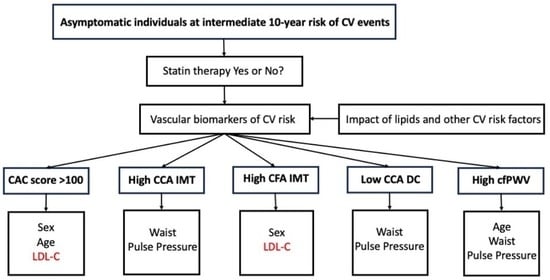
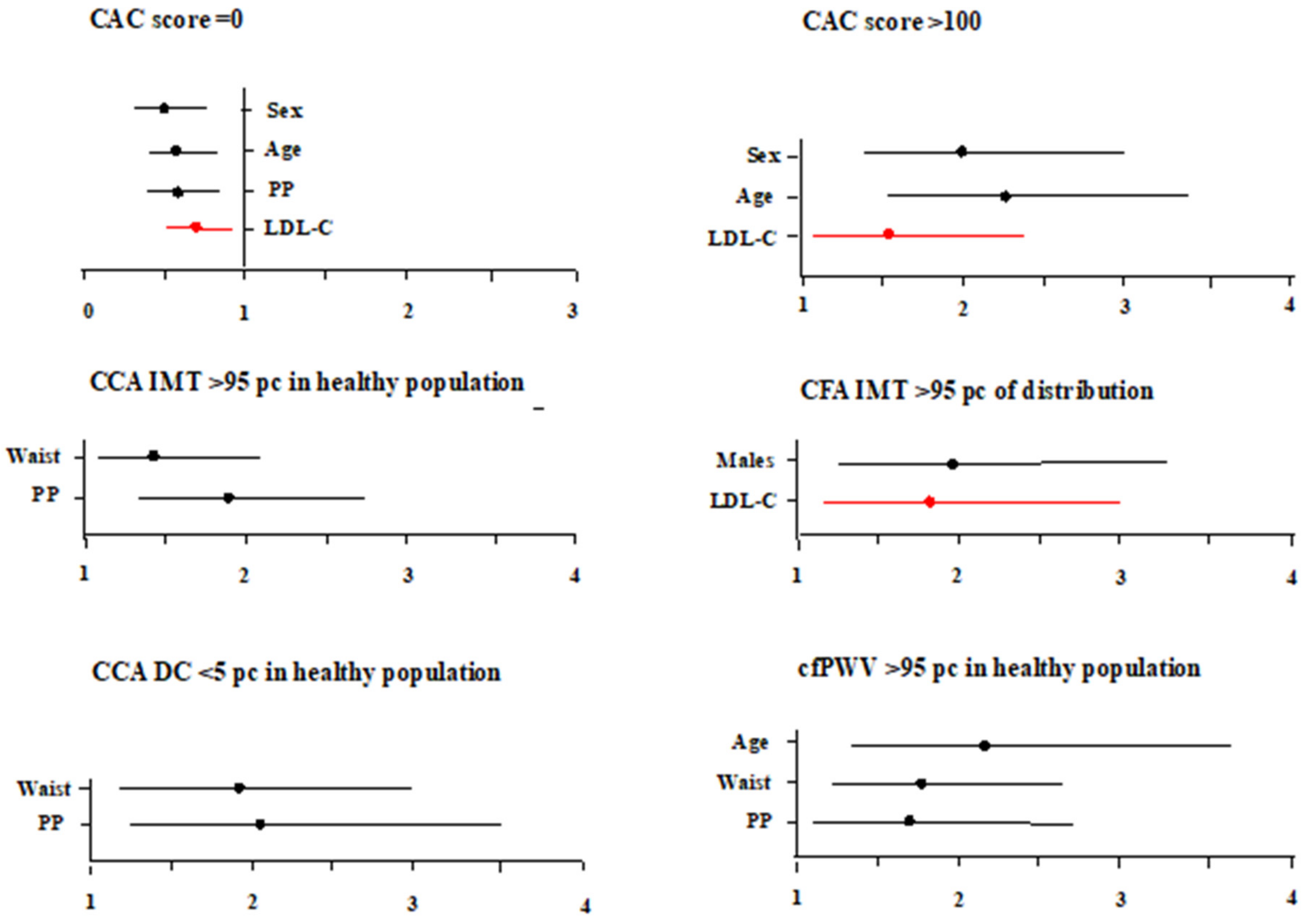
Association between Low-Density Lipoprotein Cholesterol and Vascular Biomarkers in Primary Prevention
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Protocol
2.2. Body Size and BP Measurement
2.3. Assessment of the 10-Year Risk of CV Disease
2.4. Assessment of LDL-C Plasma Level
2.5. Coronary Calcium Score (CAC)
2.6. Vascular Examination
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Study Limitations
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- von Birgelen, C.; Hartmann, M.; Mintz, G.S.; Baumgart, D.; Schmermund, A.; Erbel, R. Relation between progression and regression of atherosclerotic left main coronary artery disease and serum cholesterol levels as assessed with serial long-term (> or =12 months) follow-up intravascular ultrasound. Circulation 2003, 108, 2757–2762. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Nicholls, S.J.; Sipahi, I.; Libby, P.; Raichlen, J.S.; Ballantyne, C.M.; Davignon, J.; Erbel, R.; Fruchart, J.C.; Tardif, J.C.; et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: The ASTEROID trial. JAMA 2006, 295, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Tuzcu, E.M.; Sipahi, I.; Grasso, A.W.; Schoenhagen, P.; Hu, T.; Wolski, K.; Crowe, T.; Desai, M.Y.; Hazen, S.L.; et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007, 297, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Jeong, S.M.; Choi, S.; Kim, K.; Kim, S.M.; Lee, G.; Park, S.Y.; Kim, Y.Y.; Son, J.S.; Yun, J.M.; Park, S.M. Effect of change in total cholesterol levels on cardiovascular disease among young adults. J. Am. Heart Assoc. 2018, 7, e008819. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Sarwar, A.; Shaw, L.J.; Shapiro, M.D.; Blankstein, R.; Hoffmann, U.; Cury, R.C.; Abbara, S.M.; Brady, T.J.; Budoff, M.J.; Blumenthal, R.S.; et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc. Imaging 2009, 2, 675–688. [Google Scholar] [CrossRef]
- Lo-Kioeng-Shioe, M.S.; Rijlaarsdam-Hermsen, D.; van Domburg, R.T.; Hadamitzky, M.; Lima, J.A.C.; Hoeks, S.E.; Deckers, J.W. Prognostic value of coronary artery calcium score in symptomatic individuals: A meta-analysis of 34,000 subjects. Int. J. Cardiol. 2020, 299, 56–62. [Google Scholar] [CrossRef]
- Greenland, P.; Blaha, M.J.; Budoff, M.J.; Erbel, R.; Watson, K.E. Coronary calcium score and cardiovascular risk. J. Am. Coll. Cardiol. 2018, 72, 434–447. [Google Scholar] [CrossRef]
- McClelland, R.L.; Jorgensen, N.W.; Budoff, M.; Blaha, M.J.; Post, W.S.; Kronmal, R.A.; Bild, D.E.; Shea, S.M.; Liu, K.M.; Watson, K.E.; et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J. Am. Coll. Cardiol. 2015, 66, 1643–1653. [Google Scholar]
- Puri, R.; Nicholls, S.J.; Shao, M.; Kataoka, Y.; Uno, K.; Kapadia, S.R.; Tuzcu, E.M.; Nissen, S.E. Impact of statins on serial coronary calcification during atheroma progression and regression. J. Am. Coll. Cardiol. 2015, 65, 1273–1282. [Google Scholar] [CrossRef]
- Di Giovanni, G.; Nicholls, S.J. Intensive lipid lowering agents and coronary atherosclerosis: Insights from intravascular imaging. Am. J. Prev. Cardiol. 2022, 11, 100366. [Google Scholar] [CrossRef]
- Henein, M.; Granåsen, G.; Wiklund, U.; Schmermund, A.; Guerci, A.; Erbel, R.; Raggi, P. High dose and long-term statin therapy accelerate coronary artery calcification. Int. J. Cardiol. 2015, 184, 581–586. [Google Scholar] [CrossRef]
- Dykun, I.; Lehmann, N.; Kälsch, H.; Möhlenkamp, S.; Moebus, S.; Budde, T.; Seibel, R.; Grönemeyer, D.; Jöckel, K.H.; Erbel, R.; et al. Statin medication enhances progression of coronary artery calcification: The Heinz Nixdorf Recall Study. J. Am. Coll. Cardiol. 2016, 68, 2123–2125. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.; Huffman, M.D.; Macedo, A.F.; Moore, T.H.; Burke, M.; Davey Smith, G.; Ward, K.; Ebrahim, S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013, 1, CD004816. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.P.; Einstein, A.J.; Berrington de González, A. Coronary artery calcification screening: Estimated radiation dose and cancer risk. Arch. Intern. Med. 2009, 169, 1188–1194. [Google Scholar] [CrossRef]
- Crouse, J.R., 3rd; Raichlen, J.S.; Riley, W.A.; Evans, G.W.; Palmer, M.K.; O’Leary, D.H.; Grobbee, D.E.; Bots, M.L.; METEOR Study Group. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: The METEOR Trial. JAMA 2007, 297, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Daida, H.; Nohara, R.; Hata, M.; Kaku, K.; Kawamori, R.; Kishimoto, J.; Kurabayashi, M.; Masuda, I.; Sakuma, I.; Yamazaki, T.; et al. Can intensive lipid-lowering therapy improve the carotid intima-media thickness in Japanese subjects under primary prevention for cardiovascular disease? The JART and JART extension subanalysis. J. Atheroscler. Thromb. 2014, 21, 739–754. [Google Scholar] [CrossRef]
- Smilde; Den Berkmortel, V.; Wollersheim; Langen, V.; Kastelein; Stalenhoef. The effect of cholesterol lowering on carotid and femoral artery wall stiffness and thickness in patients with familial hypercholesterolaemia. Eur. J. Clin. Investig. 2000, 30, 473–480. [Google Scholar] [CrossRef]
- Upala, S.; Wirunsawanya, K.; Jaruvongvanich, V.; Sanguankeo, A. Effects of statin therapy on arterial stiffness: A systematic review and meta-analysis of randomized controlled trial. Int. J. Cardiol. 2017, 227, 338–341. [Google Scholar] [CrossRef]
- Bu, D.X.; Griffin, G.; Lichtman, A.H. Mechanisms for the anti-inflammatory effects of statins. Curr. Opin. Lipidol. 2011, 22, 165–170. [Google Scholar] [CrossRef]
- Davignon, J.; Jacob, R.F.; Mason, R.P. The antioxidant effects of statins. Coron. Artery Dis. 2004, 15, 251–258. [Google Scholar] [CrossRef]
- Liao, J.K.; Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, S.; Arnaboldi, L.; Gerosa, L.; Canavesi, M.; Parente, R.; Baetta, R.; Paoletti, R.; Corsini, A. Statins effect on smooth muscle cell proliferation. Semin. Vasc. Med. 2004, 4, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; Kerry, S.M.; Barbato, A.; Versiero, M.; D’Elia, L.; Cappuccio, F.P. Do statins reduce blood pressure? A meta-analysis of randomized, controlled trials. Hypertension 2007, 49, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cífková, R.; Cosentino, F.; De Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532. [Google Scholar] [PubMed]
- Tarkin, J.M.; Dweck, M.R.; Rudd, J.H.F. Imaging as a surrogate marker of drug efficacy in cardiovascular disease. Heart 2019, 105, 567–578. [Google Scholar] [CrossRef]
- Vasan, R.S. Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation 2006, 113, 2335–2362. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redón, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- NCEP. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Engelen, L.; Bossuyt, J.; Ferreira, I.; van Bortel, L.M.; Reesink, K.D.; Segers, P.; Stehouwer, C.D.; Laurent, S.; Boutouyrie, P. Reference Values for Arterial Measurements Collaboration. Reference values for local arterial stiffness. Part A: Carotid artery. J. Hypertens. 2015, 33, 1981–1996. [Google Scholar] [CrossRef]
- Engelen, L.; Ferreira, I.; Stehouwer, C.D.; Boutouyrie, P.; Laurent, S. Reference Values for Arterial Measurements Collaboration. Reference intervals for common carotid intima-media thickness measured with echotracking: Relation with risk factors. Eur. Heart J. 2013, 34, 2368–2380. [Google Scholar] [CrossRef]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.; Protogerou, A.D.; et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–458. [Google Scholar] [CrossRef]
- Baldo, M.P.; Cunha, R.S.; Molina, M.D.C.B.; Chór, D.; Griep, R.H.; Duncan, B.B.; Schmidt, M.I.; Ribeiro, A.L.P.; Barreto, S.M.; Lotufo, P.A.; et al. Carotid-femoral pulse wave velocity in a healthy adult sample: The ELSA-Brasil study. Int. J. Cardiol. 2018, 251, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Demer, L.L. Cholesterol in vascular and valvular calcification. Circulation 2001, 104, 1881–1883. [Google Scholar] [CrossRef] [PubMed]
- Parhami, F.; Morrow, A.D.; Balucan, J.; Leitinger, N.; Watson, A.D.; Tintut, Y.; Berliner, J.A.; Demer, L.L. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation: A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, Z.; Li, Y.; Chen, C.; Yang, H.; Lin, Q.; Hu, M.; Qin, X. The cell origin and role of osteoclastogenesis and osteoblastogenesis in vascular calcification. Front. Cardiovasc. Med. 2021, 8, 639740. [Google Scholar] [CrossRef]
- Shioi, A.; Ikari, Y. Plaque calcification during atherosclerosis progression and regression. J. Atheroscler. Thromb. 2018, 25, 294–303. [Google Scholar] [CrossRef]
- Lai, R.; Ju, J.; Lin, Q.; Xu, H. Coronary artery calcification under statin therapy and its effect on cardiovascular outcomes: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2020, 7, 600497. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P. Arterial stiffness and hypertension in the elderly. Front. Cardiovasc. Med. 2020, 7, 544302. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.T.; Charakida, M.; Georgiopoulos, G.; Dangardt, F.; Wade, K.H.; Rapala, A.; Bhowruth, D.J.; Nguyen, H.C.; Muthurangu, V.; Shroff, R.; et al. Determinants of intima-media thickness in the young: The ALSPAC Study. JACC Cardiovasc. Imaging 2021, 14, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Kozakova, M.; Palombo, C.; Paterni, M.; Anderwald, C.H.; Konrad, T.; Colgan, M.P.; Flyvbjerg, A.; Dekker, J. Relationship between Insulin Sensitivity Cardiovascular Risk Investigators. Body composition and common carotid artery remodeling in a healthy population. J. Clin. Endocrinol. Metab. 2008, 93, 3325–3332. [Google Scholar] [CrossRef] [PubMed]
- Bautista, L.E. Blood pressure-lowering effects of statins: Who benefits? J. Hypertens. 2009, 27, 1478–1484. [Google Scholar] [CrossRef]
- Briasoulis, A.; Agarwal, V.; Valachis, A.; Messerli, F.H. Antihypertensive effects of statins: A meta-analysis of prospective controlled studies. J. Clin. Hypertens. 2013, 15, 310–320. [Google Scholar] [CrossRef]
- Chien, K.L.; Tu, Y.K.; Hsu, H.C.; Su, T.C.; Lin, H.J.; Chen, M.F.; Lee, Y.T. Differential effects of the changes of LDL cholesterol and systolic blood pressure on the risk of carotid artery atherosclerosis. BMC Cardiovasc. Disord. 2012, 12, 66. [Google Scholar] [CrossRef]
- Steene-Johannessen, J.; Kolle, E.; Reseland, J.E.; Anderssen, S.A.; Andersen, L.B. Waist circumference is related to low-grade inflammation in youth. Int. J. Pediatr. Obes. 2010, 5, 313–319. [Google Scholar] [CrossRef]
- Ackermann, D.; Jones, J.; Barona, J.; Calle, M.C.; Kim, J.E.; LaPia, B.; Volek, J.S.; McIntosh, M.; Kalynych, C.; Najm, W.; et al. Waist circumference is positively correlated with markers of inflammation and negatively with adiponectin in women with metabolic syndrome. Nutr. Res. 2011, 3, 197–204. [Google Scholar] [CrossRef]
- Pirro, M.; Schillaci, G.; Savarese, G.; Gemelli, F.; Vaudo, G.; Siepi, D.; Bagaglia, F.; Mannarino, E. Low-grade systemic inflammation impairs arterial stiffness in newly diagnosed hypercholesterolaemia. Eur. J. Clin. Investig. 2004, 34, 335–341. [Google Scholar] [CrossRef]
- Dahlén, E.M.; Tengblad, A.; Länne, T.; Clinchy, B.; Ernerudh, J.; Nystrom, F.H.; Östgren, C.J. Abdominal obesity and low-grade systemic inflammation as markers of subclinical organ damage in type 2 diabetes. Diabetes Metab. 2014, 40, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Gariepy, J.; Salomon, J.; Denarié, N.; Laskri, F.; Mégnien, J.L.; Levenson, J.; Simon, A. Sex and topographic differences in associations between large-artery wall thickness and coronary risk profile in a French working cohort: The AXA Study. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Gariepy, J.; Simon, A.; Massonneau, M.; Linhart, A.; Levenson, J. Wall thickening of carotid and femoral arteries in male subjects with isolated hypercholesterolemia. PCVMETRA Group. Prevention Cardio-Vasculaire en Medecine du Travail. Atherosclerosis 1995, 113, 141–151. [Google Scholar] [CrossRef] [PubMed]
- de Sauvage Nolting, P.R.; de Groot, E.; Zwinderman, A.H.; Buirma, R.J.; Trip, M.D.; Kastelein, J.J. Regression of carotid and femoral artery intima-media thickness in familial hypercholesterolemia: Treatment with simvastatin. Arch. Intern. Med. 2003, 163, 1837–1841. [Google Scholar] [CrossRef]
- de Groot, E.; Jukema, J.W.; Montauban van Swijndregt, A.D.; Zwinderman, A.H.; Ackerstaff, R.G.; van der Steen, A.F.; Bom, N.; Lie, K.I.; Bruschke, A.V. B-mode ultrasound assessment of pravastatin treatment effect on carotid and femoral artery walls and its correlations with coronary arteriographic findings: A report of the Regression Growth Evaluation Statin Study (REGRESS). J. Am. Coll. Cardiol. 1998, 31, 1561–1567. [Google Scholar] [CrossRef]
| Mean ± SD, Median [IR], n (%) | Range | |
|---|---|---|
| Male: Female | 119 (46):141 (54) | |
| Age (years) | 63 ± 6 | 50–74 |
| BMI (kg/m2) | 26.9 ± 3.9 | 17.3–51.7 |
| Waist circumference (cm) | 95 ± 11 | 67–147 |
| Systolic BP (mmHg) | 136 ± 14 | 105–180 |
| Pulse pressure (mmHg) | 61 ± 13 | 30–95 |
| Total cholesterol (mmo/L) | 5.45 ± 0.78 | 3.28–6.88 |
| LDL-cholesterol (mmo/L) | 3.42 ± 0.6 | 1.66–4.85 |
| LDL-C O:NO:BH: H | 29 (11):93 (36):95 (36):43 (17) | |
| HDL-cholesterol (mmo/L) | 1.58 ± 0.37 | 0.89–2.64 |
| Triglycerides (mmo/L) | 0.89 [0.75] | 0.23–3.89 |
| Fasting glucose (mmo/L) | 5.36 ± 0.49 | 4.44–6.70 |
| Current smoking (yes) | 43 (17) | |
| Hypertension (yes) | 113 (43) | |
| Hypertensive treatment (yes) | 38 (15) |
| Mean ± SD, n (%) | Range | |
|---|---|---|
| CAC = 0 | 182 (70) | |
| CAC score > 100 | 36 (14) | |
| CCA IMT (microns) | 731 ± 135 | 459–1202 |
| CCA IMT > 95th percentile | 49 (19) | |
| CCA DC (10−3kPa−1) | 14.2 ± 4.5 | 4.31–30.2 |
| CCA DC < 5th percentile | 15 (6) | |
| CFA IMT (microns) | 736 ± 202 | 340–1671 |
| CFA IMT > 95th percentile | 25 (10) | |
| cfPWV (m/s) (n = 182) | 9.4 ± 2.4 | 4.1–23.6 |
| cfPWV > 95th percentile (n = 182) | 27 (15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozakova, M.; Morizzo, C.; Jamagidze, G.; Della Latta, D.; Chiappino, S.; Chiappino, D.; Palombo, C. Association between Low-Density Lipoprotein Cholesterol and Vascular Biomarkers in Primary Prevention. Biomedicines 2023, 11, 1753. https://doi.org/10.3390/biomedicines11061753
Kozakova M, Morizzo C, Jamagidze G, Della Latta D, Chiappino S, Chiappino D, Palombo C. Association between Low-Density Lipoprotein Cholesterol and Vascular Biomarkers in Primary Prevention. Biomedicines. 2023; 11(6):1753. https://doi.org/10.3390/biomedicines11061753
Chicago/Turabian StyleKozakova, Michaela, Carmela Morizzo, Giuli Jamagidze, Daniele Della Latta, Sara Chiappino, Dante Chiappino, and Carlo Palombo. 2023. "Association between Low-Density Lipoprotein Cholesterol and Vascular Biomarkers in Primary Prevention" Biomedicines 11, no. 6: 1753. https://doi.org/10.3390/biomedicines11061753
APA StyleKozakova, M., Morizzo, C., Jamagidze, G., Della Latta, D., Chiappino, S., Chiappino, D., & Palombo, C. (2023). Association between Low-Density Lipoprotein Cholesterol and Vascular Biomarkers in Primary Prevention. Biomedicines, 11(6), 1753. https://doi.org/10.3390/biomedicines11061753