Nanoparticle Synthesis and Their Integration into Polymer-Based Fibers for Biomedical Applications
Abstract
1. Nanoparticles
1.1. Inorganic NPs
1.1.1. Silver NPs
1.1.2. Gold NPs
1.1.3. Iron Oxide NPs
1.1.4. Zinc Oxide NPs
1.1.5. Magnesium Oxide NPs
1.1.6. Cerium Oxide NPs
1.1.7. Titanium Dioxide NPs
1.2. Silica NPs
1.3. Organic NPs
1.3.1. Polymeric micelles
1.3.2. Chitosan-Based NPs
1.3.3. Liposomes
1.3.4. Dendrimers
2. Fibers
2.1. Natural Fibers
2.2. Manufactured Fibers
2.2.1. Natural Polymers as Building Blocks for Manufactured Fibers
2.2.2. Synthetic Polymers as Building Blocks for Manufactured Fibers
2.3. Fiber Formation
3. NPs Integration into Fibers for Their Intended Biological Effects
3.1. Microbial Balance
3.2. Tissue Regeneration
3.3. Anticancer Approaches
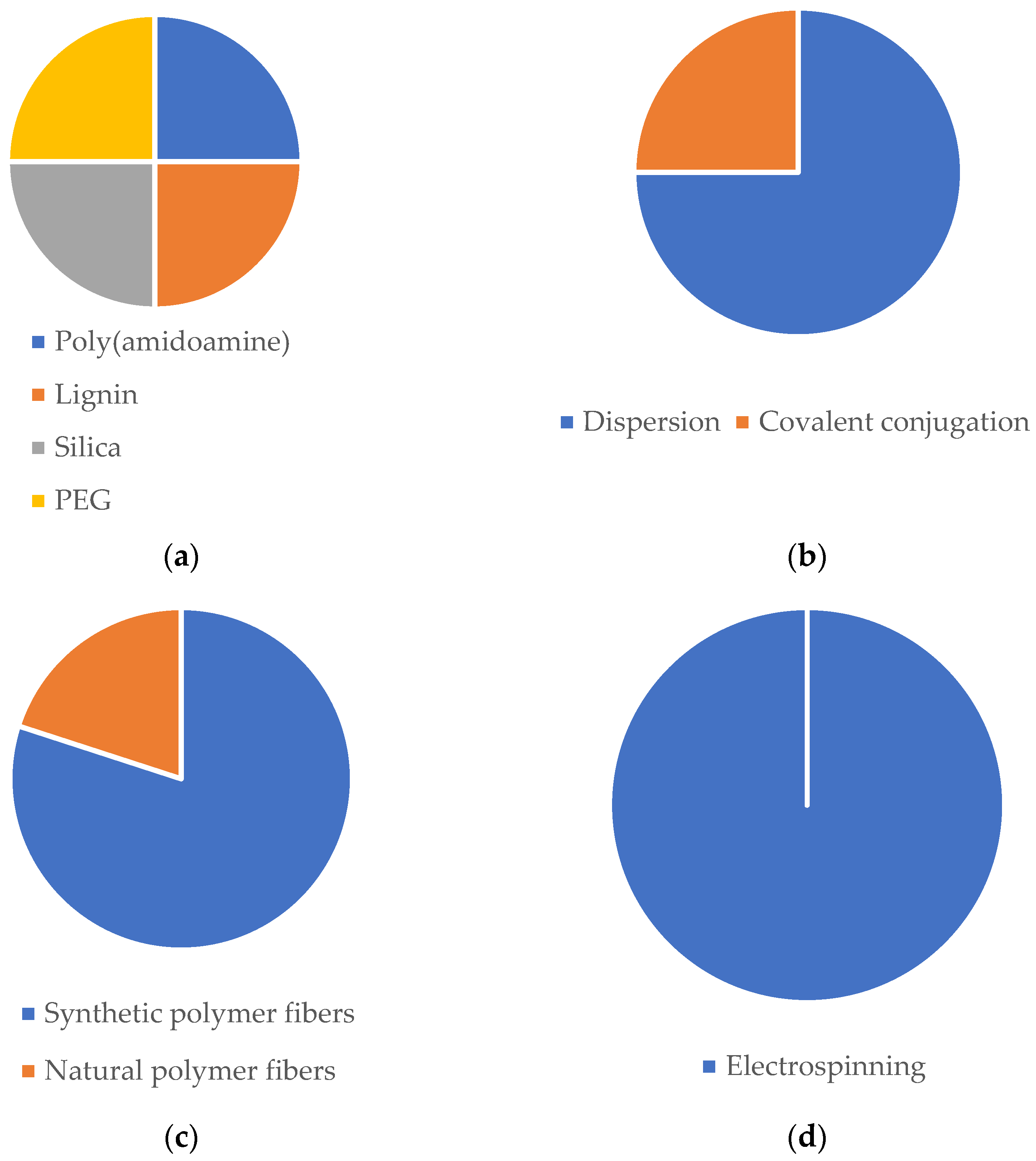
| NP | NP-Loaded Fibers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Composition | Production | Characteristics | Composition | Production Method | NP Loading | Characteristics | Architecture | Bioactivity | Administration | Intended Biomedical Effect | Ref. |
| Au/mercaptophenylboronic acid | One-pot method | Spherical; dTEM = 1.8 nm; ζ = −5.55 mV | PCL/GN | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dTEM = 560 nm | Nanofibrous mat | Improved antibacterial efficiency against S. aureus and MDR S. aureus. Non-toxic towards HUVECs and NIH 3T3 cells. No hemolysis in rat blood. 89% and 98% of mice wound closure in 14 days, both with S. aureus and MDR S. aureus infection. | Topical | Microbial balance | [222] |
| Ag and CS; Phenytoin | Reduction method | Spherical; dDLS = 53.6 nm; dTEM = 30 nm; ζ = +48 mV | PCL/PVA | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dTEM = 368 nm | Coaxial nanofibrous mat | Slowly and steady release of phenytoin (16.7% in 6h and 53.8% in 7 days). Antibacterial efficiency against S. aureus and E. coli. Survival and proliferation of 3T3 cells. The scaffold demonstrated the ability to swell to absorb wound exudates. | Topical | Microbial balance | [345] |
| ZnO; oregano essential oil | - | Commercially acquired with size ≤ 40 nm | PLCL | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dTEM = 1.04 µm | Core-shell nanofibrous mat | Antioxidant potency. Antibacterial efficiency against E. coli and S. aureus. Survival and proliferation of 3T3 cells. In vivo studies revealed 89.7% diabetic rats wound closure in 15 days without bacterial infections. | Topical | Microbial balance | [324] |
| Cerium oxide | Redox chemistry | Quasi-spherical; dTEM = 42 nm; ζ = +30.8 mV | PCL/GN | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dSEM = 486 nm | Nanofibrous mesh | Proliferation of 3T3 cells. Antioxidant properties | Topical | Microbial balance | [4] |
| Zinc doped hollow mesoporous silica nanospheres; Ciprofloxacin | Sol-gel method | Spherical; dTEM = 100 nm | PCL | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dSEM = 2 µm | Nanofibrous mat | Antibacterial activity against E. coli. No cytotoxic effects on HUVECs and HDFs. After 13 days, healthy tissue appeared in the wound area of E. coli-infected mice. | Topical | Microbial balance | [346] |
| Carboxymethyl CS; Antimicrobial peptide:OH-30 | Electrostatic droplet | Spherical; dTEM = 164.6 nm; ζ = −37.6 mV | PVA/CS | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dSEM = 327 nm | Nanofibrous mat | Cumulate release of the OH-CATH30 around 66% in 24 h. Antibacterial efficiency against E. coli and S. aureus. No cytotoxic effects towards HaCaT cells. Around 98% of mice wound closure in 12 days. | Topical | Microbial balance | [323] |
| CS; TPP; Curcumin | Ionic gelation | dTEM = 32.17 nm | PCL/CS/Curcumin | Electrospinning | Electrospraying | Bead-free; dSEM = 99.84 nm | Nanofibrous mat | Slow and sustained release of curcumin of 67.2% in 6 days. Antioxidant activity. Antibacterial activity against MRSA and E. coli (ESBL). Proliferation and survival of HDF cells. 98.5% wound closure of MRSA-infected mice wounds. | Topical | Microbial balance | [347] |
| CS; TPP; Curcumin | Ionic gelation | Spherical; dTEM = 359 nm; ζ = −10.7 mV | PCL/GN | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dSEM = 1548 µm | Nanofibrous mat | Good mechanical properties and swelling capacity. Accumulate release of curcumin of 23% in 6 h. Cytocompatibility towards EnSCs cells. In vivo studies showed 73.4% of wound closure in 14 days. | Topical | Microbial balance | [348] |
| Ag | - | Commercially acquired with size of 15 nm | PLA/Cellulose nanofibrils | Electrospinning | Vacuum filtration (Ag NPs suspension was filtrated for the PLA nanofibers) | Bead-free; dFESEM = 1.44 µm | Nanofibrous mat | Good tensile strength and hydrophilic mats. Biocompatibility towards CjECS and CECs ocular epithelial cells. Antibacterial efficiency against S. aureus and E. coli. | Transdermal | Microbial balance | [349] |
| PEGylated PLGA; Etravirine | Nanoprecipitation | dTEM = 172 nm | PVA and PVP | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dTEM (PVA) = 248 nm; dTEM (PVP) = 297 nm | Nanofibrous mat | Increase in the fluorescent signal in cervicovaginal mucus and vaginal tissue in C57/Bl6 mice in the case of topical application of the PVA/PVP-loaded NPs. Improvement in the pharmacokinetic profile of etravirine due to the sustained release of the drug. | Transdermal | Microbial balance | [329] |
| CS; Benzydamine | Ionic gelation | dDLS varying between 184 and 710 nm | PVP | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dTEM = 557 nm | Nanofibrous mat | Appropriate tensile strength and contact angles. 53.03% of drug release in 24 h and 59.66% after 48 h. | Transdermal | Microbial balance | [330] |
| Au | - | dTEM = 10 nm | PCL/GN | Electrospinning | Evaporation of gold NPs (the functional groups of gelatin were the binding sites for the evaporated NPs) | Bead-free; dTEM = 260 nm | Nanofibrous mat | Differentiation, growth, and maturation of neurons. Elaborated neuronal growth and axonal elongation, leading to more complex neuronal networks | Transdermal | Microbial balance | [14] |
| Ag | - | - | PLLA | Electrospinning | In situ reduction method (PLLA nanofibers immersed in silver nitrate, washed, and dried) | Bead-free; XRD patterns at 38.26°, 44.37° and 76.61° | Nanofibrous mat | Antibacterial activity against E. coli and S. aureus. Biocompatibility towards MC3T3 and L929 cells. | Topical | Tissue regeneration | [350] |
| Ag; CS | - | - | PLLA | Electrospinning | In situ reduction method (PLLA nanofibers immersed in silver nitrate, washed, and dried) | Bead-free; dTEM = 667.92 nm | Nanofibrous mat | Slow and steady release of Ag NPs (0.2 mg/L on day 7 and 0.25 mg/L on day 11). Antibacterial efficiency against E. coli and S. aureus. Excellent angiogenesis performance in VECs cells. | Topical | Tissue regeneration | [335] |
| Iron oxide (SPIONs); Casein | Ultrasonication | Spherical; dSEM = 36 nm | Silk-fibroin | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dSEM = 251.78 nm | Nanofibrous mat | Good mechanical properties. Biocompatibility towards ECCs. Survival and proliferation of ECCs. | Transdermal | Tissue regeneration | [351] |
| ZnO | - | Commercially acquired with size ranging between 10 and 30 nm | Outer layers: PVA, chitosan and shell protein; Middle layer: PEO, GN and ZnONPs | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dSEM = 108, 128.5, 138.5, 140, 153.7 nm | Tri-layer nanofibrous composite | Good mechanical properties and swelling reduction of three-layer nanofibers with incorporation of NPs. Accelerated proliferation of fibroblast cells. | Transdermal | Tissue regeneration | [352] |
| Iron oxide (SPIONs) | - | dTEM = 11–12 nm | PLLA | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dTEM = 1.73, 1.65, 1.96, 1.76, 2.03 µm | Nanofibrous mat | In vivo studies showed that neurons yielded a significant increase in the mean neurite outgrowth. Cytocompatibility towards neurons cells. | Intravenous injection | Tissue regeneration | [353] |
| MgO | Hydroxide precipitation and sol-gel | Hexagonal and cubical shape; dTEM = 40–60 nm | PCL | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dSEM = 0.2–0.6 µm | Nanofibrous mat | Improved mechanical properties, promotion of adhesion, proliferation, and differentiation of MG-63 cells. In vivo studies revealed good biocompatibility with an initial moderate inflammatory response near the implant site which became less intense at eighth week. | Subcutaneous implant | Tissue regeneration | [354] |
| Calcium phosphate | - | - | PLGA | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dSEM = 810 nm | Nanofibrous mat | Good biocompatibility towards rADSCs cells. Thermal treatment of NPs improved in vitro mineralization properties of nanofibers. The presence of NPs resulted in higher elasticity and ductility of nanofibers. | Transdermal | Tissue regeneration | [355] |
| Mesoporous silica; Paclitaxel; Endothelial growth factor (VEGF) | Stöber method | Pore size SEM = 3.17 nm | PLA | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dTEM = 1.26 µm | Nanofibrous mat | Promoted endothelial cell proliferation of HUVECs, inhibiting the proliferation of SMCs. In vivo studies revealed improved immediate and mid-term complete aneurysm occlusion rates, earlier endothelialization promotion and better lumen restenosis. | Transdermal | Tissue regeneration | [356] |
| Mesoporous silica; Dexamethasone | Surfactant templating | Spherical; dTEM = 100–200 nm | PLGA/GN | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; Average thickness of 0.088 mm and 0.305 mm | Bi-layer nanofibrous membrane | Good mechanical properties. Sustained release of dexamethasone (38.8% after 21 days). Proliferation of L929 cells and enhanced osteoinductive capacity. Antibacterial activity against E. coli and S. aureus. | Transdermal | Tissue regeneration | [357] |
| Mesoporous silica | Template removal | Spherical; dTEM = 70.9 nm | PLGA and PLGA/GN | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dTEM (PLGA + NPs) = 418 nm; dTEM (PLGA/gelatin + NPs) = 267 nm | Nanofibrous mat | Enhanced hydrophilicity and tensile mechanical properties of scaffold upon incorporation of NPs and gelatin. Improved cell attachment and proliferation of PC12 cells. | Transdermal | Tissue regeneration | [358] |
| Mesoporous silica | Sol-gel method | - | PLA/PANI | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dSEM = 150–300 nm | Nanofibrous mat | Biocompatibility towards C2C12 myoblasts. Controlled release of NPs from the scaffold. Promoted tissue vascularization on chicken embryo chorioallantoic membrane. | Transdermal | Tissue regeneration | [359] |
| Aldehyde cationic liposomes; IL-4 plasmid | Reverse evaporation method | dDLS varying between 70 and 280 nm | PLA/NGF | Electrospinning | Grafted by Schiff base bond | Bead-free; dTEM = 500 nm | Nanofibrous mat | Good mechanical properties. In vivo studies revealed reduced risk of further damage to motor neurons since it successfully inhibited the acute inflammatory response of spinal cord injury and encouraged nerve repair. | Transdermal | Tissue regeneration | [334] |
| Dextran glassy; bFGF | - | dSEM = 200 to 500 nm | PLLA | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dSEM = 0.27 µm | Nanofibrous mat | Encapsulation efficiency of 67.03% and no burst release and a controlled release kinetic of nearly 30 days. Promotion of cell adhesion and proliferation of C3 cells. Significantly increased tendon thickness in mice after 21 days. | Transdermal | Tissue regeneration | [360] |
| CS; Veratric acid | Ionic gelation | Spherical; dTEM = 99 nm | PCL (core)/PVP (sheath) | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dTEM = 515 nm | Coaxial nanofibrous mat | Good mechanical properties and protein adsorption. Mineralization capacity. Controlled release of veratric acid (60% release in 20 days). Biocompatibility towards mMSCs cells, and osteoblastic differentiation. | Transdermal | Tissue regeneration | [361] |
| CS; Nell-1 growth factor | Ionic gelation | Spherical; dTEM = 207 nm | PLLA-CL (core)/Collagen I (sheath) | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dTEM = 5 to 50 µm | Coaxial nanofibrous mat | Bioactivity of Nell-1 towards sao-2 cells release from the NPs-loaded scaffold was increased. hBMSCs showed elongated morphology and alignment when cultured with the NP-loaded scaffold. In vitro studies showed that Nell-1 released from the NP-loaded scaffold significantly increased the GAG content (component of hyaline cartilage. | Transdermal | Tissue regeneration | [362] |
| PCL; PLGA; Ciprofloxacin | Nanoprecipitation | dDLS = 250 nm | PEOT/PBT | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | - | Nanofibrous mat | No cytotoxicity towards HaCaT and hMSCs cells. Antibacterial activity against S. aureus and P. aeruginosa. In vitro studies showed that all ciprofloxacin-loaded NPs were able to hamper S. aureus adhesion and invasion to HaCaT cells as well as for P. aeruginosa. | Transdermal | Tissue regeneration | [363] |
| Titanium nitride | Laser ablation | - | PCL | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dSEM = 0.403 and 1.1 µm | Nanofibrous mat | Thermal analysis demonstrated that the incubation of TiN NPs in nanofibers led to slight variations in mass degradation initiation and phase behavior. In vitro studies revealed biocompatibility towards 3T3 fibroblast cell. | Transdermal | Tissue regeneration | [364] |
| Silica | Direct self-assembly | - | Cellulose | Wet-spinning | Dispersion (solubilization of NP within the coagulation bath) | - | Fibers | The incorporation of silica NPs resulted for all types of fibers in an enhancement of the strength and superior toughness. | Transdermal | Tissue regeneration | [365] |
| Holo-transferrin conjugated liposomes; SiRNA (36 nM) | - | Spherical; dTEM = 100 nm; dDLS = 117.2 nm; ζ = −11 mV | PCL/GN | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | - | Microfibrous mat | Produced liposomes showed 3:1 specificity between cancerous K562 cells in relation to healthy HUVEC. In vitro studies showed inhibition of sphingosine kinase 1 in K562 cells. | Transdermal | Anticancer approaches | [344] |
| Amine-terminated generation 5 poly(amidoamine) dendrimers | - | - | Cellulose Acetate assembled layer-by-layer with a bilayer of PDADMAC and PAA | Electrospinning | Covalent conjugation (via the 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride coupling reaction) | Bead-free; dSEM = 431.6 nm | Sandwich | Cell capture efficiencies of 36.3% and 82.7% at 40 and 60 min., respectively, in KB-HFAR cells. In vitro studies showed that the developed mat displays specificity to capture FAR-overexpressing cancer cells via ligand-receptor interactions. | Transdermal | Anticancer approaches | [366] |
| Lignin; Paclitaxel | Dissolution in tetrahydrofuran, followed by a dialysis process | Spherical; dTEM = 72 nm | PVA/PVP | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dTEM = 207 nm | Nanofibrous mat | Good thermal stability, mechanical properties, and biocompatibility towards HeLa cells with a survival rate of 21% at day 7. exhibited a long-term effective anticancer ability by promoting an apoptosis process in cell number and cytoplasmic vacuolation. | Transdermal | Anticancer approaches | [342] |
| Mesoporous silica; Curcumin | Modified Stöber method | Spherical; dDLS = 117 nm; ζ = + 3.3 mV | PCL/GN/Curcumin | Electrospinning | Dispersion (solubilization of NP within the polymeric solution) | Bead-free; dTEM = 610 nm | Nanofibrous mat | Exhibited higher toxicity towards MDA-MB-231 breast cancer cells after a period of 72 hr. incubation time, significantly more anti-migratory effect, a more pronounced effect on apoptosis induction, and reduction of the cell number and showed the greatest decrease for Bcl-2, suggesting that the two-stage curcumin discharge from the scaffold promoted cell apoptosis. | Transdermal | Anticancer approaches | [343] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ag | silver |
| Au | gold |
| BDD | boron-doped diamond |
| bFGF | fibroblast growth factor 2 |
| BV | bacterial vaginosis |
| C2C12 | myoblast cell line |
| CA | cellulose acetate |
| Ce | cerium |
| CECs | circulating endothelial cells |
| CMC | critical micelle concentration |
| CS | chitosan |
| CTAB | cetyltrimethylammonium bromide |
| CUR | curcumin |
| DLS | dynamic light scattering |
| DMAc | dimethylacetamide |
| DMF | N,N-dimethylformamide |
| DMSO | dimethylsulfoxide |
| DOX | doxorubicin |
| DSS | dioctylsodium dodecyl sulfate |
| ECCs | embryonal carcinoma cells |
| ECM | extracellular matrix |
| ELS | Electrophoretic light scattering |
| EnSCs | embryonic stem cells |
| FDA | food and drug administration |
| Fe | iron |
| FESEM | field emission scanning electron microscopy |
| GN | gelatin |
| GRAS | generally recognized as safe |
| HaCaT | immortalized human keratinocytes |
| hBMSCs | Bone-marrow-derived mesenchymal stem cells |
| HDFs | human dermal fibroblasts |
| HeLa | cervical cancer cells. |
| HMSN | hollow mesoporous silica nanoparticles |
| HR-TEM | high resolution transmission electron microscopy |
| HUVECs | human umbilical vein endothelial cells |
| IO | iron oxide |
| K562 | lymphoblast cells |
| L929 | mouse fibroblast cell line |
| MDR | multidrug-resistant |
| Mg | magnesium |
| MG-63 | human osteoblastic line |
| MgO | magnesium oxide |
| MMP | matrix metallo proteinase |
| mMSCs | MM cancer stem cells |
| MRI | magnetic resonance imaging |
| MRSA | methilicin-resistant S. aureus |
| MSNs | mesoporous silica nanoparticles |
| MTX | methotrexate |
| NADH | nicotinamide adenine dinucleotide |
| NGF | nerve growth factor |
| NIH 3T3 | fibroblast cell line |
| NPs | nanoparticles |
| PAA | poly (acrylic acid) |
| PAN | polyacrylonitrile |
| PANI | polyaniline |
| PC12 | clonal cell line derived from a pheochromocytoma of the rat adrenal medulla |
| PCL | polycaprolactone |
| PDA | polydopamine |
| PDADMA | poly(diallyldimethylammonium chloride) |
| PdI | polydispersity index |
| PDLLA | poly (dl-lactide) |
| PE | polyethylene |
| PEG | polyethylene glycol |
| PEO | polyethylene glycol |
| PEOT/PBT | poly(butylene terephthalate) |
| PICsomes | polyion complex vesicles |
| PLA | polylactic acid |
| PLCL | poly(lactide-co-epsilon-caprolactone) |
| PLDA | poly (d-lactide) |
| PLGA | poly(lactic-co-glycolic acid) |
| PLLA | poly(lactic acid) |
| PM | polymeric micelles |
| PP | polypropylene |
| PPE | personal protective equipment |
| PS | polystyrene |
| PSD | particle-size distribution |
| PTX | paclitaxel |
| PU | polyurethane |
| PVA | poly(vinyl alcohol) |
| PVP | polyvinylpyrrolidone |
| rADSCs | adipose-derived stem cells |
| RGD | Arginylglycylaspartic acid |
| SDS | sodium dodecyl sulfate |
| SEM | scanning electron microscopy |
| Si | silica |
| SMCs | smooth muscle cells |
| SSS | sodium silicate solution |
| Ta | tantalum |
| TAM | tamoxifen |
| TEM | transmission electron microscopy |
| TEOS | tetraethylorthosilicate |
| THF | tetrahydrofuran |
| Ti | titanium |
| TPP | thiamine pyrophosphate |
| VECs | vascular endothelial cells |
| VEGF | vascular endothelial growth factor |
| WHO | world health organization |
| XRD | X-ray powder diffraction |
| Zn | zinc |
| ZnO | zinc oxide |
References
- Allhoff, F. On the Autonomy and Justification of Nanoethics. Nanoethics 2007, 1, 185–210. [Google Scholar] [CrossRef]
- Shin, E.J.; Choi, S.M. Advances in Waterborne Polyurethane-Based Biomaterials for Biomedical Applications. Adv. Exp. Med. Biol. 2018, 1077, 251–283. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Joshi, C.P.; Bapat, P.; Chaubal, T.V.; Pandurangappa, R.; Jnanendrappa, N.; Gorain, B.; Khurana, S.; Kesharwani, P. The Use of Nanoparticles as Biomaterials in Dentistry. Drug Discov. Today 2019, 24, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Saiding, Q.; Cui, W. Functional Nanoparticles in Electrospun Fibers for Biomedical Applications. Nano Sel. 2021, 3, 999–1011. [Google Scholar] [CrossRef]
- Araújo, J.C.; Fangueiro, R.; Ferreira, D.P. Protective Multifunctional Fibrous Systems Based on Natural Fibers and Metal Oxide Nanoparticles. Polym. J. 2021, 13, 2654. [Google Scholar] [CrossRef]
- Peiris, T.A.N.; Weerasinghe, H.C.; Sharma, M.; Kim, J.-E.; Michalska, M.; Chandrasekaran, N.; Senevirathna, D.C.; Li, H.; Chesman, A.S.R.; Vak, D.; et al. Non-Aqueous One-Pot SnO2 Nanoparticle Inks and Their Use in Printable Perovskite Solar Cells. Chem. Mater. 2022, 2022, 5535–5545. [Google Scholar] [CrossRef]
- Anvar, A.A.; Ahari, H.; Ataee, M. Antimicrobial Properties of Food Nanopackaging: A New Focus on Foodborne Pathogens. Front. Microbiol. 2021, 12, 1945. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Rahdar, A. Functional Nanomaterials in Biomedicine: Current Uses and Potential Applications. Chem. Med. Chem. 2022, 17, e202200142. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Van Rijt, S.; Habibovic, P. Enhancing Regenerative Approaches with Nanoparticles. J. R. Soc. Interface 2017, 14, 20170093. [Google Scholar] [CrossRef]
- Baranes, K.; Shevach, M.; Shefi, O.; Dvir, T. Gold Nanoparticle-Decorated Scaffolds Promote Neuronal Differentiation and Maturation. Nano Lett. 2016, 16, 2916–2920. [Google Scholar] [CrossRef] [PubMed]
- Kairdolf, B.A.; Qian, X.; Nie, S. Bioconjugated Nanoparticles for Biosensing, in Vivo Imaging, and Medical Diagnostics. Anal. Chem. 2017, 89, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Win, K.Y.; Liu, S.; Teng, C.P.; Zheng, Y.; Han, M.Y. Surface-Functionalized Nanoparticles for Biosensing and Imaging-Guided Therapeutics. Nanoscale 2013, 5, 3127–3148. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Chen, M.; Xi, Y.; Cheng, W.; Mao, C.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; et al. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. ACS Nano 2019, 13, 10279–10293. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. ACS Publ. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Novel Hydrophilic Chitosan-polyethylene Oxide Nanoparticles as Protein Carriers. Wiley Online Libr. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Shalaby, T.; Hamad, H.; Ibrahim, E.; Mahmoud, O.; Al-Oufy, A. Electrospun Nanofibers Hybrid Composites Membranes for Highly Efficient Antibacterial Activity. Ecotoxicol. Environ. Saf. 2018, 162, 354–364. [Google Scholar] [CrossRef]
- Wang, J.; Windbergs, M. Functional Electrospun Fibers for the Treatment of Human Skin Wounds. Eur. J. Pharm. Biopharm. 2017, 119, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, R.; Lakshminarayanan, R.; Madhaiyan, K.; Barathi, V.A.; Limh, K.H.C.; Ramakrishna, S. Electrosprayed Nanoparticles and Electrospun Nanofibers Based on Natural Materials: Applications in Tissue Regeneration, Drug Delivery and Pharmaceuticals. Chem. Soc. Rev. 2015, 44, 790–814. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P.; Felgueiras, H.P. Electrospun Nanocomposites Containing Cellulose and Its Derivatives Modified with Specialized Biomolecules for an Enhanced Wound Healing. J. Nanomater. 2020, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser Ablation Synthesis in Solution and Size Manipulation of Noble Metal Nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef]
- Harra, J.; Mäkitalo, J.; Siikanen, R.; Virkki, M.; Genty, G.; Kobayashi, T.; Kauranen, M.; Mäkelä, J.M. Size-Controlled Aerosol Synthesis of Silver Nanoparticles for Plasmonic Materials. J. Nanopart. Res. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Ji, J.H.; Jung, J.H.; Kim, S.S.; Yoon, J.U.; Park, J.D.; Choi, B.S.; Chung, Y.H.; Kwon, I.H.; Jeong, J.; Han, B.S.; et al. Twenty-Eight-Day Inhalation Toxicity Study of Silver Nanoparticles in Sprague-Dawley Rats. Inhal. Toxicol. 2007, 19, 857–871. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yeh, C.S. Laser Ablation Method: Use of Surfactants to Form the Dispersed Ag Nanoparticles. Colloids Surf. A Physicochem. 2002, 197, 133–139. [Google Scholar] [CrossRef]
- Delmeé, M.; Mertz, G.; Bardon, J.; Marguier, A.; Ploux, L.; Roucoules, V.; Ruch, D. Laser Ablation of Silver in Liquid Organic Monomer: Influence of Experimental Parameters on the Synthesized Silver Nanoparticles/Graphite Colloids. J. Phys. Chem. B 2017, 121, 6646–6654. [Google Scholar] [CrossRef]
- Perito, B.; Giorgetti, E.; Marsili, P.; Muniz-Miranda, M. Antibacterial Activity of Silver Nanoparticles Obtained by Pulsed Laser Ablation in Pure Water and in Chloride Solution. Beilstein J. Nanotechnol. 2016, 7, 465. [Google Scholar] [CrossRef]
- Kinnear, C.; Moore, T.L.; Rodriguez-Lorenzo, L.; Rothen-Rutishauser, B.; Petri-Fink, A. Form Follows Function: Nanoparticle Shape and Its Implications for Nanomedicine. Chem. Rev. 2017, 117, 11476–11521. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385. [Google Scholar] [PubMed]
- Wolf, J.B.; Stawski, T.M.; Smales, G.J.; Thünemann, A.F.; Emmerling, F. Towards Automation of the Polyol Process for the Synthesis of Silver Nanoparticles. Sci. Rep. 2022, 12, 5769. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhou, C.; Wu, J.; Zhou, J.; Wang, L. A Further Insight into the Mechanism of Ag+ Biosorption by Lactobacillus Sp. Strain A09. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 1195–1200. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.J.S.; Shim, Y.Y.; Reaney, M.J.T.; Cho, J.Y. Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. J. Cancer. 2020, 12, 855. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Suresh Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of Silver Nanocrystals by Bacillus Licheniformis. Colloids Surf. B 2008, 65, 150–153. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of Nanoparticles: Technological Concepts and Future Applications. J. Nanoparticle Res. 2007, 10, 507–517. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Activity against Pathogenic Fungi in Combination with Fluconazole. Nanomed J. 2009, 5, 382–386. [Google Scholar] [CrossRef]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green Synthesis, Characterization of Gold and Silver Nanoparticles and Their Potential Application for Cancer Therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309. [Google Scholar] [CrossRef]
- Mukunthan, K.S.; Balaji, S. Cashew Apple Juice (Anacardium Occidentale L.) Speeds Up the Synthesis of Silver Nanoparticles. Sage 2012, 4, 71–79. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of Metallic Nanoparticles Using Plant Extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Sellami, H.; Khan, S.A.; Ahmad, I.; Alarfaj, A.A.; Hirad, A.H.; Al-Sabri, A.E. Green Synthesis of Silver Nanoparticles Using Olea Europaea Leaf Extract for Their Enhanced Antibacterial, Antioxidant, Cytotoxic and Biocompatibility Applications. Int. J. Mol. Sci. 2021, 22, 12562. [Google Scholar] [CrossRef]
- Fan, J.; Cheng, Y.; Sun, M. Functionalized Gold Nanoparticles: Synthesis, Properties and Biomedical Applications. Chem. Rec. 2020, 20, 1474–1504. [Google Scholar] [CrossRef] [PubMed]
- António, M.; Vitorino, R.; Daniel-da-Silva, A.L. Gold Nanoparticles-Based Assays for Biodetection in Urine. Talanta 2021, 230, 122345. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent Biomedical Applications of Gold Nanoparticles: A Review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Pereira, S.O.; Barros-Timmons, A.; Trindade, T. Biofunctionalisation of Colloidal Gold Nanoparticles via Polyelectrolytes Assemblies. Colloid Polym. Sci. 2014, 292, 33–50. [Google Scholar] [CrossRef]
- Trindade, T.; da Silva, A.L.D. Nanocomposite Particles for Bio-Applications: Materials and Bio-Interfaces; CRC Press: Boca Raton, FL, USA, 2012; Volume 4. [Google Scholar]
- Hammami, I.; Alabdallah, N.M.; Jomaa, A.A.; Kamoun, M. Gold Nanoparticles: Synthesis Properties and Applications. J. King Saud Univ.-Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Teimouri, M.; Khosravi-Nejad, F.; Attar, F.; Saboury, A.A.; Kostova, I.; Benelli, G.; Falahati, M. Gold Nanoparticles Fabrication by Plant Extracts: Synthesis, Characterization, Degradation of 4-Nitrophenol from Industrial Wastewater, and Insecticidal Activity—A Review. J. Clean. Prod. 2018, 184, 740–753. [Google Scholar] [CrossRef]
- Anjana, P.M.; Bindhu, M.R.; Rakhi, R.B. Green Synthesized Gold Nanoparticle Dispersed Porous Carbon Composites for Electrochemical Energy Storage. Mater. Sci. Energy Technol. 2019, 2, 389–395. [Google Scholar] [CrossRef]
- Zhang, T.; Dang, M.; Zhang, W.; Lin, X. Gold Nanoparticles Synthesized from Euphorbia Fischeriana Root by Green Route Method Alleviates the Isoprenaline Hydrochloride Induced Myocardial Infarction in Rats. J. Photochem. Photobiol. B Biol. 2020, 202, 111705. [Google Scholar] [CrossRef]
- Gupta, R.; Padmanabhan, P. Biogenic Synthesis and Characterization of Gold Nanoparticles by a Novel Marine Bacteria Marinobacter Algicola: Progression from Nanospheres to Various Geometrical Shapes. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 732–737. [Google Scholar] [CrossRef]
- Clarance, P.; Luvankar, B.; Sales, J.; Khusro, A.; Agastian, P.; Tack, J.C.; Al Khulaifi, M.M.; AL-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Green Synthesis and Characterization of Gold Nanoparticles Using Endophytic Fungi Fusarium Solani and Its In-Vitro Anticancer and Biomedical Applications. Saudi J. Biol. Sci. 2020, 27, 706. [Google Scholar] [CrossRef]
- Wei, M.Z.; Deng, T.S.; Zhang, Q.; Cheng, Z.; Li, S. Seed-Mediated Synthesis of Gold Nanorods at Low Concentrations of CTAB. ACS Omega 2021, 6, 9188–9195. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Erratum: Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2010, 110, 2574. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Singh, S. Recent Advances and Future Prospects of Iron Oxide Nanoparticles in Biomedicine and Diagnostics. 3 Biotech 2018, 8, 279. [Google Scholar] [CrossRef]
- Lakshmipriya, T.; Gopinath, S.C.B. Introduction to Nanoparticles and Analytical Devices. In Nanoparticles in Analytical and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–29. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Chircov, C.; Grumezescu, A.M. Magnetite Nanoparticles: Synthesis Methods—A Comparative Review. Meth. Enzymol. 2022, 199, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Arbain, R.; Othman, M.; Palaniandy, S. Preparation of Iron Oxide Nanoparticles by Mechanical Milling. Miner. Eng. 2011, 24, 1–9. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Luo, X.; Al-Antaki, A.H.M.; Alharbi, T.M.D.; Hutchison, W.D.; Zou, Y.C.; Zou, J.; Sheehan, A.; Zhang, W.; Raston, C.L. Laser-Ablated Vortex Fluidic-Mediated Synthesis of Superparamagnetic Magnetite Nanoparticles in Water under Flow. ACS Omega 2018, 3, 11172–11178. [Google Scholar] [CrossRef]
- Johnson, G.E.; Moser, T.; Engelhard, M.; Browning, N.D.; Laskin, J. Fabrication of Electrocatalytic Ta Nanoparticles by Reactive Sputtering and Ion Soft Landing. J. Chem. Phys. 2016, 145, 174701. [Google Scholar] [CrossRef]
- Lee, G.J.; Choi, E.H.; Nam, S.-H.; Lee, J.S.; Boo, J.-H.; Oh, S.D.; Choi, S.-H.; Cho, J.-H.; Yoon, M.-Y. Optical Sensing Properties of ZnO Nanoparticles Prepared by Spray Pyrolysis. J. Nanosci. Nanotechnol. 2018, 19, 1048–1051. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron Oxide Nanoparticles: Diagnostic, Therapeutic and Theranostic Applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Nekobahr, E.; Akhtari, J.; Saeedi, M.; Akbari, J.; Fathi, F. Synthesis and Characterization of Magnetite Nanoparticles by Co-Precipitation Method Coated with Biocompatible Compounds and Evaluation of in-Vitro Cytotoxicity. Toxicol. Rep. 2021, 8, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Harisinghani, M.G.; Barentsz, J.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; van de Kaa, C.H.; de la Rosette, J.; Weissleder, R. Noninvasive Detection of Clinically Occult Lymph-Node Metastases in Prostate Cancer. N. Engl. J. Med. 2003, 348, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.E.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-Large-Scale Syntheses of Monodisperse Nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Murray, C.B.; Kagan, C.R.; Bawendi, M.G. Synthesis and Characterization of Monodisperse Nanocrystals and Close-Packed Nanocrystal Assemblies. Annu. Rev. Mater. Sci. 2000, 30, 545–610. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal Oxides Nanoparticles via Sol–Gel Method: A Review on Synthesis, Characterization and Applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Lopez, S.; Hallali, N.; Lalatonne, Y.; Hillion, A.; Antunes, J.C.; Serhan, N.; Clerc, P.; Fourmy, D.; Motte, L.; Carrey, J.; et al. Magneto-Mechanical Destruction of Cancer-Associated Fibroblasts Using Ultra-Small Iron Oxide Nanoparticles and Low Frequency Rotating Magnetic Fields. Nanoscale Adv. 2022, 4, 421. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Eder, V.; Caputo, G.; Journé, C.; Ou, P.; Bolley, J.; Louedec, L.; Guenin, E.; Motte, L.; Pinna, N.; et al. USPIO Size Control through Microwave Nonaqueous Sol-Gel Method for Neoangiogenesis T2 MRI Contrast Agent. Nanomed. J. 2016, 11, 2769–2779. [Google Scholar] [CrossRef]
- Salvador, M.; Gutiérrez, G.; Noriega, S.; Moyano, A.; Blanco-López, M.C.; Matos, M. Microemulsion Synthesis of Superparamagnetic Nanoparticles for Bioapplications. Int. J. Mol. Sci. 2021, 22, 427. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.; Pavel, S. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: Focus on Their Safety and Effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic Effect of Active Ingredients in Sunscreen Products, a Contemporary Review. Toxicol. Rep. 2017, 4, 245. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. J. Mater. 2014, 7, 2833. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc Oxide Nanoparticles for Selective Destruction of Tumor Cells and Potential for Drug Delivery Applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc Oxide Nanoparticles: A Promising Nanomaterial for Biomedical Applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Xiong, H.M. ZnO Nanoparticles Applied to Bioimaging and Drug Delivery. Adv. Mater. 2013, 25, 5329–5335. [Google Scholar] [CrossRef]
- Spanhel, L.; Anderson, M.A. Semiconductor Clusters in the Sol-Gel Process: Quantized Aggregation, Gelation, and Crystal Growth in Concentrated Zinc Oxide Colloids. J. Am. Chem. Soc. 2002, 113, 2826–2833. [Google Scholar] [CrossRef]
- Han, X.; Harris, J.; Šiller, L. Synthesis of Porous Zinc-Based/Zinc Oxide Composites via Sol–Gel and Ambient Pressure Drying Routes. J. Mater. Sci. 2018, 53, 8170. [Google Scholar] [CrossRef] [PubMed]
- Majeed Khan, M.A.; Wasi Khan, M.; Alhoshan, M.; Alsalhi, M.S.; Aldwayyan, A.S. Influences of Co Doping on the Structural and Optical Properties of ZnO Nanostructured. Appl. Phys. A 2010, 100, 45–51. [Google Scholar] [CrossRef]
- Mahmood, N.B.; Saeed, F.R.; Gbashi, K.R.; Mahmood, U.S. Synthesis and Characterization of Zinc Oxide Nanoparticles via Oxalate Co-Precipitation Method. Mater. Lett. X 2022, 13, 100126. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Zhang, L.; Yuan, J.; Yan, S.; Wang, C. Low-Temperature Synthesis of ZnO Nanoparticles by Solid-State Pyrolytic Reaction. J. Nanotechnol. 2003, 14, 11–15. [Google Scholar] [CrossRef]
- Navale, S.T.; Jadhav, V.V.; Tehare, K.K.; Sagar, R.U.R.; Biswas, C.S.; Galluzzi, M.; Liang, W.; Patil, V.B.; Mane, R.S.; Stadler, F.J. Solid-State Synthesis Strategy of ZnO Nanoparticles for the Rapid Detection of Hazardous Cl2. Sens. Actuators B Chem. 2017, 238, 1102–1110. [Google Scholar] [CrossRef]
- Shen, L.; Bao, N.; Yanagisawa, K.; Domen, K.; Gupta, A.; Grimes, C.A. Direct Synthesis of ZnO Nanoparticles by a Solution-Free Mechanochemical Reaction. J. Nanotechnol. 2006, 17, 5117–5123. [Google Scholar] [CrossRef]
- Otis, G.; Ejgenberg, M.; Mastai, Y. Solvent-Free Mechanochemical Synthesis of ZnO Nanoparticles by High-Energy Ball Milling of ε-Zn(OH)2 Crystals. J. Nanomater. 2021, 11, 238. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S. Green Synthesis, Characterization and Antimicrobial Activities of Zinc Oxide Nanoparticles from the Leaf Extract of Azadirachta indica (L.). Appl. Surf. Sci. 2015, 345, 329–336. [Google Scholar] [CrossRef]
- Mahendra, C.; Murali, M.; Manasa, G.; Ponnamma, P.; Abhilash, M.R.; Lakshmeesha, T.R.; Satish, A.; Amruthesh, K.N.; Sudarshana, M.S. Antibacterial and Antimitotic Potential of Bio-Fabricated Zinc Oxide Nanoparticles of Cochlospermum religiosum (L.). Microb. Pathog. 2017, 110, 620–629. [Google Scholar] [CrossRef]
- Fu, L.; Fu, Z. Plectranthus Amboinicus Leaf Extract–Assisted Biosynthesis of ZnO Nanoparticles and Their Photocatalytic Activity. Ceram. Int. 2015, 41, 2492–2496. [Google Scholar] [CrossRef]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.M. Green Approach for Synthesis of Zinc Oxide Nanoparticles from Andrographis Paniculata Leaf Extract and Evaluation of Their Antioxidant, Anti-Diabetic, and Anti-Inflammatory Activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yao, J.; Russel, M.; Chen, K.; Wang, X. Characterization of Green Synthesized Nano-Formulation (ZnO-A. Vera) and Their Antibacterial Activity against Pathogens. Environ. Toxicol. Pharmacol. 2015, 39, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Yuvakkumar, R.; Suresh, J.; Nathanael, A.J.; Sundrarajan, M.; Hong, S.I. Novel Green Synthetic Strategy to Prepare ZnO Nanocrystals Using Rambutan (Nephelium lappaceum L.) Peel Extract and Its Antibacterial Applications. Mater. Sci. Eng. C. Mater. Biol. Appl. 2014, 41, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, P.C.; Cha, S.J.; Yang, I.J.; Sreekanth, T.V.M.; Kim, K.J.; Shin, H.M. Antioxidant and Anti-Inflammatory Activities of Zinc Oxide Nanoparticles Synthesized Using Polygala Tenuifolia Root Extract. J. Photochem. Photobiol. B. 2015, 146, 10–17. [Google Scholar] [CrossRef]
- Janaki, A.C.; Sailatha, E.; Gunasekaran, S. Synthesis, Characteristics and Antimicrobial Activity of ZnO Nanoparticles. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2015, 144, 17–22. [Google Scholar] [CrossRef]
- Dobrucka, R.; Długaszewska, J. Biosynthesis and Antibacterial Activity of ZnO Nanoparticles Using Trifolium Pratense Flower Extract. Saudi J. Biol. Sci. 2016, 23, 517–523. [Google Scholar] [CrossRef]
- Sharma, D.; Sabela, M.I.; Kanchi, S.; Mdluli, P.S.; Singh, G.; Stenström, T.A.; Bisetty, K. Biosynthesis of ZnO Nanoparticles Using Jacaranda Mimosifolia Flowers Extract: Synergistic Antibacterial Activity and Molecular Simulated Facet Specific Adsorption Studies. J. Photochem. Photobiol. B. 2016, 162, 199–207. [Google Scholar] [CrossRef]
- Qu, J.; Yuan, X.; Wang, X.; Shao, P. Zinc Accumulation and Synthesis of ZnO Nanoparticles Using Physalis alkekengi L. Environ. Pollut. 2011, 159, 1783–1788. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica Fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef]
- Hornak, J. Synthesis, Properties, and Selected Technical Applications of Magnesium Oxide Nanoparticles: A Review. Int. J. Mol. Sci. 2021, 22, 12752. [Google Scholar] [CrossRef]
- Mastuli, M.S.; Ansari, N.S.; Nawawi, M.A.; Mahat, A.M. Effects of Cationic Surfactant in Sol-Gel Synthesis of Nano Sized Magnesium Oxide. APCBEE Procedia 2012, 3, 93–98. [Google Scholar] [CrossRef]
- Wahab, R.; Ansari, S.G.; Dar, M.A.; Kim, Y.S.; Shin, H.S. Synthesis of Magnesium Oxide Nanoparticles by Sol-Gel Process. Mater. Sci. Forum 2007, 558–559, 983–986. [Google Scholar] [CrossRef]
- Boddu, V.M.; Viswanath, D.S.; Maloney, S.W. Synthesis and Characterization of Coralline Magnesium Oxide Nanoparticles. J. Am. Ceram. Soc. 2008, 91, 1718–1720. [Google Scholar] [CrossRef]
- Salman, K.D.; Abbas, H.H.; Aljawad, H.A. Synthesis and Characterization of MgO Nanoparticle via Microwave and Sol-Gel Methods. J. Phys. Conf. Ser. 2021, 1973, 012104. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef] [PubMed]
- Magnetic, Ferroelectric, and Multiferroic Metal Oxides—Biljana D. Stojanovic—Google Livros. Available online: https://books.google.pt/books?hl=pt-PT&lr=&id=ey0sDwAAQBAJ&oi=fnd&pg=PP1&ots=HDbiTHgP3e&sig=OpiPJapDIKqWd7934LmHKMSZiR4&redir_esc=y#v=onepage&q&f=false (accessed on 4 March 2022).
- Mukasyan, A.S.; Dinka, P. Novel Approaches to Solution-Combustion Synthesis of Nanomaterials. Int. J. Self-Propagating High-Temp. Synth. 2007, 16, 23–35. [Google Scholar] [CrossRef]
- Tharani, K.; Jegatha Christy, A.; Sagadevan, S.; Nehru, L.C. Fabrication of Magnesium Oxide Nanoparticles Using Combustion Method for a Biological and Environmental Cause. Chem. Phys. Lett. 2021, 763, 138216. [Google Scholar] [CrossRef]
- Devaraja, P.B.; Avadhani, D.N.; Prashantha, S.C.; Nagabhushana, H.; Sharma, S.C.; Nagabhushana, B.M.; Nagaswarupa, H.P. Synthesis, Structural and Luminescence Studies of Magnesium Oxide Nanopowder. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2014, 118, 847–851. [Google Scholar] [CrossRef]
- Al-Hazmi, F.; Alnowaiser, F.; Al-Ghamdi, A.A.; Al-Ghamdi, A.A.; Aly, M.M.; Al-Tuwirqi, R.M.; El-Tantawy, F. A New Large—Scale Synthesis of Magnesium Oxide Nanowires: Structural and Antibacterial Properties. Superlattices Microstruct. 2012, 52, 200. [Google Scholar] [CrossRef]
- Duong, T.H.Y.; Nguyen, T.N.; Oanh, H.T.; Dang Thi, T.A.; Giang, L.N.T.; Phuong, H.T.; Anh, N.T.; Nguyen, B.M.; Quang, V.T.; Le, G.T.; et al. Synthesis of Magnesium Oxide Nanoplates and Their Application in Nitrogen Dioxide and Sulfur Dioxide Adsorption. J. Chem. 2019, 2019, 4376429. [Google Scholar] [CrossRef]
- Mantzaris, N.V. Liquid-Phase Synthesis of Nanoparticles: Particle Size Distribution Dynamics and Control. Chem. Eng. Sci. 2005, 60, 4749–4770. [Google Scholar] [CrossRef]
- Rashid, H.; Mansoor, M.A.; Haider, B.; Nasir, R.; Abd Hamid, S.B.; Abdulrahman, A. Synthesis and Characterization of Magnetite Nano Particles with High Selectivity Using In-Situ Precipitation Method. Sep. Sci. Technol. 2019, 55, 1207–1215. [Google Scholar] [CrossRef]
- Frantina, Y.I.; Fajaroh, F.; Nazriati, N.; Yahmin, Y.; Sumari, S. Synthesis of MgO/CoFe2O4nanoparticles with Coprecipitation Method and Its Characterization. AIP Conf. Proc. 2021, 2330, 070003. [Google Scholar] [CrossRef]
- Verma, R.; Pathak, S.; Srivastava, A.K.; Prawer, S.; Tomljenovic-Hanic, S. ZnO Nanomaterials: Green Synthesis, Toxicity Evaluation and New Insights in Biomedical Applications. J. Alloys Compd. 2021, 876, 160175. [Google Scholar] [CrossRef]
- Younis, I.Y.; El-Hawary, S.S.; Eldahshan, O.A.; Abdel-Aziz, M.M.; Ali, Z.Y. Green Synthesis of Magnesium Nanoparticles Mediated from Rosa Floribunda Charisma Extract and Its Antioxidant, Antiaging and Antibiofilm Activities. Sci. Rep. 2021, 11, 16868. [Google Scholar] [CrossRef]
- Abdallah, Y.; Ogunyemi, S.O.; Abdelazez, A.; Zhang, M.; Hong, X.; Ibrahim, E.; Hossain, A.; Fouad, H.; Li, B.; Chen, J. The Green Synthesis of MgO Nano-Flowers Using Rosmarinus Officinalis L. (Rosemary) and the Antibacterial Activities against Xanthomonas Oryzae Pv. Oryzae. Biomed Res. Int. 2019, 2019, 5620989. [Google Scholar] [CrossRef]
- Khan, M.I.; Akhtar, M.N.; Ashraf, N.; Najeeb, J.; Munir, H.; Awan, T.I.; Tahir, M.B.; Kabli, M.R. Green Synthesis of Magnesium Oxide Nanoparticles Using Dalbergia Sissoo Extract for Photocatalytic Activity and Antibacterial Efficacy. Appl. Nanosci. 2020, 10, 2351–2364. [Google Scholar] [CrossRef]
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Oraby, H.F.; Al Hamoud, G.A.; Bukhari, S.I.; Moubayed, N.M.S. Biogenic Green Synthesis of MgO Nanoparticles Using Saussurea Costus Biomasses for a Comprehensive Detection of Their Antimicrobial, Cytotoxicity against MCF-7 Breast Cancer Cells and Photocatalysis Potentials. PLoS ONE 2020, 15, e0237567. [Google Scholar] [CrossRef]
- Sharma, G.; Soni, R.; Jasuja, N.D. Phytoassisted Synthesis of Magnesium Oxide Nanoparticles with Swertia Chirayaita. J. Taibah Univ. Sci. 2018, 11, 471–477. [Google Scholar] [CrossRef]
- Fatiqin, A.; Amrulloh, H.; Simanjuntak, W. Green Synthesis of MgO Nanoparticles Using Moringa Oleifera Leaf Aqueous Extract for Antibacterial Activity. Bull. Chem. Soc. Ethiop. 2021, 35, 161–170. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Dang, H.H.; Vo, D.V.N.; Bach, L.G.; Nguyen, T.D.; Tran, T. Van Biogenic Synthesis of MgO Nanoparticles from Different Extracts (Flower, Bark, Leaf) of Tecoma stans (L.) and Their Utilization in Selected Organic Dyes Treatment. J. Hazard. Mater. 2021, 404, 124146. [Google Scholar] [CrossRef] [PubMed]
- Suresh, J.; Yuvakkumar, R.; Sundrarajan, M.; Hong, S.I. Green Synthesis of Magnesium Oxide Nanoparticles. Adv. Mater. Res. 2014, 952, 141–144. [Google Scholar] [CrossRef]
- Kumar, S.A.; Jarvin, M.; Inbanathan, S.S.R.; Umar, A.; Lalla, N.P.; Dzade, N.Y.; Algadi, H.; Rahman, Q.I.; Baskoutas, S. Facile Green Synthesis of Magnesium Oxide Nanoparticles Using Tea (Camellia sinensis) Extract for Efficient Photocatalytic Degradation of Methylene Blue Dye. Environ. Technol. Innov. 2022, 28, 102746. [Google Scholar] [CrossRef]
- Dahle, J.T.; Arai, Y. Environmental Geochemistry of Cerium: Applications and Toxicology of Cerium Oxide Nanoparticles. Int. J. Environ. Res. Public Health 2015, 12, 1253–1278. [Google Scholar] [CrossRef]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef]
- Abuid, N.J.; Gattás-Asfura, K.M.; LaShoto, D.J.; Poulos, A.M.; Stabler, C.L. Biomedical Applications of Cerium Oxide Nanoparticles: A Potent Redox Modulator and Drug Delivery Agent. In Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–301. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X. Cerium Oxide Nanoparticle: A Remarkably Versatile Rare Earth Nanomaterial for Biological Applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Pelletier, D.A.; Suresh, A.K.; Holton, G.A.; McKeown, C.K.; Wang, W.; Gu, B.; Mortensen, N.P.; Allison, D.P.; Joy, D.C.; Allison, M.R.; et al. Effects of Engineered Cerium Oxide Nanoparticles on Bacterial Growth and Viability. Appl. Environ. Microbiol. 2010, 76, 7981. [Google Scholar] [CrossRef]
- Rojas, S.; Gispert, J.D.; Abad, S.; Buaki-Sogo, M.; Victor, V.M.; Garcia, H.; Herance, J.R. In Vivo Biodistribution of Amino-Functionalized Ceria Nanoparticles in Rats Using Positron Emission Tomography. Mol. Pharm. 2012, 9, 3543–3550. [Google Scholar] [CrossRef]
- Maria Magdalane, C.; Kaviyarasu, K.; Siddhardha, B.; Ramalingam, G. Synthesis and Characterization of CeO2 Nanoparticles by Hydrothermal Method. Mater. Today Proc. 2021, 36, 130–132. [Google Scholar] [CrossRef]
- Nyoka, M.; Choonara, Y.E.; Kumar, P.; Kondiah, P.P.D.; Pillay, V. Synthesis of Cerium Oxide Nanoparticles Using Various Methods: Implications for Biomedical Applications. J. Nanomater. 2020, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Soren, S.; Jena, S.R.; Samanta, L.; Parhi, P. Antioxidant Potential and Toxicity Study of the Cerium Oxide Nanoparticles Synthesized by Microwave-Mediated Synthesis. Appl. Biochem. Biotechnol. 2015, 177, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.W.; Yu, Y.; Simakov, D.S.A. Enhancing the Surface Area Stability of the Cerium Oxide Reverse Water Gas Shift Nanocatalyst via Reverse Microemulsion Synthesis. Catal. Today 2021, 407, 230–243. [Google Scholar] [CrossRef]
- Kalaycloǧlu, Z.; Geçim, B.; Erim, F.B. Green Synthesis of Cerium Oxide Nanoparticles from Turmeric and Kinds of Honey: Characterisations, Antioxidant and Photocatalytic Dye Degradation Activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2022, 13, 015016. [Google Scholar] [CrossRef]
- Fei Yin, Z.; Wu, L.; Gui Yang, H.; Hua Su, Y. Recent Progress in Biomedical Applications of Titanium Dioxide. Phys. Chem. Chem. Phys. 2013, 15, 4844–4858. [Google Scholar] [CrossRef]
- Albukhaty, S.; Al-Bayati, L.; Al-Karagoly, H.; Al-Musawi, S. Preparation and Characterization of Titanium Dioxide Nanoparticles and in Vitro Investigation of Their Cytotoxicity and Antibacterial Activity against Staphylococcus Aureus and Escherichia Coli. Anim. Biotechnol. 2020, 33, 864–870. [Google Scholar] [CrossRef]
- Afonso, C.; Lima, O.; Segundo, I.R.; Landi, S.; Margalho, É.; Homem, N.; Pereira, M.; Costa, M.F.M.; Freitas, E.; Carneiro, J. Effect of Iron-Doping on the Structure and Photocatalytic Activity of TiO2 Nanoparticles. Catalysts 2022, 13, 58. [Google Scholar] [CrossRef]
- Waghmode, M.S.; Gunjal, A.B.; Mulla, J.A.; Patil, N.N.; Nawani, N.N. Studies on the Titanium Dioxide Nanoparticles: Biosynthesis, Applications and Remediation. SN Appl. Sci. 2019, 1, 310. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Okoh, O.; Mungondori, H.; Taziwa, R.; Zinya, S. Synthetic Methods for Titanium Dioxide Nanoparticles: A Review. In Titanium Dioxide—Material for a Sustainable Environment; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Narayan, M.R.; Raturi, A. Deposition and Characterisation of Titanium Dioxide Films Formed by Electrophoretic Deposition. Int. J. Mater. Eng. Innov. 2012, 3, 17–31. [Google Scholar] [CrossRef]
- Sigcha-Pallo, C.; Peralta-Hernández, J.M.; Alulema-Pullupaxi, P.; Carrera, P.; Fernández, L.; Pozo, P.; Espinoza-Montero, P.J. Photoelectrocatalytic Degradation of Diclofenac with a Boron-Doped Diamond Electrode Modified with Titanium Dioxide as a Photoanode. Environ. Res. 2022, 212, 113362. [Google Scholar] [CrossRef]
- Widiyandari, H.; Purwanto, A.; Gunawan, V.; Widyanto, S.A. Synthesis of Titanium Dioxide (TiO2) Fine Particle by Flame Spray Pyrolysis (FSP) Method Using Liquid Petroleum Gas (LPG) as Fuel. Reaktor 2018, 17, 226. [Google Scholar] [CrossRef]
- Reis, K.P.; Ramanan, A.; Whittingham, M.S. Hydrothermal Synthesis of Sodium Tungstates. Chem. Mater. 2002, 2, 219–221. [Google Scholar] [CrossRef]
- Shahat, A.M.; El-Hossary, F.M.; Ghitas, A.; Abd El-Rahman, A.M.; Ebnalwaled, A.A. Low-Temperature Hydrothermal Synthesis of Titanium Dioxide Nanoparticles for Photocatalytic Applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1171, 012008. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A Review on TiO2-Based Nanotubes Synthesized via Hydrothermal Method: Formation Mechanism, Structure Modification, and Photocatalytic Applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Aguilar, T.; Carrillo-Berdugo, I.; Gómez-Villarejo, R.; Gallardo, J.J.; Martínez-Merino, P.; Piñero, J.C.; Alcántara, R.; Fernández-Lorenzo, C.; Navas, J. A Solvothermal Synthesis of TiO2 Nanoparticles in a Non-Polar Medium to Prepare Highly Stable Nanofluids with Improved Thermal Properties. J. Nanomater. 2018, 8, 816. [Google Scholar] [CrossRef] [PubMed]
- Baetzold, R.C. Chemisorption of Halogen on Copper and Silver Clusters. J. Am. Chem. Soc. 2002, 103, 6116–6120. [Google Scholar] [CrossRef]
- Dubey, R.S.; Krishnamurthy, K.V.; Singh, S. Experimental Studies of TiO2 Nanoparticles Synthesized by Sol-Gel and Solvothermal Routes for DSSCs Application. Results Phys. 2019, 14, 102390. [Google Scholar] [CrossRef]
- Collins, M.J. Future Trends in Microwave Synthesis. Future Med. Chem. 2010, 2, 151–155. [Google Scholar] [CrossRef]
- Baldassari, S.; Komarneni, S.; Mariani, E.; Villa, C. Rapid Microwave–Hydrothermal Synthesis of Anatase Form of Titanium Dioxide. J. Am. Ceram. Soc. 2005, 88, 3238–3240. [Google Scholar] [CrossRef]
- Falk, G.S.; Borlaf, M.; López-Muñoz, M.J.; Fariñas, J.C.; Rodrigues Neto, J.B.; Moreno, R. Microwave-Assisted Synthesis of TiO2 Nanoparticles: Photocatalytic Activity of Powders and Thin Films. J. Nanoparticle Res. 2018, 20, 23. [Google Scholar] [CrossRef]
- Downing, M.A.; Jain, P.K. Mesoporous Silica Nanoparticles: Synthesis, Properties, and Biomedical Applications. In Nanoparticles for Biomedical Applications: Fundamental Concepts, Biological Interactions and Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 267–281. ISBN 9780128166628. [Google Scholar]
- Castillo, R.R.; Colilla, M.; Vallet-Regí, M. Advances in Mesoporous Silica-Based Nanocarriers for Co-Delivery and Combination Therapy against Cancer. Expert Opin. Drug Deliv. 2017, 14, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Akhter, F.; Rao, A.A.; Abbasi, M.N.; Wahocho, S.A.; Mallah, M.A.; Anees-ur-Rehman, H.; Chandio, Z.A. A Comprehensive Review of Synthesis, Applications and Future Prospects for Silica Nanoparticles (SNPs). Silicon 2022, 14, 8295–8310. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Subhani, T.; Wilayat Husain, S. Synthesis of Silica Nanoparticles from Sodium Silicate under Alkaline Conditions. J. Sol-Gel Sci. Technol. 2016, 77, 753–758. [Google Scholar] [CrossRef]
- Gao, W.; Rigout, M.; Owens, H. Facile Control of Silica Nanoparticles Using a Novel Solvent Varying Method for the Fabrication of Artificial Opal Photonic Crystals. J. Nanoparticle Res. 2016, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Koźlecki, T.; Polowczyk, I.; Bastrzyk, A.; Sawiński, W. Improved Synthesis of Nanosized Silica in Water-in-Oil Microemulsions. J. Nanoparticles 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, J.; Chen, X.; Tian, S.; Li, K. Synthesis and Characterization of Silica Nanoparticles Preparing by Low-Temperature Vapor-Phase Hydrolysis of SiCl4. Ind. Eng. Chem. Res. 2014, 53, 11884–11890. [Google Scholar] [CrossRef]
- Cho, Y.S. Fabrication of Hollow or Macroporous Silica Particles by Spray Drying of Colloidal Dispersion. J. Dispers. Sci. Technol. 2016, 37, 23–33. [Google Scholar] [CrossRef]
- Cai, X.; Hong, R.Y.; Wang, L.S.; Wang, X.Y.; Li, H.Z.; Zheng, Y.; Wei, D.G. Synthesis of Silica Powders by Pressured Carbonation. Chem. Eng. J. 2009, 151, 380–386. [Google Scholar] [CrossRef]
- Pieła, A.; Żymańczyk-Duda, E.; Brzezińska-Rodak, M.; Duda, M.; Grzesiak, J.; Saeid, A.; Mironiuk, M.; Klimek-Ochab, M. Biogenic Synthesis of Silica Nanoparticles from Corn Cobs Husks. Dependence of the Productivity on the Method of Raw Material Processing. Bioorg. Chem. 2020, 99, 103773. [Google Scholar] [CrossRef] [PubMed]
- Romero, G.; Moya, S.E. Synthesis of Organic Nanoparticles. In Frontiers of Nanoscience; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; Volume 4, pp. 115–141. [Google Scholar]
- Drexler, K.E. Molecular Engineering: An Approach to the Development of General Capabilities for Molecular Manipulation. Proc. Natl. Acad. Sci. USA 1981, 78, 5275–5278. [Google Scholar] [CrossRef]
- Shatrohan Lal, R.K. Synthesis of Organic Nanoparticles and Their Applications in Drug Delivery and Food Nanotechnology: A Review. J. Nanomater. Mol. Nanotechnol. 2014, 3, 4. [Google Scholar] [CrossRef]
- Soo, P.L.; Eisenberg, A. Preparation of Block Copolymer Vesicles in Solution. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 923–938. [Google Scholar] [CrossRef]
- Bai, K.; Wang, A. Polymeric Micelles: Morphology, Synthesis, and Pharmaceutical Application. E3S Web Conf. 2021, 290, 01029. [Google Scholar] [CrossRef]
- Wakaskar, R.R. General Overview of Lipid–Polymer Hybrid Nanoparticles, Dendrimers, Micelles, Liposomes, Spongosomes and Cubosomes. J. Drug Target. 2018, 26, 311–318. [Google Scholar] [CrossRef]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the Enhanced Permeability and Retention Effect for Tumor Targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef]
- Wang, Y.; Thies-Weesie, D.M.E.; Bosman, E.D.C.; van Steenbergen, M.J.; van den Dikkenberg, J.; Shi, Y.; Lammers, T.; van Nostrum, C.F.; Hennink, W.E. Tuning the Size of All-HPMA Polymeric Micelles Fabricated by Solvent Extraction. J. Control Release 2022, 343, 338–346. [Google Scholar] [CrossRef]
- Du, J.; Armes, S.P. Preparation of Biocompatible Zwitterionic Block Copolymer Vesicles by Direct Dissolution in Water and Subsequent Silicification within Their Membranes. Langmuir 2009, 25, 9564–9570. [Google Scholar] [CrossRef]
- Kishimura, A. Development of Polyion Complex Vesicles (PICsomes) from Block Copolymers for Biomedical Applications. Polym. J. 2013, 45, 892–897. [Google Scholar] [CrossRef]
- Antunes, J.C.; Domingues, J.M.; Miranda, C.S.; Silva, A.F.G.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Bioactivity of Chitosan-Based Particles Loaded with Plant-Derived Extracts for Biomedical Applications: Emphasis on Antimicrobial Fiber-Based Systems. Mar. Drugs. 2021, 19, 359. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Chitosan Nanocomposite Coatings for Food, Paints, and Water Treatment Applications. Appl. Sci. 2019, 9, 2409. [Google Scholar] [CrossRef]
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-Based Nanoparticles: An Overview of Biomedical Applications and Its Preparation. J. Drug Deliv. Sci. Technol. 2019, 49, 66–81. [Google Scholar] [CrossRef]
- Grenha, A. Chitosan Nanoparticles: A Survey of Preparation Methods. J. Drug Target. 2012, 20, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.; Pereira, C.L.; Molinos, M.; Ferreira-Da-Silva, F.; Dessi, M.; Gloria, A.; Ambrosio, L.; Gonçalves, R.M.; Barbosa, M.A. Layer-by-Layer Self-Assembly of Chitosan and Poly(γ-Glutamic Acid) into Polyelectrolyte Complexes. Biomacromolecules 2011, 12, 4183–4195. [Google Scholar] [CrossRef]
- Essa, E.E.; Hamza, D.; Khalil, M.M.H.; Zaher, H.; Salah, D.; Alnemari, A.M.; Rady, M.H.; Momen, S.A.A. The Antibacterial Activity of Egyptian Wasp Chitosan-Based Nanoparticles against Important Antibiotic-Resistant Pathogens. Mol. 2022, 27, 7189. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric Nanoparticles: A Study on the Preparation Variables and Characterization Methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, Functionalization Strategies and Biomedical Applications of Targeted Biodegradable/Biocompatible Polymer-Based Nanocarriers for Drug Delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef]
- Natrajan, D.; Srinivasan, S.; Sundar, K.; Ravindran, A. Formulation of Essential Oil-Loaded Chitosan–Alginate Nanocapsules. J. Food Drug Anal. 2015, 23, 560–568. [Google Scholar] [CrossRef]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative Study of Encapsulated Peppermint and Green Tea Essential Oils in Chitosan Nanoparticles: Encapsulation, Thermal Stability, in-Vitro Release, Antioxidant and Antibacterial Activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Poerio, T.; Curcio, F.; Piacentini, E.; Cassano, R. Production of α-Tocopherol–Chitosan Nanoparticles by Membrane Emulsification. Mol. 2022, 27, 2319. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth Liposomes: Review of the Basic Science, Rationale, and Clinical Applications, Existing and Potential. Int. J. Nanomedicine 2006, 1, 297–315. [Google Scholar] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Benech, R.O.; Kheadr, E.E.; Laridi, R.; Lacroix, C.; Fliss, I. Inhibition of Listeria Innocua in Cheddar Cheese by Addition of Nisin Z in Liposomes or by in Situ Production in Mixed Culture. Appl. Environ. Microbiol. 2002, 68, 3683–3690. [Google Scholar] [CrossRef] [PubMed]
- Atrooz, O.M. Effects of Alkylresorcinolic Lipids Obtained from Acetonic Extract of Jordanian Wheat Grains on Liposome Properties. Int. J. Biol. Chem. 2011, 5, 314–321. [Google Scholar] [CrossRef]
- Mickova, A.; Buzgo, M.; Benada, O.; Rampichova, M.; Fisar, Z.; Filova, E.; Tesarova, M.; Lukas, D.; Amler, E. Core/Shell Nanofibers with Embedded Liposomes as a Drug Delivery System. Biomacromolecules 2012, 13, 952–962. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Int. J. Pharm. 2022, 14, 543. [Google Scholar] [CrossRef]
- Guimarães, D.; Noro, J.; Silva, C.; Cavaco-Paulo, A.; Nogueira, E. Protective Effect of Saccharides on Freeze-Dried Liposomes Encapsulating Drugs. Front. Bioeng. Biotechnol. 2019, 7, 424. [Google Scholar] [CrossRef]
- Penoy, N.; Grignard, B.; Evrard, B.; Piel, G. A Supercritical Fluid Technology for Liposome Production and Comparison with the Film Hydration Method. Int. J. Pharm. 2021, 592, 120093. [Google Scholar] [CrossRef]
- Xu, R.; Tomeh, M.A.; Ye, S.; Zhang, P.; Lv, S.; You, R.; Wang, N.; Zhao, X. Novel Microfluidic Swirl Mixers for Scalable Formulation of Curcumin Loaded Liposomes for Cancer Therapy. Int. J. Pharm. 2022, 622, 121857. [Google Scholar] [CrossRef]
- Laouini, A.; Jaafar-Maalej, C.; Sfar, S.; Charcosset, C.; Fessi, H. Liposome Preparation Using a Hollow Fiber Membrane Contactor—Application to Spironolactone Encapsulation. Int. J. Pharm. 2011, 415, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Aurelia Chis, A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Aulenta, F.; Hayes, W.; Rannard, S. Dendrimers: A New Class of Nanoscopic Containers and Delivery Devices. Eur. Polym. J. 2003, 39, 1741–1771. [Google Scholar] [CrossRef]
- Boas, U.; Heegaard, P.M.H. Dendrimers in Drug Research. Chem. Soc. Rev. 2004, 33, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Tomalia, D.A. Dendrimers in Biomedical Applications-Reflections on the Field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- López-Méndez, L.J.; Cuéllar-Ramírez, E.E.; Cabrera-Quiñones, N.C.; Rojas-Aguirre, Y.; Guadarrama, P. Convergent Click Synthesis of Macromolecular Dendritic β-Cyclodextrin Derivatives as Non-Conventional Drug Carriers: Albendazole as Guest Model. Int. J. Biol. Macromol. 2020, 164, 1704–1714. [Google Scholar] [CrossRef]
- García-Álvarez, F.; Martínez-García, M. Click Reaction in the Synthesis of Dendrimer Drug-Delivery Systems. Curr. Med. Chem. 2022, 29, 3445–3470. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Isa, I.L.M.; Zaman, W.S.W.K.; Tabata, Y.; Fauzi, M.B. The Effect of Nanoparticle-Incorporated Natural-Based Biomaterials towards Cells on Activated Pathways: A Systematic Review. Polym. J. 2022, 14, 476. [Google Scholar] [CrossRef]
- Habibzadeh, F.; Sadraei, S.M.; Mansoori, R.; Singh Chauhan, N.P.; Sargazi, G. Nanomaterials Supported by Polymers for Tissue Engineering Applications: A Review. Heliyon 2022, 8, e12193. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.W. Brief History of Fibers from Synthetic Polymers. J. Macromol. Sci. Chem. 2006, 15, 1113–1131. [Google Scholar] [CrossRef]
- Kumar, S.S.; Anbumalar, V. Selection and Evaluation of Natural Fibers—A Literature Review. Int. J. Innov. Sci. Eng. Technol. 2015, 2, 929–939. [Google Scholar]
- Houck, M.M. Identification of Textile Fibers; Woodhead Publishing: Delhi, India, 2009; p. 396. [Google Scholar]
- Salehi, S.; Koeck, K.; Scheibel, T. Spider Silk for Tissue Engineering Applications. Molecules 2020, 25, 737. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.Y.; Ho, M.P.; Lau, K.T.; Cardona, F.; Hui, D. Natural Fibre-Reinforced Composites for Bioengineering and Environmental Engineering Applications. Compos. Part B Eng. 2009, 40, 655–663. [Google Scholar] [CrossRef]
- Qin, Y. A Brief Description of Textile Fibers. In Medical Textile Materials; Woodhead Publishing: Delhi, India, 2016; pp. 23–42. [Google Scholar] [CrossRef]
- Xueliang, X. Animal Fibers. In Handbook of Fibrous Materials; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 37–74. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk Fibroin Biomaterials for Tissue Regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- van Dam, J.E.G.; Gorshkova, T.A. Cell Walls and Fibers | Fiber Formation. In Encyclopedia of Applied Plant Sciences; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 87–96. [Google Scholar] [CrossRef]
- Namvar, F.; Jawaid, M.; Tahir, P.M.; Mohamad, R.; Azizi, S.; Khodavandi, A.; Rahman, H.S.; Nayeri, M.D. Potential Use of Plant Fibres and Their Composites for Biomedical Applications. BioResources 2014, 9, 5688–5706. [Google Scholar] [CrossRef]
- Setyarini, P.H.; Cahyandari, D. Potential Natural Fiber-Reinforced Composite for Biomedical Application. IOP Conf. Ser. Mater. Sci. Eng. 2019, 494, 012018. [Google Scholar] [CrossRef]
- Khalid, H.; Suhaib, F.; Zahid, S.; Ahmed, S.; Jamal, A.; Kaleem, M.; Khan, A.S. Microwave-Assisted Synthesis and in Vitro Osteogenic Analysis of Novel Bioactive Glass Fibers for Biomedical and Dental Applications. Biomed. Mater. 2018, 14, 015005. [Google Scholar] [CrossRef]
- Houck, M.M.; Siegel, J.A. Textile Fibers. In Fundamentals of Forensic Science; Academic Press: Cambridge, MI, USA, 2015; pp. 381–404. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Yang, X.; Hou, Q.; Liu, S.; Zheng, W.; Long, Y.; Jiang, X. Mercaptophenylboronic Acid-Activated Gold Nanoparticles as Nanoantibiotics against Multidrug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2020, 12, 51148–51159. [Google Scholar] [CrossRef]
- Shevach, M.; Maoz, B.M.; Feiner, R.; Shapira, A.; Dvir, T. Nanoengineering Gold Particle Composite Fibers for Cardiac Tissue Engineering. J. Mater. Chem. B 2013, 1, 5210–5217. [Google Scholar] [CrossRef] [PubMed]
- Lobo, F.C.M.; Franco, A.R.; Fernandes, E.M.; Reis, R.L. An Overview of the Antimicrobial Properties of Lignocellulosic Materials. Molecules 2021, 26, 1749. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic Bionanocomposites: A Review of Preparation, Properties and Applications. Polym. J. 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Suhas; Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A Review as Natural, Modified and Activated Carbon Adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar]
- Wang, S.; Lu, A.; Zhang, L. Recent Advances in Regenerated Cellulose Materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Yadav, C.; Maji, P.K. Synergistic Effect of Cellulose Nanofibres and Bio- Extracts for Fabricating High Strength Sodium Alginate Based Composite Bio-Sponges with Antibacterial Properties. Carbohydr. Polym. 2019, 203, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose Acetate Electrospun Nanofibers for Drug Delivery Systems: Applications and Recent Advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef]
- Bashir, S.M.; Ahmed Rather, G.; Patrício, A.; Haq, Z.; Sheikh, A.A.; Shah, M.Z.; Singh, H.; Khan, A.A.; Imtiyaz, S.; Ahmad, S.B.; et al. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef]
- Jc, A.; Rm, G.; Ma, B. Chitosan/Poly(γ-Glutamic Acid) Polyelectrolyte Complexes: From Self- Assembly to Application in Biomolecules Delivery and Regenerative Medicine. Res. Rev. J. Mater. Sci. 2016, 4, 12–36. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polym. J. 2018, 10, 462. [Google Scholar] [CrossRef]
- Pacheco, C.; Sousa, F.; Sarmento, B. Chitosan-Based Nanomedicine for Brain Delivery: Where Are We Heading? React. Funct. Polym. 2020, 146, 104430. [Google Scholar] [CrossRef]
- Guan, G.; Abul Kalam Azad, M.; Lin, Y.; Kim, S.W.; Tian, Y.; Liu, G.; Wang, H. Biological Effects and Applications of Chitosan and Chito-Oligosaccharides. Front. Physiol. 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-Based Biomaterials for Biomedical Applications. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef]
- Kang, J.I.; Park, K.M. Advances in Gelatin-Based Hydrogels for Wound Management. J. Mater. Chem. B 2021, 9, 1503–1520. [Google Scholar] [CrossRef]
- Miranda, C.S.; Antunes, J.C.; Homem, N.C.; Felgueiras, H.P. Controlled Release of Cinnamon Leaf Oil from Chitosan Microcapsules Embedded within a Sodium Alginate/Gelatin Hydrogel-Like Film for Pseudomonas Aeruginosa Elimination. Proceedings 2020, 69, 39. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polym. J. 2017, 9, 401. [Google Scholar] [CrossRef]
- Buie, T.; McCune, J.; Cosgriff-Hernandez, E. Gelatin Matrices for Growth Factor Sequestration. Trends Biotechnol. 2020, 38, 546–557. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Negrini, N.C.; Farè, S.; Draghi, L. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. J. Mater. 2019, 12, 2476. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, Y.C.; Lee, J.H.; Jun, S.W.; Kim, C.S.; Lee, Y.; Park, J.C.; Lee, S.H.; Park, K.D.; Han, D.W. Multiphoton Imaging of Myogenic Differentiation in Gelatin-Based Hydrogels as Tissue Engineering Scaffolds. Biomater. Res. 2016, 20, 1–7. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Jayakrishnan, A. Self-Cross-Linking Biopolymers as Injectable in Situ Forming Biodegradable Scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef]
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship between Triple-Helix Content and Mechanical Properties of Gelatin Films. Biomaterials 2004, 25, 5675–5680. [Google Scholar] [CrossRef]
- Cattelan, G.; Guerrero Gerbolés, A.; Foresti, R.; Pramstaller, P.P.; Rossini, A.; Miragoli, M.; Caffarra Malvezzi, C. Alginate Formulations: Current Developments in the Race for Hydrogel-Based Cardiac Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 414. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef]
- Shakiba, M.; Rezvani Ghomi, E.; Khosravi, F.; Jouybar, S.; Bigham, A.; Zare, M.; Abdouss, M.; Moaref, R.; Ramakrishna, S. Nylon—A Material Introduction and Overview for Biomedical Applications. Polym. Adv. Technol. 2021, 32, 3368–3383. [Google Scholar] [CrossRef]
- Winnacker, M. Polyamides and Their Functionalization: Recent Concepts for Their Applications as Biomaterials. Biomater. Sci. 2017, 5, 1230–1235. [Google Scholar] [CrossRef]
- Antunes, J.C.; Moreira, I.P.; Gomes, F.; Cunha, F.; Henriques, M.; Fangueiro, R. Recent Trends in Protective Textiles against Biological Threats: A Focus on Biological Warfare Agents. Polym. J. 2022, 14, 1599. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, A.R. Technical Fibres for Heat and Flame Protection. In Handbook of technical textiles; Woodhead Publishing: Delhi, India, 2016; pp. 237–270. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Zhen, Q.; Hu, J.J.; Cui, J.Q.; Qian, X.M. Facile Preparation of PET/PA6 Bicomponent Microfilament Fabrics with Tunable Porosity for Comfortable Medical Protective Clothing. ACS Appl. Bio Mater. 2022, 5, 3509–3518. [Google Scholar] [CrossRef]
- McKeen, L.W. Polyesters. In The Effect of Creep and Other TIME Related Factors on Plastics and Elastomers; Elsevier: Amsterdam, The Netherlands, 2009; pp. 115–165. [Google Scholar] [CrossRef]
- Attia, M.F.; Brummel, B.R.; Lex, T.R.; Van Horn, B.A.; Whitehead, D.C.; Alexis, F. Recent Advances in Polyesters for Biomedical Imaging. Adv. Healthc. Mater. 2018, 7, e1800798. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Tay, Y.S.; Lim, T.T.; Wang, R.; Webster, R.D.; Hu, X. Polyacrylonitrile (PAN)-Induced Carbon Membrane with in-Situ Encapsulated Cobalt Crystal for Hybrid Peroxymonosulfate Oxidation-Filtration Process: Preparation, Characterization and Performance Evaluation. Chem. Eng. J. 2019, 373, 425–436. [Google Scholar] [CrossRef]
- Adegbola, T.A.; Agboola, O.; Fayomi, O.S.I. Review of Polyacrylonitrile Blends and Application in Manufacturing Technology: Recycling and Environmental Impact. Results Eng. 2020, 7, 100144. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, C.; Kwon, O.S. Conducting Polymer Based Nanobiosensors. Polym. J. 2016, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Chen, J.; Zeng, W.; Yu, C.; Yi, C.; Xu, Z. Facile Preparation of Uniform Nanocomposite Spheres with Loading Silver Nanoparticles on Polystyrene-Methyl Acrylic Acid Spheres for Catalytic Reduction of 4-Nitrophenol. J. Phys. Chem. C 2016, 120, 25935–25944. [Google Scholar] [CrossRef]
- Bhadra, J.; Alkareem, A.; Al-Thani, N. A Review of Advances in the Preparation and Application of Polyaniline Based Thermoset Blends and Composites. J. Polym. Res. 2020, 27, 1–20. [Google Scholar] [CrossRef]
- Liao, G. Green Preparation of Sulfonated Polystyrene/Polyaniline/Silver Composites with Enhanced Anticorrosive Properties. Int. J. Chem. 2018, 10, 81. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Rashid, S.A.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polym. J. 2021, 13, 2003. [Google Scholar] [CrossRef]
- Chen, Y. A Review of Polyaniline Based Materials as Anodes for Lithiumion Batteries. IOP Conf. Ser. Mater. Sci. Eng. 2019, 677, 022115. [Google Scholar] [CrossRef]
- Pina, C.D.; Falletta, E. Advances in Polyaniline for Biomedical Applications. Curr. Med. Chem. 2022, 29, 329–357. [Google Scholar] [CrossRef]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly (ε-Caprolactone) Fiber: An Overview. J. Eng. Fibers Fabr. 2014, 9, 74–90. [Google Scholar]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in Wound Healing and Skin Tissue Engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Goyal, A.; Khatri, K.; Vaidya, B.; Paliwal, R.; Rai, S.; Mehta, A.; Tiwari, S.; Vyas, S.; Vyas, S. Biodegradable Polymer Based Particulate Carrier(s) for the Delivery of Proteins and Peptides. Antiinflamm. Antiallergy Agents Med. Chem. 2008, 7, 240–251. [Google Scholar] [CrossRef]
- Deschamps, A.A.; Van Apeldoorn, A.A.; Hayen, H.; De Bruijn, J.D.; Karst, U.; Grijpma, D.W.; Feijen, J. In vivo and in vitro degradation of poly(ether ester) block copolymers based on poly(ethylene glycol) and poly(butylene terephthalate). Biomaterials 2004, 25, 247–258. [Google Scholar] [CrossRef] [PubMed]
- PEOT/PBT Polymers|PolyVation. Available online: https://www.polyvation.com/enabling-technologies/peotpbt-polymers/ (accessed on 12 April 2022).
- Ottenbrite, R.M.; Javan, R. Biological Structures. In Encyclopedia of Condensed Matter Physics; Elsevier: Amsterdam, The Netherlands, 2005; pp. 99–108. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Rane, M.; Parmar, J.; Tiwari, S.; Rajabi-Siahboomi, A. Application of Polyethylene Oxide in Hydrophilic Matrix Tablets. Pharma Times 2013, 45, 41–48. [Google Scholar] [CrossRef]
- Vanza, J.D.; Patel, R.B.; Dave, R.R.; Patel, M.R. Polyethylene Oxide and Its Controlled Release Properties in Hydrophilic Matrix Tablets for Oral Administration. Pharm. Dev. Technol. 2020, 10, 1169–1187. [Google Scholar] [CrossRef]
- Meruva, S.; Donovan, M.D. Polyethylene Oxide (PEO) Molecular Weight Effects on Abuse-Deterrent Properties of Matrix Tablets. AAPS PharmSciTech 2020, 21, 28. [Google Scholar] [CrossRef]
- Arora, B.; Bhatia, R.; Attri, P. Bionanocomposites: Green Materials for a Sustainable Future. In New Polymer Nanocomposites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 699–712. [Google Scholar] [CrossRef]
- Thummarungsan, N.; Paradee, N.; Pattavarakorn, D.; Sirivat, A. Influence of Graphene on Electromechanical Responses of Plasticized Poly(Lactic Acid). Polymer 2018, 138, 169–179. [Google Scholar] [CrossRef]
- Yang, C.; Yan, Z.; Lian, Y.; Wang, J.; Zhang, K. Graphene Oxide Coated Shell-Core Structured Chitosan/PLLA Nanofibrous Scaffolds for Wound Dressing. J. Biomater. Sci. Polym. Ed. 2020, 31, 622–641. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current Applications of Poly(Lactic Acid) Composites in Tissue Engineering and Drug Delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A Unique Polymer for Drug Delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A. Poly(Lactic Acid) and Poly(Lactic-Co-Glycolic) Acid Nanoparticles: Versatility in Biomedical Applications. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 199–216. [Google Scholar] [CrossRef]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The Design of Poly(Lactide-Co-Glycolide) Nanocarriers for Medical Applications. Front. Bioeng. Biotechnol. 2020, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.A.; Flores-Sahagun, T.H.S.; Ribeiro, A.M. Biodegradable Poly (l-Lactic Acid) (PLLA) and PLLA-3-Arm Blend Membranes: The Use of PLLA-3-Arm as a Plasticizer. Polym. Test. 2017, 60, 84–93. [Google Scholar] [CrossRef]
- Menyhárd, A.; Menczel, J.D.; Abraham, T. Polypropylene Fibers. In Thermal Analysis of Textiles and Fibers; The Textile Institute Book Series; Woodhead Publishing: Sawston, UK, 2020; pp. 205–222. ISBN 978-0-08-100572-9. [Google Scholar]
- Nathanael, A.J.; Oh, T.H. Biopolymer Coatings for Biomedical Applications. Polymer 2020, 12, 3061. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C. Polyurethane for Biomedical Applications: A Review of Recent Developments. In The Design and Manufacture of Medical Devices; Elsevier: Amsterdam, The Netherlands, 2012; pp. 115–151. [Google Scholar] [CrossRef]
- Mallakpour, S.; Rashidimoghadam, S. Poly(Vinyl Alcohol)/Carbon Nanotube Nanocomposites. In Biodegradable and Biocompatible Polymer Composites—Processing, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 297–315. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymer 2019, 12, 7. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in Nanoparticle Synthesis. Dalt. Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Kurakula, M.; Koteswara Rao, G.S.N. Moving Polyvinyl Pyrrolidone Electrospun Nanofibers and Bioprinted Scaffolds toward Multidisciplinary Biomedical Applications. Eur. Polym. J. 2020, 136, 109919. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical Assessment of Polyvinylpyrrolidone (PVP): As Excipient from Conventional to Controlled Delivery Systems with a Spotlight on COVID-19 Inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. The Use of Poly(N-Vinyl Pyrrolidone) in the Delivery of Drugs: A Review. Polymer 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, C.R.; King, M.W. Biotextiles: Fiber to Fabric for Medical Applications. In Resorbable Fiber-Forming Polymers for Biotextile Applications; Springer: Berlin/Heidelberg, Germany, 2014; pp. 11–22. [Google Scholar] [CrossRef]
- Miranda, C.S.; Ribeiro, A.R.M.; Homem, N.C.; Felgueiras, H.P. Spun Biotextiles in Tissue Engineering and Biomolecules Delivery Systems. J. Antibiot. 2020, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Mahdieh, Z.; Mitra, S.; Holian, A. Core-Shell Electrospun Fibers with an Improved Open Pore Structure for Size-Controlled Delivery of Nanoparticles. ACS Appl. Polym. Mater. 2020, 2, 4004–4015. [Google Scholar] [CrossRef]
- Kharaghani, D.; Khan, M.Q.; Kim, I.S. Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Wsoo, M.A.; Shahir, S.; Mohd Bohari, S.P.; Nayan, N.H.M.; Razak, S.I.A. A Review on the Properties of Electrospun Cellulose Acetate and Its Application in Drug Delivery Systems: A New Perspective. Carbohydr. Res. 2020, 491, 107978. [Google Scholar] [CrossRef]
- Asmatulu, R.; Khan, W.S. Introduction to Electrospun Nanofibers. In Synthesis and Applications of Electrospun Nanofibers; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–15. [Google Scholar] [CrossRef]
- Imura, Y.; Hogan, R.M.C.; Jaffe, M. Dry Spinning of Synthetic Polymer Fibers. In Advances in Filament Yarn Spinning of Textiles and Polymers; Woodhead Publishing: Delhi, India, 2014; pp. 187–202. [Google Scholar] [CrossRef]
- Clarkson, C.M.; Youngblood, J.P. Dry-Spinning of Cellulose Nanocrystal/Polylactic Acid Composite Fibers. Green Mater. 2018, 6, 6–14. [Google Scholar] [CrossRef]
- Qu, H.; Skorobogatiy, M. Conductive Polymer Yarns for Electronic Textiles. In Electronic Textiles; Elsevier: Amsterdam, The Netherlands, 2015; pp. 21–53. [Google Scholar] [CrossRef]
- Kramschuster, A.; Turng, L.S. Fabrication of Tissue Engineering Scaffolds. In Handbook of Biopolymers and Biodegradable Plastics: Properties, Processing and Applications; William Andrew: Norwich, NY, USA, 2013; pp. 427–446. [Google Scholar] [CrossRef]
- Rawal, A.; Mukhopadhyay, S. Melt Spinning of Synthetic Polymeric Filaments. In Advances in Filament Yarn Spinning of Textiles and Polymers; Woodhead Publishing: Delhi, India, 2014; pp. 75–99. [Google Scholar] [CrossRef]
- Jia, J.; Yao, D.; Wang, Y. Melt Spinning of Continuous Fibers by Cold Air Attenuation I: Experimental Studies. Sage 2014, 84, 593–603. [Google Scholar] [CrossRef]
- Mirabedini, A. Developing Novel Spinning Methods to Fabricate Continuous Multifunctional Fibres for Bioapplications. Doctor’s Thesis, University of Wollongong, Wollongong, Australia, 2017; pp. 1–235. [Google Scholar]
- Mathiowitz, E.; Lavin, D.M.; Hopkins, R.A. Wet Spun Microfibers: Potential in the Design of Controlled-Release Scaffolds? Future Sci. 2013, 4, 1075–1077. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Homem, N.C.; Teixeira, M.A.; Ribeiro, A.R.M.; Antunes, J.C.; Amorim, M.T.P. Physical, Thermal, and Antibacterial Effects of Active Essential Oils with Potential for Biomedical Applications Loaded onto Cellulose Acetate/Polycaprolactone Wet-Spun Microfibers. Biomolecules 2020, 10, 1129. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F. Wet-Spinning of Biomedical Polymers: From Single-Fibre Production to Additive Manufacturing of Three-Dimensional Scaffolds. Polym. Int. 2017, 66, 1690–1696. [Google Scholar] [CrossRef]
- Cong, H.P.; Ren, X.C.; Wang, P.; Yu, S.H. Wet-Spinning Assembly of Continuous, Neat and Macroscopic Graphene Fibers. Sci. Rep. 2012, 2, 613. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P.; Homem, N.C.; Teixeira, M.A.; Ribeiro, M.; Teixeira, M.O.; Antunes, J.C.; Amorim, M.T.P. Biodegradable Wet-Spun Fibers Modified with Antimicrobial Agents for Potential Applications in Biomedical Engineering. J. Phys. Conf. Ser. 2021, 1756, 012007. [Google Scholar] [CrossRef]
- Miranda, C.S.; Silva, A.F.G.; Seabra, C.L.; Reis, S.; Silva, M.M.P.; Pereira-Lima, S.M.M.A.; Costa, S.P.G.; Homem, N.C.; Felgueiras, H.P. Sodium Alginate/Polycaprolactone Co-Axial Wet-Spun Microfibers Modified with N-Carboxymethyl Chitosan and the Peptide AAPV for Staphylococcus Aureus and Human Neutrophil Elastase Inhibition in Potential Chronic Wound Scenarios. Biomater. Adv. 2023, 151, 213488. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.S.; Silva, A.F.G.; Pereira-Lima, S.M.M.A.; Costa, S.P.G.; Homem, N.C.; Felgueiras, H.P. Tunable Spun Fiber Constructs in Biomedicine: Influence of Processing Parameters in the Fibers’ Architecture. Int. J. Pharm. 2022, 14, 164. [Google Scholar] [CrossRef]
- Morris, H.; Murray, R. Medical Textiles. Text. Prog. 2020, 52, 1–127. [Google Scholar] [CrossRef]
- Tiffany, C.R.; Bäumler, A.J. Dysbiosis: From Fiction to Function. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G602–G608. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the Immune System. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining Dysbiosis and Its Influence on Host Immunity and Disease. Cell. Microbiol. 2014, 16, 1024. [Google Scholar] [CrossRef]
- Johnson, T.R.; Gómez, B.I.; McIntyre, M.K.; Dubick, M.A.; Christy, R.J.; Nicholson, S.E.; Burmeister, D.M. The Cutaneous Microbiome and Wounds: New Molecular Targets to Promote Wound Healing. Int. J. Mol. Sci. 2018, 19, 2699. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.D.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the Chronic Wound Microbiota of 2963 Patients by 16S RDNA Pyrosequencing. Wound Repair Regen. 2016, 24, 163–174. [Google Scholar] [CrossRef]
- Obagi, Z.; Damiani, G.; Grada, A.; Falanga, V. Principles of Wound Dressings: A Review. Surg. Technol. Int. 2019, 35, 1–8. [Google Scholar]
- Gizaw, M.; Thompson, J.; Faglie, A.; Lee, S.Y.; Neuenschwander, P.; Chou, S.F. Electrospun Fibers as a Dressing Material for Drug and Biological Agent Delivery in Wound Healing Applications. J. Bioeng. 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Goswami, L.; Kim, B.S. Nanomaterial-Based Therapy for Wound Healing. J. Nanomater. 2022, 12, 618. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Lee, W.H.; Gao, Z.; Qin, D.; Wang, Y.; Liu, J.; Sun, T.; Gao, Y. Wound Dressing from Polyvinyl Alcohol/Chitosan Electrospun Fiber Membrane Loaded with OH-CATH30 Nanoparticles. Carbohydr. Polym. 2020, 232, 115786. [Google Scholar] [CrossRef]
- Khan, A.u.R.; Huang, K.; Khalaji, M.S.; Yu, F.; Xie, X.; Zhu, T.; Morsi, Y.; Jinzhong, Z.; Mo, X. Multifunctional Bioactive Core-Shell Electrospun Membrane Capable to Terminate Inflammatory Cycle and Promote Angiogenesis in Diabetic Wound. Bioact. Mater. 2021, 6, 2783. [Google Scholar] [CrossRef]
- Saraf, V.S.; Sheikh, S.A.; Ahmad, A.; Gillevet, P.M.; Bokhari, H.; Javed, S. Vaginal Microbiome: Normalcy vs Dysbiosis. Arch. Microbiol. 2021, 203, 3793–3802. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- Elsherbini, A.M.; Sabra, S.A. Nanoparticles-in-Nanofibers Composites: Emphasis on Some Recent Biomedical Applications. J. Control Release 2022, 348, 57–83. [Google Scholar] [CrossRef]
- Nayak, R.; Kar, B.; Ghosh, G.; Rath, G. Current Trends in Chitosan Based Nanopharmaceuticals for Topical Vaginal Therapies. Int. J. Biol. Macromol. 2021, 193, 2140–2152. [Google Scholar] [CrossRef]
- Krogstad, E.A.; Ramanathan, R.; Nhan, C.; Kraft, J.C.; Blakney, A.K.; Cao, S.; Ho, R.J.Y.; Woodrow, K.A. Nanoparticle-Releasing Nanofiber Composites for Enhanced in Vivo Vaginal Retention. Biomaterials 2017, 144, 1–16. [Google Scholar] [CrossRef]
- Tuğcu-Demiröz, F.; Saar, S.; Kara, A.A.; Yıldız, A.; Tunçel, E.; Acartürk, F. Development and Characterization of Chitosan Nanoparticles Loaded Nanofiber Hybrid System for Vaginal Controlled Release of Benzydamine. Eur. J. Pharm. Sci. 2021, 161, 105801. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Bhandari, R.; Quirk, R.A.; Shakesheff, K.M. Recent Advances in Tissue Engineering. J. Long. Term. Eff. Med. Implant. 2017, 27, 199–232. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.S. Nanoparticles in Tissue Engineering: Applications, Challenges and Prospects. Int. J. Nanomed. 2018, 13, 5637. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and Their Applications in Tissue Engineering. Int. J. Nanomed. 2006, 1, 15. [Google Scholar] [CrossRef]
- Xi, K.; Gu, Y.; Tang, J.; Chen, H.; Xu, Y.; Wu, L.; Cai, F.; Deng, L.; Yang, H.; Shi, Q.; et al. Microenvironment-Responsive Immunoregulatory Electrospun Fibers for Promoting Nerve Function Recovery. Nat. Commun. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Liu, F.; Cheng, X.; Xiao, L.; Wang, Q.; Yan, K.; Su, Z.; Wang, L.; Ma, C.; Wang, Y. Inside-Outside Ag Nanoparticles-Loaded Polylactic Acid Electrospun Fiber for Long-Term Antibacterial and Bone Regeneration. Int. J. Biol. Macromol. 2021, 167, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 5 July 2022).
- Seebacher, N.A.; Stacy, A.E.; Porter, G.M.; Merlot, A.M. Clinical Development of Targeted and Immune Based Anti-Cancer Therapies. J. Exp. Clin. Cancer Res. 2019, 38, 1–39. [Google Scholar] [CrossRef]
- Salim, S.A.; Kamoun, E.A.; Evans, S.; El-Moslamy, S.H.; El-Fakharany, E.M.; Elmazar, M.M.; Abdel-Aziz, A.F.; Abou-Saleh, R.H.; Salaheldin, T.A. Mercaptopurine-Loaded Sandwiched Tri-Layered Composed of Electrospun Polycaprolactone/Poly(Methyl Methacrylate) Nanofibrous Scaffolds as Anticancer Carrier with Antimicrobial and Antibiotic Features: Sandwich Configuration Nanofibers, Release Study and in. Int. J. Nanomed. 2021, 16, 6937. [Google Scholar] [CrossRef]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Nanoparticle-Based Bioactive Agent Release Systems for Bone and Cartilage Tissue Engineering. Regen. Ther. 2015, 1, 109. [Google Scholar] [CrossRef]
- Cavo, M.; Serio, F.; Kale, N.R.; D’amone, E.; Gigli, G.; Del Mercato, L.L. Electrospun Nanofibers in Cancer Research: From Engineering of in Vitro 3D Cancer Models to Therapy. Biomater. Sci. 2020, 8, 4887–4905. [Google Scholar] [CrossRef]
- Peng, J.; Chen, J.; Xie, F.; Bao, W.; Xu, H.; Wang, H.; Xu, Y.; Du, Z. Herceptin-Conjugated Paclitaxel Loaded PCL-PEG Worm-like Nanocrystal Micelles for the Combinatorial Treatment of HER2-Positive Breast Cancer. Biomaterials 2019, 222, 119420. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xia, X.; Chen, J.; Xia, D.; Xu, R.; Zou, X.; Wang, H.; Liang, C. Paclitaxel-Loaded Lignin Particle Encapsulated into Electrospun PVA/PVP Composite Nanofiber for Effective Cervical Cancer Cell Inhibition. J. Nanotechnol. 2020, 32, 015101. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, W.; Sadeghi-Soureh, S.; Amirsaadat, S.; Pourpirali, R.; Alijani, S. Dual Drug Release Mechanisms through Mesoporous Silica Nanoparticle/Electrospun Nanofiber for Enhanced Anticancer Efficiency of Curcumin. J. Biomed. Mater. Res.—Part A 2022, 110, 316–330. [Google Scholar] [CrossRef]
- Al-Attar, T.; Madihally, S.V. Targeted Cancer Treatment Using a Combination of SiRNA-Liposomes and Resveratrol-Electrospun Fibers in Co-Cultures. Int. J. Pharm. 2019, 569, 118599. [Google Scholar] [CrossRef]
- Mohamady Hussein, M.A.; Guler, E.; Rayaman, E.; Cam, M.E.; Sahin, A.; Grinholc, M.; Sezgin Mansuroglu, D.; Sahin, Y.M.; Gunduz, O.; Muhammed, M.; et al. Dual-Drug Delivery of Ag-Chitosan Nanoparticles and Phenytoin via Core-Shell PVA/PCL Electrospun Nanofibers. Carbohydr. Polym. 2021, 270, 118373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, M.; Bao, F.; Xing, M.; Wang, E.; Xu, Q.; Huan, Z.; Guo, F.; Chang, J. Multifunctional Zn Doped Hollow Mesoporous Silica/Polycaprolactone Electrospun Membranes with Enhanced Hair Follicle Regeneration and Antibacterial Activity for Wound Healing. Nanoscale 2019, 11, 6315–6333. [Google Scholar] [CrossRef] [PubMed]
- Fahimirad, S.; Abtahi, H.; Satei, P.; Ghaznavi-Rad, E.; Moslehi, M.; Ganji, A. Wound Healing Performance of PCL/Chitosan Based Electrospun Nanofiber Electrosprayed with Curcumin Loaded Chitosan Nanoparticles. Carbohydr. Polym. 2021, 259, 117640. [Google Scholar] [CrossRef]
- Zahiri, M.; Khanmohammadi, M.; Goodarzi, A.; Ababzadeh, S.; Sagharjoghi Farahani, M.; Mohandesnezhad, S.; Bahrami, N.; Nabipour, I.; Ai, J. Encapsulation of Curcumin Loaded Chitosan Nanoparticle within Poly (ε-Caprolactone) and Gelatin Fiber Mat for Wound Healing and Layered Dermal Reconstitution. Int. J. Biol. Macromol. 2020, 153, 1241–1250. [Google Scholar] [CrossRef]
- Yan, D.; Yao, Q.; Yu, F.; Chen, L.; Zhang, S.; Sun, H.; Lin, J.; Fu, Y. Surface Modified Electrospun Poly(Lactic Acid) Fibrous Scaffold with Cellulose Nanofibrils and Ag Nanoparticles for Ocular Cell Proliferation and Antimicrobial Application. Mater. Sci. Eng. C 2020, 111, 110767. [Google Scholar] [CrossRef]
- Wang, J.; Zhan, L.; Zhang, X.; Wu, R.; Liao, L.; Wei, J. Silver Nanoparticles Coated Poly(L-Lactide) Electrospun Membrane for Implant Associated Infections Prevention. Front. Pharmacol. 2020, 11, 431. [Google Scholar] [CrossRef]
- Nazari, H.; Heirani-Tabasi, A.; Hajiabbas, M.; Salimi Bani, M.; Nazari, M.; Pirhajati Mahabadi, V.; Rad, I.; Kehtari, M.; Ahmadi Tafti, S.H.; Soleimani, M. Incorporation of SPION-Casein Core-Shells into Silk-Fibroin Nanofibers for Cardiac Tissue Engineering. J. Cell. Biochem. 2020, 121, 2981–2993. [Google Scholar] [CrossRef] [PubMed]
- Amirsadeghi, A.; Khorram, M.; Hashemi, S.S. Preparation of Multilayer Electrospun Nanofibrous Scaffolds Containing Soluble Eggshell Membrane as Potential Dermal Substitute. J. Biomed. Mater. Res.—Part A 2021, 109, 1812–1827. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.L.; Ganguly, D.; Zuidema, J.M.; Cardinal, T.J.; Ziemba, A.M.; Kearns, K.R.; McCarthy, S.M.; Thompson, D.M.; Ramanath, G.; Borca-Tasciuc, D.A.; et al. Injectable, Magnetically Orienting Electrospun Fiber Conduits for Neuron Guidance. ACS Appl. Mater. Interfaces 2019, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, A.; Khanna, K.; Sindhu, K.R.; Bellare, J.; Srivastava, R. Magnesium Oxide Nanoparticle-Loaded Polycaprolactone Composite Electrospun Fiber Scaffolds for Bone–Soft Tissue Engineering Applications: In-Vitro and in-Vivo Evaluation. Biomed. Mater. 2017, 12, 055011. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Xuan, L.; Zhang, Y.; Wang, D.; Ye, F.; Shi, X.; Li, Y. Synergistic Effects of Thermal Treatment and Encapsulation of Calcium Phosphate Nanoparticles on Enhancing Dimensional Stability and Osteogenic Induction Potential of Free-Standing PLGA Electrospun Membranes. Colloids Surf. B 2019, 183, 110437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Xiao, J.; Fang, T.; Hu, N.; Li, M.; Deng, L.; Cheng, Y.; Zhu, Y.; Cui, W. An Electrospun Fiber-Covered Stent with Programmable Dual Drug Release for Endothelialization Acceleration and Lumen Stenosis Prevention. Acta Biomater. 2019, 94, 295–305. [Google Scholar] [CrossRef]
- Lian, M.; Sun, B.; Qiao, Z.; Zhao, K.; Zhou, X.; Zhang, Q.; Zou, D.; He, C.; Zhang, X. Bi-Layered Electrospun Nanofibrous Membrane with Osteogenic and Antibacterial Properties for Guided Bone Regeneration. Colloids Surf. B 2019, 176, 219–229. [Google Scholar] [CrossRef]
- Mehrasa, M.; Asadollahi, M.A.; Ghaedi, K.; Salehi, H.; Arpanaei, A. Electrospun Aligned PLGA and PLGA/Gelatin Nanofibers Embedded with Silica Nanoparticles for Tissue Engineering. Int. J. Biol. Macromol. 2015, 79, 687–695. [Google Scholar] [CrossRef]
- Shokry, H.; Vanamo, U.; Wiltschka, O.; Niinimäki, J.; Lerche, M.; Levon, K.; Linden, M.; Sahlgren, C. Mesoporous Silica Particle-PLA–PANI Hybrid Scaffolds for Cell-Directed Intracellular Drug Delivery and Tissue Vascularization. Nanoscale 2015, 7, 14434–14443. [Google Scholar] [CrossRef]
- Liu, S.; Qin, M.; Hu, C.; Wu, F.; Cui, W.; Jin, T.; Fan, C. Tendon Healing and Anti-Adhesion Properties of Electrospun Fibrous Membranes Containing BFGF Loaded Nanoparticles. Biomaterials 2013, 34, 4690–4701. [Google Scholar] [CrossRef]
- Sruthi, R.; Balagangadharan, K.; Selvamurugan, N. Polycaprolactone/Polyvinylpyrrolidone Coaxial Electrospun Fibers Containing Veratric Acid-Loaded Chitosan Nanoparticles for Bone Regeneration. Colloids Surf. B 2020, 193, 111110. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hou, W.; Guo, X.; Li, J.; Hu, T.; Qiu, M.; Liu, S.; Mo, X.; Liu, X. Two-Phase Electrospinning to Incorporate Growth Factors Loaded Chitosan Nanoparticles into Electrospun Fibrous Scaffolds for Bioactivity Retention and Cartilage Regeneration. Mater. Sci. Eng. C 2017, 79, 507–515. [Google Scholar] [CrossRef]
- Günday, C.; Anand, S.; Gencer, H.B.; Munafò, S.; Moroni, L.; Fusco, A.; Donnarumma, G.; Ricci, C.; Hatir, P.C.; Türeli, N.G.; et al. Ciprofloxacin-Loaded Polymeric Nanoparticles Incorporated Electrospun Fibers for Drug Delivery in Tissue Engineering Applications. Drug Deliv. Transl. Res. 2020, 10, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Nirwan, V.P.; Filova, E.; Al-Kattan, A.; Kabashin, A.V.; Fahmi, A. Smart Electrospun Hybrid Nanofibers Functionalized with Ligand-Free Titanium Nitride (Tin) Nanoparticles for Tissue Engineering. J. Nanomater. 2021, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Andersson Trojer, M.; Olsson, C.; Bengtsson, J.; Hedlund, A.; Bordes, R. Directed Self-Assembly of Silica Nanoparticles in Ionic Liquid-Spun Cellulose Fibers. J. Colloid Interface Sci. 2019, 553, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, X.; Liu, H.; Luo, Y.; Wang, S.; Shen, M.; Zhu, M.; Shi, X. Dendrimer-Functionalized Electrospun Cellulose Acetate Nanofibers for Targeted Cancer Cell Capture Applications. J. Mater. Chem. B 2014, 2, 7384–7393. [Google Scholar] [CrossRef]
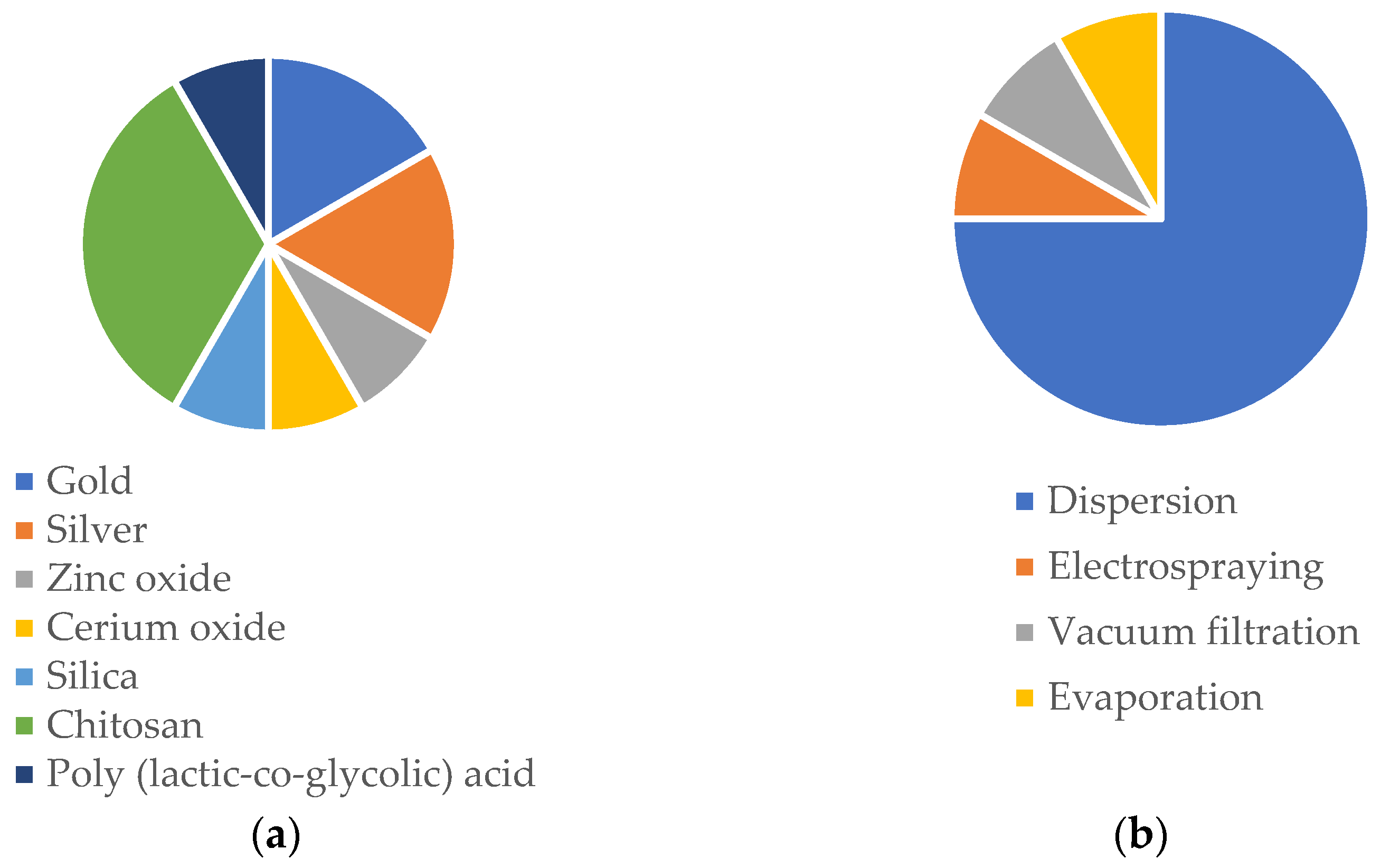

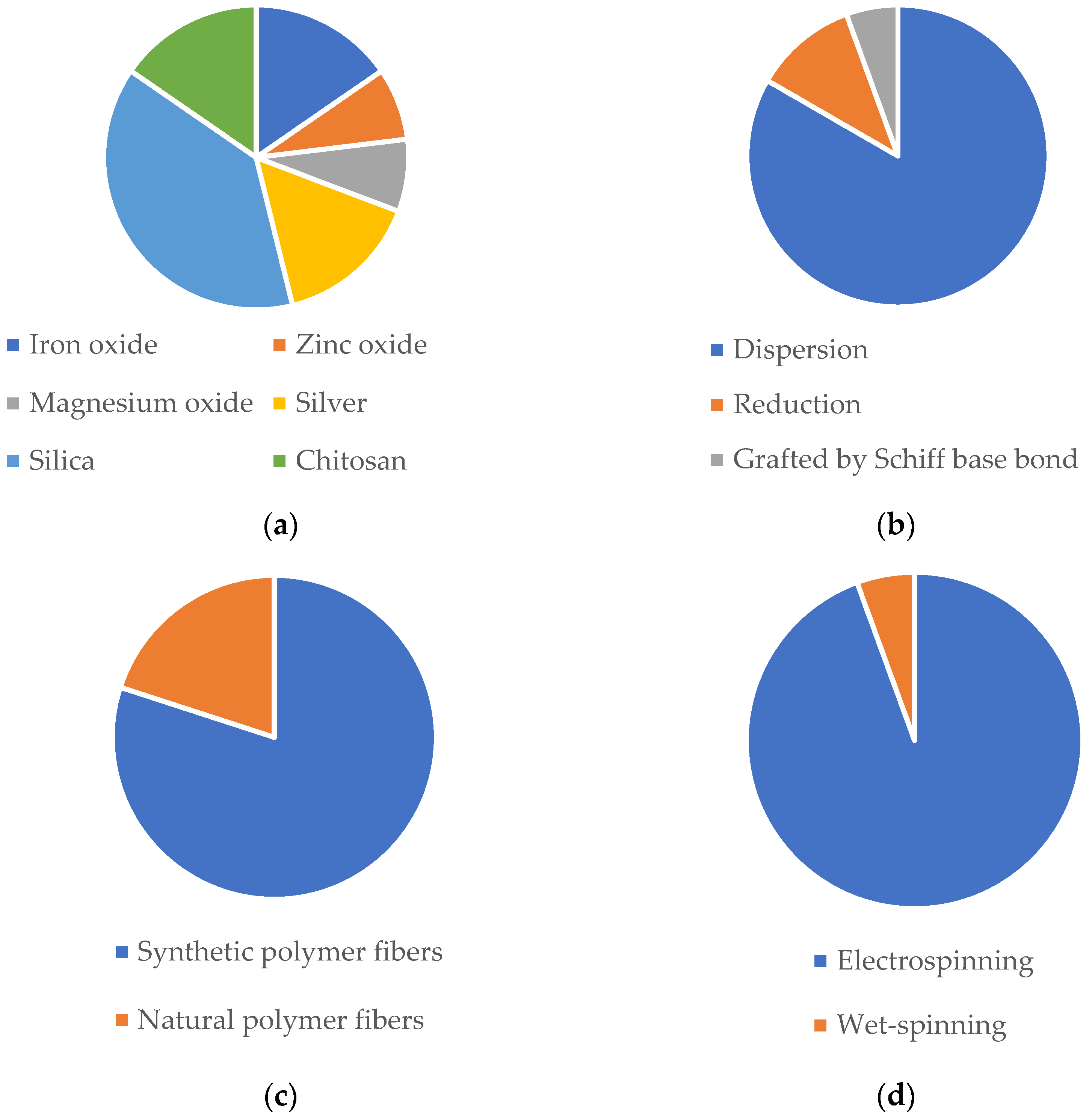
| Type | Advantages | Limitations | ||
|---|---|---|---|---|
| Nanoparticles | Inorganic | Silver |
|
|
Gold |
|
| ||
Iron oxide |
|
| ||
Zinc oxide |
|
| ||
Magnesium oxide |
|
| ||
Cerium oxide |
|
| ||
Titanium dioxide |
|
| ||
Silica |
|
| ||
| Organic | Polymeric micelles  |
|
| |
Chitosan-based |
|
| ||
Dendrimers |
|
| ||
Liposomes |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingues, J.M.; Miranda, C.S.; Homem, N.C.; Felgueiras, H.P.; Antunes, J.C. Nanoparticle Synthesis and Their Integration into Polymer-Based Fibers for Biomedical Applications. Biomedicines 2023, 11, 1862. https://doi.org/10.3390/biomedicines11071862
Domingues JM, Miranda CS, Homem NC, Felgueiras HP, Antunes JC. Nanoparticle Synthesis and Their Integration into Polymer-Based Fibers for Biomedical Applications. Biomedicines. 2023; 11(7):1862. https://doi.org/10.3390/biomedicines11071862
Chicago/Turabian StyleDomingues, Joana M., Catarina S. Miranda, Natália C. Homem, Helena P. Felgueiras, and Joana C. Antunes. 2023. "Nanoparticle Synthesis and Their Integration into Polymer-Based Fibers for Biomedical Applications" Biomedicines 11, no. 7: 1862. https://doi.org/10.3390/biomedicines11071862
APA StyleDomingues, J. M., Miranda, C. S., Homem, N. C., Felgueiras, H. P., & Antunes, J. C. (2023). Nanoparticle Synthesis and Their Integration into Polymer-Based Fibers for Biomedical Applications. Biomedicines, 11(7), 1862. https://doi.org/10.3390/biomedicines11071862








