Photodynamic Therapy Can Modulate the Nasopharyngeal Carcinoma Microenvironment Infected with the Epstein–Barr Virus: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Data Extraction and Study Question
2.3. Eligibility Criteria
2.4. Search Strategy
2.5. Risk of Bias Assessment
2.6. Meta-Analysis
3. Results
3.1. Search Results
3.2. Synthesis of Results
3.3. Risk of Bias Assessment
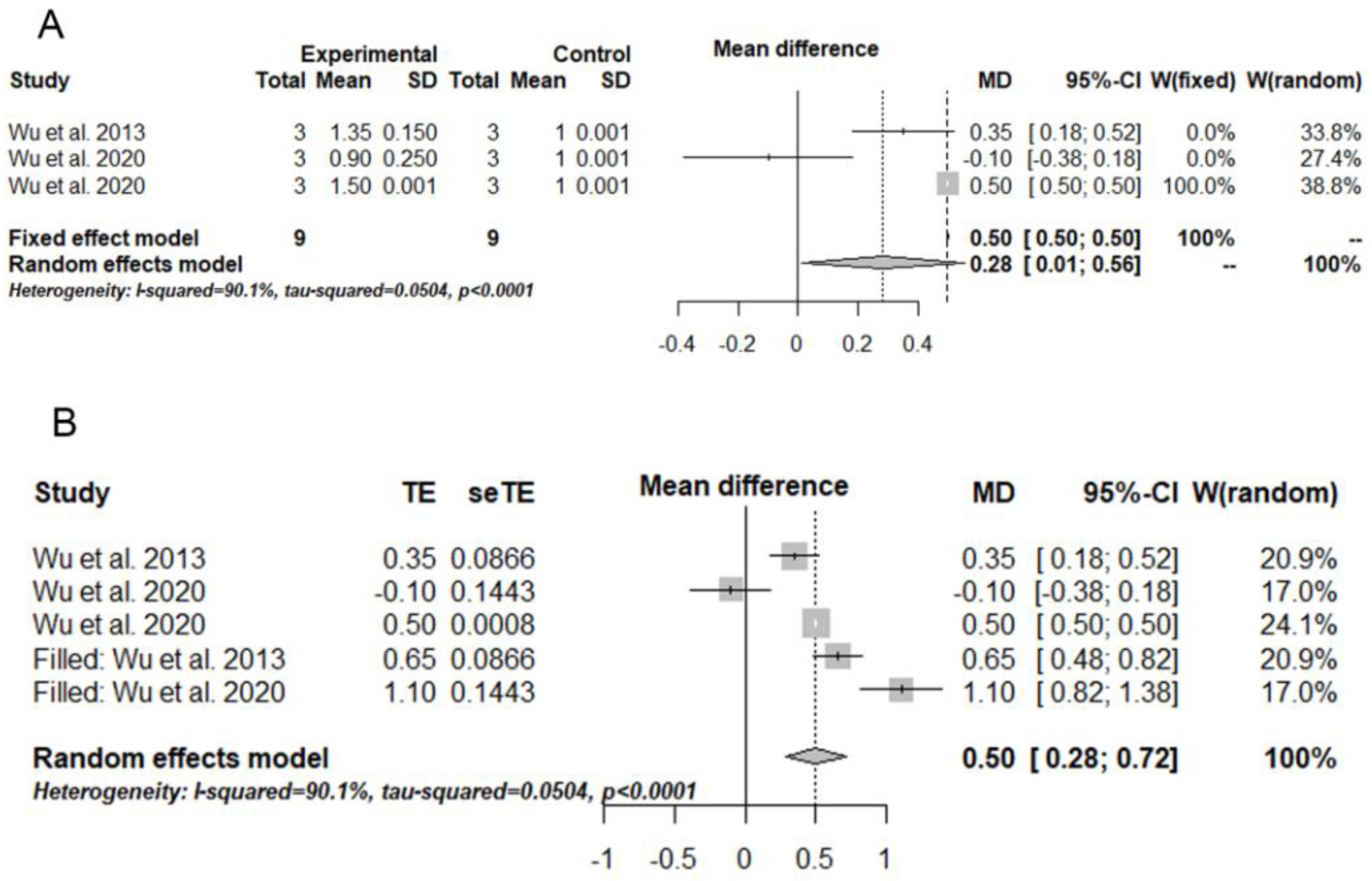
3.4. Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. A global cancer statistic, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, E.; Iacovelli, N.A.; Tombolini, V.; Rancati, T.; Polimeni, A.; De Cecco, L.; Valdagni, R.; De Felice, F. Potential role of microbiome in oncogenesis, outcome prediction and therapeutic targeting for head and neck cancer. Oral Oncol. 2019, 99, 104453. [Google Scholar] [CrossRef]
- Chang, C.M.; Yu, K.J.; Mbulaiteye, S.M.; Hildesheim, A.; Bhatia, K. The extent of genetic diversity of Epstein-Barr virus and its geographic and disease patterns: A need for reappraisal. Virus Res. 2009, 143, 209–221. [Google Scholar] [CrossRef]
- Bakkalci, D.; Jia, Y.; Winter, J.R.; Lewis, J.E.; Taylor, G.S.; Stagg, H.R. Risk factors for Epstein Barr virus-associated cancers: A systematic review, critical appraisal, and mapping of the epidemiological evidence. J. Glob. Health 2020, 10, 010405. [Google Scholar] [CrossRef]
- Sham, J.; Choy, D.; Wei, W.; Ng, M.H.; Zong, Y.-S.; Lin, Z.-X.; Guo, Y.-Q.; Luo, Y. Detection of subclinical riasopharyngeal carcinoma by fibreoptic endoscopy and multiple biopsy. Lancet 1990, 335, 371–374. [Google Scholar] [CrossRef]
- Renaud, S.; Lefebvre, A.; Mordon, S.; Moralès, O.; Delhem, N. Novel Therapies Boosting T Cell Immunity in Epstein Barr Virus-Associated Nasopharyngeal Carcinoma. Int. J. Mol. Sci. 2020, 21, 4292. [Google Scholar] [CrossRef] [PubMed]
- Young, L.S.; Dawson, C.W. Epstein-Barr virus and nasopharyngeal carcinoma. Chin. J. Cancer 2014, 33, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Hutajulu, S.H.; Kurnianda, J.; Tan, B.I.; Middeldorp, J.M. Therapeutic implications of Epstein-Barr virus infection for the treatment of nasopharyngeal carcinoma. Ther. Clin. Risk Manag. 2014, 10, 721–736. [Google Scholar] [CrossRef]
- Banko, A.; Miljanovic, D.; Lazarevic, I.; Cirkovic, A. A Systematic Review of Epstein–Barr Virus Latent Membrane Protein 1 (LMP1) Gene Variants in Nasopharyngeal Carcinoma. Pathogens 2021, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Edilova, M.I.; Abdul-Sater, A.A.; Watts, T.H. TRAF1 Signaling in Human Health and Disease. Front. Immunol. 2018, 9, 2969. [Google Scholar] [CrossRef]
- da Costa, V.G.; Marques-Silva, A.C.; Moreli, M.L. The Epstein-Barr virus latent membrane protein-1 (LMP1) 30-bp deletion and XhoI-polymorphism in nasopharyngeal carcinoma: A meta-analysis of observational studies. Syst. Rev. 2015, 4, 46. [Google Scholar] [CrossRef]
- Ahn, M.-J.; Chirovsky, D.; Kuyas, H.; Auclair, V.; Abounit, S.; Joo, S.; Shah, R.; Yang, M.-H. Global longitudinal assessment of treatment outcomes in recurrent/metastatic nasopharyngeal carcinoma: GLANCE-NPC study. Futur. Oncol. 2021, 17, 2015–2025. [Google Scholar] [CrossRef]
- Ou, D.; Blanchard, P.; El Khoury, C.; De Felice, F.; Even, C.; Levy, A.; Nguyen, F.; Janot, F.; Gorphe, P.; Deutsch, E.; et al. Induction chemotherapy with docetaxel, cisplatin and fluorouracil followed by concurrent chemoradiotherapy or chemoradiotherapy alone in locally advanced non-endemic nasopharyngeal carcinoma. Oral Oncol. 2016, 62, 114–121. [Google Scholar] [CrossRef]
- Zong, J.; Liu, Y.; Liang, Q.; Xu, H.; Chen, B.; Guo, Q.; Xu, Y.; Hu, C.; Pan, J.; Lin, S. Administration of oral maintenance chemotherapy for 1 year following definitive chemoradiotherapy may improve the survival of patients with stage N3 nasopharyngeal carcinoma. Oral Oncol. 2021, 118, 105313. [Google Scholar] [CrossRef] [PubMed]
- Stoker, S.D.; van Diessen, J.N.A.; de Boer, J.P.; Karakullukcu, B.; Leemans, C.R.; Tan, I.B. Current Treatment Options for Local Residual Nasopharyngeal Carcinoma. Curr. Treat. Options Oncol. 2013, 14, 475–491. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef]
- NTP-OHAT. OHAT Risk of Bias Rating Tool for Human and Animal Studies; Office of Health Assessment and Translation: Rockville, MD, USA, 2015. [Google Scholar]
- NTP-OHAT. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration; National Toxicology Program–Office of Health Assessment and Translation: Rockville, MD, USA, 2019. [Google Scholar]
- Ferrisse, T.M.; de Oliveira, A.B.; Surur, A.K.; Buzo, H.S.; Brighenti, F.L.; Fontana, C.R. Photodynamic therapy associated with nanomedicine strategies for treatment of human squamous cell carcinoma: A systematic review and meta-analysis. Nanomedicine 2022, 40, 102505. [Google Scholar] [CrossRef]
- Du, H.; Bay, B.H.; Mahendran, R.; Olivo, M. Endogenous expression of interleukin-8 and interleukin-10 in nasopharyngeal carcinoma cells and the effect of photodynamic therapy. Int. J. Mol. Med. 2002, 10, 73–76. [Google Scholar] [CrossRef]
- Koon, H.K.; Lo, K.W.; Leung, K.N.; Lung, M.L.; Chang, C.C.; Wong, R.N.; Leung, W.N.; Mak, N.K. Photodynamic therapy-mediated modulation of inflammatory cytokine production by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Cell Mol. Immunol. 2010, 7, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, Z.; Liu, L.; Huang, Z.; Huang, Z.; Xie, S. Differences in sensitivity to HMME-mediated photodynamic therapy between EBV+ C666-1 and EBV- CNE2 cells. Photodiagnosis Photodyn. Ther. 2010, 7, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.W.; Chu, E.S.; Huang, Z.; Xu, C.S.; Ip, C.W.; Yow, C.M. FosPeg® PDT alters the EBV miRNAs and LMP1 protein expression in EBV positive nasopharyngeal carcinoma cells. J. Photochem. Photobiol. B 2013, 127, 114–122. [Google Scholar] [CrossRef]
- Peng, Y.; He, G.; Tang, D.; Xiong, L.; Wen, Y.; Miao, X.; Hong, Z.; Yao, H.; Chen, C.; Yan, S.; et al. Lovastatin Inhibits Cancer Stem Cells and Sensitizes to Chemo- and Photodynamic Therapy in Nasopharyngeal Carcinoma. J. Cancer 2017, 8, 1655–1664. [Google Scholar] [CrossRef]
- Wu, R.W.K.; Chu, E.S.M.; Yuen, J.W.M.; Huang, Z. Comparative study of FosPeg® photodynamic effect on nasopharyngeal carcinoma cells in 2D and 3D models. J. Photochem. Photobiol. B 2020, 210, 111987. [Google Scholar] [CrossRef]
- Wu, R.W.K.; Chu, E.S.M.; Yow, C.M.N. Evaluation of the effect of 5-aminolevulinic acid hexyl ester (H-ALA) PDT on EBV LMP1 protein expression in human nasopharyngeal cells. Photodiagnosis Photodyn. Ther. 2020, 30, 101801. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Chang, Y.-L.; To, K.-F.; Mai, H.-Q.; Feng, Y.-F.; Chang, E.T.; Wang, C.-P.; Kam, M.K.M.; Cheah, S.-L.; Lee, M.; et al. A new prognostic histopathologic classification of nasopharyngeal carcinoma. Chin. J. Cancer 2016, 35, 41. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Tsao, S.W.; Yip, Y.L.; Tsang, C.M.; Pang, P.S.; Lau, V.M.; Zhang, G.; Lo, K.W. Etiological factors of nasopharyngeal carcinoma. Oral Oncol. 2014, 50, 330–338. [Google Scholar] [CrossRef]
- Bossi, P.; Chan, A.; Licitra, L.; Trama, A.; Orlandi, E.; Hui, E.; Halámková, J.; Mattheis, S.; Baujat, B.; Hardillo, J.; et al. Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment, and follow-up†. Ann. Oncol. 2021, 32, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Mayor, S. Side-effects of cancer drugs are under-reported in trials. Lancet Oncol. 2015, 16, e107. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.C.; Pinto, J.G.; Vitorio, G.D.S.; Ferreira, I.; Pacheco-Soares, C.; Mamone, L.A.; Strixino, J.F. Photodynamic effect of protoporphyrin IX in gliosarcoma 9l/lacZ cell line. Photodiagnosis Photodyn. Ther. 2022, 37, 102669. [Google Scholar] [CrossRef]
- Mkhobongo, B.; Chandran, R.; Abrahamse, H. The Role of Melanoma Cell-Derived Exosomes (MTEX) and Photodynamic Therapy (PDT) within a Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 9726. [Google Scholar] [CrossRef]
- Vallecorsa, P.; Di Venosa, G.; Gola, G.; Sáenz, D.; Mamone, L.; MacRobert, A.J.; Ramírez, J.; Casas, A. Photodynamic therapy of cutaneous T-cell lymphoma cell lines mediated by 5-aminolevulinic acid and derivatives. J. Photochem. Photobiol. B 2021, 221, 112244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, M.; He, S.; Luo, Y.; Zhao, Y.; Cheng, J.; Gong, Y.; Xie, J.; Wang, Y.; Hu, B.; et al. Necroptosis regulates tumor repopulation after radiotherapy via RIP1/RIP3/MLKL/JNK/IL8 pathway. J. Exp. Clin. Cancer Res. 2019, 38, 461. [Google Scholar] [CrossRef] [PubMed]
- Beltrán Hernández, I.; Yu, Y.; Ossendorp, F.; Korbelik, M.; Oliveira, S. Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations. J. Clin. Med. 2020, 9, 333. [Google Scholar] [CrossRef] [PubMed]
- Huis In ’t Veld, R.V.; Heuts, J.; Ma, S.; Cruz, L.J.; Ossendorp, F.A.; Jager, M.J. Current Challenges and Opportunities of Photodynamic Therapy against Cancer. Pharmaceutics 2023, 15, 330. [Google Scholar] [CrossRef]
- Teijeira, A.; Garasa, S.; Ochoa, M.C.; Villalba, M.; Olivera, I.; Cirella, A.; Eguren-Santamaria, I.; Berraondo, P.; Schalper, K.A.; de Andrea, C.E.; et al. IL8, Neutrophils, and NETs in a Collusion against Cancer Immunity and Immunotherapy. Clin. Cancer Res. 2021, 27, 2383–2393. [Google Scholar] [CrossRef]
- Allen, D.Z.; Aljabban, J.; Silverman, D.; McDermott, S.; Wanner, R.A.; Rohr, M.; Hadley, D.; Panahiazar, M. Meta-Analysis illustrates possible role of lipopolysaccharide (LPS)-induced tissue injury in nasopharyngeal carcinoma (NPC) pathogenesis. PLoS ONE 2021, 16, e0258187. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, Q.; Wei, F.; Li, X.; Zhang, W.; Fan, S.; Shi, L.; Li, X.; Gong, Z.; Ma, J.; et al. LPLUNC1 inhibits nasopharyngeal carcinoma cell growth via down-regulation of the MAP kinase and cyclin D1/E2F pathways. PLoS ONE 2013, 8, e62869. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; Shayakhmetov, D.M. Interleukin 1α and the inflammatory process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Della Vittoria Scarpati, G.; Giuliano, M.; D’Aniello, C.; Gnoni, A.; Cavaliere, C.; Licchetta, A.; Pisconti, S. Epstein-Barr virus infection and nasopharyngeal carcinoma: The other side of the coin. Anticancer Drugs 2015, 26, 1017–1025. [Google Scholar] [CrossRef]
- Perri, F.; Sabbatino, F.; Ottaiano, A.; Fusco, R.; Caraglia, M.; Cascella, M.; Longo, F.; Rega, R.A.; Salzano, G.; Pontone, M.; et al. Impact of Epstein Barr Virus Infection on Treatment Opportunities in Patients with Nasopharyngeal Cancer. Cancers 2023, 15, 1626. [Google Scholar] [CrossRef] [PubMed]
- Dawson, C.W.; Rickinson, A.B.; Young, L.S. Epstein-Barr virus latent membrane protein inhibits human epithelial cell differentiation. Nature 1990, 344, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Fhraeus, R.; Rymo, L.; Rhim, J.S.; Klein, G. Morphological transformation of human keratinocytes expressing the LMP gene of Epstein-Barr virus. Nature 1990, 345, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.E.; Earp, H.S.; Raab-Traub, N. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J. Virol. 1995, 69, 4390–4398. [Google Scholar] [CrossRef]
- Kieff, E.; Rickinson, A.B. Epstein-Barr virus and its replication. In Field’s Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott/Williams & Wilkins: Philadelphia, PA, USA, 2001; Volume 2, pp. 2511–2573. [Google Scholar]
- Lo, A.K.; Dawson, C.W.; Lung, H.L.; Wong, K.L.; Young, L.S. The Role of EBV-Encoded LMP1 in the NPC Tumor Microenvironment: From Function to Therapy. Front. Oncol. 2021, 11, 640207. [Google Scholar] [CrossRef]
- Pietruszewska, W.; Bojanowska-Poźniak, K.; Kobos, J. Matrix metalloproteinases MMP1, MMP2, MMP9 and their tissue inhibitors TIMP1, TIMP2, TIMP3 in head and neck cancer: An immunohistochemical study. Otolaryngol. Pol. 2016, 70, 32–43. [Google Scholar] [CrossRef]
- Jiang, H.; Li, H. Prognostic values of tumoral MMP2 and MMP9 overexpression in breast cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 149. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, H.; Wang, W.; Zhang, M. A size-tunable nanoplatform: Enhanced MMP2-activated chemo-photodynamic immunotherapy based on biodegradable mesoporous silica nanoparticles. Biomater. Sci. 2021, 9, 917–929. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Koukourakis, M.I.; Georgiou, I.; Kouroupi, M.; Sivridis, E. LC3A, LC3B and Beclin-1 Expression in Gastric Cancer. Anticancer Res. 2018, 38, 6827–6833. [Google Scholar] [CrossRef]
- Xiong, L.; Liu, Z.; Ouyang, G.; Lin, L.; Huang, H.; Kang, H.; Chen, W.; Miao, X.; Wen, Y. Autophagy inhibition enhances photocytotoxicity of Photosan-II in human colorectal cancer cells. Oncotarget 2017, 8, 6419–6432. [Google Scholar] [CrossRef]
- Ji, H.T.; Chien, L.T.; Lin, Y.H.; Chien, H.F.; Chen, C.T. 5-ALA mediated photodynamic therapy induces autophagic cell death via AMP-activated protein kinase. Mol. Cancer 2010, 9, 91. [Google Scholar] [CrossRef]
- Martins, W.K.; Belotto, R.; Silva, M.N.; Grasso, D.; Suriani, M.D.; Lavor, T.S.; Itri, R.; Baptista, M.S.; Tsubone, T.M. Autophagy Regulation and Photodynamic Therapy: Insights to Improve Outcomes of Cancer Treatment. Front. Oncol. 2021, 10, 610472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ji, Z.; Zhang, J.; Yang, S. Photodynamic therapy enhances skin cancer chemotherapy effects through autophagy regulation. Photodiagn Photodyn. Ther. 2019, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-binding cassette (ABC) transporter family. Hum. Genom. 2009, 3, 281–290. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef]
- Saltaji, H.; Armijo-Olivo, S.; Cummings, G.G.; Amin, M.; Da Costa, B.R.; Flores-Mir, C. Influence of blinding on treatment effect size estimate in randomized controlled trials of oral health interventions. BMC Med. Res. Methodol. 2018, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.Y.; Tang, W.P.; Zeng, Y.; Tang, W.P.; Zhao, M.L.; Deng, H.H.; Li, Q. An epithelial cell line established from poorly differentiated nasopharyngeal carcinoma. Ai Zheng 1983, 2, 70–72. [Google Scholar]
- Cheung, S.T.; Huang, D.P.; Hui, A.B.; Lo, K.W.; Ko, C.W.; Tsang, Y.S.; Wong, N.; Whitney, B.M.; Lee, J.C. Nasopharyngeal carcinoma cell line (C666-1) consistently harbouring Epstein-Barr virus. Int. J. Cancer 1999, 83, 121–126. [Google Scholar] [CrossRef]
- Huang, D.P.; Ho, J.H.C.; Poon, Y.F.; Chew, E.C.; Saw, D.; Lui, M.; Li, C.L.; Mak, L.S.; Lai, S.H.; Lau, W.H. Establishment of a cell line (NPC/HK1) from a differentiated squamous carcinoma of the nasopharynx. Int. J. Cancer 1980, 26, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]

| # | Author | Study Design | Cell Line | Sample Size | Evaluated Group | Photosensitizer | Wavelength (nm) | Irradiation Time (minutes) | Photosensitizer Incubation Time | Light Dose | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Du et al., 2002 [20] | In vitro | HK-1 CNE-2 | 6 | G1: PDT G2: no PDT | Hypericin | 585 | ND | 4 h (HK-1) 6 h (CNE-2) | 0.5 J/cm2 | IL-8 (pg/mL) HK-1/ G1: 168.80 ± 7.93 HK-1/G2: 130.80 ± 5.80 CNE-2/ G1:71.15 ± 9.81 CNE-2/G2: 60.09 ± 2.01 |
| 2 | Koon et al., 2010 [21] | In vitro | HK-1 | ND | G1: HK-1 (EBV+) G2: HK-1 (EBV-) G3: control (no PDT + EBV+) | Zn-BC-AM | 682 | ND | 24 h | 0.25–1.0 J/cm2 | Apoptosis (PI) HK-1 (EBV+): 80% HK-1 (EBV−): 60% IL-1α (pg/mL) HK-1 (EBV+): 6300 ± 250 HK-1 (EBV−): 3301 ± 500 Control/G3: 1350 ± 250 IL-1β (pg/mL) HK-1 (EBV+): 92 ± 5 HK-1 (EBV−): 55 ± 5 Control/G3: 18 ± 2 IL-8 (pg/mL) HK-1 (EBV+): 15 ± 1 HK-1 (EBV−): 0 ± 0 Control/G3: 430 ± 25 |
| 3 | Li et al., 2010 [22] | In vitro | c666-1 CNE-2 | 3 | G1: c666-1 (EBV+) G2: CNE-2 (EBV-) | HMME (7(12)-(1-methoxyethyl)-12(7)-(1-hydroxyethyl)-3,8,13,17- tetramethyl-21H,23H-porphin-2,18-dipropionic) | 630 | ND | 3 h | 0.6–14.4 J/cm2 | Phototoxicity (clonogenic assay) There were significant and similar results for G1 and G2, particularly when the intracellular uptake of HMME was balanced between the groups. |
| 4 | Wu et al., 2013 [23] | In vitro | c666-1 HK-1 CNE-2 | 3 | G1: c666-1 (EBV+) G2: HK-1 (EBV-) G3: CNE-2 (EBV-) | FosPeg | 630 | ND | 4 h | 3.0 J/cm2 | Cytotoxicity (MTT) c666-1: 69% HK-1: 77% CNE-2: 84% LMP1 mRNA expression c666-1: 8 ± 1.5 (PDT+) c666-1: 1 ± 0.0 (PDT−) EBV-miR-BART 1-5p c666-1: 0.75 ± 0.1 (PDT+) c666-1: 1.0 ± 0.0 (PDT−) EBV-miR-BART 16 c666-1: 0.6 ± 0.25 (PDT+) c666-1: 1.0 ± 0.0 (PDT−) EBV-miR-BART 17-5p c666-1: 0.75 ± 0.1 (PDT+) c666-1: 1.0 ± 0.1 (PDT−) LMP1 protein expression c666-1: 1.35 ± 0.15 (PDT+) c666-1: 1.0 ± 0.1 (PDT−) |
| 5 | Peng et al., 2017 [24] | In vitro | NPC 5-8F NPC 6-10B | ND | G1: PDT G2: PDT + Lovastatin | Photosan II | 630 | 1 | 24 h | 10 J/cm2 | Cell viability (Alamar blue) There were significant results for Lovastatin + PDT for both cell lines. |
| 6 | Wu et al., 2020 (a) [25] | In vitro | c666-1 | 3 | G1: 2D culture G2: 3D culture (MCL and MCS) | FosPeg | 652 | ND | 24 h | 20 J/cm2 | Cell viability (MTT) 2D: 95 ± 5% MCL: 60 ± 10% MCS: 70% Apoptosis (Annexin V) 2D: 30.6 ± 7.7 MCL: 31.0 ± 7.4 MCS: 27.6 ± 7.0 Necrosis (Annexin V) 2D: 16.3 ± 8.6 MCL: 9.8 ± 10.6 MCS: 13.5 ± 3.2 LC3BI protein expression 2D: 1.5 ± 1.0 MCL: 1.4 ± 1.2 MCS: 0.8 ± 0.5 LC3BII protein expression 2D: 1.8 ± 1.0 MCL: 1.25 ± 0.8 MCS: 0.8 ± 0.5 LMP1 protein expression 2D: 0.9 ± 0.25 MCL: 1.25 ± 1.0 MCS: 2.0 ± 1.25 MMP2 protein expression 2D: 0.7 ± 0.15 MCL: 1.2 ± 0.25 MCS: 1.5 ± 1.0 MMP9 protein expression 2D: 0.7 ± 0.15 MCL: 2.2 ± 0.75 MCS: 1.5 ± 0.65 ABCB1 protein expression 2D: 0.5 ± 0.25 MCL: 1.5 ± 0.65 MCS: 1 ± 0.8 ABCC1 protein expression 2D: 1.0 ± 0.25 MCL: 2.3 ± 1.2 MCS: 1.8 ± 0.1 ABCG2 protein expression 2D: 1.7 ± 0.5 MCL: 1.5 ± 0.5 MCS: 1.8 ± 2.0 |
| 7 | Wu et al., 2020 (b) [26] | In vitro | c666-1 CNE-2 | 3 | G1: c666-1 (EBV+) G2: CNE-2 (EBV-) | H-ALA (5-aminolevulinic acid hexyl ester) | 630 | ND | 4 h | 2–4 J/cm2 | Cytotoxicity (MTT) G1: 70% G2: 80% LMP1 protein expression G1: 1.5 ± 0.0 Control: 1.0 ± 0 EGRF protein expression G1: 0.75 ± 0.16 G2: 0.6 ± 0.0 Control: 1.0 ± 0.0 p-EGRF protein expression G1: 0.5 ± 0.3 G2: 0.8 ± 0.16 Control: 1.0 ± 0.0 NF-ĸB protein expression G1: 0.8 ± 0.25 G2: 0.8 ± 0.16 Control: 1.0 ± 0.0 |
| Questions/Studies | Du et al., 2002 [20] | Koon et al., 2010 [21] | Li et al., 2010 [22] | Wu et al., 2013 [23] | Peng et al., 2017 [24] | Wu et al., 2020 (a) [25] | Wu et al., 2020 (b) [26] |
|---|---|---|---|---|---|---|---|
| Was the administered dose or exposure level adequately randomized? | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Were study group allocations adequately concealed? | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Were the experimental conditions identical across study groups? | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Were research personnel blind to the study group during the investigation? | − | − | − | − | − | − | − |
| Were outcome data complete without attrition or exclusion from the analysis? | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Was the exposure characterization reliable? | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Was the outcome assessment reliable (including the blinding of evaluators)? | − | − | − | − | − | − | − |
| Were there no other potential threats to internal validity? | −− | −− | −− | −− | −− | −− | −− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fornel, D.G.; Ferrisse, T.M.; de Oliveira, A.B.; Fontana, C.R. Photodynamic Therapy Can Modulate the Nasopharyngeal Carcinoma Microenvironment Infected with the Epstein–Barr Virus: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 1344. https://doi.org/10.3390/biomedicines11051344
Fornel DG, Ferrisse TM, de Oliveira AB, Fontana CR. Photodynamic Therapy Can Modulate the Nasopharyngeal Carcinoma Microenvironment Infected with the Epstein–Barr Virus: A Systematic Review and Meta-Analysis. Biomedicines. 2023; 11(5):1344. https://doi.org/10.3390/biomedicines11051344
Chicago/Turabian StyleFornel, Diógenes Germano, Túlio Morandin Ferrisse, Analú Barros de Oliveira, and Carla Raquel Fontana. 2023. "Photodynamic Therapy Can Modulate the Nasopharyngeal Carcinoma Microenvironment Infected with the Epstein–Barr Virus: A Systematic Review and Meta-Analysis" Biomedicines 11, no. 5: 1344. https://doi.org/10.3390/biomedicines11051344
APA StyleFornel, D. G., Ferrisse, T. M., de Oliveira, A. B., & Fontana, C. R. (2023). Photodynamic Therapy Can Modulate the Nasopharyngeal Carcinoma Microenvironment Infected with the Epstein–Barr Virus: A Systematic Review and Meta-Analysis. Biomedicines, 11(5), 1344. https://doi.org/10.3390/biomedicines11051344








