Changes in the Localization of Polyamine Spermidine in the Rat Retina with Age
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissues
2.2. Immunohistochemistry
2.3. Confocal Microscopy
2.4. Semi-Quantitative Analysis of Staining Intensity
2.5. Data Analysis and Statistics
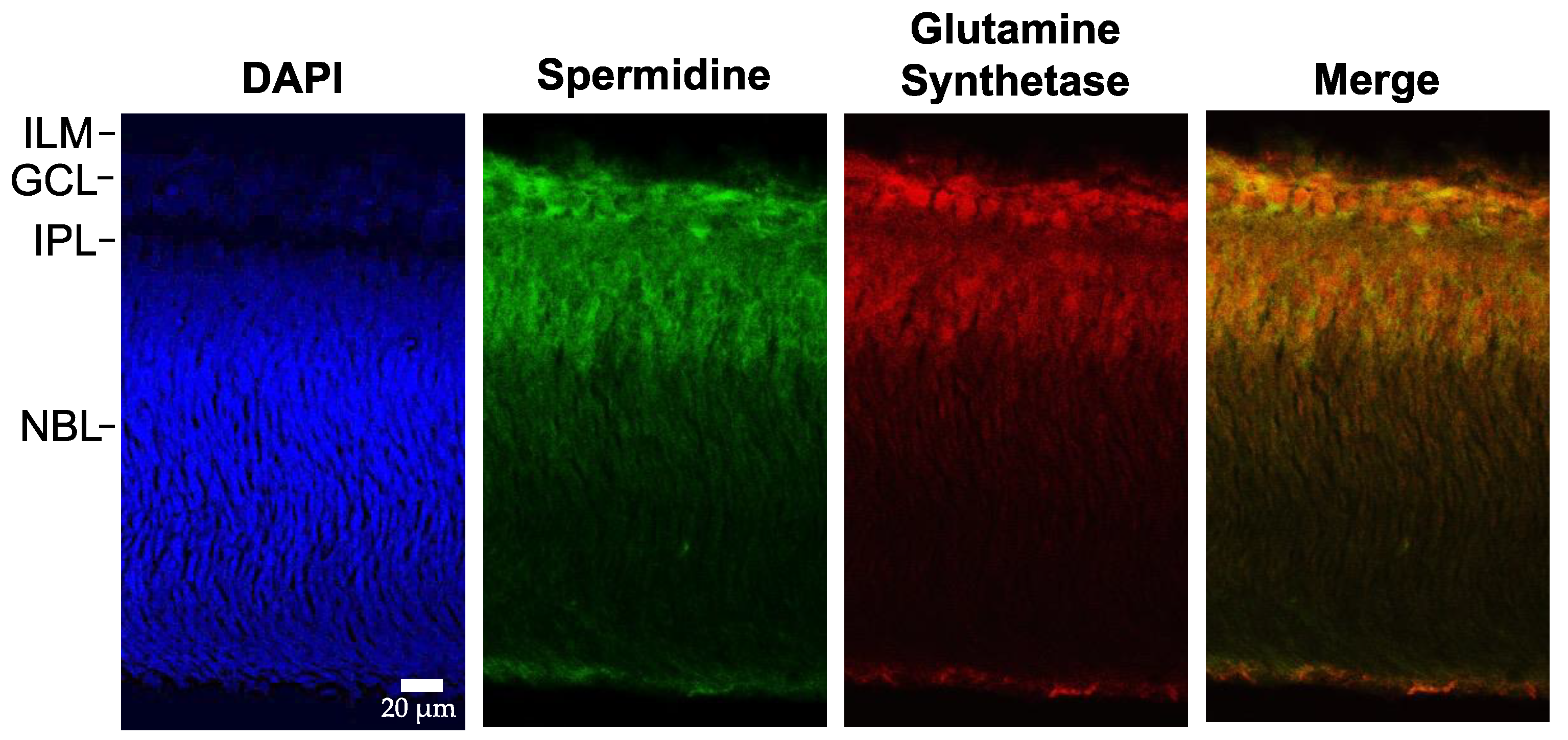
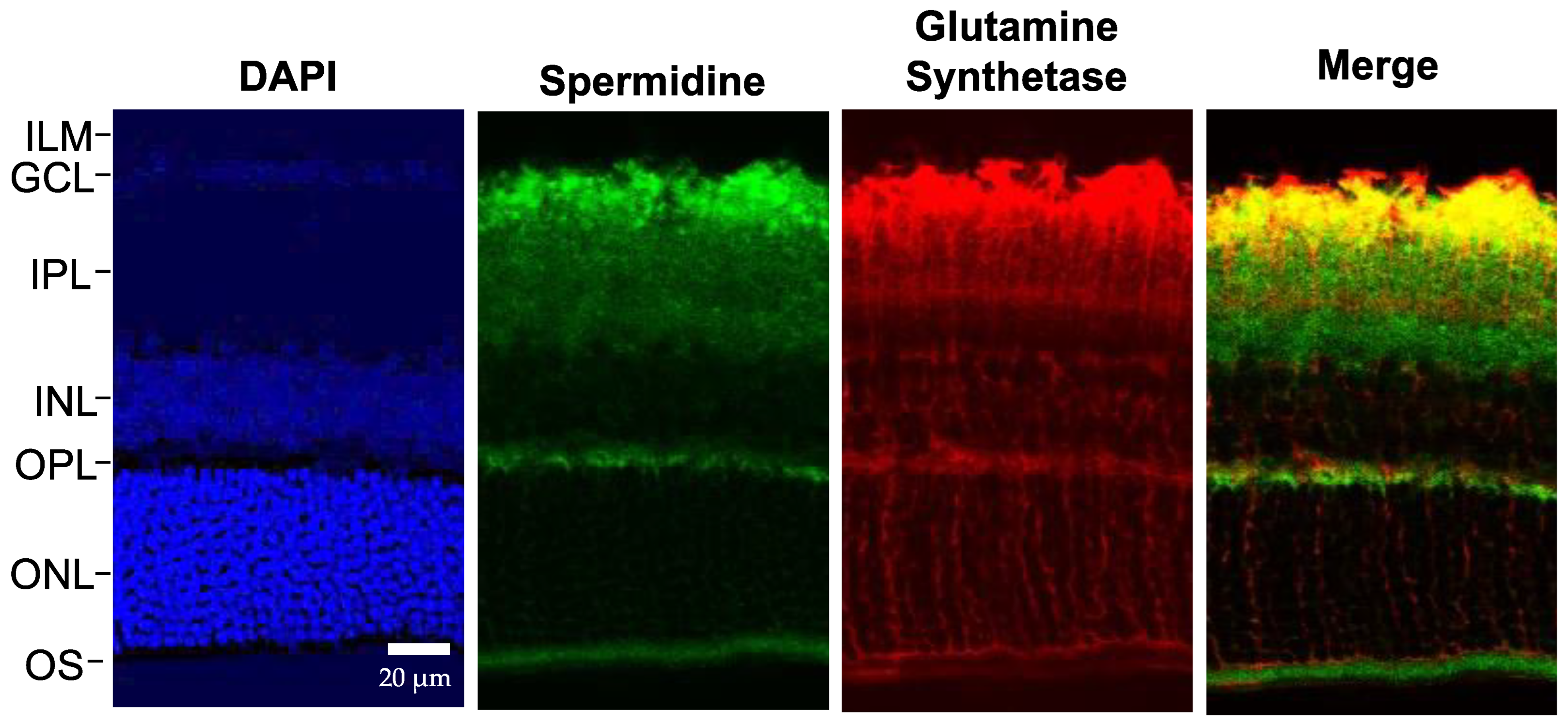
3. Results
3.1. Immunohistochemistry for Spermidine and Glutamine Synthetase
3.2. Glutamine Synthetase
3.3. Expression of the Polyamine Spermidine (SPD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watanabe, S.; Kusama-Eguchi, K.; Kobayashi, H.; Igarashi, K. Estimation of Polyamine Binding to Macromolecules and ATP in Bovine Lymphocytes and Rat Liver. J. Biol. Chem. 1991, 266, 20803–20809. [Google Scholar] [CrossRef] [PubMed]
- Graser, G.; Hartmann, T. Biosynthesis of Spermidine, a Direct Precursor of Pyrrolizidine Alkaloids in Root Cultures of Senecio vulgaris L. Planta 2000, 211, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Ientile, R.; Russo, P.; Macaione, S. Polyamine Localization and Biosynthesis in Chemically Fractionated Rat Retina. J. Neurochem. 1986, 47, 1356–1360. [Google Scholar] [CrossRef]
- Pegg, A.E. Recent Advances in the Biochemistry of Polyamines in Eukaryotes. Biochem. J. 1986, 234, 249–262. [Google Scholar] [CrossRef]
- Pegg, A.E. Regulation of Ornithine Decarboxylase. J. Biol. Chem. 2006, 281, 14529–14532. [Google Scholar] [CrossRef]
- Peters, D.; Berger, J.; Langnaese, K.; Derst, C.; Madai, V.I.; Krauss, M.; Fischer, K.-D.; Veh, R.W.; Laube, G. Arginase and Arginine Decarboxylase—Where Do the Putative Gate Keepers of Polyamine Synthesis Reside in Rat Brain? PLoS ONE 2013, 8, e66735. [Google Scholar] [CrossRef]
- Laube, G.; Bernstein, H.-G. Agmatine: Multifunctional Arginine Metabolite and Magic Bullet in Clinical Neuroscience? Biochem. J. 2017, 474, 2619–2640. [Google Scholar] [CrossRef] [PubMed]
- Rieck, J.; Skatchkov, S.N.; Derst, C.; Eaton, M.J.; Veh, R.W. Unique Chemistry, Intake, and Metabolism of Polyamines in the Central Nervous System (CNS) and Its Body. Biomolecules 2022, 12, 501. [Google Scholar] [CrossRef]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef]
- Malpica-Nieves, C.J.; Rivera-Aponte, D.E.; Tejeda-Bayron, F.A.; Mayor, A.M.; Phanstiel, O.; Veh, R.W.; Eaton, M.J.; Skatchkov, S.N. The Involvement of Polyamine Uptake and Synthesis Pathways in the Proliferation of Neonatal Astrocytes. Amino Acids 2020, 52, 1169–1180. [Google Scholar] [CrossRef]
- Wallace, H.M.; Fraser, A.V.; Hughes, A. A Perspective of Polyamine Metabolism. Biochem. J. 2003, 376, 1–14. [Google Scholar] [CrossRef]
- Jänne, J.; Alhonen, L.; Pietilä, M.; Keinänen, T.A. Genetic Approaches to the Cellular Functions of Polyamines in Mammals. Eur. J. Biochem. 2004, 271, 877–894. [Google Scholar] [CrossRef]
- Pegg, A.E. Functions of Polyamines in Mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef]
- Williams, K. Modulation and Block of Ion Channels: A New Biology of Polyamines. Cell. Signal. 1997, 9, 1–13. [Google Scholar] [CrossRef]
- Williams, K.; Romano, C.; Dichter, M.A.; Molinoff, P.B. Modulation of the NMDA Receptor by Polyamines. Life Sci. 1991, 48, 469–498. [Google Scholar] [CrossRef] [PubMed]
- Bowie, D.; Mayer, M.L. Inward Rectification of Both AMPA and Kainate Subtype Glutamate Receptors Generated by Polyamine-Mediated Ion Channel Block. Neuron 1995, 15, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Burnashev, N.; Khodorova, A.; Jonas, P.; Helm, P.J.; Wisden, W.; Monyer, H.; Seeburg, P.H.; Sakmann, B. Calcium-Permeable AMPA-Kainate Receptors in Fusiform Cerebellar Glial Cells. Science 1992, 256, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.; Baukrowitz, T.; Fakler, B. Polyamines as Gating Molecules of Inward-Rectifier K+ Channels. Eur. J. Biochem. 2000, 267, 5824–5829. [Google Scholar] [CrossRef]
- Skatchkov, S.N.; Eaton, M.J.; Krusek, J.; Veh, R.W.; Biedermann, B.; Bringmann, A.; Pannicke, T.; Orkand, R.K.; Reichenbach, A. Spatial Distribution of Spermine/Spermidine Content and K(+)-Current Rectification in Frog Retinal Glial (Müller) Cells. Glia 2000, 31, 84–90. [Google Scholar] [CrossRef]
- Nichols, C.G.; Lee, S.-J. Polyamines and Potassium Channels: A 25-Year Romance. J. Biol. Chem. 2018, 293, 18779–18788. [Google Scholar] [CrossRef]
- Scott, R.H.; Sutton, K.G.; Griffin, A.; Stapleton, S.R.; Currie, K.P. Aspects of Calcium-Activated Chloride Currents: A Neuronal Perspective. Pharmacol. Ther. 1995, 66, 535–565. [Google Scholar] [CrossRef]
- Ahern, G.P.; Wang, X.; Miyares, R.L. Polyamines Are Potent Ligands for the Capsaicin Receptor TRPV1. J. Biol. Chem. 2006, 281, 8991–8995. [Google Scholar] [CrossRef]
- Malarkey, E.B.; Parpura, V. Mechanisms of Glutamate Release from Astrocytes. Neurochem. Int. 2008, 52, 142–154. [Google Scholar] [CrossRef]
- Duan, B.; Wang, Y.-Z.; Yang, T.; Chu, X.-P.; Yu, Y.; Huang, Y.; Cao, H.; Hansen, J.; Simon, R.P.; Zhu, M.X.; et al. Extracellular Spermine Exacerbates Ischemic Neuronal Injury through Sensitization of ASIC1a Channels to Extracellular Acidosis. J. Neurosci. 2011, 31, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Gewehr, C.; Da Silva, M.A.; Dos Santos, G.T.; Rossato, M.F.; De Oliveira, S.M.; Drewes, C.C.; Pazini, A.M.; Guerra, G.P.; Rubin, M.A.; Ferreira, J. Contribution of Peripheral Vanilloid Receptor to the Nociception Induced by Injection of Spermine in Mice. Pharmacol. Biochem. Behav. 2011, 99, 775–781. [Google Scholar] [CrossRef]
- Skatchkov, S.N.; Antonov, S.M.; Eaton, M.J. Glia and Glial Polyamines. Role in Brain Function in Health and Disease. Biochem. Mosc. Suppl. Ser. A 2016, 10, 73–98. [Google Scholar] [CrossRef]
- Biedermann, B.; Skatchkov, S.N.; Brunk, I.; Bringmann, A.; Pannicke, T.; Bernstein, H.G.; Faude, F.; Germer, A.; Veh, R.; Reichenbach, A. Spermine/Spermidine Is Expressed by Retinal Glial (Müller) Cells and Controls Distinct K+ Channels of Their Membrane. Glia 1998, 23, 209–220. [Google Scholar] [CrossRef]
- Noro, T.; Namekata, K.; Kimura, A.; Guo, X.; Azuchi, Y.; Harada, C.; Nakano, T.; Tsuneoka, H.; Harada, T. Spermidine Promotes Retinal Ganglion Cell Survival and Optic Nerve Regeneration in Adult Mice Following Optic Nerve Injury. Cell Death Dis. 2015, 6, e1720. [Google Scholar] [CrossRef] [PubMed]
- Lopatin, A.N.; Makhina, E.N.; Nichols, C.G. Potassium Channel Block by Cytoplasmic Polyamines as the Mechanism of Intrinsic Rectification. Nature 1994, 372, 366–369. [Google Scholar] [CrossRef]
- Fakler, B.; Brändle, U.; Glowatzki, E.; Weidemann, S.; Zenner, H.P.; Ruppersberg, J.P. Strong Voltage-Dependent Inward Rectification of Inward Rectifier K+ Channels Is Caused by Intracellular Spermine. Cell 1995, 80, 149–154. [Google Scholar] [CrossRef]
- Lu, Z.; Ding, L. Blockade of a Retinal CGMP-Gated Channel by Polyamines. J. Gen. Physiol. 1999, 113, 35–43. [Google Scholar] [CrossRef]
- Sturman, J.A.; Ingoglia, N.A.; Lindquist, T.D. Interconversion of Putrescine, Spermidine and Spermine in Goldfish and Rat Retina. Life Sci. 1976, 19, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Laube, G.; Veh, R.W. Astrocytes, Not Neurons, Show Most Prominent Staining for Spermidine/Spermine-like Immunoreactivity in Adult Rat Brain. Glia 1997, 19, 171–179. [Google Scholar] [CrossRef]
- Skatchkov, S.N.; Woodbury-Fariña, M.A.; Eaton, M. The Role of Glia in Stress. Psychiatr. Clin. N. Am. 2014, 37, 653–678. [Google Scholar] [CrossRef]
- Valentino, T.L.; Lukasiewicz, P.D.; Romano, C. Immunocytochemical Localization of Polyamines in the Tiger Salamander Retina. Brain Res. 1996, 713, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Withrow, C.; Ashraf, S.; O’Leary, T.; Johnson, L.R.; Fitzgerald, M.E.C.; Johnson, D.A. Effect of Polyamine Depletion on Cone Photoreceptors of the Developing Rabbit Retina. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3081–3090. [Google Scholar]
- Kovács, Z.; Skatchkov, S.N.; Veh, R.W.; Szabó, Z.; Németh, K.; Szabó, P.T.; Kardos, J.; Héja, L. Critical Role of Astrocytic Polyamine and GABA Metabolism in Epileptogenesis. Front. Cell. Neurosci. 2022, 15, 787319. [Google Scholar] [CrossRef]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in Polyamines with Aging and Their Ingestion from Food and Drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef]
- Maglione, M.; Kochlamazashvili, G.; Eisenberg, T.; Rácz, B.; Michael, E.; Toppe, D.; Stumpf, A.; Wirth, A.; Zeug, A.; Müller, F.E.; et al. Spermidine Protects from Age-Related Synaptic Alterations at Hippocampal Mossy Fiber-CA3 Synapses. Sci. Rep. 2019, 9, 19616. [Google Scholar] [CrossRef]
- Schroeder, S.; Hofer, S.J.; Zimmermann, A.; Pechlaner, R.; Dammbrueck, C.; Pendl, T.; Marcello, G.M.; Pogatschnigg, V.; Bergmann, M.; Müller, M.; et al. Dietary Spermidine Improves Cognitive Function. Cell Rep. 2021, 35, 108985. [Google Scholar] [CrossRef]
- Kucheryavykh, Y.V.; Shuba, Y.M.; Antonov, S.M.; Inyushin, M.Y.; Cubano, L.; Pearson, W.L.; Kurata, H.; Reichenbach, A.; Veh, R.W.; Nichols, C.G.; et al. Complex Rectification of Müller Cell Kir Currents. Glia 2008, 56, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Kucheryavykh, Y.V.; Pearson, W.L.; Kurata, H.T.; Eaton, M.J.; Skatchkov, S.N.; Nichols, C.G. Polyamine Permeation and Rectification of Kir4.1 Channels. Channels 2007, 1, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, N.M.; Ju, S.; Verbitsky, M.; Ross, B.; Geddie, M.L.; Rockenstein, E.; Adame, A.; Muhammad, A.; Vonsattel, J.P.; Ringe, D.; et al. Polyamine Pathway Contributes to the Pathogenesis of Parkinson Disease. Proc. Natl. Acad. Sci. USA 2010, 107, 16970–16975. [Google Scholar] [CrossRef]
- Lumkwana, D.; Peddie, C.; Kriel, J.; Michie, L.L.; Heathcote, N.; Collinson, L.; Kinnear, C.; Loos, B. Investigating the Role of Spermidine in a Model System of Alzheimer’s Disease Using Correlative Microscopy and Super-Resolution Techniques. Front. Cell Dev. Biol. 2022, 10, 819571. [Google Scholar] [CrossRef]
- Jamwal, S.; Kumar, P. Spermidine Ameliorates 3-Nitropropionic Acid (3-NP)-Induced Striatal Toxicity: Possible Role of Oxidative Stress, Neuroinflammation, and Neurotransmitters. Physiol. Behav. 2016, 155, 180–187. [Google Scholar] [CrossRef]
- Yang, Q.; Zheng, C.; Cao, J.; Cao, G.; Shou, P.; Lin, L.; Velletri, T.; Jiang, M.; Chen, Q.; Han, Y.; et al. Spermidine Alleviates Experimental Autoimmune Encephalomyelitis through Inducing Inhibitory Macrophages. Cell Death Differ. 2016, 23, 1850–1861. [Google Scholar] [CrossRef]
- Virgili, M.; Crochemore, C.; Peña-Altamira, E.; Contestabile, A. Regional and Temporal Alterations of ODC/Polyamine System during ALS-like Neurodegenerative Motor Syndrome in G93A Transgenic Mice. Neurochem. Int. 2006, 48, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.L.; Khakh, B.S.; Skatchkov, S.N.; Zhou, M.; Lee, C.J.; Rouach, N. New Insights on Astrocyte Ion Channels: Critical for Homeostasis and Neuron-Glia Signaling. J. Neurosci. 2015, 35, 13827–13835. [Google Scholar] [CrossRef]
- Liang, Y.; Piao, C.; Beuschel, C.B.; Toppe, D.; Kollipara, L.; Bogdanow, B.; Maglione, M.; Lützkendorf, J.; See, J.C.K.; Huang, S.; et al. EIF5A Hypusination, Boosted by Dietary Spermidine, Protects from Premature Brain Aging and Mitochondrial Dysfunction. Cell Rep. 2021, 35, 108941. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and Lifespan Extension by the Natural Polyamine Spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Selmeci, L.; Brosnan, M.E.; Seiler, N. Recent Progress in Polyamine Research; Akadémiai Kiadó: Budapest, Hungary, 1985; ISBN 978-9-63054-243-2. [Google Scholar]
- lentile, R.; Macaione, S.; Di Giorgio, R.M. Polyamine Metabolic Defects in Inherited Disorder of Rd Mice. In Recent Progress in Polyarnine Research; Selmeci, L., Brosnan, M.E., Seiler, N., Eds.; Akadémiai Kiadó: Budapest, Hungary, 1985; pp. 401–409. [Google Scholar]
- Wirth, M.; Benson, G.; Schwarz, C.; Köbe, T.; Grittner, U.; Schmitz, D.; Sigrist, S.J.; Bohlken, J.; Stekovic, S.; Madeo, F.; et al. The Effect of Spermidine on Memory Performance in Older Adults at Risk for Dementia: A Randomized Controlled Trial. Cortex 2018, 109, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Wirth, A.; Wolf, B.; Huang, C.-K.; Glage, S.; Hofer, S.J.; Bankstahl, M.; Bär, C.; Thum, T.; Kahl, K.G.; Sigrist, S.J.; et al. Novel Aspects of Age-Protection by Spermidine Supplementation Are Associated with Preserved Telomere Length. Geroscience 2021, 43, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Malpica-Nieves, C.J.; Rivera, Y.; Rivera-Aponte, D.E.; Phanstiel, O.; Veh, R.W.; Eaton, M.J.; Skatchkov, S.N. Uptake of Biotinylated Spermine in Astrocytes: Effect of Cx43 SiRNA, HIV-Tat Protein and Polyamine Transport Inhibitor on Polyamine Uptake. Biomolecules 2021, 11, 1187. [Google Scholar] [CrossRef] [PubMed]
- Soda, K.; Kano, Y.; Chiba, F.; Koizumi, K.; Miyaki, Y. Increased Polyamine Intake Inhibits Age-Associated Alteration in Global DNA Methylation and 1,2-Dimethylhydrazine-Induced Tumorigenesis. PLoS ONE 2013, 8, e64357. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.; Horn, N.; Benson, G.; Wrachtrup Calzado, I.; Wurdack, K.; Pechlaner, R.; Grittner, U.; Wirth, M.; Flöel, A. Spermidine Intake Is Associated with Cortical Thickness and Hippocampal Volume in Older Adults. Neuroimage 2020, 221, 117132. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos, D.S.; Malpica-Nieves, C.J.; Díaz-García, A.; Eaton, M.J.; Skatchkov, S.N. Changes in the Localization of Polyamine Spermidine in the Rat Retina with Age. Biomedicines 2023, 11, 1008. https://doi.org/10.3390/biomedicines11041008
Ríos DS, Malpica-Nieves CJ, Díaz-García A, Eaton MJ, Skatchkov SN. Changes in the Localization of Polyamine Spermidine in the Rat Retina with Age. Biomedicines. 2023; 11(4):1008. https://doi.org/10.3390/biomedicines11041008
Chicago/Turabian StyleRíos, David S., Christian J. Malpica-Nieves, Amanda Díaz-García, Misty J. Eaton, and Serguei N. Skatchkov. 2023. "Changes in the Localization of Polyamine Spermidine in the Rat Retina with Age" Biomedicines 11, no. 4: 1008. https://doi.org/10.3390/biomedicines11041008
APA StyleRíos, D. S., Malpica-Nieves, C. J., Díaz-García, A., Eaton, M. J., & Skatchkov, S. N. (2023). Changes in the Localization of Polyamine Spermidine in the Rat Retina with Age. Biomedicines, 11(4), 1008. https://doi.org/10.3390/biomedicines11041008




