Development of a Transparent Transgenic Zebrafish Cellular Phenotype Tg(6xNF-kB:EGFP); Casper(roy−/−, nacre−/−) to Study NF-kB Activity
Abstract
1. Introduction
2. Methods and Materials
2.1. Fish Housing
2.2. Zebrafish Line
2.3. Equipment
2.3.1. Fluorescent Microscope
2.3.2. Confocal Microscope
2.3.3. Screening Imaging via Fluorescence and Confocal Microscopy In Vivo
2.4. Chemicals and Reagents
2.4.1. Fish Water
2.4.2. Phenyl-2-Thiourea (PTU)
2.4.3. Stress/Pain Management
2.5. Experiment Design
2.5.1. Generation of the Heterozygous and Homozygous Progeny Casper (roy−/−, nacre−/−)/NF-kB:GFP (Generations F01–F07)
2.5.2. Screening of Fluorescent Expression to Select Transgenic Phenotype through Fluorescent Microscopy
2.5.3. Confirmation and Validation of In Vivo Imaging of Homozygous Strain through Confocal Microscopy
2.5.4. Flow Cytometry Analysis for Validation of the Newly Developed Zebrafish Strain
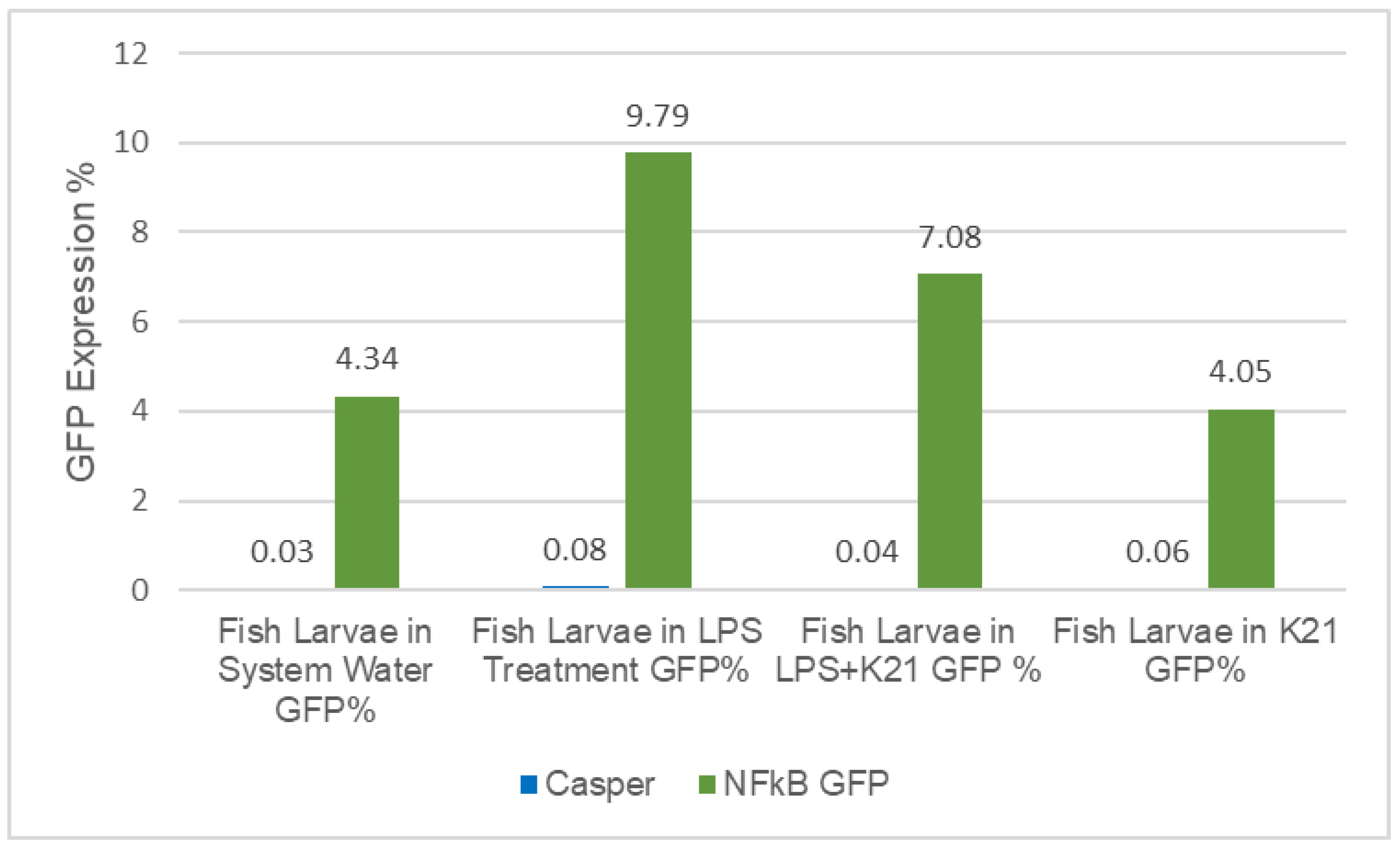
- Group 1: Control only fish system water (Casper mutant and Casper: NF-kB:GFP)
- Group 2: LPS treatment fish system water (Casper mutant and Casper: NF-kB:GFP)
- Group 3: LPS + K21 Drug treatment fish system water (Casper mutant and Casper: NF-kB:GFP)
- Group 4: K21 Drug treatment fish system water (Casper mutant and Casper: NF-kB:GFP)
3. Results
3.1. Generation of Tg(6xNF-kB:EGFP); Casper(roy−/−, nacre−/−) Strain
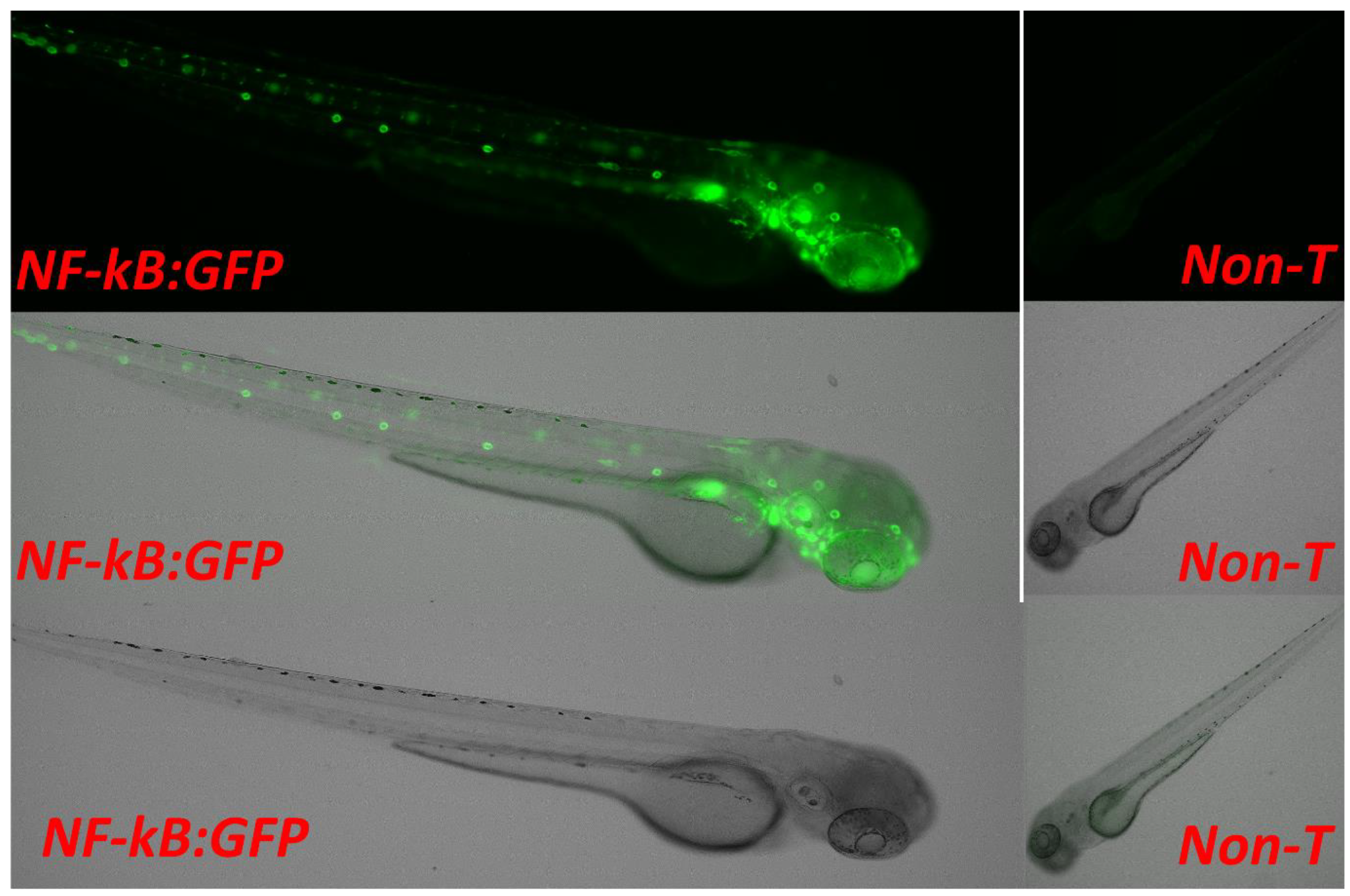
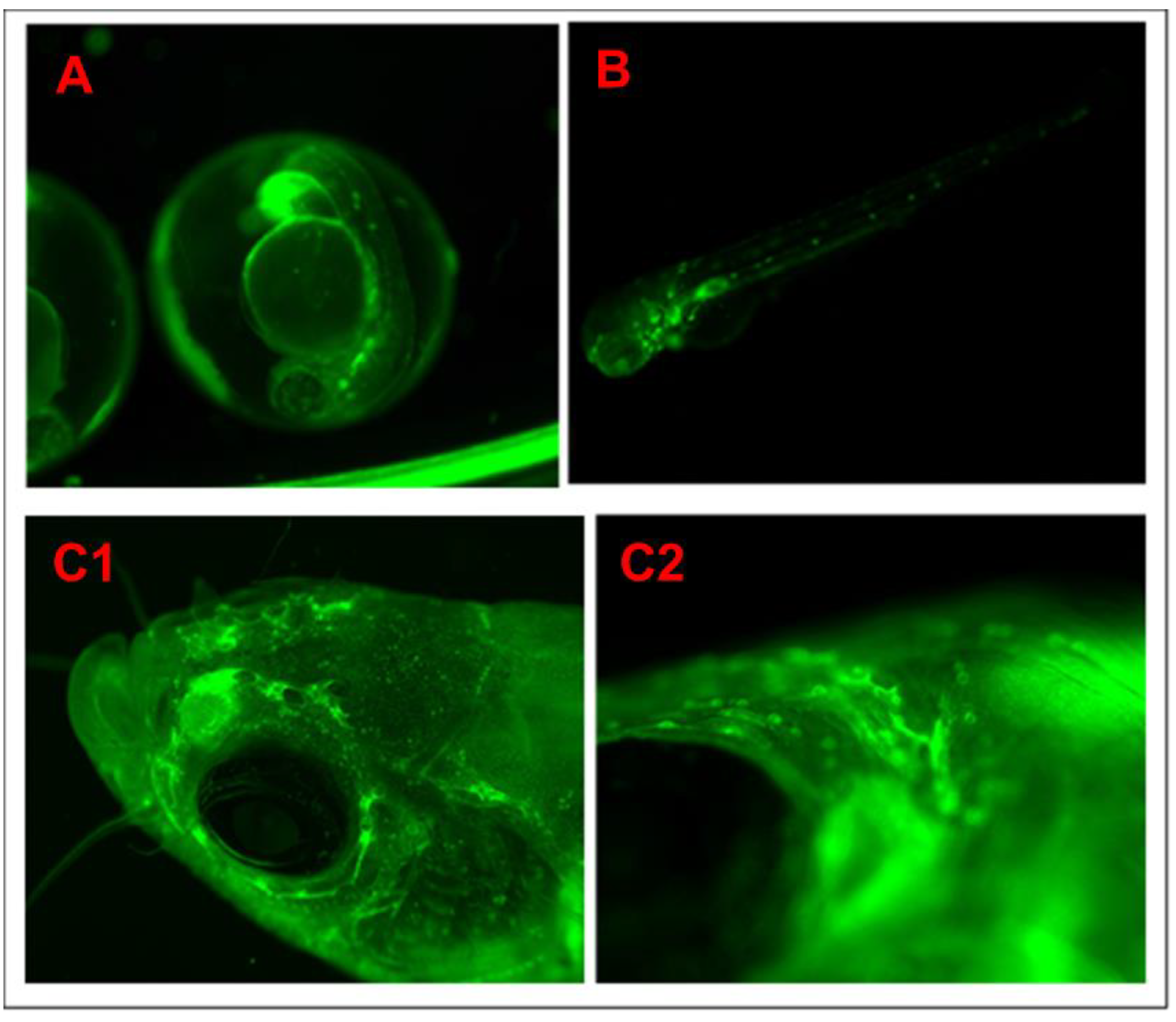
3.2. Screening and Sorting the Transgenic Progeny and In Vivo Imaging to Validate NF-kB Activity through Confocal Imaging of Tg(6xNF-kB:EGFP); Casper(roy−/−, nacre−/−) Strain
3.3. Validation of the Casper; NF-kB:GFP Strain by Evaluating the Inflammatory Response to LPS Exposure, and the Inhibitory Role of a Potential Novel Drug Candidate against LPS-Induced Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phillips, J.B.; Westerfield, M. Zebrafish models in translational research: Tipping the scales toward advancements in human health. Dis. Models Mech. 2014, 7, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Zon, L.I.; Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- White, D.T.; Eroglu, A.U.; Wang, G.; Zhang, L.; Sengupta, S.; Ding, D.; Rajpurohit, S.K.; Walker, S.L.; Ji, H.; Qian, J.; et al. ARQiv-HTS, a versatile whole-organism screening platform enabling in vivo drug discovery at high-throughput rates. Nat. Protoc. 2016, 11, 2432–2453. [Google Scholar] [CrossRef]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef]
- Kim, S.; Carlson, R.; Zafreen, L.; Rajpurohit, S.K.; Jagadeeswaran, P. Modular, easy-to-assemble, low-cost zebrafish facility. Zebrafish 2009, 6, 269–274. [Google Scholar] [CrossRef]
- Jagadeeswaran, P.; Carrillo, M.; Radhakrishnan, U.P.; Rajpurohit, S.K.; Kim, S. Laser-induced thrombosis in zebrafish. Methods Cell Biol. 2011, 101, 197–203. [Google Scholar] [CrossRef]
- Kim, S.; Radhakrishnan, U.P.; Rajpurohit, S.K.; Kulkarni, V.; Jagadeeswaran, P. Vivo-Morpholino knockdown of alphaIIb: A novel approach to inhibit thrombocyte function in adult zebrafish. Blood Cells Mol. Dis. 2010, 44, 169–174. [Google Scholar] [CrossRef]
- Carrillo, M.; Kim, S.; Rajpurohit, S.K.; Kulkarni, V.; Jagadeeswaran, P. Zebrafish von Willebrand factor. Blood Cells Mol. Dis. 2010, 45, 326–333. [Google Scholar] [CrossRef]
- Wang, G.; Rajpurohit, S.K.; Delaspre, F.; Walker, S.L.; White, D.T.; Ceasrine, A.; Kuruvilla, R.; Li, R.J.; Shim, J.S.; Liu, J.O.; et al. First quantitative high-throughput screen in zebrafish identifies novel pathways for increasing pancreatic beta-cell mass. Elife 2015, 4, e08261. [Google Scholar] [CrossRef]
- Rajpurohit, S.K.; Gopal, A.; Mon, M.Y.; Patel, N.G.; Arora, V. Development of Tg(UAS:SEC-Hsa.ANXA5-YFP,myl7:RFP); Casper(roy(-/-),nacre(-/-)) Transparent Transgenic In Vivo Zebrafish Model to Study the Cardiomyocyte Function. Cells 2021, 10, 1963. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.W. NF-kappaB in cancer: From innocent bystander to major culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar] [CrossRef]
- Karin, M.; Lin, A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Karin, M. Missing pieces in the NF-kappaB puzzle. Cell 2002, 109, S81–S96. [Google Scholar] [CrossRef] [PubMed]
- Van der Heiden, K.; Cuhlmann, S.; Luong, L.A.; Zakkar, M.; Evans, P.C. Role of nuclear factor kappaB in cardiovascular health and disease. Clin. Sci. 2010, 118, 593–605. [Google Scholar] [CrossRef]
- Hall, G.; Hasday, J.D.; Rogers, T.B. Regulating the regulator: NF-kappaB signaling in heart. J. Mol. Cell. Cardiol. 2006, 41, 580–591. [Google Scholar] [CrossRef]
- Schmitz, M.L.; Mattioli, I.; Buss, H.; Kracht, M. NF-kappaB: A multifaceted transcription factor regulated at several levels. Chembiochem 2004, 5, 1348–1358. [Google Scholar] [CrossRef]
- Correa, R.G.; Tergaonkar, V.; Ng, J.K.; Dubova, I.; Izpisua-Belmonte, J.C.; Verma, I.M. Characterization of NF-kappa B/I kappa B proteins in zebra fish and their involvement in notochord development. Mol. Cell. Biol. 2004, 24, 5257–5268. [Google Scholar] [CrossRef]
- Karra, R.; Knecht, A.K.; Kikuchi, K.; Poss, K.D. Myocardial NF-kappaB activation is essential for zebrafish heart regeneration. Proc. Natl. Acad. Sci. USA 2015, 112, 13255–13260. [Google Scholar] [CrossRef]
- Kuri, P.; Ellwanger, K.; Kufer, T.A.; Leptin, M.; Bajoghli, B. A high-sensitivity bi-directional reporter to monitor NF-kappaB activity in cell culture and zebrafish in real time. J. Cell Sci. 2017, 130, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Kanther, M.; Sun, X.; Muhlbauer, M.; Mackey, L.C.; Flynn, E.J., 3rd; Bagnat, M.; Jobin, C.; Rawls, J.F. Microbial colonization induces dynamic temporal and spatial patterns of NF-kappaB activation in the zebrafish digestive tract. Gastroenterology 2011, 141, 197–207. [Google Scholar] [CrossRef] [PubMed]





| Progeny | Strain | Transgenic Expression | Phenotypically Skin Pigmentation Background | |||||
|---|---|---|---|---|---|---|---|---|
| Generation | Heterozygous | Transgenic | Non-Transgenic | Casper | Nacre | Roy | Wild Type | |
| Homozygous | NF-kB:GFP | Normal Skin | ||||||
| 1 | F01 | Heterozygous | 39% | 61% | 0% | 0% | 0% | 100% |
| 2 | F02 | Heterozygous | 79% | 21% | 0% | 8% | 17% | 75% |
| 3 | F03 | Heterozygous | 75% | 25% | 6% | 21% | 11% | 63% |
| 4 | F04 | Heterozygous | 100% | 0% | 88% | 12% | 0% | 0% |
| 5 | F05 | Homozygous | 100% | 0% | 100% | 0% | 0% | 0% |
| 6 | F06 | Homozygous | 100% | 0% | 100% | 0% | 0% | 0% |
| 7 | F07 | Homozygous | 100% | 0% | 100% | 0% | 0% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajpurohit, S.K.; Ouellette, L.; Sura, S.; Appiah, C.; O’Keefe, A.; McCarthy, K.; Kandepu, U.; Ye Mon, M.; Kimmerling, K.; Arora, V.; et al. Development of a Transparent Transgenic Zebrafish Cellular Phenotype Tg(6xNF-kB:EGFP); Casper(roy−/−, nacre−/−) to Study NF-kB Activity. Biomedicines 2023, 11, 1985. https://doi.org/10.3390/biomedicines11071985
Rajpurohit SK, Ouellette L, Sura S, Appiah C, O’Keefe A, McCarthy K, Kandepu U, Ye Mon M, Kimmerling K, Arora V, et al. Development of a Transparent Transgenic Zebrafish Cellular Phenotype Tg(6xNF-kB:EGFP); Casper(roy−/−, nacre−/−) to Study NF-kB Activity. Biomedicines. 2023; 11(7):1985. https://doi.org/10.3390/biomedicines11071985
Chicago/Turabian StyleRajpurohit, Surendra K., Logan Ouellette, Suvarsha Sura, Chelsea Appiah, Annabelle O’Keefe, Katherine McCarthy, Umasai Kandepu, May Ye Mon, Kirk Kimmerling, Vishal Arora, and et al. 2023. "Development of a Transparent Transgenic Zebrafish Cellular Phenotype Tg(6xNF-kB:EGFP); Casper(roy−/−, nacre−/−) to Study NF-kB Activity" Biomedicines 11, no. 7: 1985. https://doi.org/10.3390/biomedicines11071985
APA StyleRajpurohit, S. K., Ouellette, L., Sura, S., Appiah, C., O’Keefe, A., McCarthy, K., Kandepu, U., Ye Mon, M., Kimmerling, K., Arora, V., & Lokeshwar, B. L. (2023). Development of a Transparent Transgenic Zebrafish Cellular Phenotype Tg(6xNF-kB:EGFP); Casper(roy−/−, nacre−/−) to Study NF-kB Activity. Biomedicines, 11(7), 1985. https://doi.org/10.3390/biomedicines11071985








