Intraluminal Therapy for Helicobacter pylori Infection—Comparison of Medicament Containing Tetracycline, Metronidazole, and Bismuth versus Amoxicillin, Metronidazole, and Clarithromycin: A Randomized Controlled Study
Abstract
1. Introduction
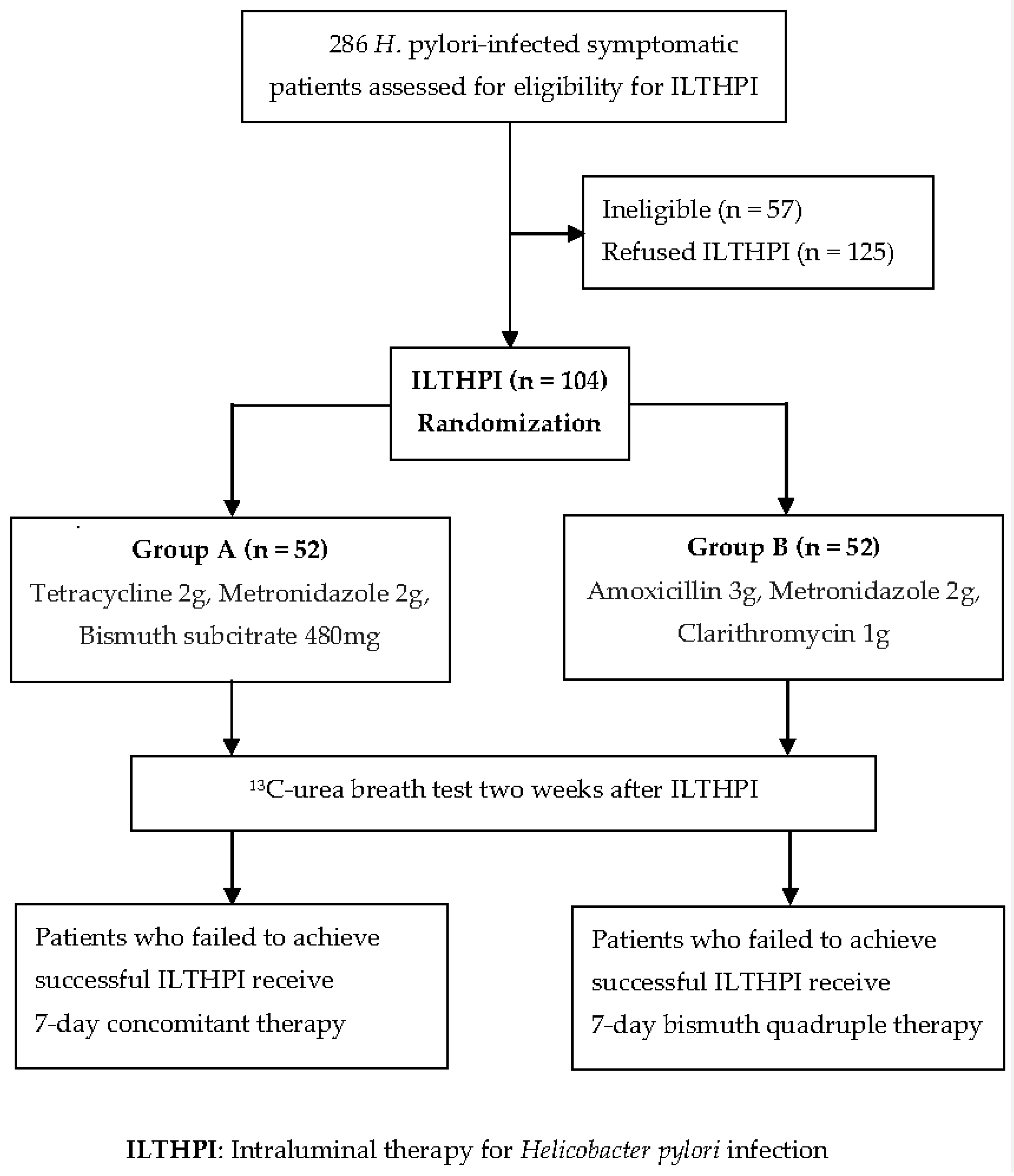
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Plummer, M.; Franceschi, S.; Vignat, J.; Forman, D.; de Martel, C. Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer 2015, 136, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Chiang, T.H.; Chou, C.K.; Tu, Y.K.; Liao, W.C.; Wu, M.S.; Graham, D.Y. Association between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016, 150, 1113–1124.e5. [Google Scholar] [CrossRef] [PubMed]
- Kusters, J.G.; van Vliet, A.H.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef]
- Ho, J.J.C.; Navarro, M.; Sawyer, K.; Elfanagely, Y.; Moss, S.F. Helicobacter pylori Antibiotic Resistance in the United States between 2011 and 2021: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2022, 117, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-M.; Tai, W.-C.; Hsu, P.-I.; Wu, D.-C.; Kuo, C.-H.; Tsay, F.-W.; Lee, C.-L.; Chen, K.-Y.; Chuah, S.-K.; on behalf of the Taiwan Acid-Related Disease and Microbiota Consortium Study Group. Trend of changes in antibiotic resistance in Helicobacter pylori from 2013 to 2019, a multicenter report from Taiwan. Ther. Adv. Gastroenterol. 2020, 13, 1756284820976990. [Google Scholar] [CrossRef]
- Liou, J.-M.; Malfertheiner, P.; Lee, Y.-C.; Sheu, B.-S.; Sugano, K.; Cheng, H.-C.; Yeoh, K.-G.; Hsu, P.-I.; Goh, K.-L.; Mahachai, V.; et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: The Taipei global consensus. Gut 2020, 69, 2093–2112. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/newsroom/detail/27-022017 (accessed on 26 February 2023).
- Liou, T.C.; Liao, P.H.; Lin, Y.C.; Chu, C.H.; Shih, S.C. Intraluminal therapy for Helicobacter pylori infection. J. Gastroenterol. Hepatol. 2019, 34, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Chen, Y.P.; Ho, C.Y.; Liu, T.W.; Chu, C.H.; Wang, H.Y.; Liou, T.C. The Impact of Gastric Juice pH on the Intraluminal Therapy for Helicobacter pylori Infection. J. Clin. Med. 2020, 9, 1852. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Liu, T.W.; Lin, Y.S.; Chen, Y.P.; Chen, M.J.; Wang, H.Y.; Liou, T.C. Factors Affecting the Intraluminal Therapy for Helicobacter pylori Infection. Microorganisms 2022, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Sheu, B.-S.; Wu, M.-S.; Chiu, C.-T.; Lo, J.-C.; Wu, D.-C.; Liou, J.-M.; Wu, C.-Y.; Cheng, H.-C.; Lee, Y.-C.; Hsu, P.-I.; et al. Consensus on the clinical management, screening-to-treat, and surveillance of Helicobacter pylori infection to improve gastric cancer control on a nationwide scale. Helicobacter 2017, 22, e12368. [Google Scholar] [CrossRef]
- Gisbert, J.P. Empirical or susceptibility-guided treatment for Helicobacter pylori infection? A comprehensive review. Ther. Adv. Gastroenterol. 2020, 13, 1756284820968736. [Google Scholar] [CrossRef]
- Sugimoto, M.; Furuta, T.; Shirai, N.; Kodaira, C.; Nishino, M.; Ikuma, M.; Ishizaki, T.; Hishida, A. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter 2007, 12, 317–323. [Google Scholar] [CrossRef]
- Erah, P.O.; Goddard, A.F.; Barrett, D.A.; Shaw, P.N.; Spiller, R.C. The stability of amoxicillin, clarithromycin and metronidazole in gastric juice: Relevance to the treatment of Helicobacter pylori infection. J. Antimicrob. Chemother. 1997, 39, 5–12. [Google Scholar] [CrossRef]
- Marcus, E.A.; Inatomi, N.; Nagami, G.T.; Sachs, G.; Scott, D.R. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment. Pharmacol. Ther. 2012, 36, 972–979. [Google Scholar] [CrossRef]
- Cheng, A.; Sheng, W.H.; Liou, J.M.; Wang, H.P.; Wu, M.S.; Lin, J.T.; Chang, S.C. Comparative in vitro antimicrobial susceptibility and synergistic activity of antimicrobial combinations against Helicobacter pylori isolates in Taiwan. J. Microbiol. Immunol. Infect. 2015, 48, 72–79. [Google Scholar] [CrossRef]
- Scarpignato, C.; Hunt, R.H. Acid suppressant therapy: A step forward with potassium-competitive acid blockers. Curr. Treat. Options Gastroenterol. 2021, 19, 94–132. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 33613, Amoxicillin. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Amoxicillin (accessed on 26 February 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 84029, Clarithromycin. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Clarithromycin (accessed on 26 February 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 4173, Metronidazole. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Metronidazole (accessed on 26 February 2023).
- Alsamman, M.A.; Vecchio, E.C.; Shawwa, K.; Acosta-Gonzales, G.; Resnick, M.B.; Moss, S.F. Retrospective analysis confirms tetracycline quadruple as best Helicobacter pylori regimen in the USA. Dig. Dis. Sci. 2019, 64, 2893–2898. [Google Scholar] [CrossRef]
- Nyssen, O.P.; Bordin, D.; Tepes, B.; Pérez-Aisa, Á.; Vaira, D.; Caldas, M.; Bujanda, L.; Castro-Fernandez, M.; Lerang, F.; Leja, M.; et al. European Registry on Helicobacter pylori management (Hp-EuReg): Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21,533 patients. Gut 2021, 70, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Nyssen, O.P.; Perez-Aisa, A.; Castro-Fernandez, M.; Pellicano, R.; Huguet, J.M.; Rodrigo, L.; Ortuño, J.; Gomez-Rodriguez, B.J.; Pinto, R.M.; Areia, M.; et al. European Registry on Helicobacter pylori management: Single-capsule bismuth quadruple therapy is effective in real-world clinical practice. United Eur. Gastroenterol. J. 2021, 9, 38–46. [Google Scholar] [CrossRef]
- Khadangi, F.; Yassi, M.; Kerachian, M.A. Review: Diagnostic accuracy of PCR-based detection tests for Helicobacter pylori in stool samples. Helicobacter 2017, 22, e12444. [Google Scholar] [CrossRef] [PubMed]
- Saracino, I.; Pavoni, M.; Zullo, A.; Fiorini, G.; Lazzarotto, T.; Borghi, C.; Vaira, D. Next Generation Sequencing for the Prediction of the Antibiotic Resistance in Helicobacter pylori: A Literature Review. Antibiotics 2021, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.F.; Dang, L.P.; Chua, D.; Sobrado, J.; Zhou, Y.; Graham, D.Y. Comparable results of Helicobacter pylori antibiotic resistance testing of stools vs gastric biopsies using next-generation sequencing. Gastroenterology 2022, 162, 2095–2097.e2. [Google Scholar] [CrossRef]
- Si, X.B.; Bi, D.Y.; Lan, Y.; Zhang, S.; Huo, L.Y. Gastric Juice-Based Genotypic Methods for Diagnosis of Helicobacter pylori Infection and Antibiotic Resistance Testing: A Systematic Review and Meta-analysis. Turk. J. Gastroenterol. 2021, 32, 53–65. [Google Scholar] [CrossRef]
- Hsieh, M.-S.; Kuo, F.-C.; Wu, M.-C.; Wang, J.-W.; Liu, C.-J.; Chu, N.-S.; Tsai, P.-Y.; Hsu, P.-I.; Wu, I.-C.; Wu, J.-Y.; et al. Tailored susceptibility-guided therapy via gastric juice PCR for the first-line H. pylori eradication, a randomized controlled trial. J. Formos. Med. Assoc. 2022, 121, 1450–1457. [Google Scholar] [CrossRef]
- Chu, Y.T.; Wang, Y.H.; Wu, J.J.; Lei, H.Y. Invasion and Multiplication of Helicobacter pylori in Gastric Epithelial Cells and Implications for Antibiotic Resistance. Infect. Immun. 2010, 78, 4157–4165. [Google Scholar] [CrossRef]
| Characteristics | Group A (n = 52) | Group B (n = 52) |
|---|---|---|
| Age (years, mean ± SD/range) * | 49.73 ± 12.96 (20–73) | 50.58 ± 11.37 (25–72) |
| Gender (M/F) * | 30/22 | 30/22 |
| NSAID ingestion * | 11 (21.2%) | 12 (23.1%) |
| Smoking * | 8 (15.4%) | 10 (19.2%) |
| Alcohol consumption * | 7 (13.5%) | 6 (11.5%) |
| Ingestion of tea * | 18 (34.6%) | 19 (36.5%) |
| Ingestion of coffee * | 22 (42.3%) | 20 (38.5%) |
| BMI (kg/m2, mean ± SD/range) * | 25.4 ± 4.5 (17.5–37.2) | 25.7 ± 4.8 (17.8–38.5) |
| Endoscopic Findings | Group A (n =52) | Group B (n = 52) |
|---|---|---|
| Normal * | 7 (13.5%) | 6 (11.5%) |
| Gastritis * | 45 (86.5%) | 46 (88.5%) |
| (antrum **/corpus **/cardia **) | (40/44/32) | (38/45/35) |
| Peptic ulcer disease * | 14 (26.9%) | 15 (28.8%) |
| Medicaments | Patients Number (Lost to Follow up) | Eradication Rate | Adverse Event |
|---|---|---|---|
| Group A † | 52 (1) | 39/51 (76.5%) * | 1/52 (1.9%) ** |
| Group B † | 52 (0) | 44/52 (84.6%) * | 2/52 (3.8%) ** |
| Patients (No.) | ILTHPI * | Oral Antibiotic Therapy †* | ILTHPI Plus Oral Antibiotic Therapy (Overall Eradication Rate) * |
|---|---|---|---|
| Group A (51) | 39/51 (76.5%) | 10/12 (83.3%) | 49/51 (96.1%) |
| Group B (52) | 44/52 (84.6%) | 7/8 (87.5%) | 51/52 (98.1%) |
| Clinical Characteristics | Group A † (n = 100) | Group B † (n = 52) |
|---|---|---|
| Age (years, mean ± SD/range) * | 52.1 ± 10.3 (24–74) | 50.58 ± 11.37 (25–72) |
| Gender (M/F) * | 47/53 | 30/22 |
| NSAID ingestion * | 21 (21.0%) | 12 (23.1%) |
| Smoking * | 17 (17.0%) | 10 (19.2%) |
| Alcohol consumption * | 7 (7.0%) | 6 (11.5%) |
| Ingestion of tea * | 30 (30.0%) | 19 (36.5%) |
| Ingestion of coffee * | 39 (39.0%) | 20 (38.5%) |
| BMI (kg/m2, mean ± SD/range) * | 25.9 ± 4.4 (17.5–36.5) | 25.7 ± 4.8 (17.8–38.5) |
| Peptic ulcer disease * | 28 (28.0%) | 15 (28.8%) |
| Gastric Juice pH ≥ 6.0 | 45 (45.0%) ** | 51 (98.1%) ** |
| Eradication rate | 51/95 (53.7%) *** | 44/52 (84.6%) *** |
| Medicament Containing | Stomach Acid Control before ILTHPI (Eradication Rate) | Adverse Events of ILTHPI | Oral Antibiotic Therapy for ILTHPI Failure (Eradication Rate) | ILTHPI Plus Oral Antibiotic Therapy for ILTHPI Failure (Overall Eradication Rate) |
|---|---|---|---|---|
| Tetracycline 2 g Metronidazole 2 g Bismuth 480 mg | Yes (76.5%; 39/51) * | 1/52 (1.9%) * | 7-day concomitant therapy (83.3%; 10/12) * | 49/51 (96.1%) * |
| Amoxicillin 3 g Metronidazole 2 g Clarithromycin 1 g | Yes (84.6%; 44/52) *† | 2/52 (3.8%) * | 7-day bismuth quadruple therapy (87.5%; 7/8) * | 51/52 (98.1%) * |
| Amoxicillin 3 g Metronidazole 2 g Clarithromycin 1 g | No (53.7%; 51/95)† | |||
| pvalue | * p = 0.427 † p = 0.0004 | * p = 1.0 | * p = 1.0 | * p = 0.618 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.-W.; Chen, Y.-P.; Ho, C.-Y.; Chen, M.-J.; Wang, H.-Y.; Shih, S.-C.; Liou, T.-C. Intraluminal Therapy for Helicobacter pylori Infection—Comparison of Medicament Containing Tetracycline, Metronidazole, and Bismuth versus Amoxicillin, Metronidazole, and Clarithromycin: A Randomized Controlled Study. Biomedicines 2023, 11, 1084. https://doi.org/10.3390/biomedicines11041084
Liu T-W, Chen Y-P, Ho C-Y, Chen M-J, Wang H-Y, Shih S-C, Liou T-C. Intraluminal Therapy for Helicobacter pylori Infection—Comparison of Medicament Containing Tetracycline, Metronidazole, and Bismuth versus Amoxicillin, Metronidazole, and Clarithromycin: A Randomized Controlled Study. Biomedicines. 2023; 11(4):1084. https://doi.org/10.3390/biomedicines11041084
Chicago/Turabian StyleLiu, Ting-Wen, Yen-Po Chen, Cheng-Yu Ho, Ming-Jen Chen, Horng-Yuan Wang, Shou-Chuan Shih, and Tai-Cherng Liou. 2023. "Intraluminal Therapy for Helicobacter pylori Infection—Comparison of Medicament Containing Tetracycline, Metronidazole, and Bismuth versus Amoxicillin, Metronidazole, and Clarithromycin: A Randomized Controlled Study" Biomedicines 11, no. 4: 1084. https://doi.org/10.3390/biomedicines11041084
APA StyleLiu, T.-W., Chen, Y.-P., Ho, C.-Y., Chen, M.-J., Wang, H.-Y., Shih, S.-C., & Liou, T.-C. (2023). Intraluminal Therapy for Helicobacter pylori Infection—Comparison of Medicament Containing Tetracycline, Metronidazole, and Bismuth versus Amoxicillin, Metronidazole, and Clarithromycin: A Randomized Controlled Study. Biomedicines, 11(4), 1084. https://doi.org/10.3390/biomedicines11041084





