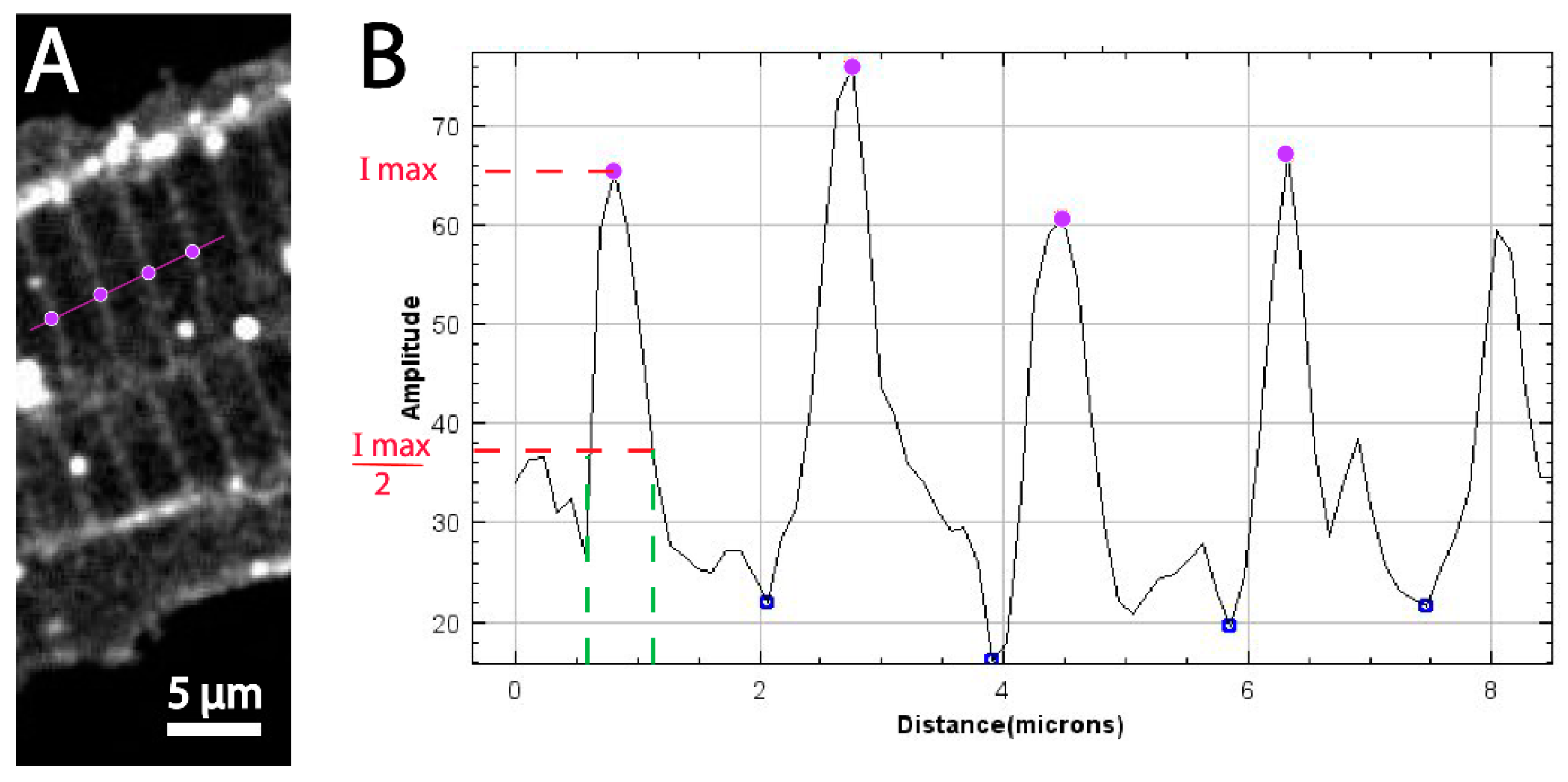
3.2. Quantitative Comparison of Muscle Features Using Confocal and Super-Resolution Microscopy
3.3. Thresholding by Density Indicates Dystroglycan Is Concentrated at the MTJ
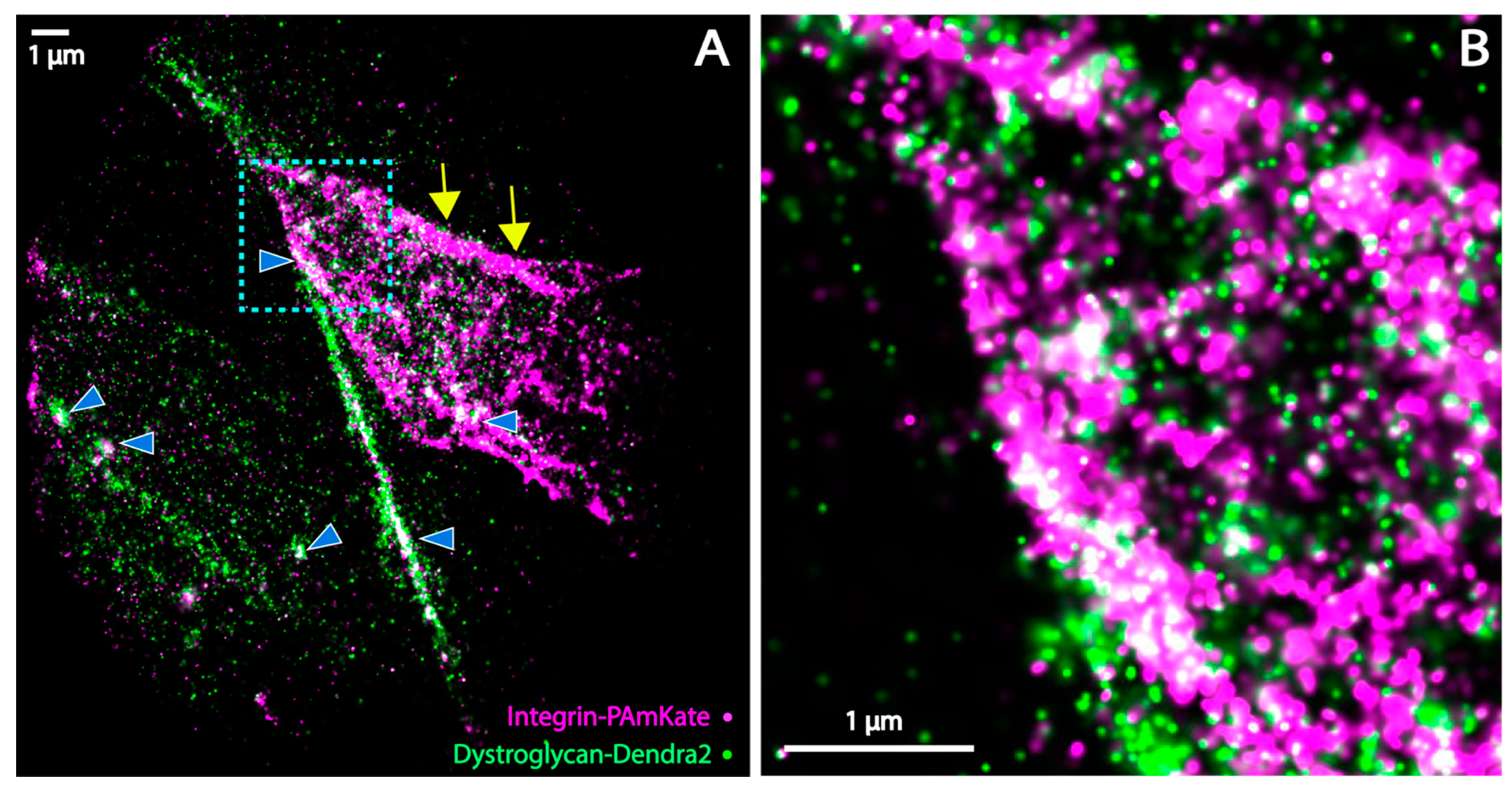
3.4. Super-Resolution Microscopy of the Co-Distribution of DG and Itga7
4. Discussion
Impact of Super Resolution on Elucidating Nanoscale Structure in the Zebrafish Model
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLeod, M.; Breen, L.; Hamilton, D.L.; Philp, A. Live strong and prosper: The importance of skeletal muscle strength for healthy ageing. Biogerontology 2016, 17, 497–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, S.; Deries, M.; Cachaco, A.S.; Bajanca, F. The extracellular matrix dimension of skeletal muscle development. Dev. Biol. 2011, 354, 191–207. [Google Scholar] [CrossRef] [Green Version]
- Goody, M.F.; Sher, R.B.; Henry, C.A. Hanging on for the ride: Adhesion to the extracellular matrix mediates cellular responses in skeletal muscle morphogenesis and disease. Dev. Biol. 2015, 401, 75–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjaer, M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.J.; Weihl, C.C.; Spencer, M.J. Molecular and cellular basis of genetically inherited skeletal muscle disorders. Nat. Rev. Mol. Cell Biol. 2021, 22, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.D.; Campbell, K.P. Dystroglycan: An extracellular matrix receptor linked to the cytoskeleton. Curr. Opin. Cell Biol. 1996, 8, 625–631. [Google Scholar] [CrossRef]
- Hayashi, Y.K.; Chou, F.L.; Engvall, E.; Ogawa, M.; Matsuda, C.; Hirabayashi, S.; Yokochi, K.; Ziober, B.L.; Kramer, R.H.; Kaufman, S.J.; et al. Mutations in the integrin alpha7 gene cause congenital myopathy. Nat. Genet. 1998, 19, 94–97. [Google Scholar] [CrossRef]
- Mohassel, P.; Foley, A.R.; Bonnemann, C.G. Extracellular matrix-driven congenital muscular dystrophies. Matrix Biol. 2018, 71–72, 188–204. [Google Scholar] [CrossRef]
- Jimenez-Mallebrera, C.; Brown, S.C.; Sewry, C.A.; Muntoni, F. Congenital muscular dystrophy: Molecular and cellular aspects. Cell. Mol. Life Sci. 2005, 62, 809–823. [Google Scholar] [CrossRef]
- Yurchenco, P.D.; McKee, K.K.; Reinhard, J.R.; Ruegg, M.A. Laminin-deficient muscular dystrophy: Molecular pathogenesis and structural repair strategies. Matrix Biol. 2018, 71–72, 174–187. [Google Scholar] [CrossRef]
- Holmberg, J.; Durbeej, M. Laminin-211 in skeletal muscle function. Cell Adhes. Migr. 2013, 7, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Gullberg, D.; Tiger, C.F.; Velling, T. Laminins during muscle development and in muscular dystrophies. Cell. Mol. Life Sci. 1999, 56, 442–460. [Google Scholar] [CrossRef]
- Goody, M.F.; Kelly, M.W.; Reynolds, C.J.; Khalil, A.; Crawford, B.D.; Henry, C.A. NAD+ biosynthesis ameliorates a zebrafish model of muscular dystrophy. PLoS Biol. 2012, 10, e1001409. [Google Scholar] [CrossRef] [Green Version]
- Sarathy, A.; Wuebbles, R.D.; Fontelonga, T.M.; Tarchione, A.R.; Mathews Griner, L.A.; Heredia, D.J.; Nunes, A.M.; Duan, S.; Brewer, P.D.; Van Ry, T.; et al. SU9516 Increases alpha7beta1 Integrin and Ameliorates Disease Progression in the mdx Mouse Model of Duchenne Muscular Dystrophy. Mol. Ther. 2017, 25, 1395–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sztal, T.E.; Sonntag, C.; Hall, T.E.; Currie, P.D. Epistatic dissection of laminin-receptor interactions in dystrophic zebrafish muscle. Hum. Mol. Genet. 2012, 21, 4718–4731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkin, D.J.; Wallace, G.Q.; Nicol, K.J.; Kaufman, D.J.; Kaufman, S.J. Enhanced expression of the alpha 7 beta 1 integrin reduces muscular dystrophy and restores viability in dystrophic mice. J. Cell Biol. 2001, 152, 1207–1218. [Google Scholar] [CrossRef]
- Rahkila, P.; Takala, T.E.; Parton, R.G.; Metsikko, K. Protein targeting to the plasma membrane of adult skeletal muscle fiber: An organized mosaic of functional domains. Exp. Cell Res. 2001, 267, 61–72. [Google Scholar] [CrossRef]
- Snow, C.J.; Henry, C.A. Dynamic formation of microenvironments at the myotendinous junction correlates with muscle fiber morphogenesis in zebrafish. Gene Expr. Patterns 2009, 9, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Bassett, D.I.; Bryson-Richardson, R.J.; Daggett, D.F.; Gautier, P.; Keenan, D.G.; Currie, P.D. Dystrophin is required for the formation of stable muscle attachments in the zebrafish embryo. Development 2003, 130, 5851–5860. [Google Scholar] [CrossRef] [Green Version]
- Postel, R.; Vakeel, P.; Topczewski, J.; Knoll, R.; Bakkers, J. Zebrafish integrin-linked kinase is required in skeletal muscles for strengthening the integrin-ECM adhesion complex. Dev. Biol. 2008, 318, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, R.; Anderson, L.V.; Voit, T. Costameric distribution of beta-Dystroglycan (43 kDa dystrophin-associated glycoprotein) in normal and dystrophin-deficient human skeletal muscle. Biochem. Soc. Trans. 1996, 24, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Kawahara, G.; Gundry, S.R.; Chen, A.T.; Lencer, W.I.; Zhou, Y.; Zon, L.I.; Kunkel, L.M.; Beggs, A.H. The zebrafish dag1 mutant: A novel genetic model for Dystroglycanopathies. Hum. Mol. Genet. 2011, 20, 1712–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eid Mutlak, Y.; Aweida, D.; Volodin, A.; Ayalon, B.; Dahan, N.; Parnis, A.; Cohen, S. A signaling hub of insulin receptor, dystrophin glycoprotein complex and plakoglobin regulates muscle size. Nat. Commun. 2020, 11, 1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, S.; Ohlendieck, K. Mass spectrometric identification of dystrophin, the protein product of the Duchenne muscular dystrophy gene, in distinct muscle surface membranes. Int. J. Mol. Med. 2017, 40, 1078–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, J.L.; Oh, J.; Chou, E.; Lee, J.A.; Holmberg, J.; Burkin, D.J.; Crosbie-Watson, R.H. Sarcospan integration into laminin-binding adhesion complexes that ameliorate muscular dystrophy requires utrophin and alpha7 integrin. Hum. Mol. Genet. 2015, 24, 2011–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, S.T.; Girirajan, T.P.K.; Mason, M.D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 2006, 91, 4258–4272. [Google Scholar]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. [Google Scholar]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006, 3, 793–795. [Google Scholar]
- Sharonov, A.; Hochstrasser, R.M. Wide-field subdiffraction imaging by accumulated binding of diffusing probes. Proc. Natl. Acad. Sci. USA 2006, 103, 18911–18916. [Google Scholar]
- Schnitzbauer, J.; Strauss, M.T.; Schlichthaerle, T.; Schueder, F.; Jungmann, R. Super-resolution microscopy with DNA-PAINT. Nat. Protoc. 2017, 12, 1198–1228. [Google Scholar] [CrossRef] [PubMed]
- Gwosch, K.C.; Pape, J.K.; Balzarotti, F.; Hoess, P.; Ellenberg, J.; Ries, J.; Hell, S.W. MINFLUX nanoscopy delivers 3D multicolor nanometer resolution in cells. Nat. Methods 2020, 17, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Gunewardene, M.S.; Subach, F.V.; Gould, T.J.; Penoncello, G.P.; Gudheti, M.V.; Verkhusha, V.V.; Hess, S.T. Superresolution Imaging of Multiple Fluorescent Proteins with Highly Overlapping Emission Spectra in Living Cells. Biophys. J. 2011, 101, 1522–1528. [Google Scholar] [PubMed] [Green Version]
- Raut, P.; Obeng, B.; Waters, H.; Zimmerberg, J.; Gosse, J.A.; Hess, S.T. Phosphatidylinositol 4,5-Bisphosphate Mediates the Co-Distribution of Influenza A Hemagglutinin and Matrix Protein M1 at the Plasma Membrane. Viruses 2022, 14, 2509. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.M.; Fujimoto, E.; Grabher, C.; Mangum, B.D.; Hardy, M.E.; Campbell, D.S.; Parant, J.M.; Yost, H.J.; Kanki, J.P.; Chien, C.B. The Tol2kit: A multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 2007, 236, 3088–3099. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.; Hess, S.T. Quantification of Mitochondrial Membrane Curvature by Three-Dimensional Localization Microscopy. iSci. Notes 2019, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Curthoys, N.M.; Mlodzianoski, M.J.; Parent, M.; Butler, M.B.; Raut, P.; Wallace, J.; Lilieholm, J.; Mehmood, K.; Maginnis, M.S.; Waters, H.; et al. Influenza Hemagglutinin Modulates Phosphatidylinositol 4,5-Bisphosphate Membrane Clustering. Biophys. J. 2019, 116, 893–909. [Google Scholar] [CrossRef] [Green Version]
- Gould, T.J.; Verkhusha, V.V.; Hess, S.T. Imaging biological structures with fluorescence photoactivation localization microscopy. Nat. Protoc. 2009, 4, 291–308. [Google Scholar]
- Gabor, K.A.; Kim, D.; Kim, C.H.; Hess, S.T. Nanoscale imaging of caveolin-1 membrane domains in vivo. PLoS ONE 2015, 10, e0117225. [Google Scholar] [CrossRef]
- The MathWorks Inc. MATLAB Version: 9.13.0 (R2022b); The MathWorks Inc.: Natick, MA, USA, 2022. [Google Scholar]
- Hoogendoorn, E.; Crosby, K.C.; Leyton-Puig, D.; Breedijk, R.M.; Jalink, K.; Gadella, T.W.; Postma, M. The fidelity of stochastic single-molecule super-resolution reconstructions critically depends upon robust background estimation. Sci. Rep. 2014, 4, 3854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egner, A.; Geisler, C.; Von Middendorff, C.; Bock, H.; Wenzel, D.; Medda, R.; Andresen, M.; Stiel, A.C.; Jakobs, S.; Eggeling, C.; et al. Fluorescence nanoscopy in whole cells by asynchronous localization of photoswitching emitters. Biophys. J. 2007, 93, 3285–3290. [Google Scholar] [PubMed] [Green Version]
- Raut, P.; Weller, S.R.; Obeng, B.; Soos, B.L.; West, B.E.; Potts, C.M.; Sangroula, S.; Kinney, M.S.; Burnell, J.E.; King, B.L.; et al. Cetylpyridinium chloride (CPC) reduces zebrafish mortality from influenza infection: Super-resolution microscopy reveals CPC interference with multiple protein interactions with phosphatidylinositol 4,5-bisphosphate in immune function. Toxicol. Appl. Pharmacol. 2022, 440, 115913. [Google Scholar] [CrossRef] [PubMed]
- Flucher, B.E.; Andrews, S.B.; Daniels, M.P. Molecular organization of transverse tubule/sarcoplasmic reticulum junctions during development of excitation-contraction coupling in skeletal muscle. Mol. Biol. Cell 1994, 5, 1105–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Gemballa, S.; Vogel, F. Spatial arrangement of white muscle fibers and myoseptal tendons in fishes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 1013–1037. [Google Scholar] [CrossRef]
- Summers, A.P.; Koob, T.J. The evolution of tendon--morphology and material properties. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Gawlik, K.I.; Durbeej, M. Skeletal muscle laminin and MDC1A: Pathogenesis and treatment strategies. Skelet. Muscle 2011, 1, 9. [Google Scholar] [CrossRef]
- Hall, T.E.; Bryson-Richardson, R.J.; Berger, S.; Jacoby, A.S.; Cole, N.J.; Hollway, G.E.; Berger, J.; Currie, P.D. The zebrafish candyfloss mutant implicates extracellular matrix adhesion failure in laminin alpha2-deficient congenital muscular dystrophy. Proc. Natl. Acad. Sci. USA 2007, 104, 7092–7097. [Google Scholar] [CrossRef]
- Thornhill, P.; Bassett, D.; Lochmuller, H.; Bushby, K.; Straub, V. Developmental defects in a zebrafish model for muscular dystrophies associated with the loss of fukutin-related protein (FKRP). Brain 2008, 131, 1551–1561. [Google Scholar] [CrossRef] [Green Version]
- Telfer, W.R.; Busta, A.S.; Bonnemann, C.G.; Feldman, E.L.; Dowling, J.J. Zebrafish models of collagen VI-related myopathies. Hum. Mol. Genet. 2010, 19, 2433–2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyon, J.R.; Steffen, L.S.; Howell, M.H.; Pusack, T.J.; Lawrence, C.; Kunkel, L.M. Modeling human muscle disease in zebrafish. Biochim. Biophys. Acta 2007, 1772, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Stickney, H.L.; Barresi, M.J.; Devoto, S.H. Somite development in zebrafish. Dev. Dyn. 2000, 219, 287–303. [Google Scholar] [CrossRef]
- Lv, C.; Gould, T.J.; Bewersdorf, J.; Zenisek, D. High-resolution optical imaging of zebrafish larval ribbon synapse protein RIBEYE, RIM2, and CaV 1.4 by stimulation emission depletion microscopy. Microsc. Microanal. 2012, 18, 745–752. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Zhang, H.; Li, Y.; Ashok, A.; Liang, R.; Zhou, W.; Peng, L. Cellular imaging of deep organ using two-photon Bessel light-sheet nonlinear structured illumination microscopy. Biomed. Opt. Express 2014, 5, 1296–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegerist, F.; Endlich, K.; Endlich, N. Novel Microscopic Techniques for Podocyte Research. Front. Endocrinol. 2018, 9, 379. [Google Scholar] [CrossRef] [Green Version]
- Legant, W.R.; Shao, L.; Grimm, J.B.; Brown, T.A.; Milkie, D.E.; Avants, B.B.; Lavis, L.D.; Betzig, E. High-density three-dimensional localization microscopy across large volumes. Nat. Methods 2016, 13, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Pennacchietti, F.; Gould, T.J.; Hess, S.T. The Role of Probe Photophysics in Localization-Based Superresolution Microscopy. Biophys. J. 2017, 113, 2037–2054. [Google Scholar] [CrossRef]
- Welf, E.S.; Naik, U.P.; Ogunnaike, B.A. A spatial model for integrin clustering as a result of feedback between integrin activation and integrin binding. Biophys. J. 2012, 103, 1379–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manley, S.; Gillette, J.M.; Patterson, G.H.; Shroff, H.; Hess, H.F.; Betzig, E.; Lippincott-Schwartz, J. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 2008, 5, 155–157. [Google Scholar] [PubMed] [Green Version]
- Axelrod, D.; Koppel, D.E.; Schlessinger, J.; Elson, E.; Webb, W.W. Mobility Measurement by Analysis of Fluorescence Photobleaching Recovery Kinetics. Biophys. J. 1976, 16, 1055–1069. [Google Scholar] [PubMed] [Green Version]



| Peak Position (μm) | Distance between Adjacent Maxima (μm) |
|---|---|
| 0.84 ± 0.29 | 1.88 ± 0.29 |
| 2.72 ± 0.29 | 1.73 ± 0.29 |
| 4.46 ± 0.29 | 1.87 ± 0.29 |
| 6.33 ± 0.29 |
| Peak Position (μm) | Distance between Adjacent Maxima (μm) |
|---|---|
| 0.5914 ± 0.07 | 1.535 ± 0.07 |
| 2.127 ± 0.07 | 1.913 ± 0.07 |
| 4.040 ± 0.07 | 1.760 ± 0.07 |
| 5.800 ± 0.07 | 1.624 ± 0.07 |
| 7.442 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shivanna, K.; Astumian, M.; Raut, P.; Ngo, V.-N.; Hess, S.T.; Henry, C. Super-Resolution Imaging Reveals the Nanoscale Distributions of Dystroglycan and Integrin Itga7 in Zebrafish Muscle Fibers. Biomedicines 2023, 11, 1941. https://doi.org/10.3390/biomedicines11071941
Shivanna K, Astumian M, Raut P, Ngo V-N, Hess ST, Henry C. Super-Resolution Imaging Reveals the Nanoscale Distributions of Dystroglycan and Integrin Itga7 in Zebrafish Muscle Fibers. Biomedicines. 2023; 11(7):1941. https://doi.org/10.3390/biomedicines11071941
Chicago/Turabian StyleShivanna, Komala, Mary Astumian, Prakash Raut, Vinh-Nhan Ngo, Samuel T. Hess, and Clarissa Henry. 2023. "Super-Resolution Imaging Reveals the Nanoscale Distributions of Dystroglycan and Integrin Itga7 in Zebrafish Muscle Fibers" Biomedicines 11, no. 7: 1941. https://doi.org/10.3390/biomedicines11071941
APA StyleShivanna, K., Astumian, M., Raut, P., Ngo, V.-N., Hess, S. T., & Henry, C. (2023). Super-Resolution Imaging Reveals the Nanoscale Distributions of Dystroglycan and Integrin Itga7 in Zebrafish Muscle Fibers. Biomedicines, 11(7), 1941. https://doi.org/10.3390/biomedicines11071941







