Artificial Intelligence in Acute Ischemic Stroke Subtypes According to Toast Classification: A Comprehensive Narrative Review
Abstract
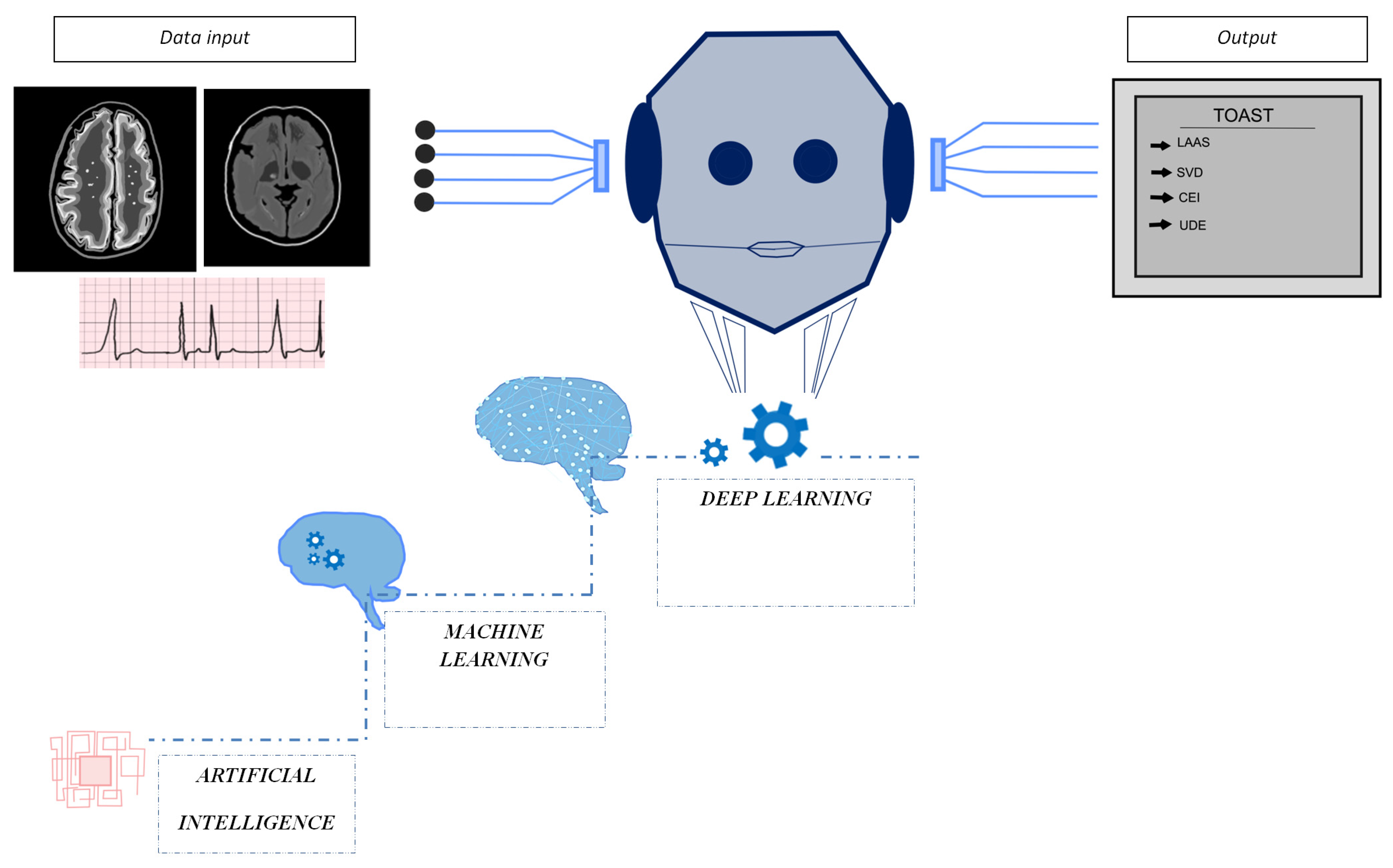
1. Introduction
2. Overview of Artificial Intelligence
3. Ischemic Stroke and Artificial Intelligence: Are You a Bot? Please Select All Images Containing Ischemic Stroke
4. Research Strategy
5. Results
5.1. Large-Artery Atherosclerosis Subtype
5.2. Cardioembolic Source Detection
5.3. Small Vessel Disease Identification
5.4. Stroke of Undetermined Etiology
6. Discussion
6.1. AI and LAAS
6.2. AI in Cardioembolic Stroke
6.3. AI in Small Vessel Disease
6.4. Stroke of Unkown Origin and AI
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Conflicts of Interest
Abbreviations
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.H.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Shafaat, O.; Bernstock, J.D.; Shafaat, A.; Yedavalli, V.S.; Elsayed, G.; Gupta, S.; Sotoudeh, E.; Sair, H.S.; Yousem, D.M.; Sotoudeh, H. Leveraging artificial intelligence in ischemic stroke imaging. J. Neuroradiol. 2022, 49, 343–351. [Google Scholar] [CrossRef]
- Soun, J.E.; Chow, D.S.; Nagamine, M.; Takhtawala, R.S.; Filippi, C.G.; Yu, W.; Chang, P.D. Artificial Intelligence and Acute Stroke Imaging. AJNR Am. J. Neuroradiol. 2021, 42, 2–11. [Google Scholar] [CrossRef]
- Zhu, G.; Jiang, B.; Chen, H.; Tong, E.; Xie, Y.; Faizy, T.D.; Heit, J.J.; Zaharchuk, G.; Wintermark, M. Artificial Intelligence and Stroke Imaging: A West Coast Perspective. Neuroimaging Clin. N. Am. 2020, 30, 479–492. [Google Scholar] [CrossRef]
- Choi, R.Y.; Coyner, A.S.; Kalpathy-Cramer, J.; Chiang, M.F.; Campbell, J.P. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 14. [Google Scholar]
- Faes, L.; Liu, X.; Wagner, S.K.; Fu, D.J.; Balaskas, K.; Sim, D.A.; Bachmann, L.M.; Keane, P.A.; Denniston, A.K. A Clinician’s Guide to Artificial Intelligence: How to Critically Appraise Machine Learning Studies. Transl. Vis. Sci. Technol. 2020, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Badillo, S.; Banfai, B.; Birzele, F.; Davydov, I.I.; Hutchinson, L.; Kam-Thong, T.; Siebourg-Polster, J.; Steiert, B.; Zhang, J.D. An Introduction to Machine Learning. Clin. Pharmacol. Ther. 2020, 107, 871–885. [Google Scholar] [CrossRef]
- Meskó, B.; Görög, M. A short guide for medical professionals in the era of artificial intelligence. NPJ Digit. Med. 2020, 3, 126. [Google Scholar] [CrossRef]
- Cui, S.; Tseng, H.H.; Pakela, J.; Ten Haken, R.K.; El Naqa, I. Introduction to machine and deep learning for medical physicists. Med. Phys. 2020, 47, e127–e147. [Google Scholar] [CrossRef]
- Yu, K.H.; Beam, A.L.; Kohane, I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018, 2, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Ivanovic, M.D.; Atanasoski, V.; Shvilkin, A.; Hadzievski, L.; Maluckov, A. Deep Learning Approach for Highly Specific Atrial fibrillation and Flutter Detection based on RR Intervals. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 1780–1783. [Google Scholar]
- Zhang, Y.; Zhou, Y.; Zhang, D.; Song, W. A Stroke Risk Detection: Improving Hybrid Feature Selection Method. J. Med. Internet Res. 2019, 21, e12437. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Du, X.; Zhang, P.; Hu, G.; Xie, G.; Guo, S.; Xu, M.; Xie, X. Integrated Machine Learning Approaches for Predicting Ischemic Stroke and Thromboembolism in Atrial Fibrillation. AMIA Annu. Symp. Proc. 2016, 2016, 799. [Google Scholar] [PubMed]
- Li, X.; Sun, Z.; Du, X.; Liu, H.; Hu, G.; Xieet, G. Bootstrap-based Feature Selection to Balance Model Discrimination and Predictor Significance: A Study of Stroke Prediction in Atrial Fibrillation. AMIA Annu. Symp. Proc. 2017, 2017, 1130–1139. [Google Scholar] [PubMed]
- Karlsson, L.O.; Nilsson, S.; Bång, M.; Nilsson, L.; Charitakis, E.; Janzon, M. A clinical decision support tool for improving adherence to guidelines on anticoagulant therapy in patients with atrial fibrillation at risk of stroke: A cluster-randomized trial in a Swedish primary care setting (the CDS-AF study). PLoS Med. 2018, 15, e1002528. [Google Scholar] [CrossRef]
- Stanciu, A.; Banciu, M.; Sadighi, A.; Marshall, K.A.; Holland, N.R.; Abedi, V.; Zand, R. A predictive analytics model for differentiating between transient ischemic attacks (TIA) and its mimics. BMC Med. Inform. Decis. Mak. 2020, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- Abedi, V.; Goyal, N.; Tsivgoulis, G.; Hosseinichimeh, N.; Hontecillas, R.; Bassaganya-Riera, J.; Elijovich, L.; Metter, J.E.; Alexandrov, A.W.; Liebeskind, D.S.; et al. Novel Screening Tool for Stroke Using Artificial Neural Network. Stroke 2017, 48, 1678–1681. [Google Scholar] [CrossRef]
- Alawieh, A.; Zaraket, F.; Alawieh, M.B.; Chatterjee, A.R.; Spiotta, A. Using machine learning to optimize selection of elderly patients for endovascular thrombectomy. J. Neurointerv. Surg. 2019, 11, 847–851. [Google Scholar] [CrossRef]
- Skandha, S.S.; Gupta, S.K.; Saba, L.; Johri, A.M.; Khanna, N.N.; Mavrogeni, S.; Laird, J.R.; Pareek, G.; Miner, M.; Sfikakis, P.P.; et al. 3-D optimized classification and characterization artificial intelligence paradigm for cardiovascular/stroke risk stratification using carotid ultrasound-based delineated plaque: AtheromaticTM 2.0. Comput. Biol. Med. 2020, 125, 103958. [Google Scholar] [CrossRef]
- Mei1, Y.; Hu, R.; Lin, J.; Xu, H.Y.; Wu, L.Y.; Li, H.P.; Ye, Z.M.; Qin, C. Diagnosis of Middle Cerebral Artery Stenosis Using Transcranial Doppler Images Based on Convolutional Neural Network. World Neurosurg. 2022, 161, e118–e125. [Google Scholar] [CrossRef]
- Sheth, S.A.; Lopez-Rivera, V.; Barman, A.; Grotta, J.C.; Yoo, A.J.; Lee, S.; Inam, M.E.; Savitz, S.I.; Giancardo, L. Machine Learning-Enabled Automated Determination of Acute Ischemic Core from Computed Tomography Angiography. Stroke 2019, 501, 3093–3100. [Google Scholar] [CrossRef]
- Amukotuwa, S.A.; Straka, M.; Smith, H.; Chandra, R.V.; Dehkharghani, S.; Fischbein, N.J.; RBammer, R. Automated Detection of Intracranial Large Vessel Occlusions on Computed Tomography Angiography. Stroke 2019, 500, 2790–2798. [Google Scholar] [CrossRef]
- Kasasbeh, A.S.; Christensen, S.; Parsons, M.W.; Campbell, B.; Albers, G.W.; Lansberg, M.J. Artificial Neural Network Computer Tomography Perfusion Prediction of Ischemic Core. Stroke 2019, 50, 1578–1581. [Google Scholar] [CrossRef]
- Ho, K.C.; Speier, W.; El-Saden, S.; et Arnold, C.W. Classifying Acute Ischemic Stroke Onset Time using Deep Imaging Features. AMIA Annu. Symp. Proc. 2018, 2017, 892–901. [Google Scholar]
- Wu, O.; Winzeck, S.; Giese, A.K.; Etherton, M.R.; Bouts, M.J.R.J.; Donahue, K.; Schirmer, M.D.; Irie, R.E.; Mocking, S.J.T.; McIntosh, E.C. Big Data Approaches to Phenotyping Acute Ischemic Stroke Using Automated Lesion Segmentation of Multi-Center Magnetic Resonance Imaging Data. Stroke 2019, 50, 1734–1741. [Google Scholar] [CrossRef]
- Nielsen, A.; Hansen, M.B.; Tietze, A.; Mouridsen, A. Prediction of Tissue Outcome and Assessment of Treatment Effect in Acute Ischemic Stroke Using Deep Learning. Stroke 2018, 49, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Giese, A.K.; Schirmer, M.D.; Dalca, A.V.; Sridharan, R.; Donahue, K.L.; Nardin, M.; Irie, R.; McIntosh, E.C.; Mocking, S.J.T.; Xu, X.; et al. White matter hyperintensity burden in acute stroke patients differs by ischemic stroke subtype. Neurology 2020, 95, e79–e88. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Castro, V.; Del C Valdés Hernández, M.; Chappell, F.M.; Armitage, P.A.; Makin, S.; Wardlaw, J.M. Reliability of an automatic classifier for brain enlarged perivascular spaces burden and comparison with human performance. Clin. Sci. 2017, 131, 1465–1481. [Google Scholar] [CrossRef] [PubMed]
- Rebrova, O.I.; Maksimova, M.I.; Piradov, M.A. The neural network algorithm for diagnosis of ischemic stroke pathogenetic subtypes. Nevrol. Psikhiatr. Korsakova 2004, 12 (Suppl. S12), 23–28. [Google Scholar]
- Cheon, S.; Kim, J.; Lim, J. The Use of Deep Learning to Predict Stroke Patient Mortality. Int. J. Environ. Res. Public Health 2019, 161, 1876. [Google Scholar] [CrossRef]
- Abedi, V.; Avula, V.; Razavi, S.M.; Bavishi, S.; Chaudhary, D.; Shahjouei, S.; Wang, M.; Griessenauer, C.J.; Li, J.; Zand, R. Predicting short and long-term mortality after acute ischemic stroke using EHR. J. Neurol. Sci. 2021, 427, 117560. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Yoon, J.G.; Park, H.; Kim, J.D.; Nam, H.S.; Heo, J.H. Machine Learning—Based Model for Prediction of Outcomes in Acute Stroke. Stroke 2019, 50, 1263–1265. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, B.; Gong, E.; Li, Y.; Zhu, G.; Michel, P.; Wintermark, M.; Zaharchuk, G. Use of gradient boosting machine learning to predict patient outcome in acute ischemic stroke on the basis of imaging, demographic, and clinical information. Am. J. Roentgenol. 2019, 22, 44–51. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Huang, H.; Peng, C.; Ge, Y.; Wu, H.; Wang, J.; Xiong, G.; Yi, Y. Extreme Gradient Boosting Model Has a Better Performance in Predicting the Risk of 90-Day Readmissions in Patients with Ischaemic Stroke. J. Stroke Cerebrovasc. Dis. 2019, 282, 104441. [Google Scholar] [CrossRef]
- Chan, K.L.; Leng, X.; Zhang, W.; Dong, W.; Qiu, Q.; Yang, J.; Soo, Y.; Wong, K.S.; Leung, T.W.; Liu, J. Early Identification of High-Risk TIA or Minor Stroke Using Artificial Neural Network. Front. Neurol. 2019, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Chen, C.H.; Tseng, Y.J.; Tsai, Y.T.; Chang, C.Y.; Wang, H.S.; Chen, C.K. Predicting post-stroke activities of daily living through a machine learning-based approach on initiating rehabilitation. Int. J. Med. Inform. 2018, 111, 159–164. [Google Scholar] [CrossRef]
- Macharzina, R.R.; Kocher, S.; Messe, S.R.; Rutkowski, T.; Hoffmann, F.; Vogt, M.; Vach, W.; Fan, N.; Rastan, A.; Neumann, F.J.; et al. 4-dimensionally guided 3-dimensional color-Doppler ultrasonography quantify carotid artery stenosis with high reproducibility and accuracy. JACC Cardiovasc. Imaging 2018, 11, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, L.; Marino, S.; Bramanti, P.; Sottile, F. Validation of a computer aided diagnosis system for the automatic identification of carotid Atherosclerosis. Ultrasound Med. Biol. 2015, 41, 509–516. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. 2012 Imagenet classification with deep convolutional neural networks. Adv. Neural Inf. Process. Syst. 2012, 25, 1097–1105. [Google Scholar]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, J. Rethinking the inception architecture for computer vision. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 2818–2826. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Huang, G.; Liu, Z.; Van Der Maaten, L.; Weinberger, K.Q. Densely connected convolutional networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 4700–4708. [Google Scholar]
- Lo, C.M.; Hung, P.H. Assessing ischemic stroke with convolutional image features in carotid color Doppler. Ultrasound Med. Biol. 2021, 47, 2266–2276. [Google Scholar] [CrossRef]
- Kordzadeh, A.; Askari, A.; Abbassi, H.A.; Sanoudos, N.; Mohaghegh, V.; Shirvani, H. Artificial intelligence and duplex ultrasound for detection of carotid artery disease. Vascular 2022, 127, 17085381221107465. [Google Scholar] [CrossRef]
- Cimflova, P.; Golan, R.; Ospel, J.M.; Sojoudi, A.; Duszynski, C.; Elebute, I.; El-Hariri, H.; Mousavi, S.H.; Souto Maior Neto, L.A.; Pinky, N.; et al. Validation of a machine learning software tool for automated large vessel occlusion detection in patients with suspected acute stroke. Neuroradiology 2022, 64, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Buckler, A.J.; Gotto, A.M., Jr.; Rajeev, A.; Nicolaou, A.; Sakamoto, A.; St Pierre, S.; Phillips, M.; Virmani, R.; Villines, T.C. Atherosclerosis risk classification with computed tomography angiography: A radiologic-pathologic validation study. Atherosclerosis 2022, 366, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Leys, D.; Pruvo, J.P.; Godefroy, O.; Rondepierre, P.; Leclerc, X. Prevalence and Significance of hyperdense middle cerebral artery in acute stroke. Stroke 1992, 23, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Manelfe, C.; Larrue, V.; von Kummer, R.; Bozzao, L.; Ringleb, P.; Bastianello, S.; Iweins, F.; Lesaffre, E. Association of hyperdense middle cerebral artery sign with clinical outcome in patients treated with tissue plasminogen activator. Stroke 1999, 30, 769–772. [Google Scholar] [CrossRef]
- Kirchhof, K.; Welzel, T.; Mecke, C.; Zoubaa, S.; Sartor, K. Differentiation of white, mixed, and red thrombi: Value of CT in estimation of the prognosis of thrombolysis—Phantom study. Radiology 2003, 228, 126–130. [Google Scholar] [CrossRef]
- Barber, P.A.; Demchuk, A.M.; Hudon, M.E.; Pexman, J.H.; Hill, M.D.; Buchan, A.M. Hyperdense sylvian fissure MCA “dot” sign: A CT marker of acute ischemia. Stroke 2001, 32, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Leary, M.C.; Kidwell, C.S.; Villablanca, J.P.; Starkman, S.; Jahan, R.; Duckwiler, G.R.; Gobin, Y.P.; Sykes, S.; Gough, K.J.; Ferguson, K.; et al. Validation of computed tomographic middle cerebral artery “dot” sign: An angiographic correlation study. Stroke 2003, 34, 2636–2640. [Google Scholar] [CrossRef]
- Takahashi, N.; Lee, Y.; Tsai, D.Y.; Matsuyama, E.; Kinoshita, T.; Ishii, K. An automated detection method for the MCA dot sign of acute stroke in unenhanced CT. Radiol. Phys. Technol. 2014, 7, 79–88. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Tsang, A.C.O.; Yu, P.L.H.; Tsui, E.L.H.; Woo, P.P.S.; Lui, C.S.M.; Leung, G.K.K. Automated Hierarchy Evaluation System of Large Vessel Occlusion in Acute Ischemia Stroke. Front. Neuroinform 2020, 14, 13. [Google Scholar] [CrossRef]
- Chung, J.W.; Kim, Y.C.; Cha, J.; Choi, E.H.; Kim, B.M.; Seo, W.K.; Kim, G.M.; Bang, O.Y. Characterization of clot composition in acute cerebral infarct using machine learning techniques. Ann. Clin. Transl. Neurol. 2019, 6, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Barreira, C.; Bouslama, M.; Haussen, D.C.; Haussen, D.C.; Al-Bayati, A.; Pisani, L.; Liberato, B.; Bhatt, N.; Frankel, M.R.; Nogueira, R.G. 2E-108 Aladin study: Automated large artery occlusion detection in stroke imaging study—A multicenter analysis. J. Neurointerv. Surg. 2018, 10, A101–A102. [Google Scholar]
- Hassan, A.E.; Ringheanu, V.M.; Rabah, R.R.; Preston, L.; Tekle, W.G.; Qureshi, A.I. Early experience utilizing artificial intelligence shows significant reduction in transfer times and length of stay in a hub and spoke model. Interv. Neuroradiol. 2020, 26, 615–622. [Google Scholar] [CrossRef]
- Dermot, H.; Mallon, D.H.; Taylor, E.J.R.; Sheeka, A.; Doig, D.; Lobotesis, K. Comparison of automated ASPECTS, large vessel occlusion detection and CTP analysis provided by Brainomix and RapidAI in patients with suspected ischaemic stroke. J. Stroke Cerebrovasc. Dis. 2022, 31, 106702. [Google Scholar]
- Rav, R.A.; Peterson, B.A.; Seymour, S.E.; Mokin, M.; Waqas, M.; Hoi, Y.; Davies, J.M.; Levy, E.I.; Siddiqui, A.H.; Ionita, C.N. Validation of an artificial intelligence-driven large vessel occlusion detection algorithm for acute ischemic stroke patients. Neuroradiol. J. 2021, 34, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Liu, C.; Li, Z.; Wang, Y. Incorporating artificial intelligence into stroke care and research. Stroke 2020, 51, e351–e354. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, G.; Neuberger, U.; Mahmutoglu, M.A.; Mahmutoglu, M.A.; Foltyn, M.; Herweh, C.; Nagel, S.; Schönenberger, S.; Heiland, S.; Ulfertet, C.; et al. Multimodal predictive modeling of endovascular treatment outcome for acute ischemic stroke using machine-learning. Stroke 2020, 51, 3541–3551. [Google Scholar] [CrossRef]
- Fiehler, J.; Thomalla, G.; Bernhardt, M.; Kniep, H.; Berlis, A.; Dorn, F.; Eckert, B.; Kemmling, A.; Langner, S.; Remonda, L.; et al. ERASER. Stroke 2019, 50, 1275–1278. [Google Scholar] [CrossRef]
- Hamann, J.; Herzog, L.; Wehrli, C.; Dobrocky, T.; Bink, A.; Piccirelli, M.; Panos, L.; Kaesmacher, J.; Fischer, U.; Stippich, C.; et al. Machine-learning-based outcome prediction in stroke patients with middle cerebral artery-M1 occlusions and early Thrombectomy. Eur. J. Neurol. 2021, 28, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Oishi, N.; Ishii, A.; Ogura, T.; Sunohara, T.; Chihara, H.; Fukumitsu, R.; Okawa, M.; Yamanaet, N. Predicting clinical outcomes of large vessel occlusion before mechanical Thrombectomy using machine learning. Stroke 2019, 50, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Oishi, N.; Ishii, A.; Ono, I.; Ogura, T.; Sunohara, T.; Chihara, H.; Fukumitsu, R.; Okawa, M.; Yamana, N.; et al. Deep learning-derived high-level neuroimaging featurespredict clinical outcomes for large vessel occlusion. Stroke 2020, 51, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Barber, P.A.; Demchuk, A.M.; Zhang, J.; Buchan, A.M. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet 2000, 355, 1670–1674. [Google Scholar] [CrossRef]
- Yedavalli, V.S.; Tong, E.; Martin, D.; Yeom, K.W.; Forkert, N.D. Artificial intelligence in stroke imaging: Current and future perspectives. Clin. Imaging 2021, 69, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Mokin, M.; Primiani, C.T.; Siddiqui, A.H.; Turk, A.S. ASPECTS (Alberta Stroke Program Early CT Score) measurement using hounsfield unit values when selecting patients for stroke thrombectomy. Stroke 2017, 48, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Goebel, J.; Stenzel, E.; Guberina, N.; Wanke, I.; Koehrmann, M.; Kleinschnitz, C.; Umutlu, L.; Forsting, M.; Moenninghoff, C.; Radbruch, A. Automated ASPECT rating: Comparison between the Frontier ASPECT Score software and the Brainomix software. Neuroradiology 2018, 60, 1267–1272. [Google Scholar] [CrossRef]
- Guberina, N.; Dietrich, U.; Radbruch, A.; Goebel, J.; Deuschl, C.; Ringelstein, A.; Köhrmann, M.; Kleinschnitz, C.; Forsting, M.; Mönninghoff, C. Detection of early infarction signs with machine learning-based diagnosis by means of the Alberta Stroke Program Early CT score (ASPECTS) in the clinical routine. Neuroradiology 2018, 60, 889–901. [Google Scholar] [CrossRef]
- Dou, Q.; Chen, H.; Yu, L.; Zhao, L.; Qin, J.; Wang, D.; Mok, V.C.T.; Shi, L.; Heng, P.A. Automatic detection of cerebral microbleeds from MR images via 3D convolutional neural networks. IEEE Trans. Med. Imaging 2016, 35, 1182–1195. [Google Scholar] [CrossRef]
- Grunwald, I.Q.; Kulikovski, J.; Reith, W.; Gerry, S.; Namias, R.; Politi, M.; Papanagiotou, P.; Essig, M.; Mathur, S.; Joly, O.; et al. Collateral automation for triage in stroke: Evaluating automated scoring of collaterals in acute stroke on computed tomography scans. Cereb. Dis. 2019, 47, 217–222. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, D.; Lou, M.; Liebeskind, D.; Scalzo, F. Prediction of hemorrhagic transformation severity in acute stroke from source perfusion MRI. IEEE Trans. Biomed. Eng. 2018, 65, 2058–2065. [Google Scholar] [CrossRef]
- Ferro, J.M. Cardioembolic stroke: An update. Lancet Neurol. 2003, 2, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.W.; Lip, G.Y.H. Clinical outcomes of acute stroke patients with atrial fibrillation. Expert Rev. Cardiovasc. 2009, 7, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, K.G.; Tae, Y.; Chang, M.; Park, S.J.; Park, K.M.; On, Y.K.; Kim, J.S.; Lee, Y.; Jang, S. An Artificial Intelligence Algorithm With 24-h Holter Monitoring for the Identification of Occult Atrial Fibrillation During Sinus Rhythm. Front. Cardiovasc. Med. 2022, 9, 906780. [Google Scholar] [CrossRef]
- Kashou, A.H.; May, A.M.; Noseworthy, P.A. Artificial Intelligence-Enabled ECG: A Modern Lens on an Old Technology. Curr. Cardiol. Rep. 2020, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Yang, M.; Zhang, L. 12-Lead ECG arrhythmia classification using cascaded convolutional neural network and expert feature. J. Electrocardiol. 2021, 67, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, R.; Nishio, M.; Do, R.K.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef]
- Kiranyaz, S.; Ince, T.; Gabbouj, M. Real-time patient-specific ECG classification by 1-D convolutional neural networks. IEEE Trans. Biomed. Eng. 2016, 63, 664–675. [Google Scholar] [CrossRef]
- Acharya, U.R.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adam, M.; Gertych, A.; Tan, R.S. A deep convolutional neural network model to classify heartbeats. Comput. Biol. Med. 2017, 89, 389–396. [Google Scholar] [CrossRef]
- Awni, Y.H.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar]
- Oh, S.L.; Ng, E.Y.K.; Tan, R.S.; Acharya, U.R. Automated beat-wise arrhythmia diagnosis using modified Unet on extended electrocardiographic recordings with heterogeneous arrhythmia types. Comput. Biol. Med. 2019, 105, 92–101. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Attia, Z.I.; Brewer, L.C.; Hayes, S.N.; Yao, X.; Kapa, S.; Friedman, P.A.; Lopez-Jimenez, F. Assessing and mitigating bias in medical artificial intelligence: The effects of race and ethnicity on a deep learning model for ECG analysis. Circ. Arrhythm. Electrophysiol. 2020, 13, e007988. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ko, D.; Khurshid, S.; Trisini Lipsanopoulos, A.T.; Ashburner, J.M.; Harrington, L.X.; Rost, N.S.; Atlas, S.J.; Singer, D.E.; McManus, D.D.; et al. Automated Electronic Phenotyping of Cardioembolic Stroke. Stroke 2021, 52, 181–189. [Google Scholar] [CrossRef]
- Jeong, H.G.; Kim, B.J.; Kim, T.; Kang, J.; Kim, J.Y.; Kim, J.; Kim, J.T.; Park, J.M.; Kim, J.G.; Hong, J.H.; et al. Classification of cardioembolic stroke based on a deep neural network using chest radiographs. EBioMedicine 2021, 69, 103466. [Google Scholar] [CrossRef]
- Rocon, C.; Tabassian, M.; Tavares de Melo, M.D.; de Araujo Filho, J.A.; Grupi, C.J.; Parga Filho, J.R.; Bocchi, E.A.; D’hooge, J.; Salemi, V.M.C. Biventricular imaging markers to predict outcomes in non-compaction cardiomyopathy: A machine learning study. ESC Heart Fail. 2020, 7, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, V.V.A.; Hora, T.F.; Guerra, A.A.; Silva, G.S.; de Paiva Bezerra, R.; Oliveira-Filho, J.; Santos, L.S.B.; de Melo, E.S.; Alves de Andrade, L.P.; de Oliveira Junior, W.A.; et al. Artificial Inteligence-Based Decision for the Prediction of Cardioembolism in Patients with Chagas Disease and Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 106034. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Markus, H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 2010, 341, c3666. [Google Scholar] [CrossRef] [PubMed]
- Karel, M.F.A.; Roosen, M.G.C.H.; Tullemans, B.M.E.; Zhang, C.E.; Staals, J.; Cosemans, J.M.E.M.; Koenen, R.R. Characterization of cerebral small vessel disease by neutrophil and platelet activation markers using artificial intelligence. J. Neuroimmunol. 2022, 367, 577863. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Lambert, C.; Sam Narean, J.; Benjamin, P.; Zeestraten, E.; Barrick, T.R.; Markus, H.S. Characterising the grey matter correlates of leukoaraiosis in cerebral small vessel disease. Neuroimage Clin. 2015, 9, 194–205. [Google Scholar] [CrossRef]
- Ciulli, S.; Citi, L.; Salvadori, E.; Valenti, R.; Poggesi, A.; Inzitari, D.; Mascalchi, M.; Toschi, N.; Pantoni, L.; Diciotti, S. Prediction of impaired performance in trail making test in MCI patients with small vessel disease using DTI data. IEEE J. Biomed. Health Inform. 2016, 20, 1026–1033. [Google Scholar] [CrossRef]
- Potter, G.M.; Doubal, F.N.; Jackson, C.A.; Chappell, F.M.; Sudlow, C.L.; Dennis, M.S.; Wardlaw, J.M. Enlarged perivascular spaces and cerebral small vessel disease. Int. J. Stroke 2015, 10, 376–381. [Google Scholar] [CrossRef]
- Cai, K.; Tain, R.; Das, S.; Damen, F.C.; Sui, Y.; Valyi-Nagy, T.; Elliott, M.A.; Zhou, X.J. The feasibility of quantitative MRI of perivascular spaces at 7T. J. Neurosci. Methods 2015, 256, 151–156. [Google Scholar] [CrossRef]
- Shan, W.; Duan, Y.; Zheng, Y.; Wu, Z.; Chan, S.W.; Wang, Q.; Gao, P.; Liu, Y.; He, K.; Wang, Y. Segmentation of Cerebral Small Vessel Diseases-White Matter Hyperintensities Based on a Deep Learning System. Front. Med. 2021, 8, 681183. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.-W.; Kwon, H.-M.; Jeong, H.Y.; Park, J.H.; Kim, S.H.; Jeong, S.M.; Yoo, T.G.; Kim, S. High neutrophil to lymphocyte ratio is associated with white matter hyperintensity in a healthy population. J. Neurol. Sci. 2017, 380, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Folco, E.J.; Mawson, T.L.; Vromman, A.; Bernardes-Souza, B.; Franck, G.; Persson, O.; Nakamura, M.; Newton, G.; Luscinskas, F.W.; Libby, P. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1α and cathepsin G. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, F.E.; De Kleine, M.; Frijns, C.; Fijnheer, R.; van Gijn, J.; Kappelle, L.J. Endothelial cell activation is associated with cerebral white matter lesions in patients with cerebrovascular disease. Ann. N. Y. Acad. Sci. J. 2002, 977, 306–314. [Google Scholar] [CrossRef]
- Fornage, M.; Chiang, Y.A.; O’Meara, E.S.; Psaty, B.M.; Reiner, A.P.; Siscovick, D.S.; Tracy, R.P.; Longstreth, W.T. Biomarkers of inflammation and MRI-defined small vessel disease of the brain: The cardiovascular health study. Stroke 2008, 39, 1952–1959. [Google Scholar] [CrossRef]
- Hassan, A.; Hunt, B.J.; O’Sullivan, M.; Parmar, K.; Bamford, J.M.; Briley, D.; Brown, M.M.; Thomas, D.J.; Markus, H.S. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain 2003, 126, 424–432. [Google Scholar] [CrossRef]
- Wang, X.; Lyu, J.; Meng, Z.; Wu, X.; Chen, W.; Wang, G.; Niu, Q.; Li, X.; Bian, Y.; Han, D.; et al. Small vessel disease burden predicts functional outcomes in patients with acute ischemic stroke using machine learning. CNS Neurosci. Ther. 2023, 29, 1024–1033. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Spring, M.; Dorian, P.; Panzov, V.; Thorpe, K.E.; Hall, J.; Vaid, H.; O’Donnell, M.; Laupacis, A.; Côté, R.; et al. Atrial fibrillation in patients with cryptogenic stroke. N. Engl. J. Med. 2014, 370, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Sanna, T.; Diener, H.C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.A.; Mollman Rymer, M.; Thijs, V.; Rogers, T.; Beckers, F.; et al. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 2014, 370, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Sharma, M.; Mundl, H.; Kasner, S.E.; Bangdiwala, S.I.; Berkowitz, S.D.; Swaminathan, B.; Lavados, P.; Wang, Y.; Wanget, Y.; et al. Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. N. Engl. J. Med. 2018, 378, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, H.S.; Nakayama, H.; Reith, J.; et Olsen, T.S. Stroke recurrence: Predictors, severity, and prognosis. The Copenhagen Stroke Study. Neurology 1997, 48, 891–895. [Google Scholar] [CrossRef]
- Diener, H.C.; Sacco, R.L.; Easton, J.D.; Granger, C.B.; Bernstein, R.A.; Uchiyama, S.; Kreuzer, J.; Cronin, L.; Cotton, D.; Grauer, C.; et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N. Engl. J. Med. 2019, 380, 1906–1917. [Google Scholar] [CrossRef]
- Kamel, H.; Navi, B.B.; Parikh, N.S.; Merkler, A.E.; Okin, P.M.; Devereux, R.B.; Weinsaft, J.W.; Kim, J.; Cheung, J.W.; Kim, L.K.; et al. Machine Learning Prediction of Stroke Mechanism in Embolic Strokes of Undetermined Source. Stroke 2020, 51, e203–e210. [Google Scholar] [CrossRef]
- Ntaios, G.; Weng, S.F.; Perlepe, K.; Akyea, R.; Condon, L.; Lambrou, D.; Sirimarco, G.; Strambo, D.; Eskandari, A.; Karagkiozi, E.; et al. Data-driven machine-learning analysis of potential embolic sources in embolic stroke of undetermined source. Eur. J. Neurol. 2021, 28, 192–201. [Google Scholar] [CrossRef]
- Luo, D.; Yang, Z.; Zhang, G.; Shen, Q.; Zhang, H.; Lai, J.; Hu, H.; He, J.; Wu, S.; Zhang, C. Machine learning in a real-world PFO study: Analysis of data from multi-centers in China. BMC Med. Inform. Decis. Mak. 2022, 22, 305. [Google Scholar] [CrossRef]
- Esenwa, C.; Cheng, N.T.; Luna, J.; Willey, J.; Boehme, A.K.; Kirchoff-Torres, K.; Labovitz, D.; Liberman, A.L.; Mabie, P.; Moncrieffe, K.; et al. Biomarkers of Coagulation and Inflammation in COVID-19-Associated Ischemic Stroke. Stroke 2021, 52, e706–e709. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miceli, G.; Basso, M.G.; Rizzo, G.; Pintus, C.; Cocciola, E.; Pennacchio, A.R.; Tuttolomondo, A. Artificial Intelligence in Acute Ischemic Stroke Subtypes According to Toast Classification: A Comprehensive Narrative Review. Biomedicines 2023, 11, 1138. https://doi.org/10.3390/biomedicines11041138
Miceli G, Basso MG, Rizzo G, Pintus C, Cocciola E, Pennacchio AR, Tuttolomondo A. Artificial Intelligence in Acute Ischemic Stroke Subtypes According to Toast Classification: A Comprehensive Narrative Review. Biomedicines. 2023; 11(4):1138. https://doi.org/10.3390/biomedicines11041138
Chicago/Turabian StyleMiceli, Giuseppe, Maria Grazia Basso, Giuliana Rizzo, Chiara Pintus, Elena Cocciola, Andrea Roberta Pennacchio, and Antonino Tuttolomondo. 2023. "Artificial Intelligence in Acute Ischemic Stroke Subtypes According to Toast Classification: A Comprehensive Narrative Review" Biomedicines 11, no. 4: 1138. https://doi.org/10.3390/biomedicines11041138
APA StyleMiceli, G., Basso, M. G., Rizzo, G., Pintus, C., Cocciola, E., Pennacchio, A. R., & Tuttolomondo, A. (2023). Artificial Intelligence in Acute Ischemic Stroke Subtypes According to Toast Classification: A Comprehensive Narrative Review. Biomedicines, 11(4), 1138. https://doi.org/10.3390/biomedicines11041138






