Whole Blood Spermine/Spermidine Ratio as a New Indicator of Sarcopenia Status in Older Adults
Abstract
1. Introduction
2. Materials and Methods
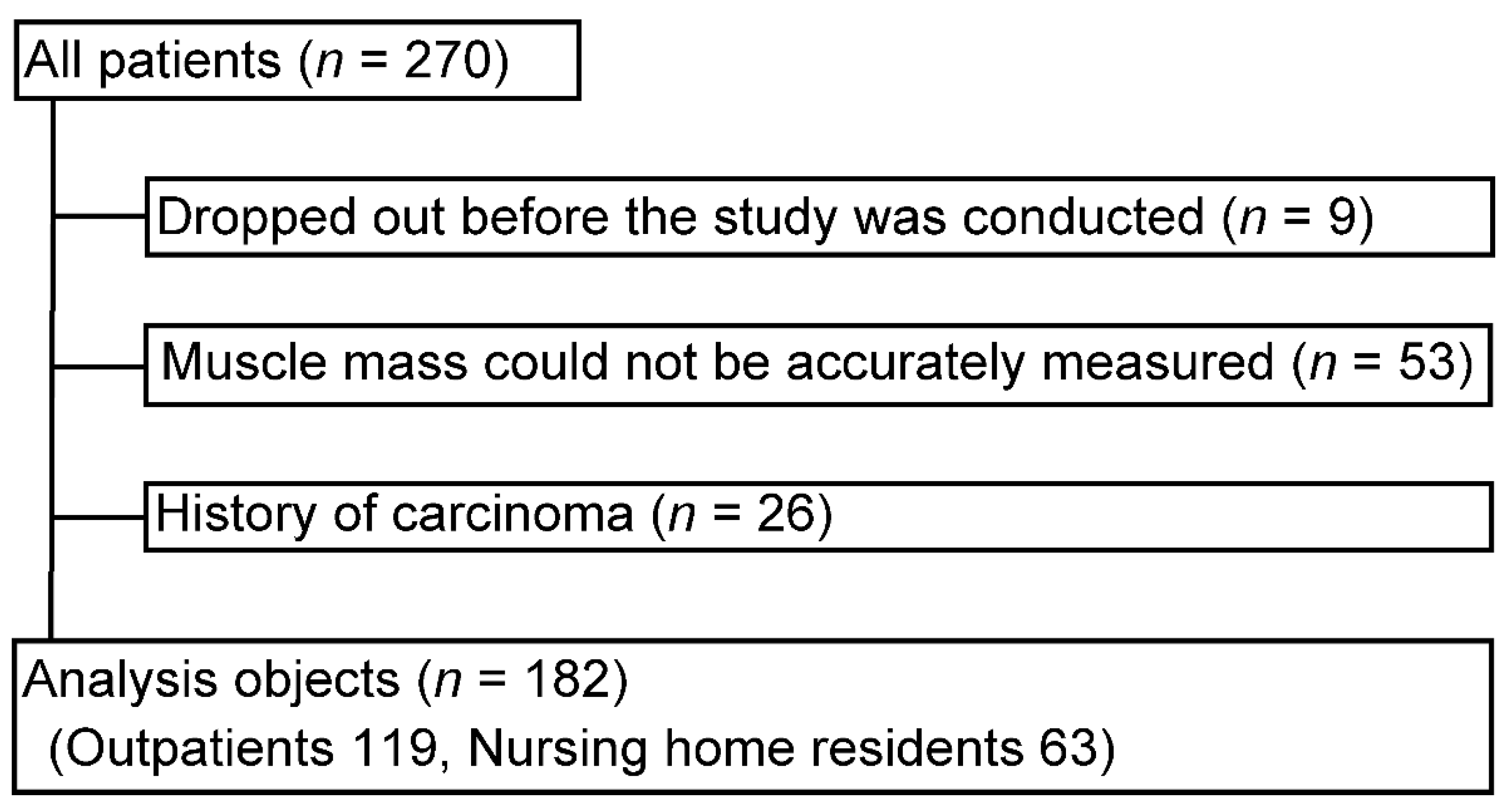
2.1. Study Design and Participants
2.2. Data and Sample Collection
2.3. Definition of Sarcopenia
2.4. Blood and Biochemical Tests
2.5. Determination of Polyamine Concentrations in Whole Blood
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Background of the Study Participants
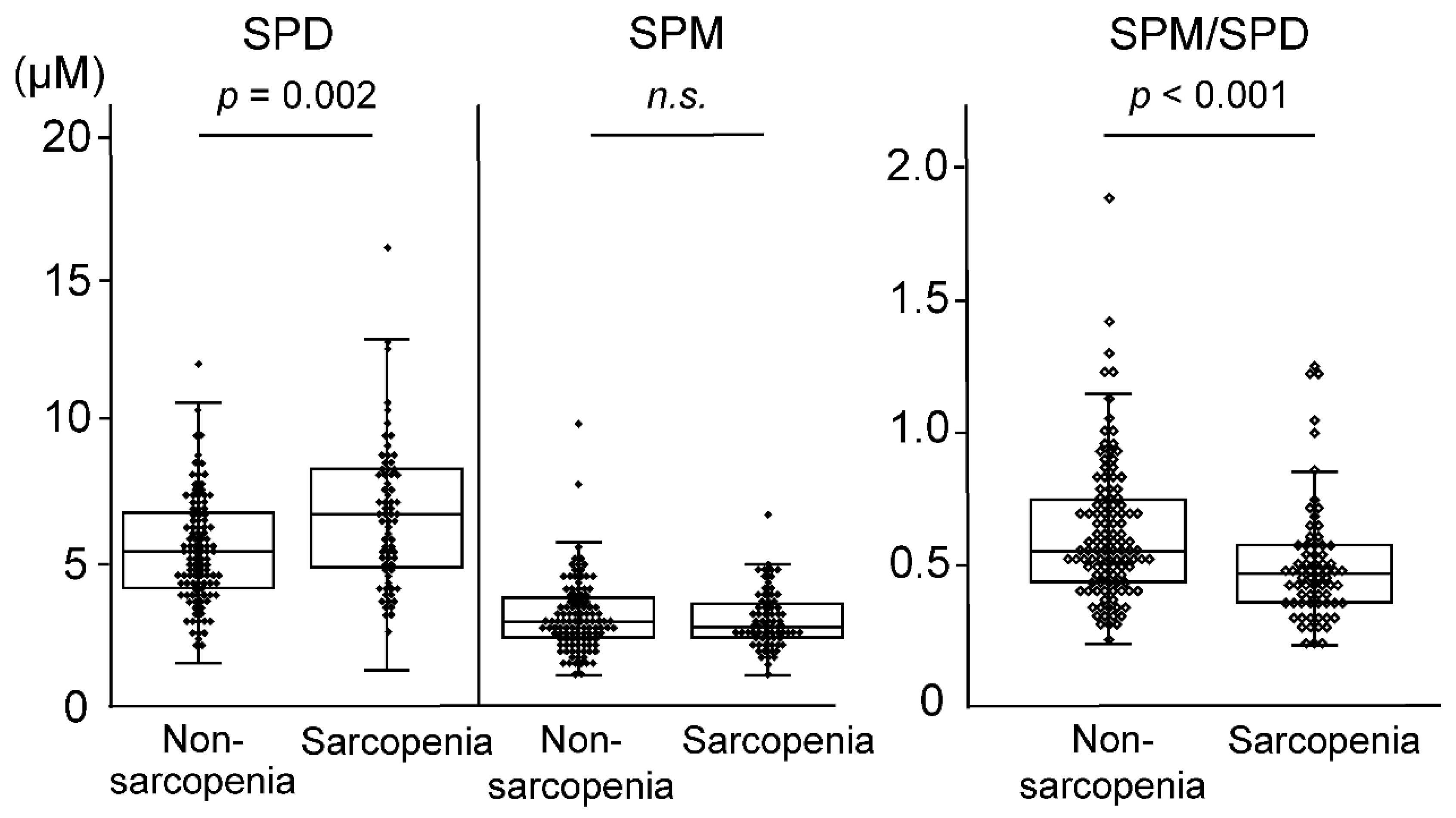
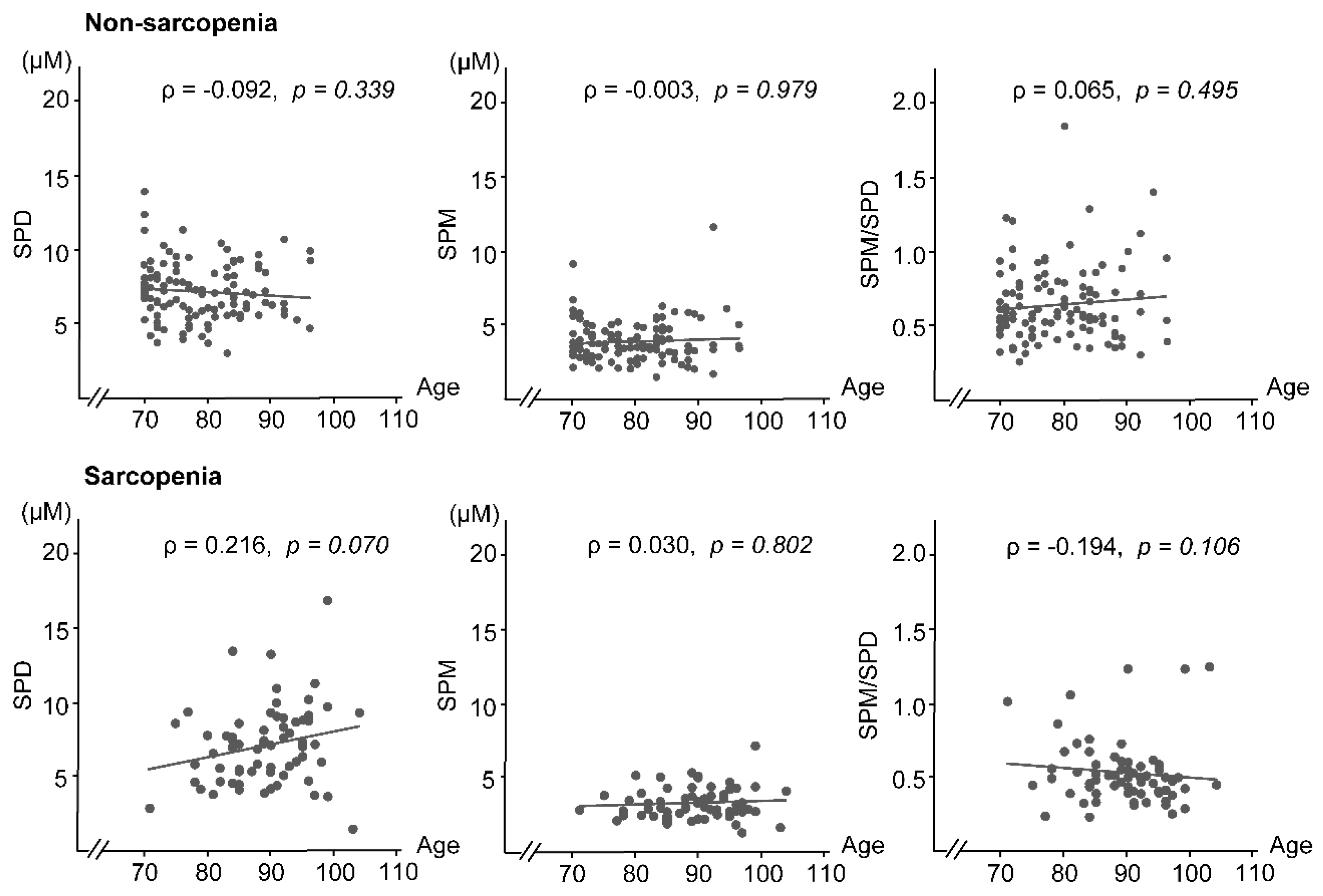
3.3. Blood Polyamines
3.4. Binomial Multivariate Logistic Regression Analysis
3.5. Factors Associated with Sarcopenia Progression
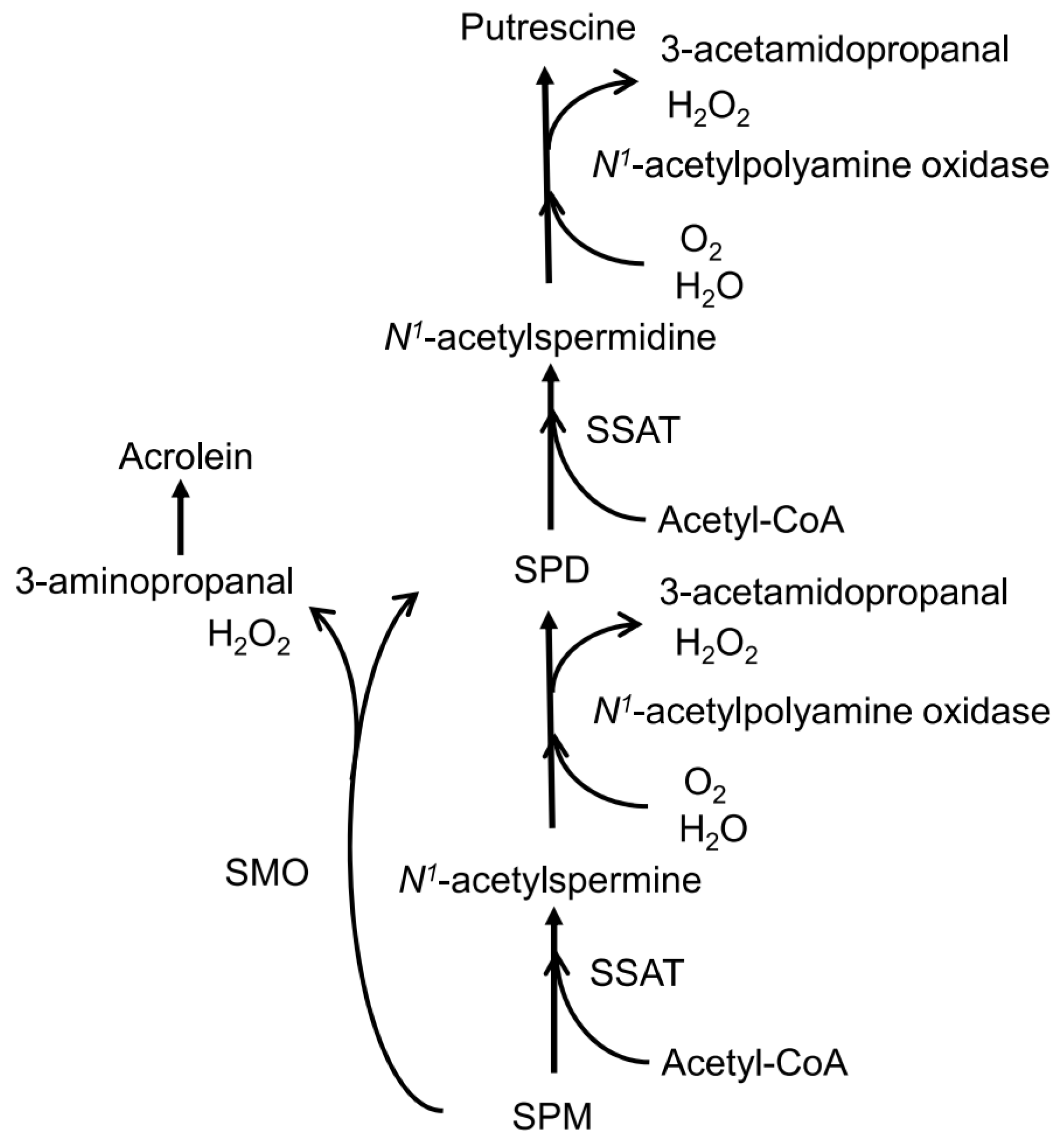
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Marshall, L.M.; Michael, Y.; Dam, T.T.; Ensrud, K.E.; Barrett-Connor, E.; Orwoll, E.S. Frailty in older men: Prevalence, progression, and relationship with mortality. J. Am. Geriatr. Soc. 2007, 55, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.J.; Fields, D.A.; Byrne, N.M.; Hunter, G.R. Resistance training improves metabolic economy during functional tasks in older adults. J. Strength Cond. Res. 2007, 21, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Czerwinski, S.; Van Kan, G.A.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging 2008, 12, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Topinková, E. Aging, disability and frailty. Ann. Nutr. Metab. 2008, 52, 6–11. [Google Scholar] [CrossRef]
- Fallah, N.; Mitnitski, A.; Searle, S.D.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. Transitions in frailty status in older adults in relation to mobility: A multistate modeling approach employing a deficit count. J. Am. Geriatr. Soc. 2011, 59, 524–529. [Google Scholar] [CrossRef]
- Sternberg, Z.; Podolsky, R.; Nir, A.; Yu, J.; Nir, R.; Halvorsen, S.W.; Quinn, J.F.; Kaye, J.; Kolb, C. Elevated spermidine serum levels in mild cognitive impairment, a potential biomarker of progression to Alzheimer dementia, a pilot study. J. Clin. Neurosci. 2022, 100, 169–174. [Google Scholar] [CrossRef]
- Saiki, S.; Sasazawa, Y.; Fujimaki, M.; Kamagata, K.; Kaga, N.; Taka, H.; Li, Y.; Souma, S.; Hatano, T.; Imamichi, Y.; et al. A metabolic profile of polyamines in Parkinson’s disease: A promising biomarker. Ann. Neurol. 2019, 86, 251–263. [Google Scholar] [CrossRef]
- Graham, S.F.; Chevallier, O.P.; Elliott, C.T.; Hölscher, C.; Johnston, J.; McGuinness, B.; Kehoe, P.G.; Passmore, A.P.; Green, B.D. Untargeted metabolomic analysis of human plasma indicates differentially affected polyamine and L-arginine metabolism in mild cognitive impairment subjects converting to Alzheimer’s disease. PLoS ONE 2015, 10, e0119452. [Google Scholar] [CrossRef]
- Inoue, K.; Tsutsui, H.; Akatsu, H.; Hashizume, Y.; Matsukawa, N.; Yamamoto, T.; Toyo’oka, T. Metabolic profiling of Alzheimer’s disease brains. Sci. Rep. 2013, 3, 2364. [Google Scholar] [CrossRef]
- Gomes-Trolin, C.; Nygren, I.; Aquilonius, S.M.; Askmark, H. Increased red blood cell polyamines in ALS and Parkinson’s disease. Exp. Neurol. 2002, 177, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Soda, K. Overview of polyamines as nutrients for human healthy long life and effect of increased polyamine intake on DNA methylation. Cells 2022, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Pekar, T.; Wendzel, A.; Flak, W.; Kremer, A.; Pauschenwein-Frantsich, S.; Gschaider, A.; Wantke, F.; Jarisch, R. Spermidine in dementia: Relation to age and memory performance. Wien Klin Wochenschr. 2020, 132, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Jaenne, J.; Raina, A.; Siimes, M. Spermidine and spermine in rat tissues at different ages. Acta Physiol. Scand. 1964, 62, 352–358. [Google Scholar] [CrossRef]
- Soda, K.; Uemura, T.; Sanayama, H.; Igarashi, K.; Fukui, T. Polyamine-rich diet elevates blood spermine levels and inhibits pro-inflammatory status: An interventional study. Med. Sci. 2021, 9, 22. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Nakamura, T.; Kasono, K.; Kawakami, M.; Konishi, F. Spermine, a natural polyamine, suppresses LFA-1 expression on human lymphocyte. J. Immunol. 2005, 175, 237–245. [Google Scholar] [CrossRef]
- Elworthy, P.; Hitchcock, E. Polyamine levels in red blood cells from patient groups of different sex and age. Biochim. Biophys. Acta 1989, 993, 212–216. [Google Scholar] [CrossRef]
- Chaisiri, P.; Harper, M.E.; Blamey, R.W.; Peeling, W.B.; Griffiths, K. Plasma spermidine concentrations in patients with tumours of the breast or prostate or testis. Clin. Chim. Acta 1980, 104, 367–375. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Sakuragi, M.; Takao, K.; Lefor, A.; Konishi, F. Long-term oral polyamine intake increases blood polyamine concentrations. J. Nutr. Sci. Vitaminol. 2009, 55, 361–366. [Google Scholar] [CrossRef]
- Soda, K.; Dobashi, Y.; Kano, Y.; Tsujinaka, S.; Konishi, F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp. Gerontol. 2009, 44, 727–732. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Chiba, F.; Koizumi, K.; Miyaki, Y. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS ONE 2013, 8, e64357. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Soda, K.; Konishi, F. Suppression of LFA-1 expression by spermine is associated with enhanced methylation of ITGAL, the LFA-1 promoter area. PLoS ONE 2013, 8, e56056. [Google Scholar] [CrossRef] [PubMed]
- Holbert, C.E.; Dunworth, M.; Foley, J.R.; Dunston, T.T.; Stewart, T.M.; Casero, R.A., Jr. Autophagy induction by exogenous polyamines is an artifact of bovine serum amine oxidase activity in culture serum. J. Biol. Chem. 2020, 295, 9061–9068. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K.; Hamasaki, H.; Miura, A.; Kakegawa, T.; Hirose, S.; Matsuzaki, S. Formation of a compensatory polyamine by Escherichia coli polyamine-requiring mutants during growth in the absence of polyamines. J. Bacteriol. 1986, 166, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef]
- Yu, J.H.; Kim, R.E.Y.; Jung, J.M.; Park, S.Y.; Lee, D.Y.; Cho, H.J.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; et al. Sarcopenia is associated with decreased gray matter volume in the parietal lobe: A longitudinal cohort study. BMC Geriatr. 2021, 21, 622. [Google Scholar] [CrossRef]
- Devereux, W.; Wang, Y.; Stewart, T.M.; Hacker, A.; Smith, R.; Frydman, B.; Valasinas, A.L.; Reddy, V.K.; Marton, L.J.; Ward, T.D.; et al. Induction of the PAOh1/SMO polyamine oxidase by polyamine analogues in human lung carcinoma cells. Cancer Chemother. Pharmacol. 2003, 52, 383–390. [Google Scholar] [CrossRef]
- Wang, Y.; Devereux, W.; Woster, P.M.; Stewart, T.M.; Hacker, A.; Casero, R.A., Jr. Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 2001, 61, 5370–5373. [Google Scholar]
- Tao, Q.; Ang, T.F.A.; DeCarli, C.; Auerbach, S.H.; Devine, S.; Stein, T.D.; Zhang, X.; Massaro, J.; Au, R.; Qiu, W.Q. Association of chronic low-grade inflammation with risk of Alzheimer disease in ApoE4 carriers. JAMA Netw. Open 2018, 1, e183597. [Google Scholar] [CrossRef]
- Holmes, C.; Butchart, J. Systemic inflammation and Alzheimer’s disease. Biochem. Soc. Trans. 2011, 39, 898–901. [Google Scholar] [CrossRef]
- Lee, Y.J.; Han, S.B.; Nam, S.Y.; Oh, K.W.; Hong, J.T. Inflammation and Alzheimer’s disease. Arch. Pharm. Res. 2010, 33, 1539–1556. [Google Scholar] [CrossRef]
- Pegg, A.E. Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, S.; Sakata, K.; Kashiwagi, K.; Ueda, S.; Iwasaki, S.; Shirahata, A.; Igarashi, K. Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem. Biophys. Res. Commun. 2001, 282, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Khan, M.A.; Moskal, J.R. The concept of “aldehyde load” in neurodegenerative mechanisms: Cytotoxicity of the polyamine degradation products hydrogen peroxide, acrolein, 3-aminopropanal, 3-acetamidopropanal and 4-aminobutanal in a retinal ganglion cell line. Brain Res. 2007, 1145, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.; Kashiwagi, K.; Sharmin, S.; Ueda, S.; Irie, Y.; Murotani, N.; Igarashi, K. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem. Biophys. Res. Commun. 2003, 305, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Protein-conjugated acrolein as a biochemical marker of brain infarction. Mol. Nutr. Food Res. 2011, 55, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Uemura, T.; Kashiwagi, K. Assessing acrolein for determination of the severity of brain stroke, dementia, renal failure, and Sjögren’s syndrome. Amino Acids. 2020, 52, 119–127. [Google Scholar] [CrossRef]
- Cooper, K.D.; Shukla, J.B.; Rennert, O.M. Polyamine distribution in cellular compartments of blood and in aging erythrocytes. Clin. Chim. Acta 1976, 73, 71–88. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kitada, Y.; Naito, Y. Endothelial function is improved by inducing microbial polyamine production in the gut: A randomized placebo-controlled trial. Nutrients 2019, 11, 1188. [Google Scholar] [CrossRef]
| Characteristics | All Patients | Non-Sarcopenia | Sarcopenia | pValue * |
| No. of participants (M/F) | 182 (68/114) | 111 (52/59) | 71 (16/55) | <0.001 |
| Age (years) | 83 (76–90) | 78 (73–84) | 90 (84–95) | <0.001 |
| No. of N.H. residents (%) | 63 (35) | 6 (5) | 57 (80) | <0.001 |
| Past history (%) | ||||
| CVDs | 8 (4) | 6 (5) | 2 (3) | 0.407 |
| CeVDs | 39 (21) | 12 (11) | 27 (38) | <0.001 |
| Dementia | 71 (39) | 16 (14) | 55 (77) | <0.001 |
| Bone fracture | 23 (13) | 8 (7) | 15 (21) | 0.006 |
| BMI (kg/m2) | 22.2 ± 3.5 | 23.8 ± 2.7 | 19.7 ± 3.1 | <0.001 |
| SMI (kg/m2) | 6.5 (4.9–8.0) | 7.7 (6.5–8.5) | 4.6 (3.6–5.3) | <0.001 |
| WS (m/s) | 0.87 (0–1.16) | 1.1 (0.87–1.28) | 0 (0–0) | <0.001 |
| HGS (kg) | 15.8 (7.4–25.6) | 22.7 (16.5–31.5) | 6.4 (0–11.0) | <0.001 |
| Laboratory data | ||||
| SPD (µM) | 5.93 (4.60–7.68) | 5.63 (4.36–7.05) | 7.02(5.18–8.69) | 0.002 |
| SPM (µM) | 3.15 (2.67–3.98) | 3.19 (2.62–4.07) | 3.04 (2.72–3.91) | 0.701 |
| SPM/SPD | 0.54 (0.43–0.70) | 0.57 (0.46–0.75) | 0.49 (0.39–0.60) | <0.001 |
| Hb (g/dL) | 12.9 (11.6–13.9) | 13.5 (12.0–14.3) | 12.0 (11.3–13.2) | <0.001 |
| Alb (g/dL) | 4.0 (3.6–4.3) | 4.2 (4.0–4.4) # | 3.5 (3.3–3.8) | <0.001 |
| eGFR (mL/min/1.73 m2) | 58.0 (47.5–69.7) | 57.0 (45.9–66.8) | 60.0 (48.1–80.2) | 0.046 |
| LDL-C (mg/dL) | 111 (95–129) | 111 (94–128) | 106 (97–131) | 0.661 |
| HDL-C (mg/dL) | 60 (47–72) | 61 (47–74) | 57 (46–69) | 0.179 |
| TG (mg/dL) | 113 (84–160) | 128 (90–184) | 100 (76–131) | <0.001 |
| HbA1c (%) | 5.8 (5.6–6.2) | 6.0 (5.7–6.4) # | 5.6 (5.4–5.8) | <0.001 |
| Variables | OR | OR (95% CI) | p Value |
|---|---|---|---|
| Sex (M:1/F:0) | 1.677 | 0.455–6.181 | 0.437 |
| Age | 1.115 | 1.021–217 | 0.016 |
| CVDs | 0.631 | 0.028–14.126 | 0.772 |
| CeVDs | 0.910 | 0.188–4.415 | 0.907 |
| Dementia | 5.427 | 1.089–27.044 | 0.039 |
| Bone fracture | 0.321 | 0.054–1.900 | 0.210 |
| BMI | 0.730 | 0.573–0.930 | 0.011 |
| SPD | 1.481 | 1.073–2.044 | 0.017 |
| SPM | 0.502 | 0.299–0.842 | 0.009 |
| Hb | 0.842 | 0.598–1.185 | 0.323 |
| Alb | 0.115 | 0.019–0.704 | 0.019 |
| eGFR | 1.050 | 1.011–1.089 | 0.010 |
| LDL-C | 0.984 | 0.959–1.010 | 0.219 |
| HDL-C | 0.994 | 0.953–1.036 | 0.768 |
| TG | 0.998 | 0.989–1.007 | 0.690 |
| HbA1c | 0.241 | 0.078–0.744 | 0.013 |
| Variables | OR | 95% CI | p Value |
|---|---|---|---|
| Sex (M:1/F:0) | 1.759 | 0.487–6.355 | 0.389 |
| Age | 1.114 | 1.024–1.211 | 0.012 |
| CVDs | 0.615 | 0.030–12.811 | 0.754 |
| CeVDs | 1.059 | 0.230–4.870 | 0.941 |
| Dementia | 4.213 | 0.998–17.789 | 0.050 |
| Bone fracture | 0.356 | 0.064–1.969 | 0.237 |
| BMI | 0.720 | 0.566–0.917 | 0.008 |
| SPM/SPD | 0.033 | 0.002–0.557 | 0.018 |
| Hb | 0.829 | 0.574–1.198 | 0.318 |
| Alb | 0.120 | 0.020–0.710 | 0.019 |
| eGFR | 1.048 | 1.010–1.086 | 0.011 |
| LDL-C | 0.985 | 0.961–1.009 | 0.221 |
| HDL-C | 0.996 | 0.956–1.037 | 0.830 |
| TG | 0.998 | 0.990–1.007 | 0.693 |
| HbA1c | 0.240 | 0.083–0.697 | 0.009 |
| Characteristics | Healthy | Semi-Sarcopenia | Sarcopenia | |||
|---|---|---|---|---|---|---|
| p Value † | p Value † | p Value †† | ||||
| No. of patients (M/F) | 56 (37/19) | 55 (15/40) | <0.05 | 71 (16/55) | <0.05 | n.s |
| Age (years) | 73 (71–79) | 84 (77–88) | <0.001 | 90 (84–95) | <0.001 | <0.001 |
| No. of N.H. residents (%) | 0 (0) | 6 (11) | <0.05 | 57 (80) | <0.05 | <0.05 |
| Past history (%) | ||||||
| CVDs | 2 (4) | 4 (7) | n.s | 2 (3) | n.s | n.s |
| CeVDs | 2 (4) | 10 (18) | <0.05 | 27 (38) | <0.05 | <0.05 |
| Dementia, n (%) | 1 (2) | 15 (27) | <0.05 | 55 (77) | <0.05 | <0.05 |
| Bone fracture | 2 (4) | 6 (11) | n.s | 15 (21) | <0.05 | n.s |
| BMI (kg/m2) | 23.4 ± 2.5 | 23.7 ± 3.0 | 0.915 | 19.7 ± 3.1 | <0.001 | <0.001 |
| SMI (kg/m2) | 8.3 (7.6–8.9) | 6.9 (6.2–8.0) | <0.001 | 4.6 (3.6–5.3) | <0.001 | <0.001 |
| WS (m/s) | 1.25 (1.14–1.35) | 0.87 (0.69–0.98) | <0.001 | 0 (0–0) | <0.001 | <0.001 |
| HGS (kg) | 30.0 (23.2–34.2) | 16.7 (12.1–20.3) | <0.001 | 6.4 (0–11.0) | <0.001 | <0.001 |
| Laboratory findings | ||||||
| SPD (µM) | 5.07 (3.96–6.74) | 5.95 (4.97–7.74) | 0.087 | 7.02 (5.18–8.69) | <0.001 | 0.087 |
| SPM (µM) | 3.05 (2.43–3.85) | 3.29 (2.92–4.49) | 0.150 | 3.04 (2.72–3.91) | 0.894 | 0.281 |
| SPM/SPD | 0.60 (0.49–0.75) | 0.55 (0.43–0.75) | 0.722 | 0.49 (0.39–0.60) | 0.010 | 0.087 |
| Hb (g/dL) | 13.9 (13.0–14.6) | 12.7 (11.5–13.8) | 0.008 | 12.0 (11.3–13.2) | <0.001 | 0.079 |
| Alb (g/dL) | 4.2 (4.1–4.4) a | 4.1 (3.8–4.4) | 0.010 | 3.5 (3.3–3.8) | <0.001 | <0.001 |
| eGFR (mL/min/1.73 m2) | 60.5 (49.7–70.9) | 54.4 (42.4–60.2) | 0.023 | 60.0 (48.1–80.2) | 0.556 | 0.003 |
| LDL-C (mg/dL) | 111 (94–129) | 111 (94–128) | 0.988 | 106 (97–131) | 0.616 | 0.715 |
| HDL-C (mg/dL) | 63 (50–76) | 60 (46–74) | 0.619 | 57 (46–69) | 0.205 | 0.756 |
| TG (mg/dL) | 128 (85–193) | 128 (93–179) | 0.803 | 100 (76–131) | 0.046 | 0.035 |
| HbA1c (%) | 6.1 (5.7–6.6) | 6.0 (5.6–6.3) b | 0.207 | 5.6 (5.4–5.8) | <0.001 | 0.002 |
| Variables | Semi-Sarcopenia (n = 54) a | Sarcopenia (n = 71) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Sex (M:1/F:0) | 0.090 | 0.021–0.378 | 0.001 | 0.259 | 0.044–1.533 | 0.136 |
| Age | 1.329 | 1.159–1.524 | <0.001 | 1.403 | 1.203–1.636 | <0.001 |
| CVDs | 6.635 | 0.274–160.6 | 0.245 | 4.637 | 0.068–315.6 | 0.476 |
| CeVDs | 18.94 | 1.813–197.9 | 0.014 | 12.63 | 0.855–186.6 | 0.065 |
| Dementia | 3.092 | 0.224–42.66 | 0.399 | 14.80 | 0.792–276.8 | 0.071 |
| Bone fracture | 0.696 | 0.039–12.29 | 0.805 | 0.188 | 0.007–4.988 | 0.317 |
| BMI | 1.068 | 0.795–1.435 | 0.662 | 0.750 | 0.523–1.076 | 0.118 |
| SPD | 1.625 | 1.110–2.379 | 0.013 | 2.218 | 1.393–3.533 | 0.001 |
| SPM | 1.024 | 0.528–1.986 | 0.944 | 0.487 | 0.214–1.105 | 0.085 |
| Hb | 1.033 | 0.732–1.456 | 0.854 | 0.811 | 0.540–1.217 | 0.311 |
| Alb | 0.411 | 0.042–4.068 | 0.447 | 0.040 | 0.003–0.617 | 0.021 |
| eGFR | 1.004 | 0.959–1.052 | 0.852 | 1.066 | 1.006–1.129 | 0.030 |
| LDL-C | 0.981 | 0.954–1.008 | 0.171 | 0.964 | 0.930–0.999 | 0.042 |
| HDL-C | 1.006 | 0.963–1.051 | 0.794 | 1.003 | 0.948–1.060 | 0.930 |
| TG | 1.008 | 1.000–1.016 | 0.065 | 1.006 | 0.995–1.018 | 0.265 |
| HbA1c | 0.389 | 0.137–1.110 | 0.078 | 0.087 | 0.019–0.389 | 0.001 |
| Variables | Semi-Sarcopenia (n = 54) a | Sarcopenia (n = 71) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Sex (M:1/F:0) | 0.104 | 0.026–0.422 | 0.002 | 0.298 | 0.052–1.703 | 0.173 |
| Age | 1.281 | 1.132–1.450 | <0.001 | 1.358 | 1.178–1.565 | <0.001 |
| CVDs | 7.036 | 0.267–185.4 | 0.242 | 5.703 | 0.085–384.3 | 0.418 |
| CeVDs | 26.54 | 2.065–341.1 | 0.012 | 21.12 | 1.176–379.3 | 0.038 |
| Dementia | 7.408 | 0.491–111.8 | 0.148 | 24.28 | 1.328–443.7 | 0.031 |
| Bone fracture | 0.953 | 0.053–17.04 | 0.974 | 0.290 | 0.012–7.302 | 0.452 |
| BMI | 1.081 | 0.804–1.453 | 0.608 | 0.753 | 0.522–1.085 | 0.128 |
| SPM/SPD | 0.057 | 0.004–0.796 | 0.033 | 0.002 | <0.001–0.091 | 0.001 |
| Hb | 1.017 | 0.688–1.502 | 0.934 | 0.779 | 0.488–1.244 | 0.295 |
| Alb | 0.392 | 0.042–3.695 | 0.413 | 0.043 | 0.003–0.628 | 0.021 |
| eGFR | 0.999 | 0.957–1.043 | 0.958 | 1.054 | 0.999–1.111 | 0.055 |
| LDL-C | 0.981 | 0.955–1.007 | 0.156 | 0.967 | 0.934–1.000 | 0.051 |
| HDL-C | 1.006 | 0.965–1.050 | 0.769 | 1.006 | 0.953–1.062 | 0.822 |
| TG | 1.010 | 1.002–1.018 | 0.016 | 1.008 | 0.998–1.019 | 0.125 |
| HbA1c | 0.486 | 0.177–1.337 | 0.162 | 0.110 | 0.026–0.459 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanayama, H.; Ito, K.; Ookawara, S.; Uemura, T.; Sakiyama, Y.; Sugawara, H.; Tabei, K.; Igarashi, K.; Soda, K. Whole Blood Spermine/Spermidine Ratio as a New Indicator of Sarcopenia Status in Older Adults. Biomedicines 2023, 11, 1403. https://doi.org/10.3390/biomedicines11051403
Sanayama H, Ito K, Ookawara S, Uemura T, Sakiyama Y, Sugawara H, Tabei K, Igarashi K, Soda K. Whole Blood Spermine/Spermidine Ratio as a New Indicator of Sarcopenia Status in Older Adults. Biomedicines. 2023; 11(5):1403. https://doi.org/10.3390/biomedicines11051403
Chicago/Turabian StyleSanayama, Hidenori, Kiyonori Ito, Susumu Ookawara, Takeshi Uemura, Yoshio Sakiyama, Hitoshi Sugawara, Kaoru Tabei, Kazuei Igarashi, and Kuniyasu Soda. 2023. "Whole Blood Spermine/Spermidine Ratio as a New Indicator of Sarcopenia Status in Older Adults" Biomedicines 11, no. 5: 1403. https://doi.org/10.3390/biomedicines11051403
APA StyleSanayama, H., Ito, K., Ookawara, S., Uemura, T., Sakiyama, Y., Sugawara, H., Tabei, K., Igarashi, K., & Soda, K. (2023). Whole Blood Spermine/Spermidine Ratio as a New Indicator of Sarcopenia Status in Older Adults. Biomedicines, 11(5), 1403. https://doi.org/10.3390/biomedicines11051403







