Nanomedicine in the Management of Alzheimer’s Disease: State-of-the-Art
Abstract
1. Introduction
2. Conventional Therapeutic Approaches and Their Limitations
3. Application of Nanomedicines in the Management of Alzheimer’s
4. Conclusions
5. Future Prospective
Author Contributions
Funding
Conflicts of Interest
References
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2006, 366, 2112–2117. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G. A Century of Alzheimer’s Disease. Science 2006, 314, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Sloane, P.D.; Zimmerman, S.; Suchindran, C.; Reed, P.; Wang, L.; Boustani, M.; Sudha, S. The Public Health Impact of Alzheimer’s Disease, 2000–2050: Potential Implication of Treatment Advances. Annu. Rev. Public Health 2002, 23, 213–231. [Google Scholar] [CrossRef]
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimer’s Dement. 2022, 19, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Arya, M.; Kaithwas, G.; Singh, G.; Saraf, S.A. Sucrose stearate as a biosurfactant for development of rivastigmine containing nanostructured lipid carriers and assessment of its activity against dementia in C. elegans model. J. Drug Deliv. Sci. Technol. 2018, 49, 219–226. [Google Scholar] [CrossRef]
- Montine, T.J.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2011, 123, 1–11. [Google Scholar] [CrossRef]
- Murman, D.L.; Colenda, C.C. The Economic Impact of Neuropsychiatric Symptoms in Alzheimer’s Disease. Pharmacoeconomics 2005, 23, 227–242. [Google Scholar] [CrossRef]
- Oesterling, B.M.; Gulati, A.; Joshi, M.D. Nanocarrier-Based Approaches for Treatment and Detection of Alzheimer’s Disease. J. Nanosci. Nanotechnol. 2014, 14, 137–156. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Haque, S.E.; Iqubal, A.; Iqubal, M.K.; Fazal, S.A.; Pottoo, F.H. Nutraceuticals and their Derived Nano-Formulations for the Prevention and Treatment of Alzheimer’s Disease. Curr. Mol. Pharmacol. 2021, 15, 23–50. [Google Scholar] [CrossRef]
- Uddin, S.; Kabir, T.; Jeandet, P.; Mathew, B.; Ashraf, G.M.; Perveen, A.; Bin-Jumah, M.N.; Mousa, S.A.; Abdel-Daim, M.M. Novel Anti-Alzheimer’s Therapeutic Molecules Targeting Amyloid Precursor Protein Processing. Oxidative Med. Cell. Longev. 2020, 2020, 7039138. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lin, B.; Shao, W.; Zhu, Z.; Ji, T.; Yang, C. In Vitro and In Vivo Studies on the Transport of PEGylated Silica Nanoparticles across the Blood–Brain Barrier. ACS Appl. Mater. Interfaces 2014, 6, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Masserini, M. Nanoparticles for Brain Drug Delivery. ISRN Biochem. 2013, 2013, 238428. [Google Scholar] [CrossRef] [PubMed]
- Mikitsh, J.L.; Chacko, A.-M. Pathways for Small Molecule Delivery to the Central Nervous System across the Blood-Brain Barrier. Perspect. Med. Chem. 2014, 6, 11–24. [Google Scholar] [CrossRef]
- Al Asmari, A.K.; Ullah, Z.; Tariq, M.; Fatani, A. Preparation, characterization, and In Vivo evaluation of intranasally administered liposomal formulation of donepezil. Drug Des. Dev. Ther. 2016, 10, 205–215. [Google Scholar] [CrossRef]
- Patel, T.; Zhou, J.; Piepmeier, J.M.; Saltzman, W.M. Polymeric nanoparticles for drug delivery to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 701–705. [Google Scholar] [CrossRef]
- Tosi, G.; Bortot, B.; Ruozi, B.; Dolcetta, D.; Vandelli, M.; Forni, F.; Severini, G. Potential Use of Polymeric Nanoparticles for Drug Delivery across the Blood-Brain Barrier. Curr. Med. Chem. 2013, 20, 2212–2225. [Google Scholar] [CrossRef]
- Xu, Y.; Du, Y. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int. J. Pharm. 2002, 250, 215–226. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Mutlu, G.; Okur, N.Ü. Alzheimer’s Disease and its Related Dementia Types: A Review on Their Management via Nanotechnology Based Therapeutic Strategies. Curr. Alzheimer Res. 2021, 17, 1239–1261. [Google Scholar] [CrossRef]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef]
- Olazarán, J.; Reisberg, B.; Clare, L.; Cruz, I.; Peña-Casanova, J.; Del Ser, T.; Woods, B.; Beck, C.; Auer, S.; Lai, C.; et al. Nonpharmacological Therapies in Alzheimer’s Disease: A Systematic Review of Efficacy. Dement. Geriatr. Cogn. Disord. 2010, 30, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Harilal, S.; Jose, J.; Parambi, D.G.T.; Kumar, R.; Mathew, G.E.; Uddin, S.; Kim, H.; Mathew, B. Advancements in nanotherapeutics for Alzheimer’s disease: Current perspectives. J. Pharm. Pharmacol. 2019, 71, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging carriers for drug delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef]
- Revi, M. Alzheimer’s Disease Therapeutic Approaches. In GeNeDis 2018: Genetics and Neurodegeneration; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 105–116. [Google Scholar] [CrossRef]
- Khalin, I.; Alyautdin, R.; Ismail, N.M.; Haron, M.H.; Kuznetsov, D. Nanoscale drug delivery systems and the blood–brain barrier. Int. J. Nanomed. 2014, 9, 795–811. [Google Scholar] [CrossRef] [PubMed]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef]
- Ling, T.S.; Chandrasegaran, S.; Xuan, L.Z.; Suan, T.L.; Elaine, E.; Nathan, D.V.; Chai, Y.H.; Gunasekaran, B.; Salvamani, S. The Potential Benefits of Nanotechnology in Treating Alzheimer’s Disease. BioMed Res. Int. 2021, 2021, 5550938. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Ayaz, M.; Ovais, M.; Ahmad, I.; Sadiq, A.; Khalil, A.T.; Ullah, F. Biosynthesized metal nanoparticles as potential Alzheimer’s disease therapeutics. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 31–42. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, N.; Yang, X.; Ling, G.; Zhang, P. The roles of gold nanoparticles in the detection of amyloid-β peptide for Alzheimer’s disease. Colloid Interface Sci. Commun. 2022, 46, 100579. [Google Scholar] [CrossRef]
- Hou, K.; Zhao, J.; Wang, H.; Li, B.; Li, K.; Shi, X.; Wan, K.; Ai, J.; Lv, J.; Wang, D.; et al. Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of Alzheimer’s disease. Nat. Commun. 2020, 11, 4790. [Google Scholar] [CrossRef]
- Liu, X.-G.; Zhang, L.; Lu, S.; Liu, D.-Q.; Huang, Y.-R.; Zhu, J.; Zhou, W.-W.; Yu, X.-L.; Liu, R.-T. Superparamagnetic iron oxide nanoparticles conjugated with Aβ oligomer-specific scFv antibody and class A scavenger receptor activator show therapeutic potentials for Alzheimer’s Disease. J. Nanobiotechnology 2020, 18, 160. [Google Scholar] [CrossRef]
- Yang, X.; He, C.; Li, J.; Chen, H.; Ma, Q.; Sui, X.; Tian, S.; Ying, M.; Zhang, Q.; Luo, Y.; et al. Uptake of silica nanoparticles: Neurotoxicity and Alzheimer-like pathology in human SK-N-SH and mouse neuro2a neuroblastoma cells. Toxicol. Lett. 2014, 229, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wan, X.; Zheng, X.; Shao, X.; Liu, Q.; Zhang, Q.; Qian, Y. Dual-functional nanoparticles targeting amyloid plaques in the brains of Alzheimer’s disease mice. Biomaterials 2014, 35, 456–465. [Google Scholar] [CrossRef]
- Javidi, S.; Razavi, B.M.; Hosseinzadeh, H. A review of Neuropharmacology Effects of Nigella sativa and Its Main Component, Thymoquinone. Phytotherapy Res. 2016, 30, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Yang, L.; Liu, Y.; Zhou, X.; Sun, J.; Liu, J. Sialic acid (SA)-modified selenium nanoparticles coated with a high blood–brain barrier permeability peptide-B6 peptide for potential use in Alzheimer’s disease. Acta Biomater. 2015, 25, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Zhang, Y.; Jin, X.; Li, Y.; Zhang, L. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer’s disease. J. Photochem. Photobiol. B Biol. 2019, 190, 98–102. [Google Scholar] [CrossRef]
- Kwon, H.J.; Cha, M.-Y.; Kim, D.; Kim, D.K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer’s Disease. ACS Nano 2016, 10, 2860–2870. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Tiseo, P.J.; Perdomo, C.; Friedhoff, L.T. Metabolism and elimination of 14C-donepezil in healthy volunteers: A single-dose study. Br. J. Clin. Pharmacol. 1998, 46, 19–24. [Google Scholar] [CrossRef]
- Shaygannejad, V.; Janghorbani, M.; Ashtari, F.; Zanjani, H.A.; Zakizade, N. Effects of Rivastigmine on Memory and Cognition in Multiple Sclerosis. Can. J. Neurol. Sci. 2008, 35, 476–481. [Google Scholar] [CrossRef]
- Topal, G.R.; Mészáros, M.; Porkoláb, G.; Szecskó, A.; Polgár, T.F.; Siklós, L.; Deli, M.A.; Veszelka, S.; Bozkir, A. ApoE-Targeting Increases the Transfer of Solid Lipid Nanoparticles with Donepezil Cargo across a Culture Model of the Blood–Brain Barrier. Pharmaceutics 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 14, 4449–4460. [Google Scholar] [CrossRef] [PubMed]
- Almuhayawi, M.S.; Ramadan, W.S.; Harakeh, S.; Al Jaouni, S.K.; Bharali, D.J.; Mousa, S.A.; Almuhayawi, S.M. The potential role of pomegranate and its nano-formulations on cerebral neurons in aluminum chloride induced Alzheimer rat model. Saudi J. Biol. Sci. 2020, 27, 1710–1716. [Google Scholar] [CrossRef]
- Sathya, S.; Shanmuganathan, B.; Manirathinam, G.; Ruckmani, K.; Devi, K.P. α-Bisabolol loaded solid lipid nanoparticles attenuates Aβ aggregation and protects Neuro-2a cells from Aβ induced neurotoxicity. J. Mol. Liq. 2018, 264, 431–441. [Google Scholar] [CrossRef]
- Dara, T.; Vatanara, A.; Sharifzadeh, M.; Khani, S.; Vakilinezhad, M.A.; Vakhshiteh, F.; Meybodi, M.N.; Malvajerd, S.S.; Hassani, S.; Mosaddegh, M.H. Improvement of memory deficits in the rat model of Alzheimer’s disease by erythropoietin-loaded solid lipid nanoparticles. Neurobiol. Learn. Mem. 2019, 166, 107082. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Re, F.; Canovi, M.; Beeg, M.; Gregori, M.; Sesana, S.; Sonnino, S.; Brogioli, D.; Musicanti, C.; Gasco, P.; et al. Lipid-based nanoparticles with high binding affinity for amyloid-β1–42 peptide. Biomaterials 2010, 31, 6519–6529. [Google Scholar] [CrossRef]
- Plaza-Oliver, M.; Santander-Ortega, M.J.; Lozano, M.V. Current approaches in lipid-based nanocarriers for oral drug delivery. Drug Deliv. Transl. Res. 2021, 11, 471–497. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Moreira, J.N.; Lobo, J.M.S.; Silva, A.C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of In Vivo studies. Acta Pharm. Sin. B 2021, 11, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Gupta, U.; Ujjwal, R.R.; Yadav, A.K. Nano-lipidic formulation and therapeutic strategies for Alzheimer’s disease via intranasal route. J. Microencapsul. 2021, 38, 572–593. [Google Scholar] [CrossRef] [PubMed]
- Zorkina, Y.; Abramova, O.; Ushakova, V.; Morozova, A.; Zubkov, E.; Valikhov, M.; Melnikov, P.; Majouga, A.; Chekhonin, V. Nano Carrier Drug Delivery Systems for the Treatment of Neuropsychiatric Disorders: Advantages and Limitations. Molecules. 2020, 25, 5294. [Google Scholar] [CrossRef]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Malvajerd, S.S.; Azadi, A.; Izadi, Z.; Kurd, M.; Dara, T.; Dibaei, M.; Zadeh, M.S.; Javar, H.A.; Hamidi, M. Brain Delivery of Curcumin Using Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Preparation, Optimization, and Pharmacokinetic Evaluation. ACS Chem. Neurosci. 2019, 10, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Malvajerd, S.S.; Izadi, Z.; Azadi, A.; Kurd, M.; Derakhshankhah, H.; Zadeh, M.S.; Javar, H.A.; Hamidi, M. Neuroprotective Potential of Curcumin-Loaded Nanostructured Lipid Carrier in an Animal Model of Alzheimer’s Disease: Behavioral and Biochemical Evidence. J. Alzheimer’s Dis. 2019, 69, 671–686. [Google Scholar] [CrossRef]
- Bernardi, A.; Frozza, R.L.; Meneghetti, A.; Hoppe, J.B.; Battastini, A.M.O.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C.G. Indomethacin-loaded lipid-core nanocapsules reduce the damage triggered by Aβ1-42 in Alzheimer’s disease models. Int. J. Nanomed. 2012, 7, 4927–4942. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin Loaded-PLGA Nanoparticles Conjugated with Tet-1 Peptide for Potential Use in Alzheimer’s Disease. PLoS ONE 2012, 7, e32616. [Google Scholar] [CrossRef]
- Jeon, S.G.; Cha, M.-Y.; Kim, J.-I.; Hwang, T.W.; Kim, K.A.; Kim, T.H.; Song, K.C.; Kim, J.-J.; Moon, M. Vitamin D-binding protein-loaded PLGA nanoparticles suppress Alzheimer’s disease-related pathology in 5XFAD mice. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 297–307. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, A.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z.; Sun, K. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 705–718. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona-Ule, N.; Silva, A.M.; Souto, E.B.; et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: In Vitro and In Vivo characterization. J. Nanobiotechnol. 2018, 16, 32. [Google Scholar] [CrossRef]
- Vilella, A.; Belletti, D.; Sauer, A.K.; Hagmeyer, S.; Sarowar, T.; Masoni, M.; Stasiak, N.; Mulvihill, J.J.; Ruozi, B.; Forni, F.; et al. Reduced plaque size and inflammation in the APP23 mouse model for Alzheimer’s disease after chronic application of polymeric nanoparticles for CNS targeted zinc delivery. J. Trace Elements Med. Biol. 2018, 49, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, A.S.; Farid, R.M.; ElGamal, S.S. Complexation as an approach to entrap cationic drugs into cationic nanoparticles administered intranasally for Alzheimer’s disease management: Preparation and detection in rat brain. Drug Dev. Ind. Pharm. 2015, 41, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, M.; Alrobaian, M.M.; Alghamdi, S.A.; Warsi, M.H.; Sultana, S.; Khan, R.A. Brain targeted Polysorbate-80 coated PLGA thymoquinone nanoparticles for the treatment of Alzheimer’s disease, with biomechanistic insights. J. Drug Deliv. Sci. Technol. 2021, 61, 102214. [Google Scholar] [CrossRef]
- Sempf, K.; Arrey, T.; Gelperina, S.; Schorge, T.; Meyer, B.; Karas, M.; Kreuter, J. Adsorption of plasma proteins on uncoated PLGA nanoparticles. Eur. J. Pharm. Biopharm. 2013, 85, 53–60. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Martinez, C.S.; Alonso, S.D.V.; Prieto, M.J. Combined Therapy for Alzheimer’s Disease: Tacrine and PAMAM Dendrimers Co-Administration Reduces the Side Effects of the Drug without Modifying its Activity. AAPS PharmSciTech 2020, 21, 110–114. [Google Scholar] [CrossRef]
- Ni, Y.-N.; Kong, L.; Li, X.-T.; Xiao, H.-H.; Wu, Y.-T.; Liang, X.-C.; Lin, Y.; Li, W.-Y.; Deng, Y.; Li, Y.; et al. Multifunctional osthole liposomes and brain targeting functionality with potential applications in a mouse model of Alzheimer’s disease. J. Liposome Res. 2020, 31, 267–278. [Google Scholar] [CrossRef]
- Arumugam, K.; Subramanian, G.; Mallayasamy, S.; Averineni, R.; Reddy, M.; Udupa, N. A study of rivastigmine liposomes for delivery into the brain through intranasal route. Acta Pharm. 2008, 58, 287–297. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Luo, G. Ligustrazine Phosphate Ethosomes for Treatment of Alzheimer’s Disease, In Vitro and in Animal Model Studies. AAPS PharmSciTech 2012, 13, 485–492. [Google Scholar] [CrossRef]
- Froelich, A.; Osmałek, T.; Jadach, B.; Puri, V.; Michniak-Kohn, B. Microemulsion-Based Media in Nose-to-Brain Drug Delivery. Pharmaceutics 2021, 13, 201. [Google Scholar] [CrossRef]
- Wen, M.M.; Ismail, N.I.K.; Nasra, M.M.A.; El-Kamel, A.H. Repurposing ibuprofen-loaded microemulsion for the management of Alzheimer’s disease: Evidence of potential intranasal brain targeting. Drug Deliv. 2021, 28, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
- Jogani, V.V.; Shah, P.J.; Mishra, P.; Mishra, A.K.; Misra, A. Intranasal Mucoadhesive Microemulsion of Tacrine to Improve Brain Targeting. Alzheimer Dis. Assoc. Disord. 2008, 22, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Cong, W.; Wang, Y.; Liu, Q.; Luo, G. Microemulsion-based patch for transdermal delivery of huperzine A and ligustrazine phosphate in treatment of Alzheimer’s disease. Drug Dev. Ind. Pharm. 2011, 38, 752–761. [Google Scholar] [CrossRef]
- Md, S.; Gan, S.Y.; Haw, Y.H.; Ho, C.L.; Wong, S.; Choudhury, H. In Vitro neuroprotective effects of naringenin nanoemulsion against β-amyloid toxicity through the regulation of amyloidogenesis and tau phosphorylation. Int. J. Biol. Macromol. 2018, 118, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Nigam, K.; Srivastava, S.; Tyagi, A.; Dang, S. Memantine nanoemulsion: A new approach to treat Alzheimer’s disease. J. Microencapsul. 2020, 37, 355–365. [Google Scholar] [CrossRef]
- Kaur, A.; Nigam, K.; Bhatnagar, I.; Sukhpal, H.; Awasthy, S.; Shankar, S.; Tyagi, A.; Dang, S. Treatment of Alzheimer’s diseases using donepezil nanoemulsion: An intranasal approach. Drug Deliv. Transl. Res. 2020, 10, 1862–1875. [Google Scholar] [CrossRef]
- Xie, Z.; Liao, Q.; Xu, X.; Yao, M.; Wan, J.; Liu, D. Rapid and sensitive determination of donepezil in human plasma by liquid chromatography/tandem mass spectrometry: Application to a pharmacokinetic study. Rapid Commun. Mass Spectrom. 2006, 20, 3193–3198. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Zueva, I.V.; Petrov, K.A.; Lukashenko, S.S.; Nizameev, I.R.; Kulik, N.V.; Voloshina, A.D.; Almasy, L.; Kadirov, M.K.; Masson, P.; et al. Mixed cationic liposomes for brain delivery of drugs by the intranasal route: The acetylcholinesterase reactivator 2-PAM as encapsulated drug model. Colloids Surf. B Biointerfaces 2018, 171, 358–367. [Google Scholar] [CrossRef]
- Sharma, A. Liposomes in drug delivery: Progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Rocha, S. Targeted Drug Delivery Across the Blood Brain Barrier in Alzheimer’s Disease. Curr. Pharm. Des. 2013, 19, 6635–6646. [Google Scholar] [CrossRef] [PubMed]
- Régina, A.; Demeule, M.; Ché, C.; Lavallée, I.; Poirier, J.; Gabathuler, R.; Béliveau, R.; Castaigne, J.-P. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br. J. Pharmacol. 2008, 155, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Shao, X.; Zhang, C.; Tan, Y.; Liu, Q.; Wan, X.; Zhang, Q.; Xu, S.; Jiang, X. Intranasal H102 Peptide-Loaded Liposomes for Brain Delivery to Treat Alzheimer’s Disease. Pharm. Res. 2015, 32, 3837–3849. [Google Scholar] [CrossRef] [PubMed]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, In Vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Vasava, P.; Khan, S.L.; Siddiqui, F.A.; Islam, F.; Chopra, H.; Bin Emran, T. Ethosomes: A novel drug carrier. Ann. Med. Surg. 2022, 82, 104595. [Google Scholar] [CrossRef]
- Ghalamfarsa, G.; Hojjat-Farsangi, M.; Mohammadnia-Afrouzi, M.; Anvari, E.; Farhadi, S.; Yousefi, M.; Jadidi-Niaragh, F. Application of nanomedicine for crossing the blood–brain barrier: Theranostic opportunities in multiple sclerosis. J. Immunotoxicol. 2016, 13, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Gong, E.J.; Park, H.R.; Kim, M.E.; Piao, S.; Lee, E.; Jo, D.-G.; Chung, H.Y.; Ha, N.-C.; Mattson, M.P.; Lee, J. Morin attenuates tau hyperphosphorylation by inhibiting GSK3β. Neurobiol. Dis. 2011, 44, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Talegaonkar, S.; Azeem, A.; Ahmad, F.; Khar, R.; Pathan, S.; Khan, Z. Microemulsions: A Novel Approach to Enhanced Drug Delivery. Recent Patents Drug Deliv. Formul. 2008, 2, 238–257. [Google Scholar] [CrossRef]
- Fortuna, A.; Alves, G.; Serralheiro, A.; Sousa, J.; Falcão, A. Intranasal delivery of systemic-acting drugs: Small-molecules and biomacromolecules. Eur. J. Pharm. Biopharm. 2014, 88, 8–27. [Google Scholar] [CrossRef]
- Imbimbo, B.P.; Solfrizzi, V.; Panza, F. Are NSAIDs useful to treat Alzheimer’s disease or mild cognitive impairment? Front. Aging Neurosci. 2010, 2, 19. [Google Scholar] [CrossRef]
- Patel, P.A.; Patil, S.C.; Kalaria, D.R.; Kalia, Y.N.; Patravale, V.B. Comparative In Vitro and In Vivo evaluation of lipid based nanocarriers of Huperzine A. Int. J. Pharm. 2013, 446, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Nirale, P.; Paul, A.; Yadav, K.S. Nanoemulsions for targeting the neurodegenerative diseases: Alzheimer’s, Parkinson’s and Prion’s. Life Sci. 2020, 245, 117394. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.P.; Pawara, D.D.; Gudewar, C.S.; Tekade, A.R. Nanostructured cubosomes in an in situ nasal gel system: An alternative approach for the controlled delivery of donepezil HCl to brain. J. Liposome Res. 2018, 29, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
| Topic | Information | References |
|---|---|---|
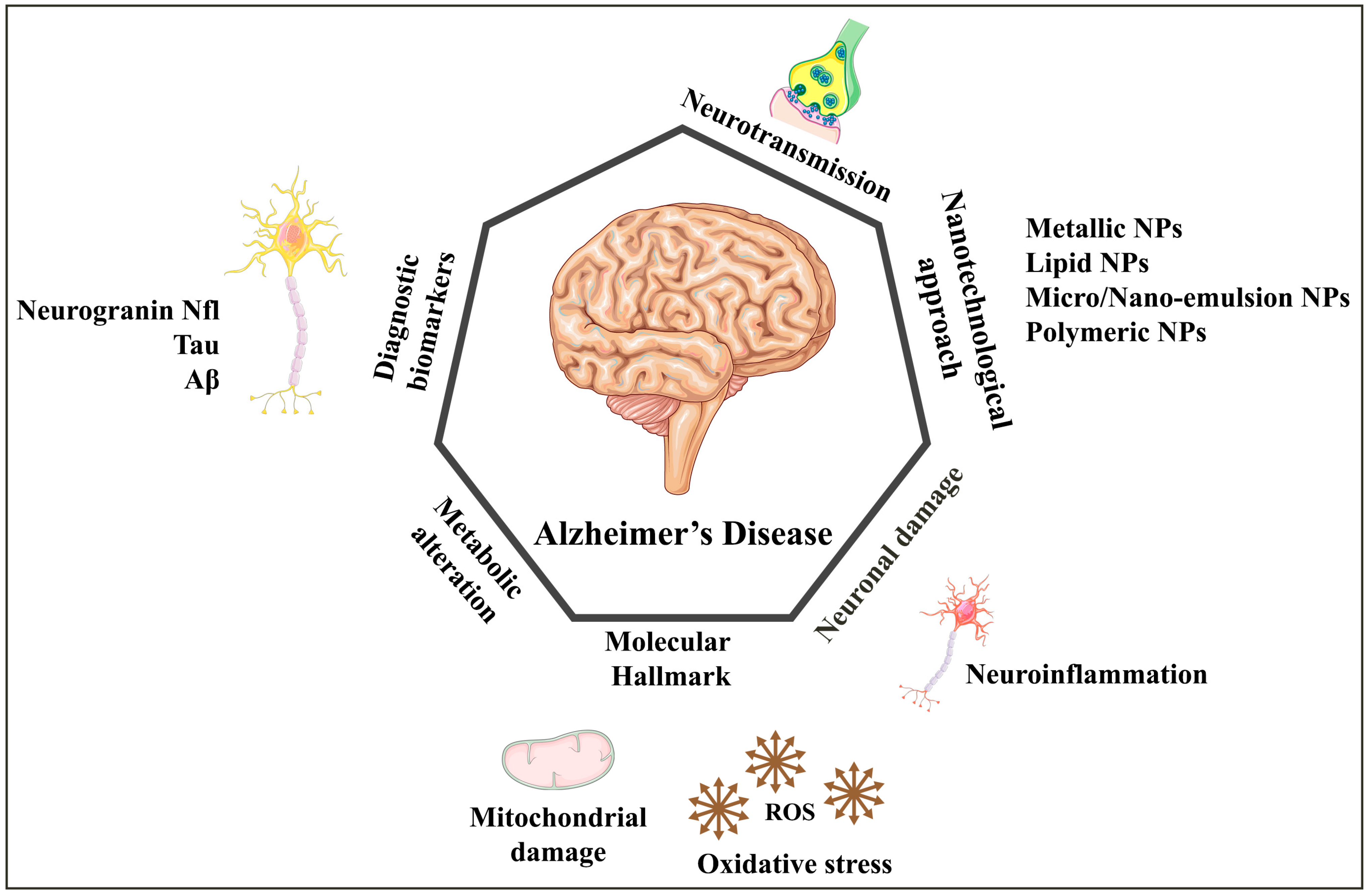
| Alzheimer’s disease (AD) | Progressive neurological condition that causes irreversible dementia syndrome. Incidence increases with age: currently affects 32.6 million people worldwide. Expected to reach 78 million by 2030 and 139 million by 2050 without new treatments. | [1,4] |
| Pathology of AD | Caused by extracellular aggregation of amyloid beta (Aβ) plaques and accumulation of intraneuronal neurofibrillary tangles of tau (τ) protein | [7] |
| Risk factors for AD | Associated with cardiovascular risk factors such as hypertension, diabetes, atherogenic dyslipidaemia, and obesity—environmental exposure to toxicants, genetic factors, mutation, trauma, and metabolic diseases can also cause AD. | [8,9] |
| Blood–brain barrier (BBB) | It is one of the most challenging physiological barriers. The drug movement in the brain parenchyma is obstructed by a physical interface, the BBB, present between the central nervous system (CNS) and peripheral circulation. Lipophilic molecules smaller than 400 Da can cross the BBB. | [12] |
| Conventional treatment of AD | Non-pharmacological and pharmacological approaches: Non-pharmacological approaches such as sleep, physical activity, and music therapy can also help. Symptomatic treatment with NMDA receptor antagonists and cholinesterase inhibitors (CHEIs) are common pharmacological approaches. | [20] |
| Food and Drug Administration (FDA)-approved drugs for AD | Aduhelm (aducanumab-avwa), Aricept (donepezil hydrochloride), Excelon patch (rivastigmine transdermal system), Namenda (memantine HCl), Namzaric (memantine hydrochloride extended-release + donepezil hydrochloride), and Razadyne (galantamine hydrobromide). | [21,22] |
| Current treatments for AD | Only reduce symptoms. Temporarily enhance cognitive abilities. Lack brain specialization and cause adverse effects. | [22] |
| Limitations of conventional treatment | First-pass metabolism and unfavorable pharmacokinetics—lower bioavailability and high dosage requirements. Physicochemical properties can affect drug effectiveness: bioactives may have suboptimal therapeutic effects via the oral route. | [23,24] |
| Nanotechnology and AD | Nanotechnology can aid in early detection and drug development for AD. Nanocarriers have advantages over conventional treatment. Drug-loaded nanocarriers can improve drug delivery to the brain across the BBB. Further details are described in Table 2. | [25] |
| Drugs | Nanocarriers | Outcomes/Benefits | References |
|---|---|---|---|
| DPL | Liposome | Donepezil via oral drug delivery cannot cross BBB. Donepezil-loaded liposome are made and intranasally administered to rapidly cross the BBB and shows improved bioavailability and reduces the systemic toxicity of it against AD. | [15] |
| A oligomer-specific scFv-AbW20 | superparamagnetic iron oxide NPs (SPIONs) | This study found have promise results against AD and exceptional early diagnostic potential. | [32] |
| Silicon dioxide | Silicon nanoparticle | Significantly induced cellular apoptosis, elevate the level of intracellular ROS in dose-dependent manner, decrease the cell viability, enhanced phosphorylation of tau at Ser262 and Ser396. | [33] |
| Sialic acid | Selenium (Se)-NPs | The loading of Sialic acid into Se-NPs increase the permeation through BBB and reduces the aggregation of Aβ in the animal model of AD. | [36] |
| Curcumin | Selenium NPs encapsulated into PLGA nanospheres | This study found strong inhibition against Aβ aggregation and can be used as targeted drug delivery in treating AD. | [37] |
| Triphenyl phosphonium | Cerium nanoparticles | TPP-ceria NPs effectively penetrate mitochondria to scavenge ROS to reduce oxidative stress. | [38] |
| Donepezil (DPL) | SLNs | Intranasal administration of DP-SLNs significantly increase the concentration of drug in brain over the i.v. administration of DPL solution. Further, the scintigraphy study observed localization of DPL-SLNs into the rabbit’s brain. | [43] |
| Pomegranate extract | LNPs | This study found higher antioxidant effects and decreased NFTs and Aβ deposition in the aluminium chloride-induced rat model of AD. | [45] |
| α-Bisabolol | LNPs | Protect the neuro-2a cells from inhibited Aβ aggregation and Aβ induced neurotoxicity | [46] |
| Erythropoietin (EPO) | Solid lipid nanoparticles (SLNs) | Erythropoietin (EPO) helps neuronal survival and regulates AD, but very limited BBB permeation, due to hydrophilicity and high molecular weight and rapid clearance from the blood stream. EPO-encapsulated SLNs overcomes abovementioned issues and decrease oxidative stress and Aβ deposition and show increased spatial memory. | [47] |
| Curcumin | NLCs | This study found enhanced curcumin bioavailability in the brain and reduces the hallmark of Aβ in AD | [51] |
| Curcumin | SLNs | The study found to reduces the behavioural dysfunction and reverses several neurotransmitters into the brain against animal model of AD. | [55] |
| Curcumin | Liposomes | It can deliver the drug to CNS, permeate the BBB, and show better anti-Alzheimer’s effect in animal model. | [56] |
| Indomethacin (Ind) | Lipid nano capsules (LNCs) | This study has been investigated that Ind loaded LNCs inhibit neuroinflammation induced by Aβ1-42 in organotypic hippocampal cultures and decrease A-induced cell death in AD animal model | [57] |
| Vitamin D | PLGA-NPs | Vitamin D observed neuroprotective effect, but poor solubility and bioavailability. The vitamin D loaded PLGA-NPs studied on murine AD model, results to decreased neuronal apoptosis and enhanced cognitive function was observed. | [60] |
| Huperzine A | PLGA-NPs | Huperzine A was loaded into PLGA-NP conjugated with lactoferrin, showed enhanced release kinetics, and significantly decreased AD symptoms. | [61] |
| Memantine | Polymer-based NPs (PBNPs) | Memantine loaded PBNPs shows effective an anti-inflammatory and anti-Alzheimer’s effect against AD animal model. | [62] |
| Zinc and sitagliptin | PBNPs | It shows improved cognitive dysfunction and reduced neuroinflammation when studied for their anti-Alzheimer effect against AD animal model. | [63] |
| Thymoquinone (TQ) | PLGA-NPs | TQ-containing PLGA NPs with polysorbate-80 (P-80) could be a safe and effective way to transport NPs across the BBB and into the brain. The PLGA-NPs are shielded from being opsonized and cleared by the body because of the P-80 surfactant coating. TQ works by reducing the production of superoxide radicals primarily through blocking the enzyme xanthine-oxidase. | [66] |
| Tacrine | Dendrimers with a poly (propylene imine) core and a maltose histidine shell (G4HisMal) | Tacrine loaded into generation 4.0 and PAMAM dendrimers has been employed and has improved biocompatibility and reduced the toxicity of the drug used to treat the AD. | [68] |
| Osthole (Coumarin derivative) | Liposome | This study found increased intracellular uptake by APP-SH-SY5Y cells and exerted a cytoprotective effect. Prolonged the cycle time and elevate the accumulation of Osthole in the brain | [69] |
| Rivastigmine | Liposome | Increase the concentration and exposure in the brain. | [70] |
| Ligustrazine phosphate | Ethosome | Drug penetration and deposition significantly higher over the plain drug. | [71] |
| Morin hydrate | ME | The morin hydrate solution given by a parenteral route has several drawbacks, such as safety issues, low patient compliance, and expensive medication. Avoiding the BBB, intranasal delivery of morin hydrate-loaded ME is a potential strategy, and it offers an advantage as it is non-invasive. | [72] |
| Ibuprofen | Microemulsion (ME) | A novel repurposing strategy and route of administration are presented in this study for the treatment of AD. The in vivo result in rats found uptake of a novel ibuprofen loaded ME nearly four times higher than that of the intravenous and ten times than that of the oral administrations. | [73] |
| Tacrine | ME | The intranasal administration of tacrine-loaded ME results in the quickest memory recovery in scopolamine-induced amnesic mice. | [74] |
| Huperzine A | ME | Huperzine A loaded ME improves cognitive function in mice compared to oral suspension. | [75] |
| Naringenin | Nanoemulsion | The study outcome shows that nanoemulsion of naringenin could be used to overcome Aβ neurotoxicity and amyloid genesis. | [76] |
| Memantine | Nanoemulsion | Memantine loaded nanoemulsion using homogenization and ultrasonication was studied for its anti-Alzheimer effect. It was given by intranasal route. This nanoemulsion crosses the BBB and increases the anti-AD effect compared with the conventional dosage form. | [77] |
| DPL | Nanoemulsion | Using labrasol and glycerol at a concentration of 10% w/w, a nanoemulsion containing donepezil hydrochloride was developed. Donepezil hydrochloride nanoemulsion has the potential to treat AD, due to its antioxidant and radical scavenging effects. | [78] |
| Deferoxamine | Nanoemulsion gel | Deferoxamine delivered via nanogels made of chitosan and tripolyphosphate shows an effective therapeutic action against AD. | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mir Najib Ullah, S.N.; Afzal, O.; Altamimi, A.S.A.; Ather, H.; Sultana, S.; Almalki, W.H.; Bharti, P.; Sahoo, A.; Dwivedi, K.; Khan, G.; et al. Nanomedicine in the Management of Alzheimer’s Disease: State-of-the-Art. Biomedicines 2023, 11, 1752. https://doi.org/10.3390/biomedicines11061752
Mir Najib Ullah SN, Afzal O, Altamimi ASA, Ather H, Sultana S, Almalki WH, Bharti P, Sahoo A, Dwivedi K, Khan G, et al. Nanomedicine in the Management of Alzheimer’s Disease: State-of-the-Art. Biomedicines. 2023; 11(6):1752. https://doi.org/10.3390/biomedicines11061752
Chicago/Turabian StyleMir Najib Ullah, Shehla Nasar, Obaid Afzal, Abdulmalik Saleh Alfawaz Altamimi, Hissana Ather, Shaheen Sultana, Waleed H. Almalki, Pragya Bharti, Ankit Sahoo, Khusbu Dwivedi, Gyas Khan, and et al. 2023. "Nanomedicine in the Management of Alzheimer’s Disease: State-of-the-Art" Biomedicines 11, no. 6: 1752. https://doi.org/10.3390/biomedicines11061752
APA StyleMir Najib Ullah, S. N., Afzal, O., Altamimi, A. S. A., Ather, H., Sultana, S., Almalki, W. H., Bharti, P., Sahoo, A., Dwivedi, K., Khan, G., Sultana, S., Alzahrani, A., & Rahman, M. (2023). Nanomedicine in the Management of Alzheimer’s Disease: State-of-the-Art. Biomedicines, 11(6), 1752. https://doi.org/10.3390/biomedicines11061752