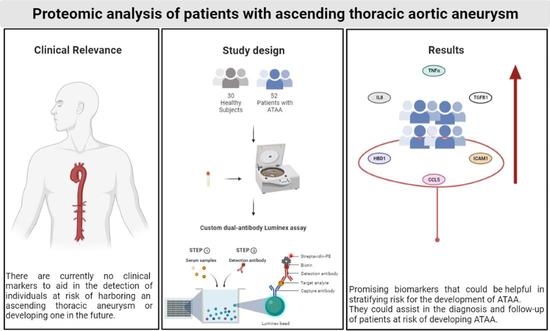
Targeted Proteomic Analysis of Patients with Ascending Thoracic Aortic Aneurysm
Abstract
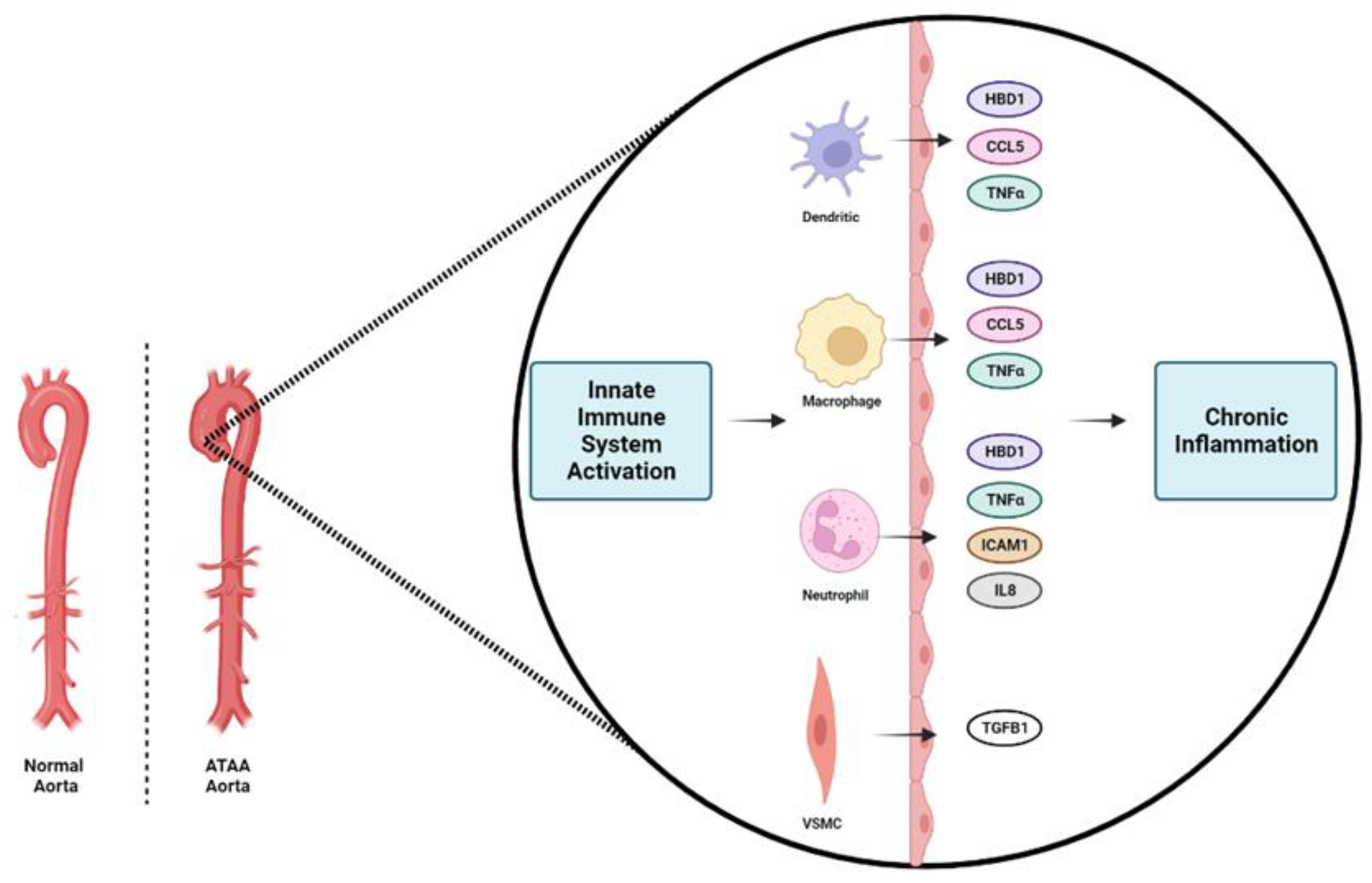
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Definition of ATAA
2.3. Blood Sampling
2.4. Proteomics
2.5. Luminex Assay Principle
2.6. Statistical Analysis
3. Results
3.1. Study Population
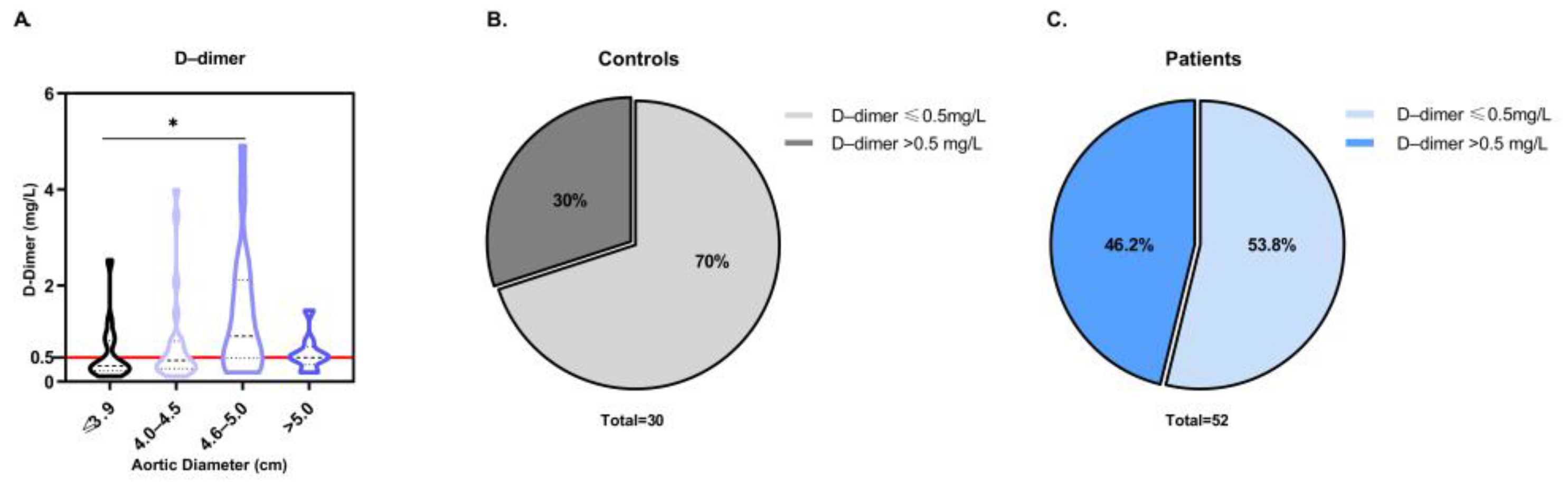
3.2. Evaluation of D-Dimer as a Potential Diagnostic Biomarker for ATAA
3.3. Targeted Proteomic Analysis for the Investigation of Potential Diagnostic Biomarkers for ATAA
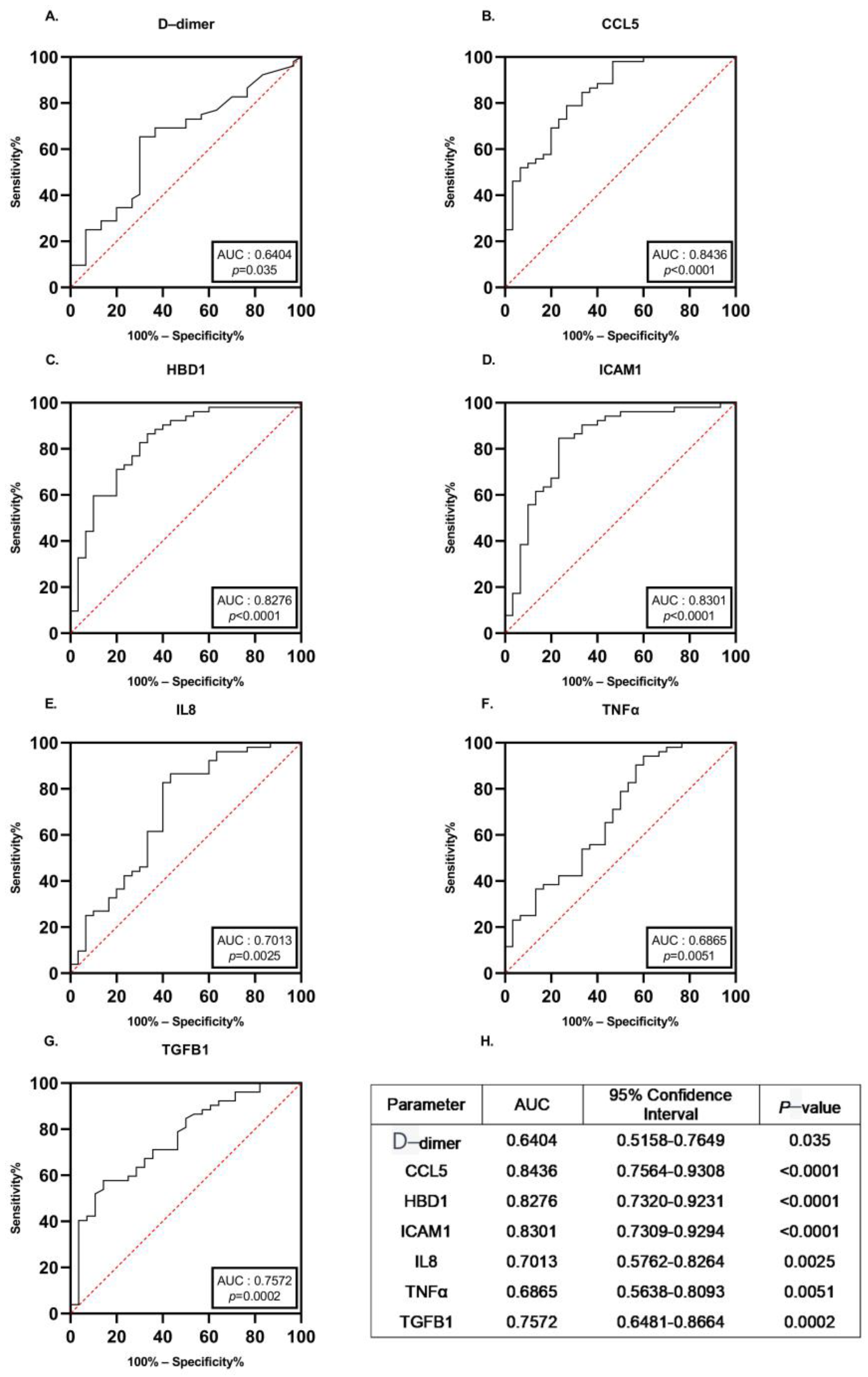
3.4. Diagnostic Performance of the Identified Proteins
3.5. Targeted Proteomic Analysis to Investigate the Specificity of the Potential Biomarkers for the ATAA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeyeldin, A.A.; Velasquez, C.A.; Mahmood, S.U.B.; Brownstein, A.J.; Zafar, M.A.; Ziganshin, B.A.; Elefteriades, J.A. Thoracic aortic aneurysm: Unlocking the “silent killer” secrets. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 1–11. [Google Scholar] [CrossRef]
- Bossone, E.; LaBounty, T.M.; Eagle, K.A. Acute aortic syndromes: Diagnosis and management, an update. Eur. Heart J. 2018, 39, 739–749d. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Preventza, O.; Hamilton, B.I.J.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease. J. Am. Coll. Cardiol. 2022, 80, e223–e393. [Google Scholar] [CrossRef]
- Cheung, K.; Boodhwani, M.; Chan, K.; Beauchesne, L.; Dick, A.; Coutinho, T. Thoracic Aortic Aneurysm Growth: Role of Sex and Aneurysm Etiology. J. Am. Heart Assoc. 2017, 6, e003792. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; De Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed]
- WISQARS (Web-based Injury Statistics Query and Reporting System)|Injury Center|CDC. 2021. Available online: https://www.cdc.gov/injury/wisqars/index.html (accessed on 9 November 2022).
- Coady, M.A.; Rizzo, J.A.; Goldstein, L.J.; Elefteriades, J.A. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol. Clin. 1999, 17, 615–635. [Google Scholar] [CrossRef]
- A Decade of Discovery in the Genetic Understanding of Thoracic Aortic Disease—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0828282X1501541X?casa_token=wsO6MWbHU80AAAAA:TVZoTIPiUm-OC5_UlBmZOLDB1bPXfnNYvscmHX_SC_hzfOGv4xSF0zJm3HdpQ2Nn5dDJuXvEjw (accessed on 11 November 2022).
- Sidloff, D.; Choke, E.; Stather, P.; Bown, M.; Thompson, J.; Sayers, R. Mortality From Thoracic Aortic Diseases and Associations With Cardiovascular Risk Factors. Circulation 2014, 130, 2287–2294. [Google Scholar] [CrossRef]
- Landenhed, M.; Engström, G.; Gottsäter, A.; Caulfield, M.P.; Hedblad, B.; Newton-Cheh, C.; Melander, O.; Smith, J.G. Risk Profiles for Aortic Dissection and Ruptured or Surgically Treated Aneurysms: A Prospective Cohort Study. J. Am. Heart Assoc. 2015, 4, e001513. [Google Scholar] [CrossRef] [PubMed]
- Huusko, T.; Salonurmi, T.; Taskinen, P.; Liinamaa, J.; Juvonen, T.; Pääkkö, P.; Savolainen, M.; Kakko, S. Elevated messenger RNA expression and plasma protein levels of osteopontin and matrix metalloproteinase types 2 and 9 in patients with ascending aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2013, 145, 1117–1123. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Montemurro, R.; Butrico, L.; Caliò, F.G.; Mastrangelo, D.; Scarcello, E.; Gallelli, L.; Buffone, G.; de Franciscis, S. The role of matrix metalloproteinases and neutrophil gelatinase-associated lipocalin in central and peripheral arterial aneurysms. Surgery 2015, 157, 155–162. [Google Scholar] [CrossRef]
- Elefteriades, J.A. Natural history of thoracic aortic aneurysms: Indications for surgery, and surgical versus nonsurgical risks. Ann. Thorac. Surg. 2002, 74, S1877–S1880; discussion S1892–S1898. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Barbacioru, C.C.; Shiffman, D.; Balasubramanian, S.; Iakoubova, O.; Tranquilli, M.; Albornoz, G.; Blake, J.; Mehmet, N.N.; Ngadimo, D.; et al. Gene Expression Signature in Peripheral Blood Detects Thoracic Aortic Aneurysm. PLoS ONE 2007, 2, e1050. [Google Scholar] [CrossRef] [PubMed]
- van Bogerijen, G.H.; Tolenaar, J.L.; Grassi, V.; Lomazzi, C.; Segreti, S.; Rampoldi, V.; Elefteriades, J.A.; Trimarchi, S. Biomarkers in TAA-the Holy Grail. Prog. Cardiovasc. Dis. 2013, 56, 109–115. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Di Bartolomeo, R.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [PubMed]
- Brownstein, A.J.; Ziganshin, B.A.; Kuivaniemi, H.; Body, S.C.; Bale, A.E.; Elefteriades, J.A. Genes Associated with Thoracic Aortic Aneurysm and Dissection: An Update and Clinical Implications. Aorta (Stamford) 2017, 5, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Elefteriades, J.A.; Mukherjee, S.K.; Mojibian, H. Discrepancies in Measurement of the Thoracic Aorta. J. Am. Coll. Cardiol. 2020, 76, 201–217. [Google Scholar] [CrossRef]
- Elefteriades, J.A.; Farkas, E.A. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J. Am. Coll. Cardiol. 2010, 55, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Abdulkareem, N.; Skroblin, P.; Jahangiri, M.; Mayr, M. Proteomics in aortic aneurysm--what have we learnt so far? Proteom. Clin. Appl. 2013, 7, 504–515. [Google Scholar] [CrossRef]
- Wu, J.; Wang, W.; Chen, Z.; Xu, F.; Zheng, Y. Proteomics applications in biomarker discovery and pathogenesis for abdominal aortic aneurysm. Expert. Rev. Proteom. 2021, 18, 305–314. [Google Scholar] [CrossRef]
- Serhatli, M.; Baysal, K.; Acilan, C.; Tuncer, E.; Bekpinar, S.; Baykal, A.T. Proteomic study of the microdissected aortic media in human thoracic aortic aneurysms. J. Proteome Res. 2014, 13, 5071–5080. [Google Scholar] [CrossRef]
- Cai, H.; Pan, B.; Xu, J.; Liu, S.; Wang, L.; Wu, K.; Yang, P.; Huang, J.; Wang, W. D-Dimer Is a Diagnostic Biomarker of Abdominal Aortic Aneurysm in Patients With Peripheral Artery Disease. Front. Cardiovasc. Med. 2022, 9, 890228. Available online: https://www.frontiersin.org/articles/10.3389/fcvm.2022.890228 (accessed on 16 November 2022).
- Linkins, L.A.; Takach Lapner, S. Review of D-dimer testing: Good, Bad, and Ugly. Int. J. Lab. Hematol. 2017, 39 (Suppl. S1), 98–103. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Guo, D.C.; Sun, W.; Papke, C.L.; Duraisamy, S.; Estrera, A.L.; Safi, H.J.; Ahn, C.; Buja, L.M.; Arnett, F.C.; et al. Characterization of the inflammatory cells in ascending thoracic aortic aneurysms in patients with Marfan syndrome, familial thoracic aortic aneurysms, and sporadic aneurysms. J. Thorac. Cardiovasc. Surg. 2008, 136, 922–929.e1. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Yang, D.; Chen, W.; Kan, H.; Xu, F.; Zhang, H.; Wang, W.; Ji, L.; Zheng, Y. The potential role of chemotaxis and the complement system in the formation and progression of thoracic aortic aneurysms inferred from the weighted gene coexpression network analysis. J. Transl. Med. 2021, 19, 49. [Google Scholar] [CrossRef]
- Shen, Y.H.; LeMaire, S.A. Molecular pathogenesis of genetic and sporadic aortic aneurysms and dissections. Curr. Probl. Surg. 2017, 54, 95–155. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.R.; Clayton, N.P.; Carta, L.; Galatioto, J.; Chiu, E.; Smaldone, S.; Nelson, C.A.; Cheng, S.H.; Wentworth, B.M.; Ramirez, F. Dimorphic effects of transforming growth factor-β signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, L.; Phoon, C.K.L.; Robertson, I.; Dabovic, B.; Ramirez, F.; Rifkin, D.B. Genetic analysis of the contribution of LTBP-3 to thoracic aneurysm in Marfan syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 14012–14017. [Google Scholar] [CrossRef] [PubMed]
- Boileau, C.; Guo, D.C.; Hanna, N.; Regalado, E.S.; Detaint, D.; Gong, L.; Varret, M.; Prakash, S.K.; Li, A.H.; d’Indy, H.; et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat. Genet. 2012, 44, 916–921. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; Shayakhmetov, D.M. Interleukin 1α and the inflammatory process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Du, X.M.; Liu, L.W.; Du, Z.K.; Gu, R.X.; Han, Y.L.; Wang, X.Z. Association of TNF-α gene variations with thoracic aortic dissection risk in a Chinese Han population. Int. Angiol. 2016, 35, 418–424. [Google Scholar] [PubMed]
- Bernhard, S.; Hug, S.; Stratmann, A.E.P.; Erber, M.; Vidoni, L.; Knapp, C.L.; Thomaß, B.D.; Fauler, M.; Nilsson, B.; Ekdahl, K.N.; et al. Interleukin 8 Elicits Rapid Physiological Changes in Neutrophils That Are Altered by Inflammatory Conditions. J. Innate Immun. 2021, 13, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Lenk, G.M.; Tromp, G.; Weinsheimer, S.; Gatalica, Z.; Berguer, R.; Kuivaniemi, H. Whole genome expression profiling reveals a significant role for immune function in human abdominal aortic aneurysms. BMC Genom. 2007, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef]
- Puchenkova, O.A.; Soldatov, V.O.; Belykh, A.E.; Bushueva, O.; Piavchenko, G.A.; Venediktov, A.A.; Shakhpazyan, N.K.; Deykin, A.V.; Korokin, M.V.; Pokrovskiy, M.V. Cytokines in Abdominal Aortic Aneurysm: Master Regulators with Clinical Application. BiomarkInsights 2022, 17, 11772719221095676. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ning, H.; Men, H.; Hou, R.; Fu, M.; Zhang, H.; Liu, J. Regulation of CCL5 Expression in Smooth Muscle Cells Following Arterial Injury. PLoS ONE 2012, 7, e30873. [Google Scholar] [CrossRef]
- Li, Y.; Han, S.; Wu, B.; Zhong, C.; Shi, Y.; Lv, C.; Fu, L.; Zhang, Y.; Lang, Q.; Liang, Z.; et al. CXCL11 Correlates with Immune Infiltration and Impacts Patient Immunotherapy Efficacy: A Pan-Cancer Analysis. Front. Immunol. 2022, 13, 951247. [Google Scholar] [CrossRef]
- Middleton, R.K.; Lloyd, G.M.; Bown, M.J.; Cooper, N.J.; London, N.J.; Sayers, R.D. The pro-inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: A protein array study. J. Vasc. Surg. 2007, 45, 574–580. [Google Scholar] [CrossRef]
- Semple, F.; Dorin, J.R. β-Defensins: Multifunctional Modulators of Infection, Inflammation and More? JIN 2012, 4, 337–348. [Google Scholar] [CrossRef]



| Characteristics | Controls | Patients | p-Value |
|---|---|---|---|
| Gender (f:m) | 13:17 | 22:30 | 0.2048 |
| Age (years) | 71.97 ± 11.28 | 71.27 ± 11.56 | 0.8952 |
| Height (cm) | 165.40 ± 11.09 | 168.50 ± 8.26 | 0.3204 |
| Weight (kg) | 80.23 ± 13.97 | 15.60 ± 15.60 | 0.8951 |
| BMI (kg/m2) | 29.47 ± 4.56 | 28.52 ± 4.80 | 0.3789 |
| BSA (m2) | 1.85 ± 0.18 | 1.92 ± 0.18 | 0.1756 |
| Systolic Blood Pressure (mmHg) | 127 ± 11 | 129 ± 12 | 0.4243 |
| Diastolic Blood Pressure (mmHg) | 69 ± 10 | 71 ± 10 | 0.3572 |
| Heart Rate (bpm) | 77 ± 13 | 75.75 ± 11.82 | 0.7066 |
| Smokers a | 11 (36.67%) | 25 (48.08%) | 0.3616 |
| Hypertension | 19 (63.33%) | 40 (76.92%) | 0.2100 |
| Hyperlipidemia | 8 (36.67%) | 19 (36.54%) | 0.4660 |
| Coronary artery disease | 3 (6.67%) | 6 (11.54%) | >0.9999 |
| Ascending aorta diameter (cm) | 3.39 ± 0.36 | 4.65 ± 0.32 | <0.0001 |
| Aortic arch diameter (cm) | 2.79 ± 0.74 | 2.93 ± 0.58 | 0.2201 |
| Descending aorta diameter (cm) | 2.50 ± 0.45 | 2.69 ± 0.33 | 0.0888 |
| Characteristics | Controls | Patients | p-Value |
|---|---|---|---|
| Glucose (mg/dL) | 133.80 ± 41.49 | 124.70 ± 39.82 | 0.248 |
| Total Cholesterol (mg/dL) | 163.09 ± 33.37 | 157.26 ± 20.84 | 0.178 |
| HDL-C (mg/dL) | 49.66 ± 16.48 | 47.26 ± 12.18 | 0.6914 |
| LDL-C (mg/dL) | 113.30 ± 36.09 | 104.40 ± 34.37 | 0.2616 |
| Triglycerides (mg/dL) | 111.50 ± 40.42 | 125.50 ± 66.63 | 0.676 |
| Hemoglobin (g/dL) | 13.25 ± 1.686 | 13.84 ± 3.701 | 0.4215 |
| WBC (cells/mm3 × 1000) | 8.71 ± 2.85 | 7.71 ± 1.9 | 0.1127 |
| Creatinine (mg/dL) | 1.00 ± 0.35 | 1.01 ± 0.35 | 0.942 |
| SGOT (IU/L) | 21.30 ± 7.54 | 22.75 ± 17.42 | 0.1322 |
| Urea (mg/dL) | 56.09 ± 33.37 | 43.26 ± 20.84 | 0.0789 |
| Uric acid (mg/dL) | 6.16 ± 1.81 | 5.75 ± 1.90 | 0.4437 |
| SGPT (IU/L) | 18.88 ± 11.96 | 19.66 ± 17.24 | 0.769 |
| γGT (IU/L) | 22.96 ± 19.59 | 29.44 ± 48.93 | 0.8767 |
| ALP (U/L) | 73.37 ± 24.49 | 74.37 ± 38.50 | 0.718 |
| Blood amylase (U/mL) | 54.56 ± 14.21 | 69.26 ± 30.72 | 0.1363 |
| Potassium (mEq/L) | 4.51 ± 0.79 | 4.25 ± 0.41 | 0.0699 |
| Sodium (mEq/L) | 137.60 ± 6.07 | 140.30 ± 2.6 | 0.1285 |
| N-terminal prohormone of brain natriuretic peptide (NT-proBNP) (pg/dL) | 347.0 ± 252.2 | 425.5 ± 284.5 | 0.5806 |
| CRP (mg/dL) | 1.71 ± 1.66 | 3.74 ± 7.37 | 0.736 |
| Troponine T (ng/mL) | 0.06 ± 0.09 | 0.05 ± 0.13 | 0.0724 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daskalopoulou, A.; Giotaki, S.G.; Toli, K.; Minia, A.; Pliaka, V.; Alexopoulos, L.G.; Deftereos, G.; Iliodromitis, K.; Dimitroulis, D.; Siasos, G.; et al. Targeted Proteomic Analysis of Patients with Ascending Thoracic Aortic Aneurysm. Biomedicines 2023, 11, 1273. https://doi.org/10.3390/biomedicines11051273
Daskalopoulou A, Giotaki SG, Toli K, Minia A, Pliaka V, Alexopoulos LG, Deftereos G, Iliodromitis K, Dimitroulis D, Siasos G, et al. Targeted Proteomic Analysis of Patients with Ascending Thoracic Aortic Aneurysm. Biomedicines. 2023; 11(5):1273. https://doi.org/10.3390/biomedicines11051273
Chicago/Turabian StyleDaskalopoulou, Aphrodite, Sotiria G. Giotaki, Konstantina Toli, Angeliki Minia, Vaia Pliaka, Leonidas G. Alexopoulos, Gerasimos Deftereos, Konstantinos Iliodromitis, Dimitrios Dimitroulis, Gerasimos Siasos, and et al. 2023. "Targeted Proteomic Analysis of Patients with Ascending Thoracic Aortic Aneurysm" Biomedicines 11, no. 5: 1273. https://doi.org/10.3390/biomedicines11051273
APA StyleDaskalopoulou, A., Giotaki, S. G., Toli, K., Minia, A., Pliaka, V., Alexopoulos, L. G., Deftereos, G., Iliodromitis, K., Dimitroulis, D., Siasos, G., Verikokos, C., & Iliopoulos, D. (2023). Targeted Proteomic Analysis of Patients with Ascending Thoracic Aortic Aneurysm. Biomedicines, 11(5), 1273. https://doi.org/10.3390/biomedicines11051273