Sex Differences in the Anti-Hypertensive Effect of Calcium-Channel Blockers: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
- To study differences and similarities between males and females in the effect of antihypertensive medication on cardiac function and structure.
- To determine the representation of females in studies on the effect of antihypertensive drugs on CVD for the past century.
2.2. Guidelines
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
| Study | Patient | Ethnicity | CCB Treatment(Administration) | Mean Dose (mg/Day) | % Max Dose * | Subjects CCB (n) | Control Group ** | Controls (n) | Age (Years + SD) | Intervention Duration(Days) | Study Design | Extracted Variables | Mentioned Method(s) of Measurement | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | M | F | Total | M | F | ||||||||||||
| Mehlum (2020) [45] | HTN, DM, HF, MI, LVH | W, B, A | Amlodipine (oral) | 5 | 0.5 | 7477 | 4305 | 3172 | Valsartan | 7519 | 4332 | 3187 | 67.2 (8.1) | 180 | RCT | SBP, DBP, HR, MAP | Sphygmomanometry |
| Baysal (2017) [44] | HTN | - | Amlodipine (oral) | 10 | 1 | 38 | 22 | 16 | Telmisartan | 39 | 22 | 17 | 48.0 (10) | 30 | RCT | SBP, DBP | Sphygmomanometry, ECG, echo |
| Thuc Sinh (2015) [43] | HTN, DM | - | Amlodipine (oral) | 10 | 1 | 52 | 34 | 18 | - | - | - | - | 65.3 (10.7) | 28 | RCT | SBP, DBP, | Sphygmomanometry |
| Lercanidipine (oral) | 20 | 1 | 52 | 32 | 20 | ||||||||||||
| Lindqvist (2007) [42] | HTN | - | Amlodipine (oral) | 7.5 | 0.75 | 14 | 11 | 3 | - | - | - | - | 59 (***) | 42 | RCT | HR, CO | Catheterization, ECG, echo |
| Mibefradil (oral) | 75 | 0.75 | 14 | 11 | 3 | ||||||||||||
| Petrella (2000) [53] | HF | - | Verapamil (oral) | 240 | 0.33 | 10 | 10 | 0 | - | - | - | - | 73.0 (4.0) | 26 | Prospective cohort | HR | Echo |
| Burggraaf (1998) [34] | Healthy | - | Nifedipine (oral) | 20 | 0.33 | 9 | 9 | 0 | Captopril | 9 | 9 | - | 18–35 (***) | 0.125 | RCT, crossover | HR, MAP | Echo |
| Gottdiener (1998) [40] | HTN | W, B | Diltiazem (oral) | 240 | 0.67 | 185 | 185 | 0 | Atenolol, captopril, clonidine, hydrochlorothiazide or prazosin | 920 | 920 | 0 | 58.8 (10) | 730 | RCT | SBP, DBP, LVM, HR | Sphygmomanometry |
| Goldsmith (1997) [41] | HF | - | Amlodipine (oral) | 7.5 | 0.75 | 7 | 7 | 0 | Placebo | 7 | 7 | 0 | 56 (***) | 10 | RCT, crossover | MAP, HR | Sphygmo-manometry, echo |
| Tomiyama (1997) [33] | HT | - | Nifedipine (oral) | 30 | 0.5 | 13 | 13 | 0 | Acebutolol | 9 | 9 | 0 | 46 (7) | 1095 | Retrospective cohort | SBP, DBP, LVEF | Sphygmo-manometry, echo |
| Seki (1996) [32] | MI | - | Nifedipine (sublingual) | 10 | 0.17 | 8 | 7 | 1 | - | - | - | - | 63 (10) | 0.021 | Prospective cohort | HR, LVEF | Catheterization |
| Naritomi (1995) [55] | HTN | - | Nitrendipine (oral) | 10 | 0.25 | 10 | 7 | 3 | - | - | - | - | 60.5 (***) | 56 | Prospective cohort | SBP, DBP | Sphygmo-manometry |
| Risoe (1993) [31] | HF, MI | - | Nifedipine (sublingual) | 20 | 0.33 | 8 | 4 | 0 | Untreated | 4 | 4 | 0 | **** | 0.03 | RCT | HR | Catheterization |
| Heywood (1991) [39] | HF, CAD | - | Diltiazem (iv) | 25 | 0.07 | 9 | 9 | 0 | - | - | - | - | 68 (9) | 0.02 | Prospective cohort | SBP, DBP, CO, HR | Sphygmo-manometry, echo, ecg, catheterization |
| Sheiban (1991) [30] | HTN | - | Nifedipine (oral) | 52 | 0.87 | 7 | 7 | 0 | Untreated | 10 | 10 | 0 | 41 (8.1) | 180 | RCT | LVEF | Echo, ecg, sphygmo-manometry |
| Lacidipine (oral) | 5 | 0.83 | 8 | 8 | 0 | ||||||||||||
| Bekheit (1990) [29] | MI | - | Diltiazem (oral) | 180 | 0.5 | 9 | 0 | 0 | Metoprolol | 8 | 8 | 0 | 62 (13) | 6 | RCT | SBP, DBP, HR | Ecg, sphygomomanometry |
| Nifedipine (oral) | 30 | 0.5 | 10 | 0 | 0 | ||||||||||||
| Senda (1990) [38] | HTN, LVH | - | Diltiazem (oral) | 180 | 0.5 | 9 | 6 | 3 | - | - | - | - | 60 (***) | 180 | Prospective cohort | LVEF | Echo, ecg, sphygomomanomatry |
| Setaro (1990) [52] | HF, HTN, CAD, MI, DM | W, B | Verapamil (orally) | 256 | 0.36 | 20 | 20 | 0 | Placebo (4 day washout interval after verapamil) | 20 | 20 | 0 | 68 (5) | 32 | RCT, crossover | SBP, DBP, HR, LVEF | Echo, ECG, sphygomomanometry |
| Binetti (1989) [57] | HF, CAD, | - | Felodipine (iv) | 0.85 | 0.85 | 10 | 10 | 0 | - | - | - | - | 53 (***) | 0.042 | Prospective cohort | SBP, DBP, CO, MAP, HR | Catheterization, ecg, sphygomomanometry |
| Crawford (1989) [28] | HF | - | Nifedipine (oral) | 66 | 1.1 | 10 | 10 | 0 | Digoxin, hydralazin (same patients, after CCB) | 10 | 10 | 0 | 54 (***) | 30 | RCT | SBP, LVEF, HR | Ecg, |
| La Rovere (1989) [50] | MI | - | Nicardipine (iv and oral) | 5 (iv) | 0.01 (iv) | 10 | 10 | 0 | - | - | - | - | 54 (9) | 0.0069 (iv) | Prospective cohort | HR | Ecg, |
| 60 (oral) | 0.17 (oral) | 21 (oral) | |||||||||||||||
| McGrath (1989) [58] | HF | - | Isradipine(oral) | 15 | 0.75 | 9 | 9 | 0 | Placebo | 9 | 9 | 0 | 54 (***) | 84 | RCT | CO, LVEF, HR | Catheterization |
| Szlachcic (1989) [37] | HTN | - | Diltiazem (oral) | 240 | 0.67 | 13 | 13 | 0 | Placebo | 11 | 11 | 0 | 48 (10) | 112 | RCT | SBP, DBP | Echo, sphygomomanometry, ecg |
| Bostr.m (1988) [36] | MI | - | Diltiazem (oral) | 120 | 0.33 | 12 | 12 | 0 | - | - | - | - | 61 (***) | 0.104 | Prospective cohort | SBP, HR | Ecg, sphygomomanometry |
| 180 | 0.5 | 14 | |||||||||||||||
| Fisman (1988) [59] | MI, CAD | - | Gallopamil (oral) | 75 | 0.38 | 9 | 9 | 0 | Placebo | 6 | 6 | 0 | 60.3 (5.5) | 21 | RCT | LVEF | Echo |
| 112.5 | 0.56 | 9 | 9 | 0 | |||||||||||||
| 150 | 0.75 | 8 | 8 | 0 | |||||||||||||
| Mookherjee (1988) [27] | HF | - | Nifedipine (sublingual) | 80 | 1.33 | 12 | 12 | 0 | - | - | - | - | 55–77 (***) | 1 | Prospective cohort | LVEF, HR | Echo, catheterization |
| Burlew (1987) [49] | HF, CAD, HTN | - | Nicardipine (oral) | 75 | 0.21 | 10 | 7 | 3 | - | - | - | - | 54 (***) | 9 | Prospective cohort | HR | Catheterization, ecg |
| Fagard (1987) [56] | HTN | - | Felodipine (oral) | 6 | 0.6 | 10 | 10 | 0 | - | - | - | - | 41 (9) | 0.06 | Prospective cohort | HR, CO | Echo, catheterization |
| Giles (1987) [54] | HTN, LVH | W, B | Nitrendipine (oral) | 20 | 0.5 | 9 | 9 | 0 | Hydrochlorothiazide | 9 | 0 | 0 | 66 (3) | 56 | RCT | SBP, DBP, HR, LVM, | Echo, sphygomomanometry |
| Sheiban (1987) [26] | HTN | - | Nifedipine (oral) | 30 | 0.5 | 8 | 8 | 0 | Captopril | 8 | 8 | 0 | 38 (10) | 180 | RCT | SBP, DBP, LVEF, HR | Echo, sphygomomanometry |
| Lahiri (1986) [48] | HF, MI | - | Nicardipine (oral) | 10 | 0.03 | 10 | 10 | 0 | - | - | - | - | 63 (***) | 0.02 | Prospective cohort | LVEF | Sphygomomanometry |
| 90 | 0.25 | 28 | |||||||||||||||
| Ortiz (1986) [51] | HTN | - | Verapamil (oral) | 240 | 0.33 | 18 | 5 | 13 | - | - | - | - | 53.6 (***) | 0.125 | Prospective cohort | SBP, DBP, HR, LVEF | Echo, auscultation method |
| Kubo (1985) [24] | HF | - | Nifedipine (oral) | 10 | 0.17 | 7 | 7 | 0 | - | - | - | - | 60 (***) | 0.08 | Prospective cohort | HR | Catheterization, |
| Nakamura (1985) [25] | HF | - | Nifedipine (sublingual) | 20 | 0.33 | 8 | 8 | 0 | - | - | - | - | 55 (***) | 0.02 | Prospective cohort | HR | Echo, ecg |
| Silke (1984) [47] | CAD | - | Nicardipine (iv) | 1.25 | 0.003 | 10 | 10 | 0 | - | - | - | - | 47 (***) | 0.08 | Prospective cohort | HR | Ecg |
| 2.5 | 0.007 | ||||||||||||||||
| 5 | 0.01 | ||||||||||||||||
| 10 | 0.03 | ||||||||||||||||
| Suwa (1984) [35] | LVH, HF | - | Diltiazem (iv and oral) | 10 (iv) | 0.03 | 13 | 11 | 2 | Propranol | 13 | 11 | 2 | 43 (***) | 0.02 (iv) | Prospective cohort | SBP, DBP, HR | Ecg, echo, sphygomomanometry |
| 180 (oral) | 0.5 | 14 (oral) | |||||||||||||||
| Amende (1983) [22] | CAD | - | Nifedipine (iv) | 0.1 (iv) | 0.002 | 8 | 8 | 0 | - | - | - | - | 53.5 (***) | 0.007 | Prospective cohort | HR | Ecg |
| Fujita (1983) [46] | HTN | - | Nicardipine (oral) | 60 | 0.17 | 10 | 10 | 0 | - | - | - | - | 52.1 (1.7) | 120 | Prospective cohort | HR | Echo, sphygomomanometry |
| Paulus (1983) [23] | LVH, HF | - | Nifedipine (sublingual) | 10 | 0.17 | 10 | 3 | 7 | Nitroprusside | 10 | 3 | 7 | 48.3 (***) | 0.02 | RCT | HR | Echo, ecg |
3.3. Quality Assessment
3.4. Systolic Blood Pressure
3.5. Diastolic Blood Pressure
3.6. Mean Arterial Pressure
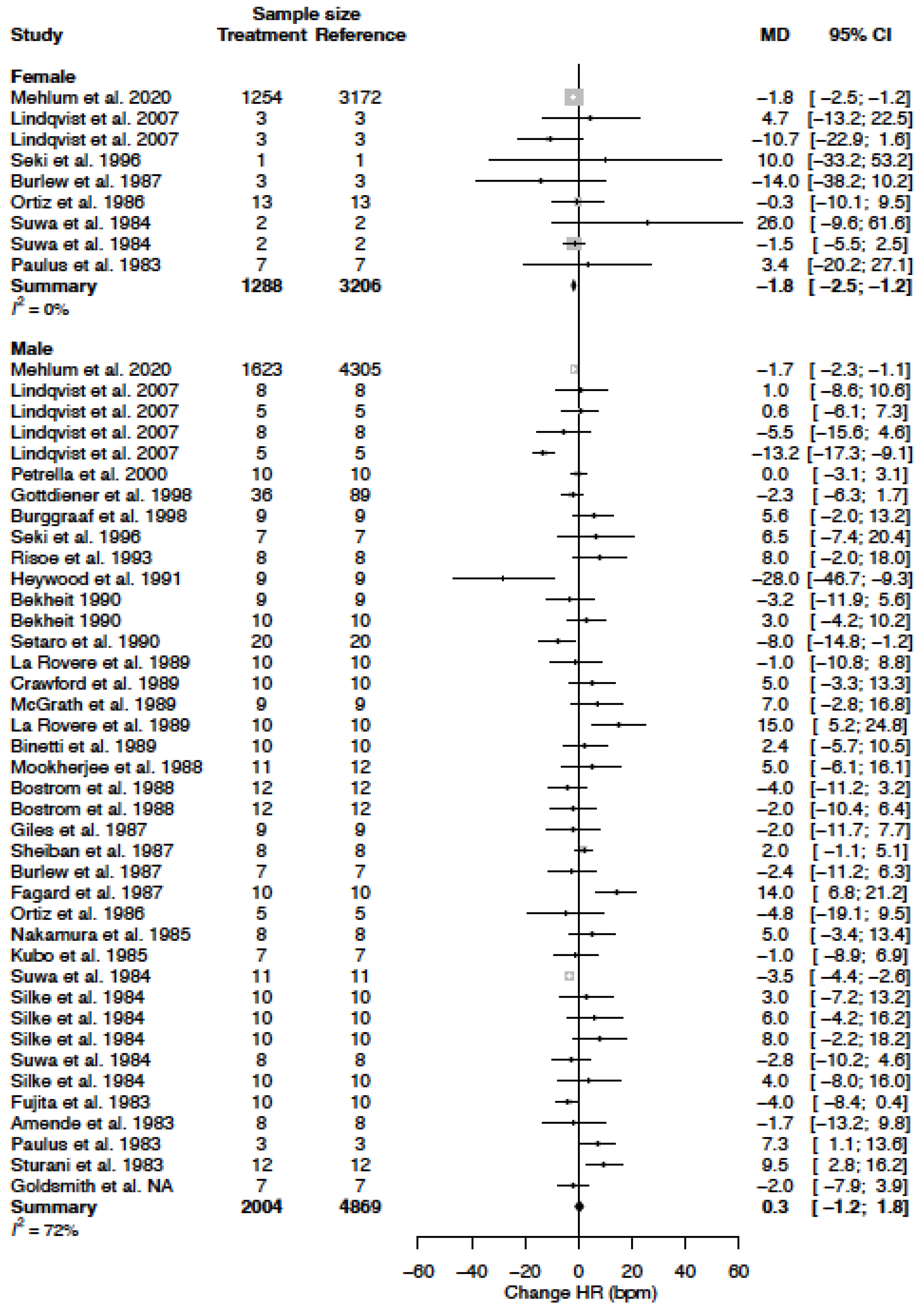
3.7. Heart Rate
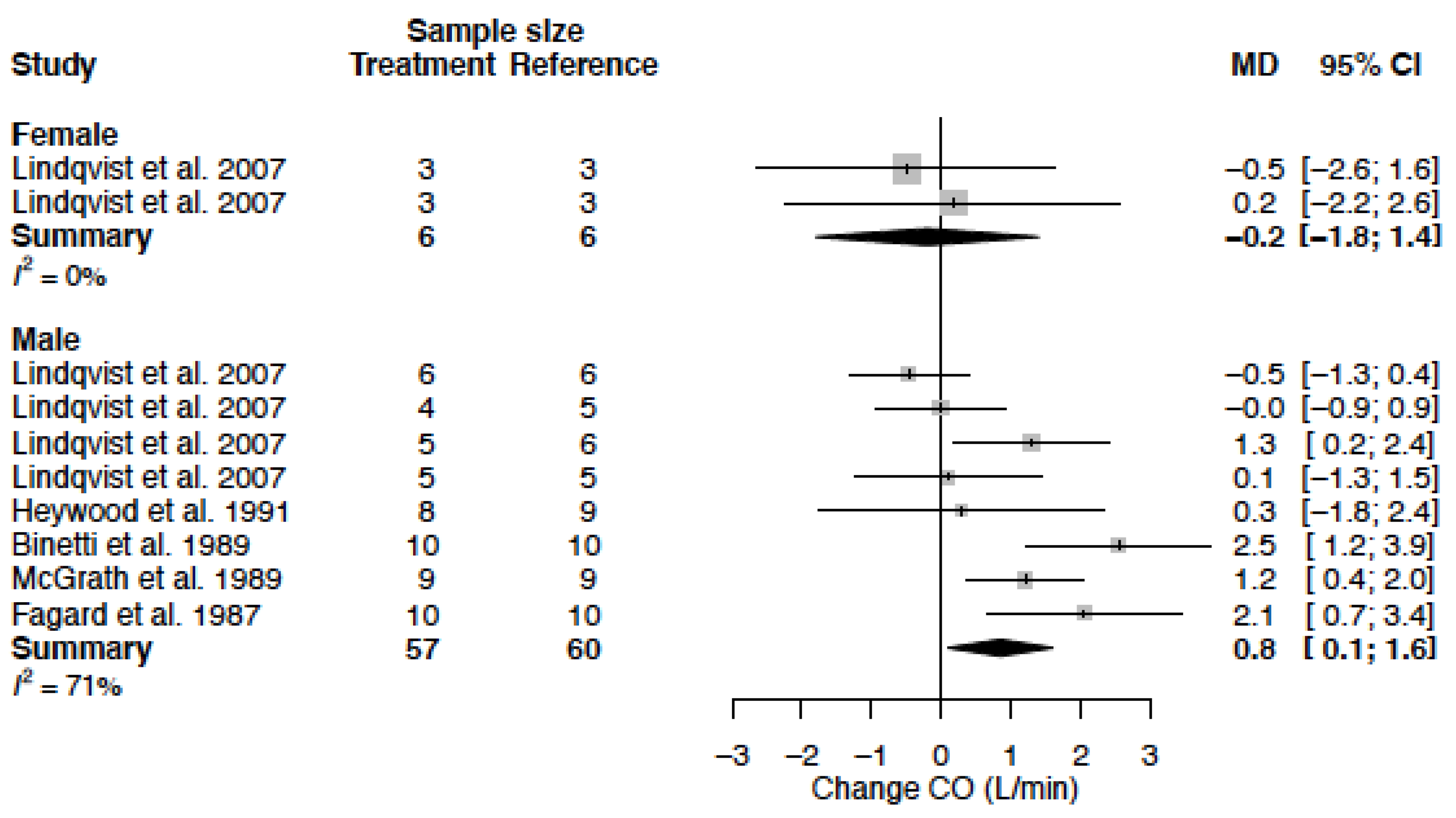
3.8. Cardiac Output
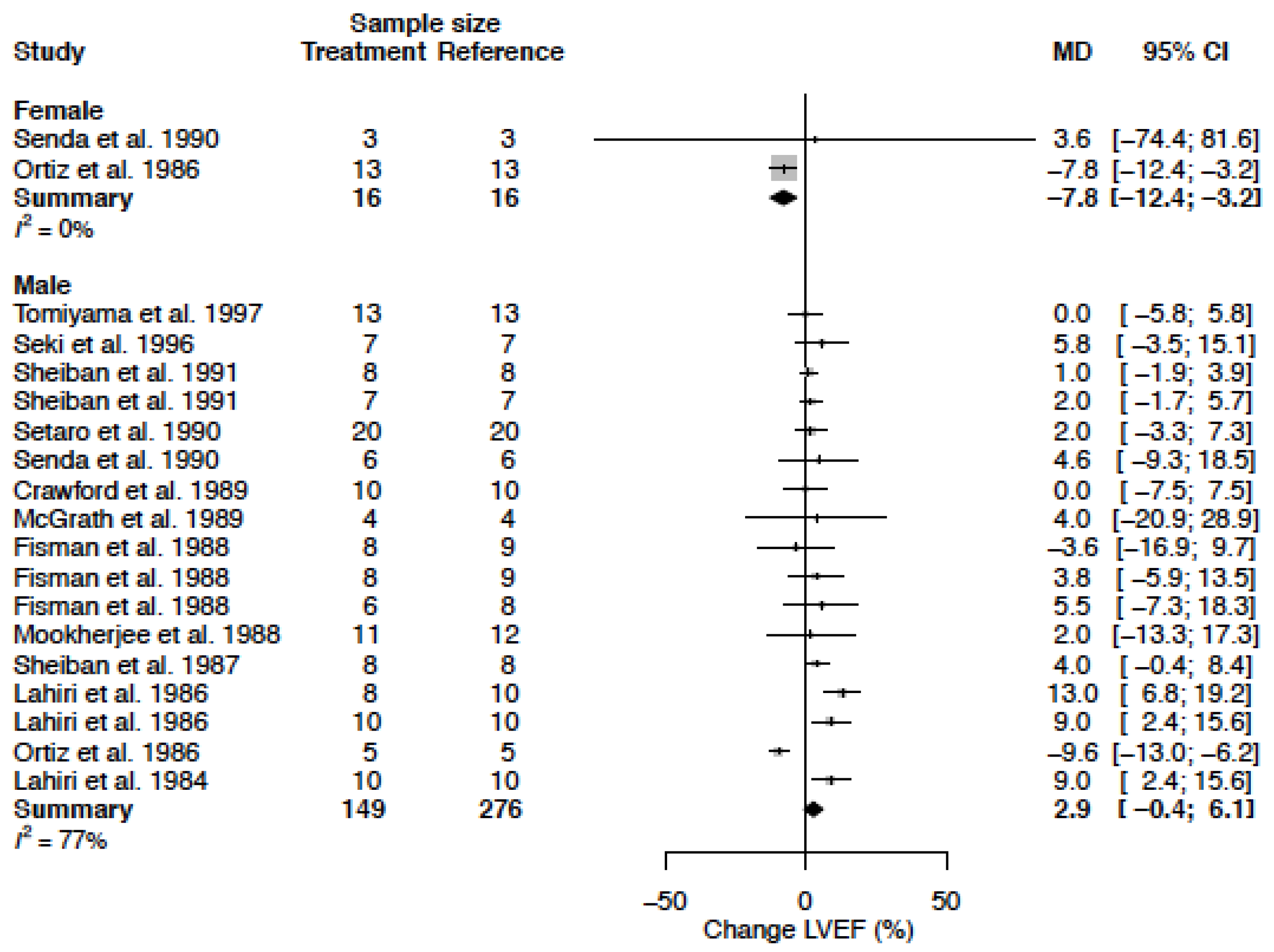
3.9. Left Ventricular Ejection Fraction
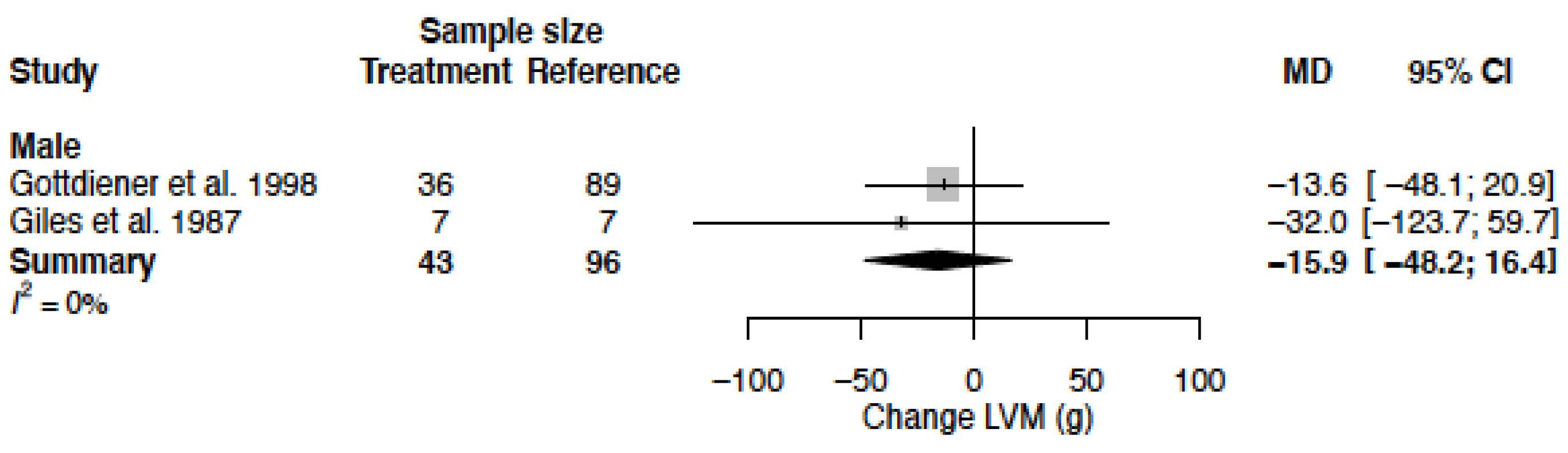
3.10. Left Ventricular Mass
4. Discussion
Strengths and Limitations
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs). 2021. Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 8 July 2022).
- World Health Organization. Hypertension; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Di Giosia, P.; Passacquale, G.; Petrarca, M.; Giorgini, P.; Marra, A.M.; Ferro, A. Gender differences in cardiovascular prophylaxis: Focus on antiplatelet treatment. Pharmacol. Res. 2017, 119, 36–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, R.M.; Varbiro, S.; Pucci, G.; Nemcsik, J.; Lonnebakken, M.T.; Kublickiene, K.; Schluchter, H.; Park, C.; Mozos, I.; Guala, A.; et al. Vascular function in hypertension: Does gender dimension matter? J. Hum. Hypertens. 2023. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Muntner, P.; Bosworth, H.B.; Whelton, P.K. Prevention and Control of Hypertension: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1278–1293. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chandramouli, C.; Allocco, B.; Gong, E.; Lam, C.S.P.; Yan, L.L. Women’s Participation in Cardiovascular Clinical Trials From 2010 to 2017. Circulation 2020, 141, 540–548. [Google Scholar] [CrossRef]
- Melloni, C.; Berger, J.S.; Wang, T.Y.; Gunes, F.; Stebbins, A.; Pieper, K.S.; Dolor, R.J.; Douglas, P.S.; Mark, D.B.; Newby, L.K. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Mosca, L.; Linfante, A.H.; Benjamin, E.J.; Berra, K.; Hayes, S.N.; Walsh, B.W.; Fabunmi, R.P.; Kwan, J.; Mills, T.; Simpson, S.L. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation 2005, 111, 499–510. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Seeland, U. Sex and gender differences in clinical medicine. In Handbook of Experimental Pharmacology; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Tamargo, J.; Rosano, G.; Walther, T.; Duarte, J.; Niessner, A.; Kaski, J.C.; Ceconi, C.; Drexel, H.; Kjeldsen, K.; Savarese, G.; et al. Gender differences in the effects of cardiovascular drugs. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 163–182. [Google Scholar] [CrossRef] [Green Version]
- Mozos, I.; Maidana, J.P.; Stoian, D.; Stehlik, M. Gender Differences of Arterial Stiffness and Arterial Age in Smokers. Int. J. Environ. Res. Public Health 2017, 14, 565. [Google Scholar] [CrossRef] [Green Version]
- McKeever, R.G.; Hamilton, R.J. Calcium Channel Blockers; StatPearls Publishing: St. Petersburg, FL, USA, 2021. [Google Scholar]
- Ueno, K.; Sato, H. Sex-related differences in pharmacokinetics and pharmacodynamics of anti-hypertensive drugs. Hypertens. Res. 2012, 35, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Katz, A.M. Pharmacology and mechanisms of action of calcium-channel blockers. J. Clin. Hypertens. 1986, 2, 28S–37S. [Google Scholar]
- Tocci, G.; Battistoni, A.; Passerini, J.; Musumeci, M.B.; Francia, P.; Ferrucci, A.; Volpe, M. Calcium channel blockers and hypertension. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 121–130. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- JPT Higgins, J.T. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarzer, G. Meta: An R package for meta-analysis. R News 2007, 7, 40–45. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Amende, I.; Simon, R.; Hood, W.P., Jr.; Hetzer, R.; Lichtlen, P.R. Intracoronary nifedipine in human beings: Magnitude and time course of changes in left ventricular contraction/relaxation and coronary sinus blood flow. J. Am. Coll. Cardiol. 1983, 2, 1141–1145. [Google Scholar] [CrossRef]
- Paulus, W.J.; Lorell, B.H.; Craig, W.E.; Wynne, J.; Murgo, J.P.; Grossman, W. Comparison of the effects of nitroprusside and nifedipine on diastolic properties in patients with hypertrophic cardiomyopathy: Altered left ventricular loading or improved muscle inactivation? J. Am. Coll. Cardiol. 1983, 2, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, S.H.; Fox, S.C.; Prida, X.E.; Cody, R.J. Combined hemodynamic effects of nifedipine and nitroglycerin in congestive heart failure. Am. Heart J. 1985, 110, 1032–1034. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ikeda, T.; Takata, S.; Yamamoto, M.; Kitamura, T.; Hattori, N. Effects of nifedipine on forearm vascular resistance and venous capacitance in normal subjects and in patients with congestive heart failure. Int. J. Cardiol. 1985, 9, 27–36. [Google Scholar] [CrossRef]
- Sheiban, I.; Arcaro, G.; Covi, G.; Accardi, R.; Zenorini, C.; Lechi, A. Regression of cardiac hypertrophy after antihypertensive therapy with nifedipine and captopril. J. Cardiovasc. Pharmacol. 1987, 10 (Suppl. 10), S187–S191. [Google Scholar] [CrossRef]
- Mookherjee, S.; Ashutosh, K.; Dunsky, M.; Hill, N.; Vardan, S.; Smulyan, H.; Warner, R. Nifedipine in chronic cor pulmonale: Acute and relatively long-term effects. Clin. Pharmacol. Ther. 1988, 44, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.H.; Wilson, R.S.; O’Rourke, R.A.; Vittitoe, J.A. Effect of digoxin and vasodilators on left ventricular function in aortic regurgitation. Int. J. Cardiol. 1989, 23, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Bekheit, S.; Tangella, M.; El-Sakr, A.; Rasheed, Q.; Craelius, W.; El-Sherif, N. Use of heart rate spectral analysis to study the effects of calcium channel blockers on sympathetic activity after myocardial infarction. Am. Heart J. 1990, 119, 79–85. [Google Scholar] [CrossRef]
- Sheiban, I.; Arosio, E.; Montesi, G.; Tonni, S.; Montresor, G.; Lechi, A. Remodeling of left ventricular geometry and function induced by lacidipine and nifedipine SR in mild-to-moderate hypertension. J. Cardiovasc. Pharmacol. 1991, 17 (Suppl. 4), S68–S74. [Google Scholar] [CrossRef]
- Risoe, C.; Smiseth, O.A.; Rootwelt, K.; Sire, S.; Simonsen, S. Effect of nifedipine on splanchnic and pulmonary vascular capacitance. Clin. Physiol. 1993, 13, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Katayama, K.; Hiro, T.; Yano, M.; Miura, T.; Kohno, M.; Fujii, T.; Matsuzaki, M. The effect of nifedipine on ventriculoarterial coupling in old myocardial infarction. Jpn. Circ. J. 1996, 60, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Tomiyama, H.; Doba, N.; Kushiro, T.; Yamashita, M.; Yoshida, H.; Kanmatsuse, K.; Kajiwara, N.; Hinohara, S. Effects of long-term antihypertensive therapy on physical fitness of men with mild hypertension. Hypertens. Res. 1997, 20, 105–111. [Google Scholar] [CrossRef]
- Burggraaf, J.; Schoemaker, R.C.; Kroon, J.M.; Cohen, A.F. The influence of nifedipine and captopril on liver blood flow in healthy subjects. Br. J. Clin. Pharmacol. 1998, 45, 447–451. [Google Scholar] [CrossRef] [Green Version]
- Suwa, M.; Hirota, Y.; Kawamura, K. Improvement in left ventricular diastolic function during intravenous and oral diltiazem therapy in patients with hypertrophic cardiomyopathy: An echocardiographic study. Am. J. Cardiol. 1984, 54, 1047–1053. [Google Scholar] [CrossRef]
- Bostrom, P.A.; Lilja, B.; Johansson, B.W.; Meier, K. The effect of oral diltiazem on left ventricular performance in postinfarction patients. Clin. Cardiol. 1988, 11, 739–742. [Google Scholar] [CrossRef]
- Szlachcic, J.; Tubau, J.F.; Vollmer, C.; Massie, B.M. Effect of diltiazem on left ventricular mass and diastolic filling in mild to moderate hypertension. Am. J. Cardiol. 1989, 63, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Senda, Y.; Tohkai, H.; Kimura, M.; Shida, Y.; Tutumi, S.; Koshiyama, H.; Tanaka, T.; Shimaji, Y.; Nogita, T. ECG-gated cardiac scan and echocardiographic assessments of left ventricular hypertrophy: Reversal by 6-month treatment with diltiazem. J. Cardiovasc. Pharmacol. 1990, 16, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Heywood, J.T.; Graham, B.; Marais, G.E.; Jutzy, K.R. Effects of intravenous diltiazem on rapid atrial fibrillation accompanied by congestive heart failure. Am. J. Cardiol. 1991, 67, 1150–1152. [Google Scholar] [CrossRef]
- Gottdiener, J.S.; Reda, D.J.; Williams, D.W.; Materson, B.J.; Cushman, W.; Anderson, R.J. Effect of single-drug therapy on reduction of left atrial size in mild to moderate hypertension: Comparison of six antihypertensive agents. Circulation 1998, 98, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldsmith, S.R. Effect of amlodipine on norepinephrine kinetics and baroreflex function in patients with congestive heart failure. Am. Heart J. 1997, 134, 13–19. [Google Scholar] [CrossRef]
- Lindqvist, M.; Kahan, T.; Melcher, A.; Ekholm, M.; Hjemdahl, P. Long-term calcium antagonist treatment of human hypertension with mibefradil or amlodipine increases sympathetic nerve activity. J. Hypertens. 2007, 25, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.S.; Huynh, V.M.; Tran, V.H. Effects of lercanidipine versus amlodipine in hypertensive patients with cerebral ischemic stroke. Curr. Med. Res. Opin. 2015, 31, 163–170. [Google Scholar] [CrossRef]
- Baysal, S.S.; Pirat, B.; Okyay, K.; Bal, U.A.; Ulucam, M.Z.; Oztuna, D.; Muderrisoglu, H. Treatment-associated change in apelin concentration in patients with hypertension and its relationship with left ventricular diastolic function. Anatol. J. Cardiol. 2017, 17, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehlum, M.H.; Liestol, K.; Kjeldsen, S.E.; Wyller, T.B.; Julius, S.; Rothwell, P.M.; Mancia, G.; Parati, G.; Weber, M.A.; Berge, E. Blood Pressure-Lowering Profiles and Clinical Effects of Angiotensin Receptor Blockers Versus Calcium Channel Blockers. Hypertension 2020, 75, 1584–1592. [Google Scholar] [CrossRef]
- Fujita, T.; Noda, H. Hemodynamic changes associated with long-term antihypertensive therapy with new calcium antagonist. Jpn. Heart J. 1983, 24, 587–593. [Google Scholar] [CrossRef] [Green Version]
- Silke, B.; Verma, S.P.; Nelson, G.I.; Hussain, M.; Taylor, S.H. Haemodynamic dose-response effects of i.v. nicardipine in coronary artery disease. Br. J. Clin. Pharmacol. 1984, 18, 717–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahiri, A.; Robinson, C.W.; Kohli, R.S.; Caruana, M.P.; Raftery, E.B. Acute and chronic effects of nicardipine on systolic and diastolic left ventricular performance in patients with heart failure: A pilot study. Clin. Cardiol. 1986, 9, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Burlew, B.S.; Gheorghiade, M.; Jafri, S.M.; Goldberg, A.D.; Goldstein, S. Acute and chronic hemodynamic effects of nicardipine hydrochloride in patients with heart failure. Am. Heart J. 1987, 114, 793–804. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Mortara, A.; Opasich, C.; Specchia, G. Acute and chronic effects of nicardipine on rest and exercise haemodynamics in post-myocardial infarction patients with latent cardiac failure. Eur. Heart J. 1989, 10, 429–436. [Google Scholar] [CrossRef]
- Ortiz, J.; Matsumoto, A.; Monaco, C.A.; Barretto, A.C. Left ventricular function after a single large dose of verapamil. Am. J. Cardiol. 1986, 57, 30D–34D. [Google Scholar] [CrossRef] [PubMed]
- Setaro, J.F.; Zaret, B.L.; Schulman, D.S.; Black, H.R.; Soufer, R. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am. J. Cardiol. 1990, 66, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Petrella, R.J.; Cunningham, D.A.; Paterson, D.H. Left ventricular diastolic filling and cardiovascular functional capacity in older men. Exp. Physiol. 2000, 85, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Giles, T.D.; Sander, G.E.; Roffidal, L.C.; Thomas, M.G.; Given, M.B.; Quiroz, A.C. Comparison of nitrendipine and hydrochlorothiazide for systemic hypertension. Am. J. Cardiol. 1987, 60, 103–106. [Google Scholar] [CrossRef]
- Naritomi, H.; Shimizu, T.; Miyashita, K.; Oe, H.; Sawada, T. Pilot study on the effects of nitrendipine on cerebral blood flow in hypertensive patients with a history of cerebral infarction. Curr. Ther. Res. 1995, 56, 649–655. [Google Scholar] [CrossRef]
- Fagard, R.; Staessen, J.; Amery, A. The use of Doppler echocardiography to assess the acute haemodynamic response to felodipine and metoprolol in hypertensive patients. J. Hypertens. 1987, 5, 143–149. [Google Scholar] [CrossRef]
- Binetti, G.; Rubino, I.; Varani, E.; Spadoni, R.; Ferretti, R.M.; Cervi, V.; Magnani, B. Felodipine in severe chronic congestive heart failure: Acute effects on central hemodynamics and regional blood flow distribution. Cardiovasc. Drugs Ther. 1989, 3, 903–911. [Google Scholar] [CrossRef] [PubMed]
- McGrath, B.P.; Newman, R.; Older, P. Hemodynamic study of short- and long-term isradipine treatment in patients with chronic ischemic congestive heart failure. Am. J. Med. 1989, 86, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Fisman, E.Z.; Pines, A.; Ben-Ari, E.; Shiner, R.J.; Drory, Y.; Friedman, B.A.; Kellermann, J.J. Echocardiographic evaluation of the effects of gallopamil on left ventricular function. Clin. Pharmacol. Ther. 1988, 44, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kompas, F. Amlodipine (Dihydropyridinen), Dosering. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/a/amlodipine#dosering (accessed on 1 July 2022).
- Kompas, F. Lercanidipine (Dihydropyridinen), Dosering. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/l/lercanidipine#doseringen (accessed on 1 July 2022).
- Welker, H.A. Single- and multiple-dose mibefradil pharmacokinetics in normal and hypertensive subjects. J. Pharm. Pharmacol. 1998, 50, 983–987. [Google Scholar] [CrossRef]
- Kompas, F. Nicardipine (Dihydropyridinen), Dosering. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/n/nicardipine#dosering (accessed on 1 July 2022).
- Kompas, F. Verapamil (Calciumantagonisten, Overige), Dosering. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/v/verapamil#dosering (accessed on 1 July 2022).
- Kompas, F. Nifedipine (Dihydropyridinen), Dosering. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/n/nifedipine (accessed on 1 July 2022).
- Kompas, F. Diltiazem (Calciumantagonisten, Overige), Dosering. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/d/diltiazem (accessed on 1 July 2022).
- Mroczek, W.J.; Burris, J.F. Nitrendipine monotherapy in severe hypertension. Angiology 1988, 39 (1 Pt 2), 81–86. [Google Scholar]
- Kompas, F. Lacidipine (Dihydropyridinen), Dosering. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/l/lacidipine#dosering (accessed on 1 July 2022).
- Kompas, F. Felodipine (Dihydropyridinen), Dosering. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/f/felodipine#dosering (accessed on 1 July 2022).
- Sundstedt, C.D.; Ruegg, P.C.; Keller, A.; Waite, R. A multicenter evaluation of the safety, tolerability, and efficacy of isradipine in the treatment of essential hypertension. Am. J. Med. 1989, 86, 98–102. [Google Scholar] [CrossRef]
- Rose, E.L.; Lahiri, A.; Raftery, E.B. Antianginal Efficacy of Sustained Release Gallopamil. Drug Investig. 2012, 5, 212–221. [Google Scholar] [CrossRef]
- Rossello, X.; Ferreira, J.P.; Pocock, S.J.; McMurray, J.J.V.; Solomon, S.D.; Lam, C.S.P.; Girerd, N.; Pitt, B.; Rossignol, P.; Zannad, F. Sex differences in mineralocorticoid receptor antagonist trials: A pooled analysis of three large clinical trials. Eur. J. Heart Fail. 2020, 22, 834–844. [Google Scholar] [CrossRef]
- Song, J.J.; Ma, Z.; Wang, J.; Chen, L.X.; Zhong, J.C. Gender Differences in Hypertension. J. Cardiovasc. Transl. Res. 2020, 13, 47–54. [Google Scholar] [CrossRef]
- Hirst, G.D.; Edwards, F.R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol. Rev. 1989, 69, 546–604. [Google Scholar] [CrossRef]
- Wier, W.G.; Morgan, K.G. Alpha1-adrenergic signaling mechanisms in contraction of resistance arteries. Rev. Physiol. Biochem. Pharmacol. 2003, 150, 91–139. [Google Scholar] [CrossRef] [PubMed]
- Kneale, B.J.; Chowienczyk, P.J.; Cockcroft, J.R.; Coltart, D.J.; Ritter, J.M. Vasoconstrictor sensitivity to noradrenaline and NG-monomethyl-L-arginine in men and women. Clin. Sci. 1997, 93, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Harvey, R.E.; Barnes, J.N.; Charkoudian, N.; Curry, T.B.; Eisenach, J.H.; Hart, E.C.; Joyner, M.J. Forearm vasodilator responses to a beta-adrenergic receptor agonist in premenopausal and postmenopausal women. Physiol. Rep. 2014, 2, e12032. [Google Scholar] [CrossRef] [PubMed]
- Loria, A.S.; Brinson, K.N.; Fox, B.M.; Sullivan, J.C. Sex-specific alterations in NOS regulation of vascular function in aorta and mesenteric arteries from spontaneously hypertensive rats compared to Wistar Kyoto rats. Physiol. Rep. 2014, 2, e12125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Gburi, S.; Deussen, A.; Zatschler, B.; Weber, S.; Kunzel, S.; El-Armouche, A.; Lorenz, K.; Cybularz, M.; Morawietz, H.; Kopaliani, I. Sex-difference in expression and function of beta-adrenoceptors in macrovessels: Role of the endothelium. Basic Res. Cardiol. 2017, 112, 29. [Google Scholar] [CrossRef]
- Fischer, M.; Baessler, A.; Schunkert, H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc. Res. 2002, 53, 672–677. [Google Scholar] [CrossRef] [Green Version]
- Komukai, K.; Mochizuki, S.; Yoshimura, M. Gender and the renin-angiotensin-aldosterone system. Fundam. Clin. Pharmacol. 2010, 24, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, L.M.; Nematbakhsh, M.; Kett, M.M.; Teichman, E.; Sampson, A.K.; Widdop, R.E.; Evans, R.G.; Denton, K.M. Gender differences in pressure-natriuresis and renal autoregulation: Role of the Angiotensin type 2 receptor. Hypertension 2011, 57, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.D.; Hilliard, L.M.; Head, G.A.; Jones, E.S.; Widdop, R.E.; Denton, K.M. Sex differences in the pressor and tubuloglomerular feedback response to angiotensin II. Hypertension 2012, 59, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, M.A.; Sullivan, J.C. Hypertension: What’s sex got to do with it? Physiology 2013, 28, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, J.C.; Rodriguez-Miguelez, P.; Zimmerman, M.A.; Harris, R.A. Differences in angiotensin (1–7) between men and women. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1171–H1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, X.; Qu, H.Y.; Jiang, S.; Zhang, J.; Fu, L.; Buggs, J.; Pang, B.; Wei, J.; Liu, R. Role of Kidneys in Sex Differences in Angiotensin II-Induced Hypertension. Hypertension 2017, 70, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Wilbert-Lampen, U.; Seliger, C.; Trapp, A.; Straube, F.; Plasse, A. Female sex hormones decrease constitutive endothelin-1 release via endothelial sigma-1/cocaine receptors: An action independent of the steroid hormone receptors. Endothelium 2005, 12, 185–191. [Google Scholar] [CrossRef]
- Barton, M.; Meyer, M.R. Postmenopausal hypertension: Mechanisms and therapy. Hypertension 2009, 54, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Orshal, J.M.; Khalil, R.A. Gender, sex hormones, and vascular tone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R233–R249. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.S.; Vongpatanasin, W. Estrogen and hypertension. Curr. Hypertens. Rep. 2006, 8, 368–376. [Google Scholar] [CrossRef]
- Dadashzadeh, S.; Javadian, B.; Sadeghian, S. The effect of gender on the pharmacokinetics of verapamil and norverapamil in human. Biopharm. Drug. Dispos. 2006, 27, 329–334. [Google Scholar] [CrossRef]
- Wolbold, R.; Klein, K.; Burk, O.; Nussler, A.K.; Neuhaus, P.; Eichelbaum, M.; Schwab, M.; Zanger, U.M. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology 2003, 38, 978–988. [Google Scholar] [CrossRef]
- George, J.; Byth, K.; Farrell, G.C. Age but not gender selectively affects expression of individual cytochrome P450 proteins in human liver. Biochem. Pharmacol. 1995, 50, 727–730. [Google Scholar] [CrossRef]
- Schmucker, D.L.; Woodhouse, K.W.; Wang, R.K.; Wynne, H.; James, O.F.; McManus, M.; Kremers, P. Effects of age and gender on in vitro properties of human liver microsomal monooxygenases. Clin. Pharmacol. Ther. 1990, 48, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.L.; Wu, C.Y.; Benet, L.Z. Sex-related differences in the clearance of cytochrome P450 3A4 substrates may be caused by P-glycoprotein. Clin. Pharmacol. Ther. 2002, 72, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A.; Sowers, J.R.; DiBona, G.F.; Gaffney, M.; Wein, M. Sex- and age-related antihypertensive effects of amlodipine. The Amlodipine Cardiovascular Community Trial Study Group. Am. J. Cardiol. 1996, 77, 713–722. [Google Scholar] [CrossRef]
- Kalibala, J.; Pechere-Bertschi, A.; Desmeules, J. Gender Differences in Cardiovascular Pharmacotherapy-the Example of Hypertension: A Mini Review. Front. Pharmacol. 2020, 11, 564. [Google Scholar] [CrossRef]
- Kang, D.; Verotta, D.; Schwartz, J.B. Population analyses of amlodipine in patients living in the community and patients living in nursing homes. Clin. Pharmacol. Ther. 2006, 79, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, D.J.; Harmatz, J.S.; von Moltke, L.L.; Wright, C.E.; Shader, R.I. Age and gender effects on the pharmacokinetics and pharmacodynamics of triazolam, a cytochrome P450 3A substrate. Clin. Pharmacol. Ther. 2004, 76, 467–479. [Google Scholar] [CrossRef]






| Male | Female | cMD Male | cMD Female | |
|---|---|---|---|---|
| SBP | 0.3503 | 0.1627 | - | - |
| DBP | 0.7937 | 0.3185 | - | - |
| MAP | 0.2550 | - | - | - |
| HR | 0.0283 | 0.6273 | −2.09 (−3.60; −0.58) | - |
| CO | 0.4162 | - | - | - |
| LVEF | 0.1651 | - | - | - |
| LVM | - | - | - | - |
| Random Sequence Allocation (Selection Bias) | Allocation Concealment (Selection Bias) | Incomplete Outcome Data (Attrition Bias) | Measure-ments Outcomes (Detection Bias) | Selective Reporting (Reporting Bias) | Overall Bias | |
|---|---|---|---|---|---|---|
| Lindqvist et al. (2007) [42] | Low | High | High | Low | High | High |
| McGrath et al. (1989) [58] | Low | Low | Low | Low | Low | Low |
| Heywood et al. (1991) [39] | High | Some concerns | Low | Some concerns | Low | High |
| Binetti et al. (1989) [57] | High | Some concerns | Low | Low | Low | High |
| Fagard et al. (1987) [56] | High | Some concerns | Low | Low | Low | High |
| Mehlum et al. (2020) [45] | High | Low | Low | Low | Low | High |
| Baysal et al. (2017) [44] | Low | High | Low | High | High | High |
| Thuc Sinh et al. (2015) [43] | High | Some concerns | Low | Some concerns | Low | High |
| Gottdiener et al. (1998) [40] | Low | Low | Low | Low | Low | Low |
| Tomiyama et al. (1997) [33] | Some concerns | Low | Low | Low | Low | Some concerns |
| Naritomi et al. (1995) [55] | High | Low | Low | Low | Low | High |
| Setaro et al. (1990) [52] | Low | Low | Low | Low | Low | Low |
| Bekheit et al. (1990) [29] | High | Low | Low | Low | Low | High |
| Szlachcic et al. (1989) [37] | Low | Low | Low | Low | Some concerns | Some concerns |
| Sheiban et al. (1987) [26] | Low | Some concerns | Low | Low | Low | Some concerns |
| Giles et al. (1987) [54] | Low | Low | Low | Low | Low | Low |
| Ortiz et al. (1986) [51] | High | Low | Low | Low | Low | High |
| Suwa et al. (1984) [35] | High | Some concerns | Low | Low | Low | High |
| Seki et al. (1996) [32] | High | Low | Low | Low | Low | High |
| Sheiban et al. (1991) [30] | High | Low | Low | Low | Low | High |
| Senda et al. (1990) [38] | High | Low | Low | Low | Low | High |
| Crawford et al. (1989) [28] | High | Some concerns | Low | Low | High | High |
| Fisman et al. (1988) [59] | Low | Low | Low | Low | Low | Low |
| Mookherjee et al. (1988) [27] | High | Low | Low | Low | Low | High |
| Lahiri et al. (1986) [48] | High | Low | Low | Low | Low | High |
| Petrella et al. (2000) [53] | High | Low | Low | Low | Low | High |
| Burggraaf et al. (1998) [34] | Low | Low | Low | Low | Low | Low |
| Risoe et al. (1993) [31] | High | Low | Low | Low | Low | High |
| La Rovere et al. (1989) [50] | High | Some concerns | Low | Low | Low | High |
| Bostrom et al. (1988) [36] | High | Some concerns | Low | Low | Low | High |
| Burlew et al. (1987) [49] | High | Low | Low | Low | Some concerns | High |
| Kubo et al. (1985) [24] | High | Some concerns | Low | Low | Some concerns | High |
| Silke et al. (1984) [47] | High | Low | Low | Low | Low | High |
| Fujita et al. (1983) [46] | Some concerns | Some concerns | Low | Low | Low | Some concerns |
| Amende et al. (1983) [22] | High | Some concerns | Low | Low | Low | High |
| Paulus et al. (1983) [23] | High | Some concerns | Low | Low | Low | High |
| Goldsmith et al. (1997) [41] | High | Low | Low | Low | Low | High |
| Nakamura et al. (1985) [25] | High | Some concerns | Low | Low | Low | High |
| Parameter | Females | Males | |
|---|---|---|---|
| SBP (mmHg) | MD % | −27.6 (−36.4; −18.8) −17.1 (−22.5; −11.6) | −14.4 (−19.0; −9.9) −9.8 (−12.9; −6.7) |
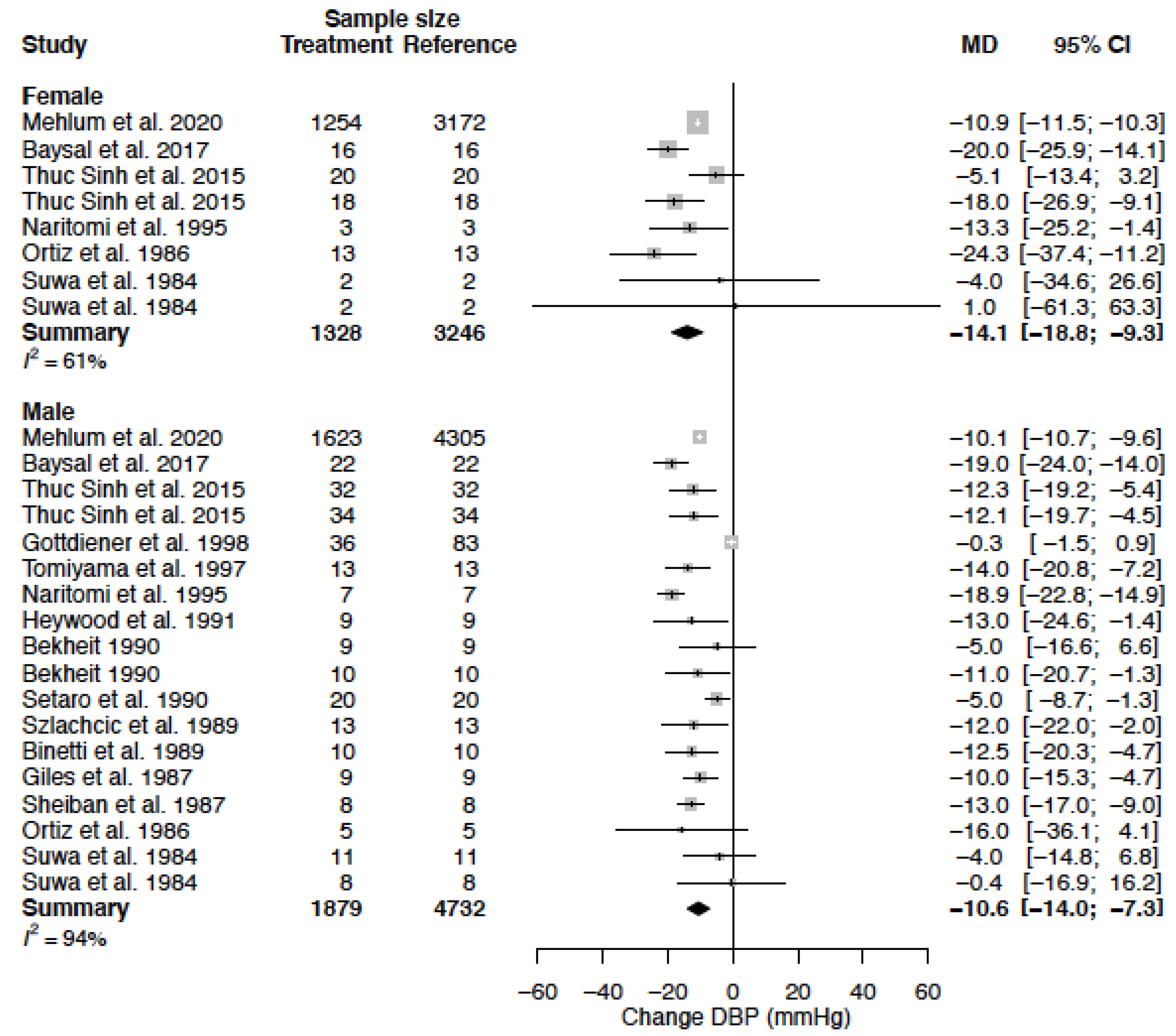
| DBP (mmHg) | MD % | −14.1 (−18.8; −9.3) −15.2 (−20.3; −10.1) | −10.6 (−14.0; −7.3) −11.2 (−14.8; −7.7) |
| MAP (mmHg) | MD % | −13.9 (−14.5; −13.2) −12.5 (−13.1; −12.0) | −8.7 (−14.1; −3.3) −8.9 (−14.5; −3.4) |
| HR (bpm) | MD % | −1.8 (−2.5; −1.2) −2.5 (−3.4; −1.6) | 0.3 (−1.2; 1.8) 0.4 (−1.7; 2.4) |
| CO (L/min) | MD % | −0.2 (−1.8; 1.4) −4.0 (−35.8; 27.7) | 0.8 (0.1; 1.6) 18.2 (2.1; 34.2) |
| LVEF (%) | MD % | −7.8 (−12.4; −3.2) −11.4 (−18.0; −4.7) | 2.9 (−0.4; 6.1) 5.3 (−0.7; 11.3) |
| LVM (g) | MD % | - - | −15.9 (−48.2; 16.4) −4.9 (−15.0; 5.1) |
| Sources of Heterogeneity | SBP | DBP | MAP | HR | CO | LVEF |
|---|---|---|---|---|---|---|
| Diltiazem | 0.0033 | 0.0169 | - | 0.3344 | 0.6689 | - |
| Felodipine | 0.4463 | 0.8744 | 0.8968 | 0.0013 | 0.0179 | - |
| Isradipine | - | - | - | 0.1107 | 0.4874 | 0.9644 |
| Gallopamil | - | - | - | - | - | 0.7769 |
| Mibefradil | - | - | - | <0.0001 | 0.0469 | - |
| Lacidipine | - | - | - | - | - | 0.6467 |
| Nicardipine | - | - | - | 0.1601 | - | 0.4624 |
| Lercanidipine | 0.7665 | 0.2490 | - | - | - | - |
| Nifedipine | 0.3323 | 0.8709 | 0.0059 | 0.0005 | - | 0.7478 |
| Nitrendipine | 0.8774 | 0.8796 | - | 0.9458 | - | - |
| Verapamil | 0.4007 | 0.4838 | - | 0.8234 | - | 0.2200 |
| Low quality | 0.0765 | 0.0954 | 0.8467 | 0.8485 | - | 0.8701 |
| Moderate quality | 0.2036 | 0.8437 | 0.8034 | 0.3195 | 0.0015 | 0.8598 |
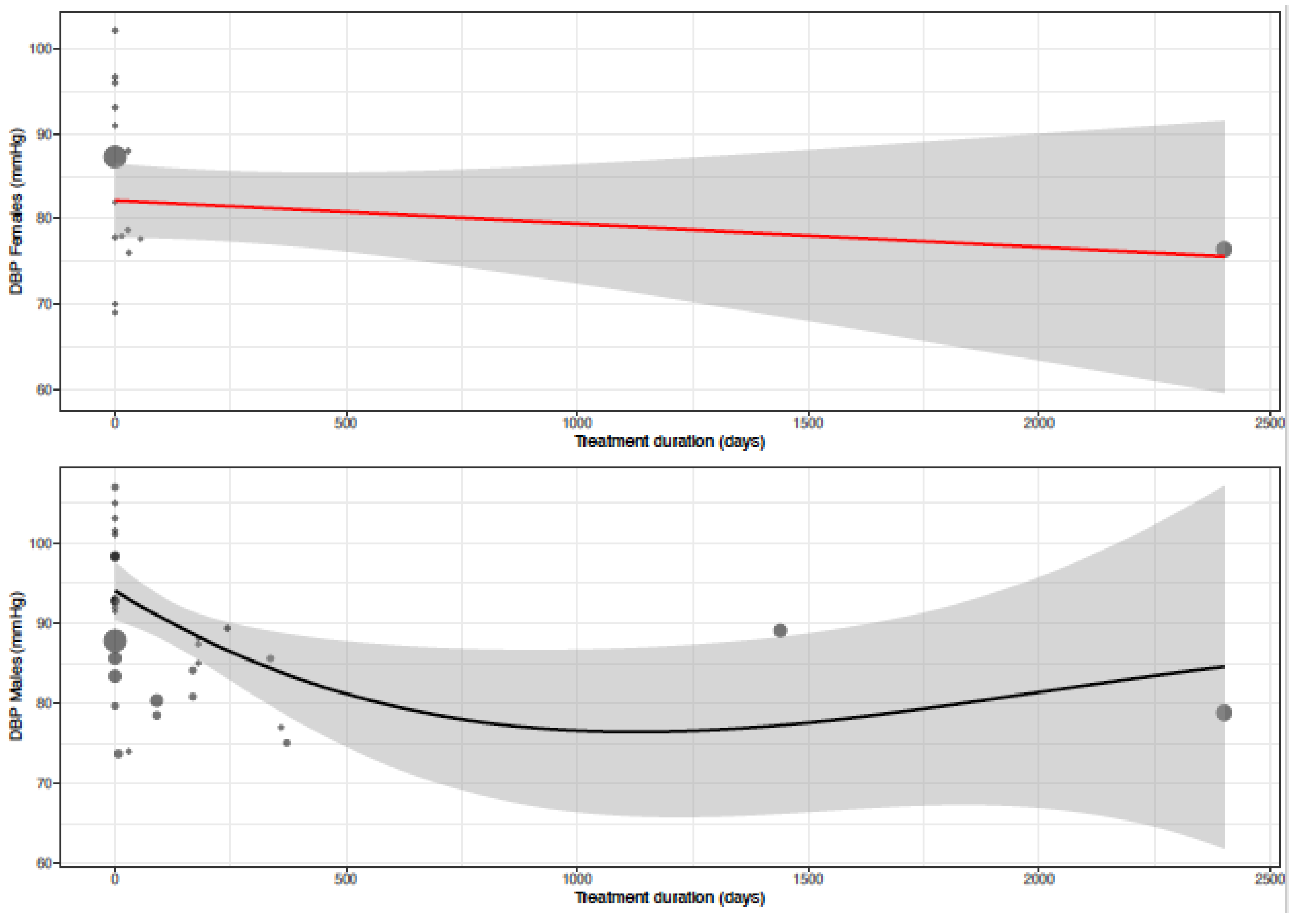
| Treatment duration | 0.5907 | 0.6152 | 0.3061 | 0.5232 | 0.0075 | 0.6331 |
| % max dose | 0.1842 | 0.7350 | 0.8570 | 0.2724 | 0.0605 | 0.2966 |
| Parameter | Females | Males | |
|---|---|---|---|
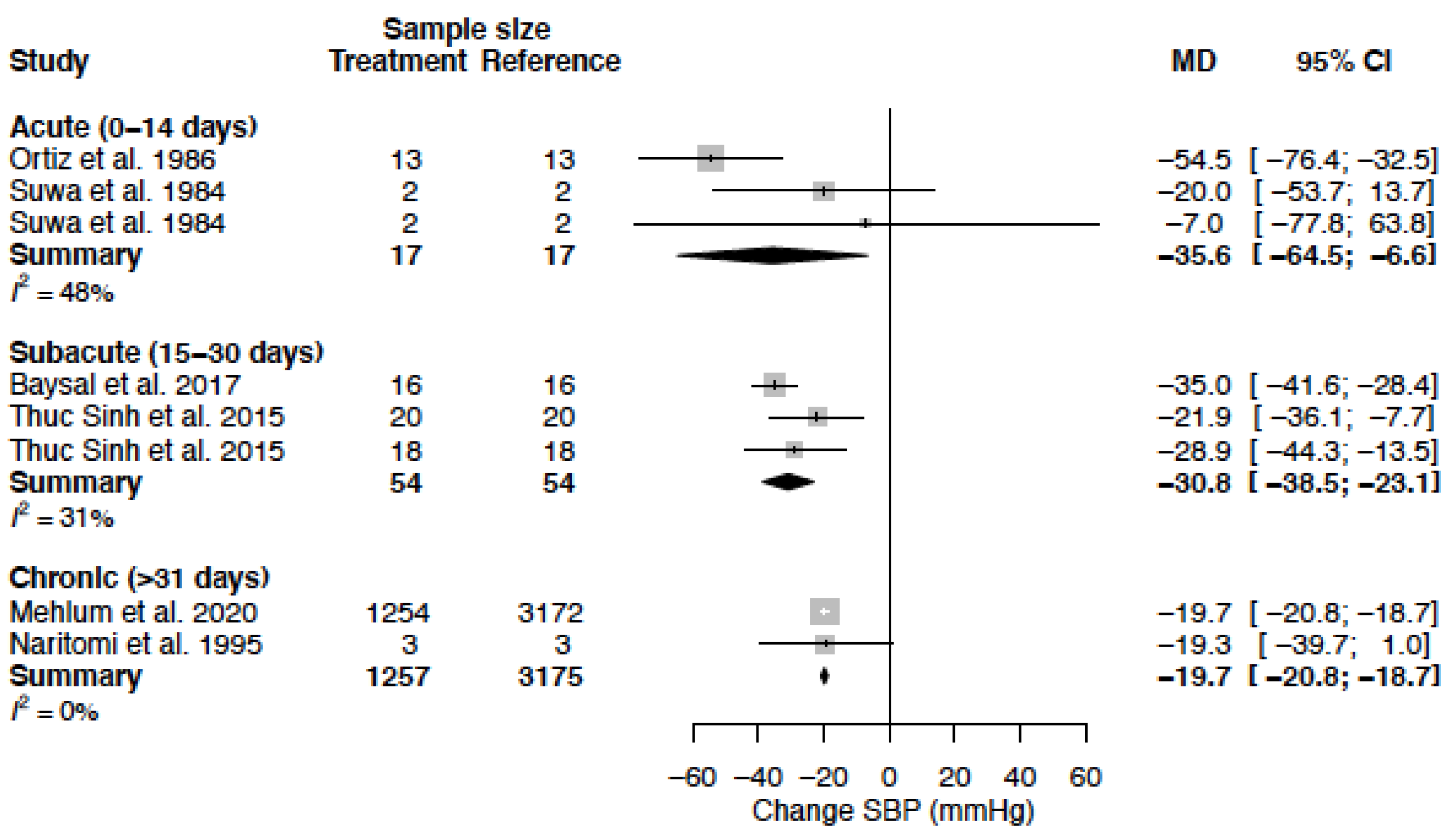
| SBP (mmHg) | MD acute | −35.6 (−64.5; −6.6) | −9.4 (−15.2; −3.7) |
| MD sub-acute | −30.8 (−38.5; −23.1) | −21.8 (−28.8; −14.7) | |
| MD chronic | −19.7 (−20.8; −18.7) | −15.1 (−23.8; −6.4) | |
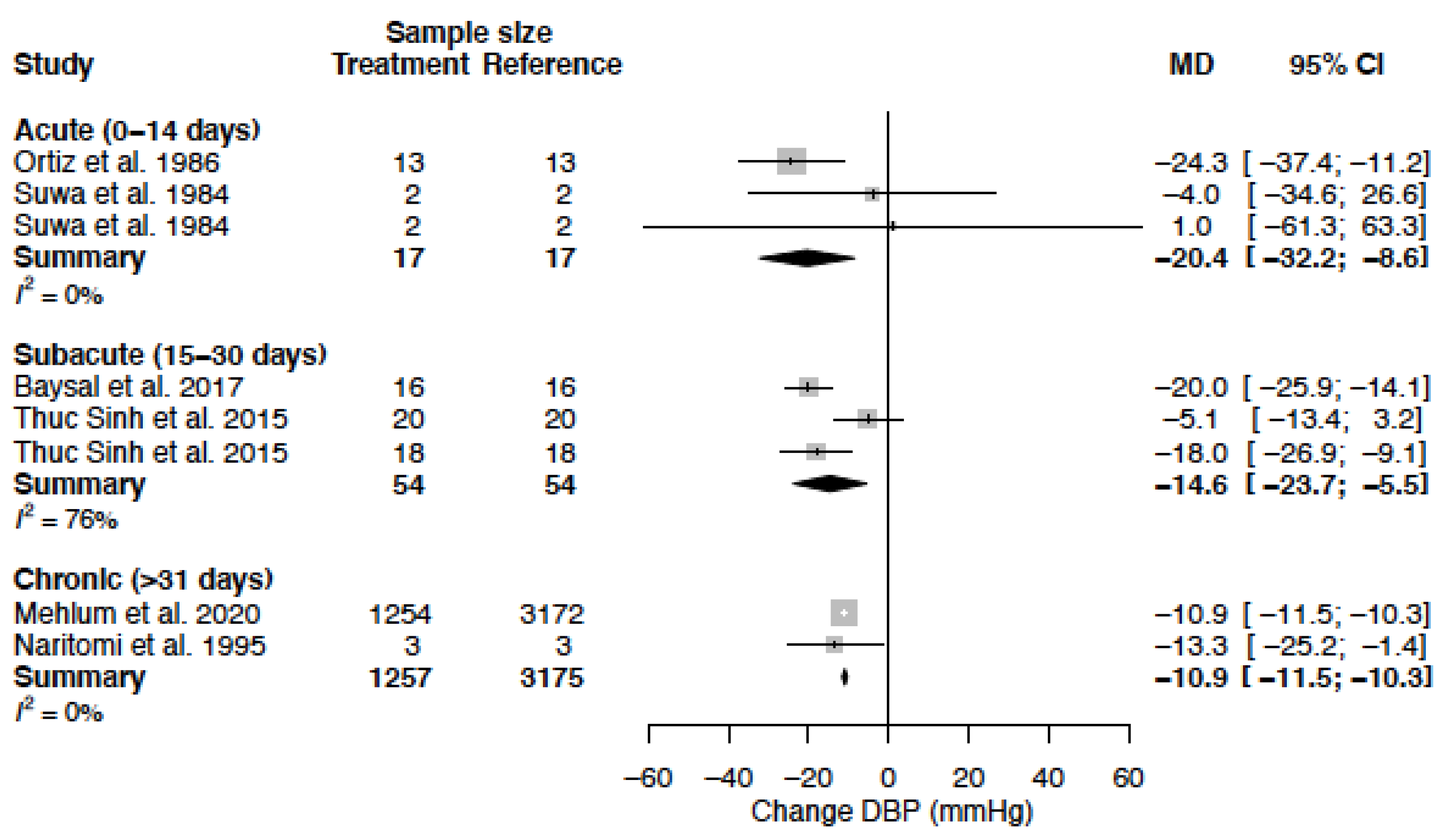
| DBP (mmHg) | MD acute | −20.4 (−32.2; −8.6) | −6.9 (−9.7; −4.1) |
| MD sub-acute | −14.6 (−23.7; −5.5) | −15.1 (−20.0; −10.2) | |
| MD chronic | −10.9 (−11.5; −10.3) | −10.9 (−16.1; −5.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Luik, E.M.; Vaes, E.W.P.; Vesseur, M.A.M.; Wilmes, N.; Meijs, D.A.M.; Laven, S.A.J.S.; Mohseni-Alsalhi, Z.; de Haas, S.; Spaanderman, M.E.A.; Ghossein-Doha, C. Sex Differences in the Anti-Hypertensive Effect of Calcium-Channel Blockers: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 1622. https://doi.org/10.3390/biomedicines11061622
van Luik EM, Vaes EWP, Vesseur MAM, Wilmes N, Meijs DAM, Laven SAJS, Mohseni-Alsalhi Z, de Haas S, Spaanderman MEA, Ghossein-Doha C. Sex Differences in the Anti-Hypertensive Effect of Calcium-Channel Blockers: A Systematic Review and Meta-Analysis. Biomedicines. 2023; 11(6):1622. https://doi.org/10.3390/biomedicines11061622
Chicago/Turabian Stylevan Luik, Eveline M., Esmée W. P. Vaes, Maud A. M. Vesseur, Nick Wilmes, Daniek A. M. Meijs, Sophie A. J. S. Laven, Zenab Mohseni-Alsalhi, Sander de Haas, Marc E. A. Spaanderman, and Chahinda Ghossein-Doha. 2023. "Sex Differences in the Anti-Hypertensive Effect of Calcium-Channel Blockers: A Systematic Review and Meta-Analysis" Biomedicines 11, no. 6: 1622. https://doi.org/10.3390/biomedicines11061622
APA Stylevan Luik, E. M., Vaes, E. W. P., Vesseur, M. A. M., Wilmes, N., Meijs, D. A. M., Laven, S. A. J. S., Mohseni-Alsalhi, Z., de Haas, S., Spaanderman, M. E. A., & Ghossein-Doha, C. (2023). Sex Differences in the Anti-Hypertensive Effect of Calcium-Channel Blockers: A Systematic Review and Meta-Analysis. Biomedicines, 11(6), 1622. https://doi.org/10.3390/biomedicines11061622






