Hybrid Argon Plasma Coagulation for Barrett’s Esophagus and for Colonic Mucosal Resection—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Collection
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Literature Search and Study Selection
3.2. Characteristics of Included Studies
3.2.1. Studies on Barrett’s Esophagus
3.2.2. Studies on Colonic Endoscopic Mucosal Resection
3.3. Hybrid Argon Plasma Coagulation for BE
3.3.1. Endoscopic and Histologic Remission
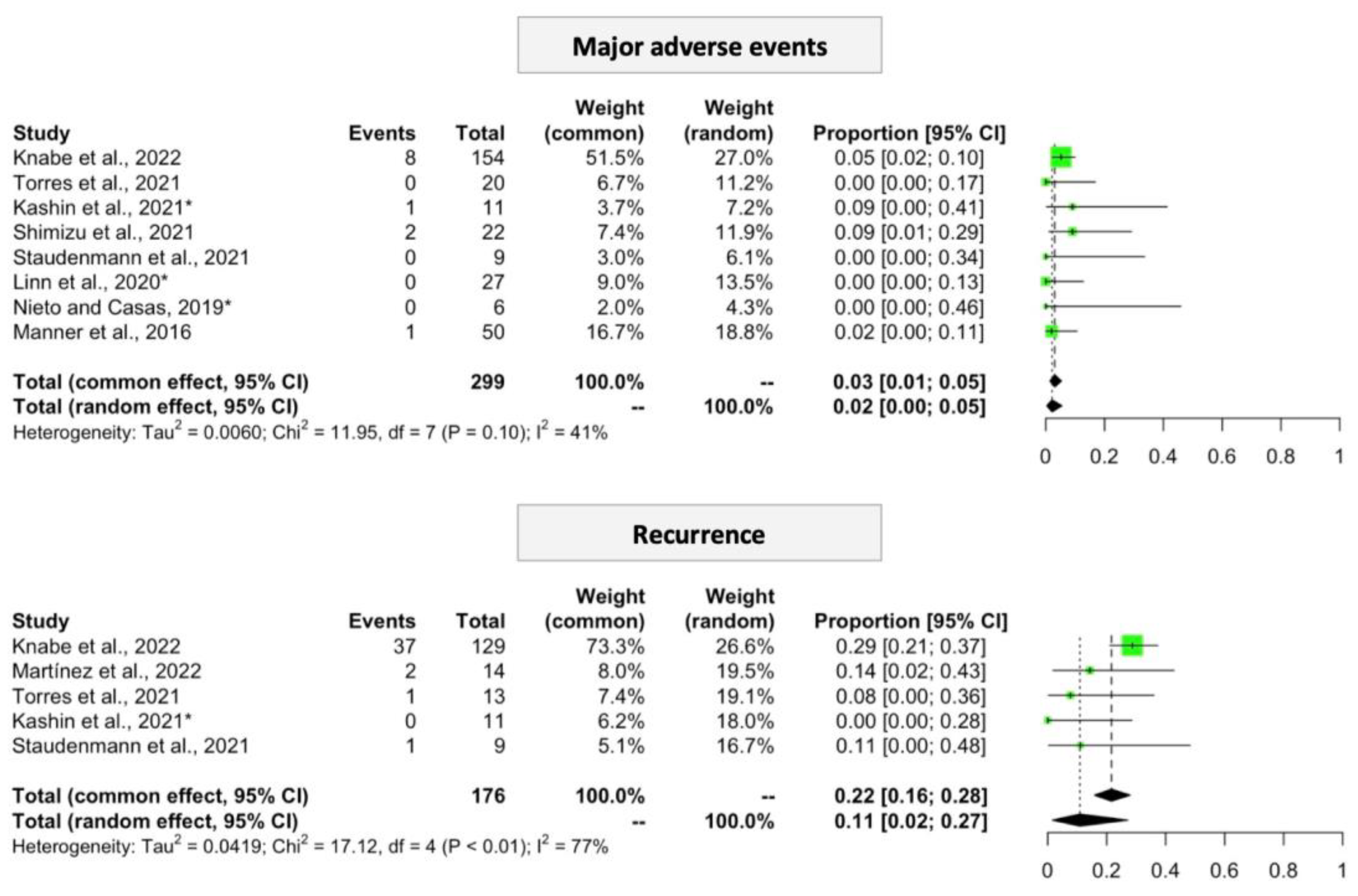
3.3.2. Procedure-Related Adverse Events
3.3.3. Recurrence
3.4. Hybrid Argon Plasma Coagulation after Colonic EMR
3.4.1. Procedure-Related Adverse Events
3.4.2. Recurrence
3.5. Publication Bias and Reporting Quality
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nieuwenhuis, E.A.; van Munster, S.N.; Curvers, W.L.; Weusten, B.L.A.M.; Alvarez Herrero, L.; Bogte, A.; Alkhalaf, A.; Schenk, B.E.; Koch, A.D.; Spaander, M.C.W.; et al. Impact of Expert Center Endoscopic Assessment of Confirmed Low Grade Dysplasia in Barrett’s Esophagus Diagnosed in Community Hospitals. Endoscopy 2022, 54, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Weusten, B.; Bisschops, R.; Coron, E.; Dinis-Ribeiro, M.; Dumonceau, J.-M.; Esteban, J.-M.; Hassan, C.; Pech, O.; Repici, A.; Bergman, J.; et al. Endoscopic Management of Barrett’s Esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017, 49, 191–198. [Google Scholar] [CrossRef]
- van Munster, S.N.; Pouw, R.E.; Sharma, V.K.; Meijer, S.L.; Weusten, B.L.A.M.; Bergman, J.J.G.H.M. Radiofrequency Vapor Ablation for Barrett’s Esophagus: Feasibility, Safety and Proof of Concept in a Stepwise Study with in Vitro, Animal, and the First in-Human Application. Endoscopy 2021, 53, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Staudenmann, D.A.; Skacel, E.P.; Tsoutsman, T.; Kaffes, A.J.; Saxena, P. Safety and Long-Term Efficacy of Hybrid-Argon Plasma Coagulation for the Treatment of Barrett’s Esophagus: An Australian Pilot Study. Int. J. Gastrointest. Interv. 2021, 10, 128–132. [Google Scholar] [CrossRef]
- Overwater, A.; Elias, S.G.; Schoon, E.J.; Bergman, J.J.G.H.M.; Pouw, R.E.; Weusten, B.L.A.M. The Course of Pain and Dysphagia after Radiofrequency Ablation for Barrett’s Esophagus-Related Neoplasia. Endoscopy 2022, 55, 255–260. [Google Scholar]
- Shaheen, N.J.; Sharma, P.; Overholt, B.F.; Wolfsen, H.C.; Sampliner, R.E.; Wang, K.K.; Galanko, J.A.; Bronner, M.P.; Goldblum, J.R.; Bennett, A.E.; et al. Radiofrequency Ablation in Barrett’s Esophagus with Dysplasia. N. Engl. J. Med. 2009, 360, 2277–2288. [Google Scholar] [CrossRef]
- Fasullo, M.; Shah, T.; Patel, M.; Mutha, P.; Zfass, A.; Lippman, R.; Smallfield, G. Outcomes of Radiofrequency Ablation Compared to Liquid Nitrogen Spray Cryotherapy for the Eradication of Dysplasia in Barrett’s Esophagus. Dig. Dis. Sci. 2022, 67, 2320–2326. [Google Scholar] [CrossRef]
- Manner, H.; May, A.; Kouti, I.; Pech, O.; Vieth, M.; Ell, C. Efficacy and Safety of Hybrid-APC for the Ablation of Barrett’s Esophagus. Surg. Endosc. 2016, 30, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.N.; Chehade, N.E.H.; Tavangar, A.; Choi, A.; Monachese, M.; Chang, K.J.; Samarasena, J.B. Hybrid Argon Plasma Coagulation in Barrett’s Esophagus: A Systematic Review and Meta-Analysis. Clin. Endosc. 2023, 56, 38–49. [Google Scholar] [CrossRef]
- Motchum, L.; Levenick, J.M.; Djinbachian, R.; Moyer, M.T.; Bouchard, S.; Taghiakbari, M.; Repici, A.; Deslandres, É.; von Renteln, D. EMR Combined with Hybrid Argon Plasma Coagulation to Prevent Recurrence of Large Nonpedunculated Colorectal Polyps (with Videos). Gastrointest. Endosc. 2022, 96, 840–848. [Google Scholar] [CrossRef]
- Motz, V.L.; Lester, C.; Moyer, M.T.; Maranki, J.L.; Levenick, J.M. Hybrid Argon Plasma Coagulation-Assisted Endoscopic Mucosal Resection for Large Sessile Colon Polyps to Reduce Local Recurrence: A Prospective Pilot Study. Endoscopy 2022, 54, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Levenick, J.M.; Groff, A.J.; Manzo, C.; Lester, C.; Maranki, J.L. Hybrid APC Colon EMR, A Novel Approach to Reduce Local Recurrence. Tech. Innov. Gastrointest. Endosc. 2022, 24, 10–15. [Google Scholar] [CrossRef]
- Chandan, S.; Facciorusso, A.; Ramai, D.; Deliwala, S.; Mohan, B.P.; Kassab, L.L.; Draganov, P.V.; Othman, M.O.; Kochhar, G.S. Snare Tip Soft Coagulation (STSC) after Endoscopic Mucosal Resection (EMR) of Large (>20 Mm) Non Pedunculated Colorectal Polyps: A Systematic Review and Meta-Analysis. Endosc. Int. Open 2022, 10, E74–E81. [Google Scholar] [CrossRef] [PubMed]
- Kemper, G.; Turan, A.S.; Schoon, E.J.; Schrauwen, R.W.M.; Epping, L.S.M.; Gerges, C.; Beyna, T.; Neuhaus, H.; Gündug, U.; Siersema, P.D.; et al. Endoscopic Techniques to Reduce Recurrence Rates after Colorectal EMR: Systematic Review and Meta-Analysis. Surg. Endosc. 2021, 35, 5422–5429. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbach, T.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Gupta, S.; Lieberman, D.; Robertson, D.J.; Shaukat, A.; Syngal, S.; Rex, D.K. Endoscopic Removal of Colorectal Lesions-Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest. Endosc. 2020, 91, 486–519. [Google Scholar] [CrossRef]
- Manner, H.; Neugebauer, A.; Scharpf, M.; Braun, K.; May, A.; Ell, C.; Fend, F.; Enderle, M.D. The Tissue Effect of Argon-Plasma Coagulation with Prior Submucosal Injection (Hybrid-APC) versus Standard APC: A Randomized Ex-Vivo Study. United Eur. Gastroenterol. J. 2014, 2, 383–390. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; The Cochrane Collaboration, Ed.; Cochrane: London, UK, 2011. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement—Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid. Based. Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Elli, L.; Casazza, G.; Locatelli, M.; Branchi, F.; Ferretti, F.; Conte, D.; Fraquelli, M. Use of Enteroscopy for the Detection of Malignant and Premalignant Lesions of the Small Bowel in Complicated Celiac Disease: A Meta-Analysis. Gastrointest. Endosc. 2017, 86, 264–273.e1. [Google Scholar] [CrossRef]
- Knabe, M.; Beyna, T.; Rösch, T.; Bergman, J.; Manner, H.; May, A.; Schachschal, G.; Neuhaus, H.; Kandler, J.; Weusten, B.; et al. Hybrid APC in Combination With Resection for the Endoscopic Treatment of Neoplastic Barrett’s Esophagus: A Prospective, Multicenter Study. Am. J. Gastroenterol. 2022, 117, 110–119. [Google Scholar] [CrossRef]
- Kashin, S.V.; Kuvaev, R.; Nadezhin, A.S.; Kraynova, E.A.; Nekhaykova, N. The New Hybrid Argon Plasma Coagulation (Hybrid APC) for Endoscopic Ablation of Barrett’s Esophagus (BE): The Results of the Pilot Trial. Gastrointest. Endosc. 2016, 83, AB495. [Google Scholar] [CrossRef]
- Shimizu, T.; Samarasena, J.B.; Fortinsky, K.J.; Hashimoto, R.; El Hage Chehade, N.; Chin, M.A.; Moosvi, Z.; Chang, K.J. Benefit, Tolerance, and Safety of Hybrid Argon Plasma Coagulation for Treatment of Barrett’s Esophagus: US Pilot Study. Endosc. Int. Open 2021, 9, E1870–E1876. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.M.; Quintanilla, R.A.B.; Torres, M.C.A.; Morera, N.D. Endoscopic Treatment of Barrett’s Esophagus with Low- and High-Grade Dysplasia. Med. Electrónica 2022, 26, 637–656. [Google Scholar]
- Linn, B.; Mangels-Dick, T.; Clemens, M.A.; Hanada, Y.; Roy, B.; Genere, J.R.; Wang, K.K. Hybrid Argon Plasma Coagulation and Radiofrequency Ablation in Barrett’s Esophagus. Gastrointest. Endosc. 2020, 91, AB413. [Google Scholar] [CrossRef]
- Torres, M.C.; Quintanilla, R.A.B.; Megías, E.M.d.O.; Escobar, V.M.A.; Contino, N.C.A.; Menocal, J.L.G.; Jiménez, F.N.P. Hybrid-APC Therapeutic Response in Patients with Low-Grade Dysplasia in Barrett’s Esophagus. Arch. Cuba. Gastroenterol. 2021, 1, 159–168. [Google Scholar]
- Trindade, A.J.; Wee, D.; Wander, P.; Stewart, M.; Lee, C.; Benias, P.C.; McKinley, M.J. Successful Treatment of Refractory Barrett’s Neoplasia with Hybrid Argon Plasma Coagulation: A Case Series. Endoscopy 2020, 52, 812–813. [Google Scholar] [CrossRef] [PubMed]
- Nieto, J.; Casas, D. Salvage Hybrid APC After Failed Radiofrequency Ablation and Cryotherapy for Barrett’s Esophagus. Am. J. Gastroenterol. 2019, 114, S544–S545. [Google Scholar] [CrossRef]
- Orman, E.S.; Li, N.; Shaheen, N.J. Efficacy and Durability of Radiofrequency Ablation for Barrett’s Esophagus: Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1245–1255. [Google Scholar] [CrossRef]
- Tariq, R.; Enslin, S.; Hayat, M.; Kaul, V. Efficacy of Cryotherapy as a Primary Endoscopic Ablation Modality for Dysplastic Barrett’s Esophagus and Early Esophageal Neoplasia: A Systematic Review and Meta-Analysis. Cancer Control 2020, 27, 1073274820976668. [Google Scholar] [CrossRef]
- Guthikonda, A.; Cotton, C.C.; Madanick, R.D.; Spacek, M.B.; Moist, S.E.; Ferrell, K.; Dellon, E.S.; Shaheen, N.J. Clinical Outcomes Following Recurrence of Intestinal Metaplasia After Successful Treatment of Barrett’s Esophagus with Radiofrequency Ablation. Am. J. Gastroenterol. 2017, 112, 87–94. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Singh, S.; Ragunathan, K.; Katzka, D.A.; Wang, K.K.; Iyer, P.G. Risk of Recurrence of Barrett’s Esophagus after Successful Endoscopic Therapy. Gastrointest. Endosc. 2016, 83, 1090–1106.e3. [Google Scholar] [CrossRef] [PubMed]
- Hamade, N.; Desai, M.; Thoguluva Chandrasekar, V.; Chalhoub, J.; Patel, M.; Duvvuri, A.; Gorrepati, V.S.; Jegadeesan, R.; Choudhary, A.; Sathyamurthy, A.; et al. Efficacy of Cryotherapy as First Line Therapy in Patients with Barrett’s Neoplasia: A Systematic Review and Pooled Analysis. Dis. Esophagus 2019, 32, doz040. [Google Scholar] [CrossRef]
- Mohan, B.P.; Krishnamoorthi, R.; Ponnada, S.; Shakhatreh, M.; Jayaraj, M.; Garg, R.; Law, J.; Larsen, M.; Irani, S.; Ross, A.; et al. Liquid Nitrogen Spray Cryotherapy in Treatment of Barrett’s Esophagus, Where Do We Stand? A Systematic Review and Meta-Analysis. Dis. Esophagus 2019, 32, doy130. [Google Scholar] [CrossRef] [PubMed]
- Wronska, E.; Polkowski, M.; Orlowska, J.; Mroz, A.; Wieszczy, P.; Regula, J. Argon Plasma Coagulation for Barrett’s Esophagus with Low-Grade Dysplasia: A Randomized Trial with Long-Term Follow-up on the Impact of Power Setting and Proton Pump Inhibitor Dose. Endoscopy 2021, 53, 123–132. [Google Scholar] [PubMed]
- Estevinho, M.M.; Pinho, R. Hybrid Argon Plasma Coagulation after Endoscopic Mucosal Resection—Some Caveats in the Comparison with Snare Tip Soft Coagulation. Gastrointest. Endosc. 2022; in press. [Google Scholar] [CrossRef]



| Study | Study Type | Population | Prior Treatment Modalities | Hybrid APC Details | Surveillance | N | Follow-Up | Endoscopic Remission | Histologic Remission | Recurrence | Procedure-Related Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Knabe et al., 2022 [21] | Prospective, multicentric, single arm | Patients with macroscopically invisible neoplastic BE | EMR (89.6%), treatment-naïve (10.4%) | Submucosal injection of sodium chloride 0.9% with ERBEjet 2 (ERBE, Germany), prior to ablation (first 60–70 W; then 40 W for the remaining islets); mean 2.7 sessions (range 1–5) | Endoscopy with 4-quadrant biopsies from former BE, neo-Z line and neosquamous epithelium (every 1–2 cm) at 3, 6, 12 and 24 months | 154 (80.9% male; mean age 64.2 [range 42–84]) | 24 months | 136/148 = 92.6% | 129/148 = 87.2% | 37/129 = 29.2% | 1/154 = 0.6% (perforation), 6/154 = 3.9% (stricture), 1/154 = 0.6% (bleeding); 31/154 = 20.1% (minor events) |
| Martínez et al., 2022 [24] | Retrospective cohort study, unicentric, double arm | Patients with biopsy-proven BE + low-grade flat dysplasia | Treatment-naïve patients | Submucosal injection with ERBEjet2 prior to ablation (60 W, effect 2 in the first section; 50 W, effect 2 in subsequent sections) | Endoscopy at 6 and 12 months | 29 (58.6% male, mean age 50.5 [range 27–81] | 12 months | 12/14 = 85.7% versus 12/15 = 80.0% (EMR) | NR | 2/14 = 14.3% versus 3/15 = 20.0% (EMR) | NR |
| Torres et al., 2021 [26] | Retrospective, cohort study, unicentric, single arm | Patients with biopsy-proven BE + low-grade flat dysplasia | NR | Hybrid-APC in the areas of dysplasia (2 quadrants per session); 60 W and effect 2 (first session), 50 W and effect 2 (subsequent); mean 1.5 sessions (range 1–4) | Endoscopy with biopsies from former BE at 3 and 6 months | 20 (55.0% male, mean age 50.5 [range 27–81]) | 6 months | 18/20 = 90.0% | 18/20 = 90.0% | 1/13 = 7.7% | 0/20 = 0.0% |
| Kashin et al., 2021 (abstract) [22] | Prospective, unicentric, single arm | Patients with biopsy-proven BE + flat low-grade dysplasia | Treatment-naïve (54.5%), EMR (45.5%) | Submucosal injection with ERBEjet2 prior to ablation (first with 60 W, effect 2; remaining islets treated with 40 W, effect 2); mean of 1.6 sessions (range 1–3) | Endoscopy with 4-quadrant biopsies from former BE at 3, 6 months and then annually | 11 (45.5% male, mean age 46 [range 25–63]) | Median 4.5 months | 11/11 = 100.0% | 11/11 = 100.0% | 0/11 = 0.0% | 1/11 = 9.0% (stricture) |
| Shimizu et al., 2021 [23] | Prospective, unicentric, single arm | Patients with residual BE (54.4% with flat neoplasia) | Treatment-naïve (36.0%), EMR (22.7%), RFA (50.0%), and cryotherapy (13.6%) | Submucosal injection of 0.9% methylene blue solution with ERBEjet2, 40–50 W, effect 2, prior to ablation (first with 60 W, effect 2; remaining islets treated with 40 W, effect 2); up to 3 sessions (range 2–4) | Endoscopy with biopsies at the neo Z-line and biopsies in at least one level in the area of the former BE at 3 months | 22 (81.8% male, mean age 67.8 [range 49–83]) | Average 134.7 days | NR | 19/22 = 86.4% | NR | 2/22 = 9.1% (strictures) |
| Staudenmann et al., 2021 [4] | Prospective, unicentric, single arm | Patients with biopsy-proven BE, with flat low- or high-grade dysplasia, or T1a adenocarcinoma | Treatment-naïve (72.8%), RFA (9.1%) or EMR (18.2%) | Submucosal injection of 0.9% sodium chloride solution with ERBEjet2, effect 50, prior to ablation (60–70 W, effect 2) | Endoscopy with biopsies at the neo Z-line and biopsies in at least one level in the area of the former BE at 3, 6, 9, 12, 18, 24 months | 9 (72.7% male, mean age 68.2 ± 8.0 years-old) | 24 months | 9/9 = 100.0% | 8/9 = 88.9% | 1/9 = 11.1% | 0/9 = 0.0%;1/9 = 11.1% (minor event) |
| Linn et al., 2020 (abstract) [25] | Retrospective cohort study, unicentric, double arm | Patients with residual BE | NR | NR | Endoscopy with 4-quadrant biopsies from former BE at 3 and 6 months | 54 (83.0% male, mean age 66.5 years-old) | 6 months | NR | 24/27 = 88.9% versus 20/27 = 74.1% | NR | 0/27 = 0.0% versus 12/27 = 44.4% (for RFA, 4 strictures) |
| Trindade et al., 2020 [27] | Retrospective, case reports, unicentric | Refractory residual BE (in 2 cases with non-visible neoplasia) | RFA, 60.0% also refractory to cryotherapy | Submucosal injection with ERBEjet, effect 50, prior to ablation (40 W in the EMR defect, 60 W in the remaining nondysplastic mucosa); mean 2.2 sessions (range 2–3) | Endoscopy 3 and 6 months later; no further details | 5 (60.0% male; mean age 66.8 [range 51–76]) | Not defined | 5/5 = 100.0% | 5/5 = 100.0% | NR | NR |
| Nieto and Casas, 2019 (abstract) [28] | Retrospective cohort study, unicentric, single arm | BE with persistent flat dysplasia | Failed RFA and cryotherapy | Hybrid APC (no further details), mean 2.3 sessions | Endoscopy 6 months later; no further details | 6 (84.0% male, mean age 63) | 6 months | 6/6 = 100% | 6/6 = 100% | NR | 0/6 = 0.0% |
| Manner et al., 2016 [8] | Prospective, unicentric, single arm | Patients with residual non-neoplastic BE | EMR (100%) | Submucosal injection of sodium chloride 0.9% with ERBEjet, prior to ablation (first with 50–60 W, effect 2; then 40 W for the remaining islets); median sessions 3.5 (range 1–10) | Endoscopy with 4 quadrant biopsies from former BE, neo-Z line and neosquamous epithelium at 3 months (every 2 cm) | 50 (92.0% male; mean age 62.0 [range 42–79]) | 3 months | 48/50 = 96.0% (PP) | 39/50 = 78.0% | NR | 1/50 = 2% (stricture), 11/50 = 22% (minor events) |
| Study | Study Type | Population | Hybrid APC Details | Comparator | Surveillance | N | Follow-Up | Polyps’ Characteristics | Recurrence | Procedure-Related Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| Levenick et al., 2022 [12] | Retrospective cohort study, unicentric, double arm | Patients who underwent EMR for non-pedunculated colonic polyps > 20 mm | Submucosal injection (normal saline and contrast agent) with ERBEjet effect 30–50, prior to thermal ablation (flow of 0.8 L/min, 40 W; done on both eschar base and peripheral edges) | Standard EMR (n = 29 polyps) | Surveillance colonoscopy at 6-months | 48 (54.2% male, mean age 66.1); 59 polyps removed (30 with APC-assisted EMR) | 6 months | Mean size 31.6 mm (SD 13.7), 66.1% in the right colon; en-bloc resection in 28.8%; lift adequacy differed (non-lifting in 10.3% of the sEMR group versus 3.3% of the hAPC) | 0/30 = 0.0% versus 6/29 = 20.7% (standard EMR) | 2/25 = 8.0% versus 4/23 = 17.4% for sEMR (bleeding) |
| Motchum et al., 2022 [10] | Prospective, multicentric, single arm | Patients who underwent EMR for non-pedunculated colonic polyps > 20 mm | Submucosal injection with ERBEjet, effect 30–50, prior to ablation (flow of 0.8 L/min, 40 W); the ablation of the peripheral edges was done in all patients; eschar surface was only ablated in 78% (complete in 20%, partial in 58%) | No | Surveillance colonoscopy at 4–6 months | 84 (53.6% male, median age 66.3 [range 18–89]); 101 polyps removed | 6 months | Median polyp size 30.9 mm (range 20–60 mm), 83.0% in the right colon, en-bloc resection in 6.0%; non-lifting in 8.0%; prophylactic clipping in 13.1% | 2.2% (2/91) | 2/84 = 2.4% (bleeding); 1/84 = 1.2% (microperforation) |
| Motz et al., 2022 [11] | Prospective, unicentric, single arm | Patients who underwent EMR for non-pedunculated colonic polyps > 20 mm | Submucosal injection (sodium chloride [0.9%] ± hetastarch), prior to thermal ablation (flow of 0.8 L/min, 40 W; done on both eschar base and peripheral edges) | No | Surveillance colonoscopy at 6-months | 32 (62.5% male, mean age 64.6 [range 50–78]); 35 polyps removed | 6 months | Median polyp size 27.0 mm (IQR 14.5); 65.9% in the right colon; non-lifting in 4.6%; prophylactic clipping in 82.5% | 0/35 = 0.0% | 3/32 = 7.5% (bleeding) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estevinho, M.M.; Pinho, R.; Silva, J.C.; Correia, J.; Mesquita, P.; Freitas, T. Hybrid Argon Plasma Coagulation for Barrett’s Esophagus and for Colonic Mucosal Resection—A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 1139. https://doi.org/10.3390/biomedicines11041139
Estevinho MM, Pinho R, Silva JC, Correia J, Mesquita P, Freitas T. Hybrid Argon Plasma Coagulation for Barrett’s Esophagus and for Colonic Mucosal Resection—A Systematic Review and Meta-Analysis. Biomedicines. 2023; 11(4):1139. https://doi.org/10.3390/biomedicines11041139
Chicago/Turabian StyleEstevinho, Maria Manuela, Rolando Pinho, João Carlos Silva, João Correia, Pedro Mesquita, and Teresa Freitas. 2023. "Hybrid Argon Plasma Coagulation for Barrett’s Esophagus and for Colonic Mucosal Resection—A Systematic Review and Meta-Analysis" Biomedicines 11, no. 4: 1139. https://doi.org/10.3390/biomedicines11041139
APA StyleEstevinho, M. M., Pinho, R., Silva, J. C., Correia, J., Mesquita, P., & Freitas, T. (2023). Hybrid Argon Plasma Coagulation for Barrett’s Esophagus and for Colonic Mucosal Resection—A Systematic Review and Meta-Analysis. Biomedicines, 11(4), 1139. https://doi.org/10.3390/biomedicines11041139





