Integrative Analysis of the Predictive Value of Perilipin Family on Clinical Significance, Prognosis and Immunotherapy of Glioma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Expression Levels of PLINs in Pan-Cancer and Gliomas
2.3. Correlation of PLINs Expression with Survival Prognosis and Clinicopathological Features of Glioma
2.4. Genetic Alterations of PLINs Family in Glioma
2.5. Association Analysis of PLINs Expression with Tumor Immune Microenvironment in Glioma
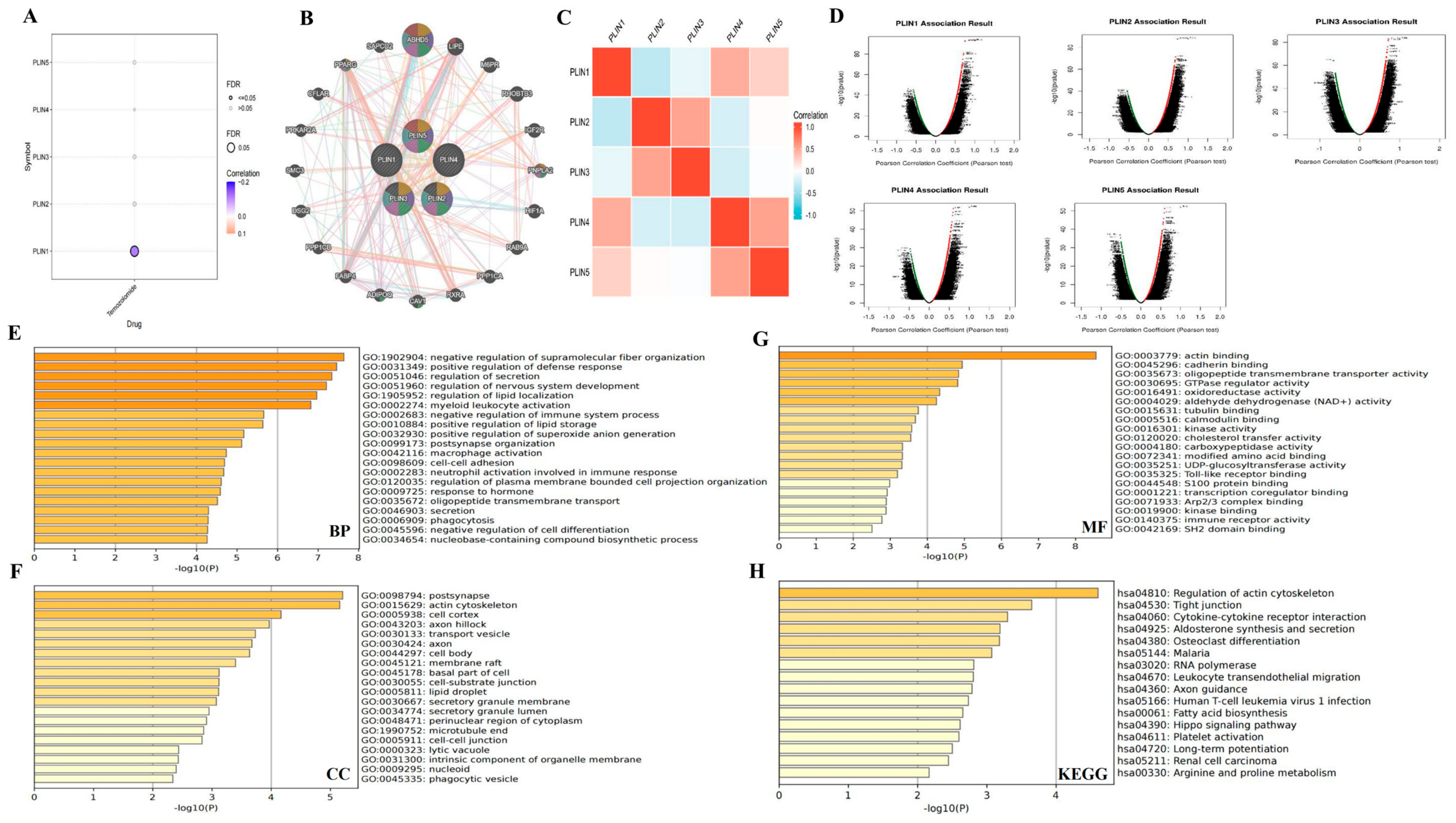
2.6. Correlation and Functional Enrichment Analysis of PLINs Family
2.7. Drug Sensitivity Analysis
3. Results
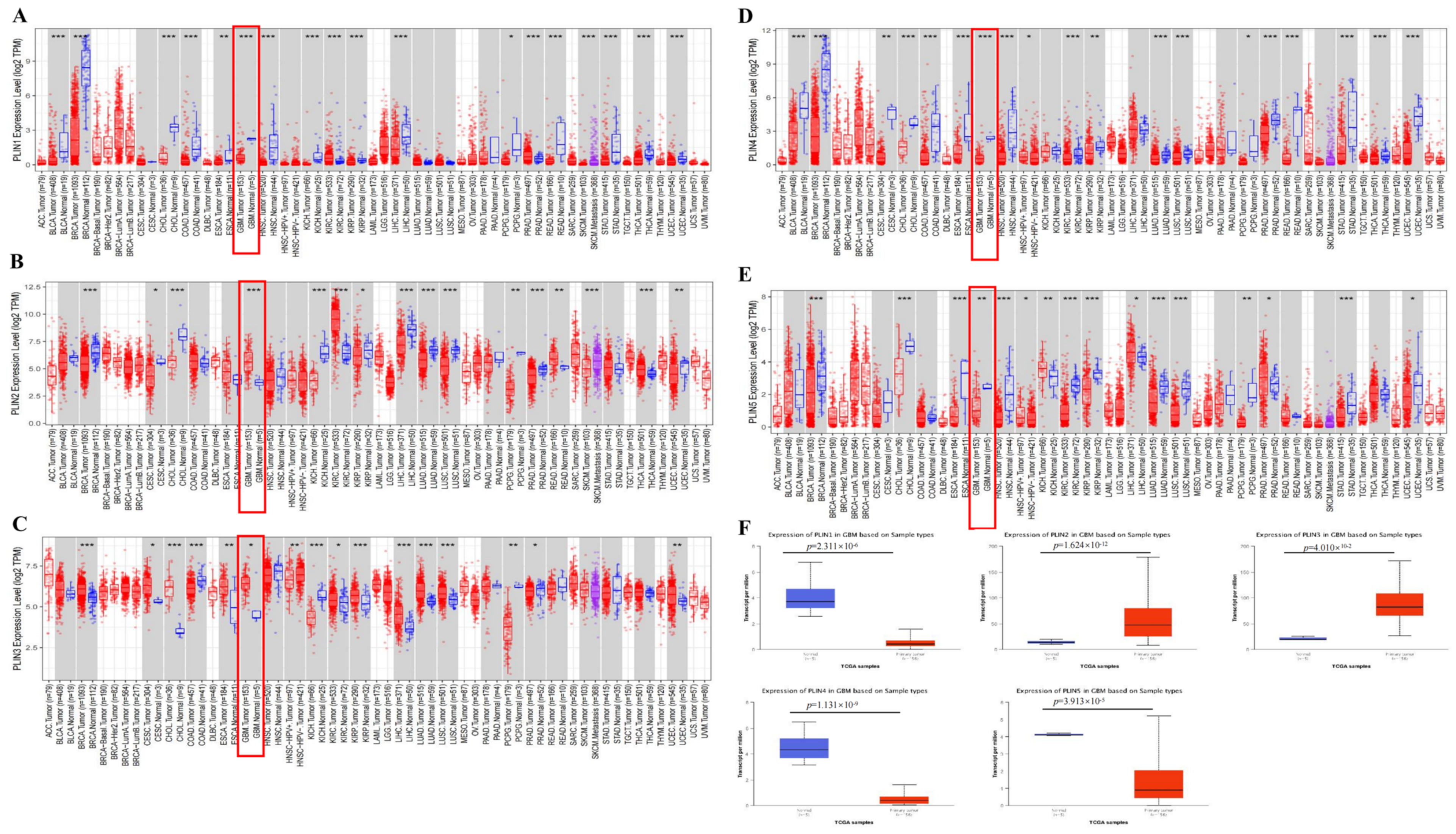
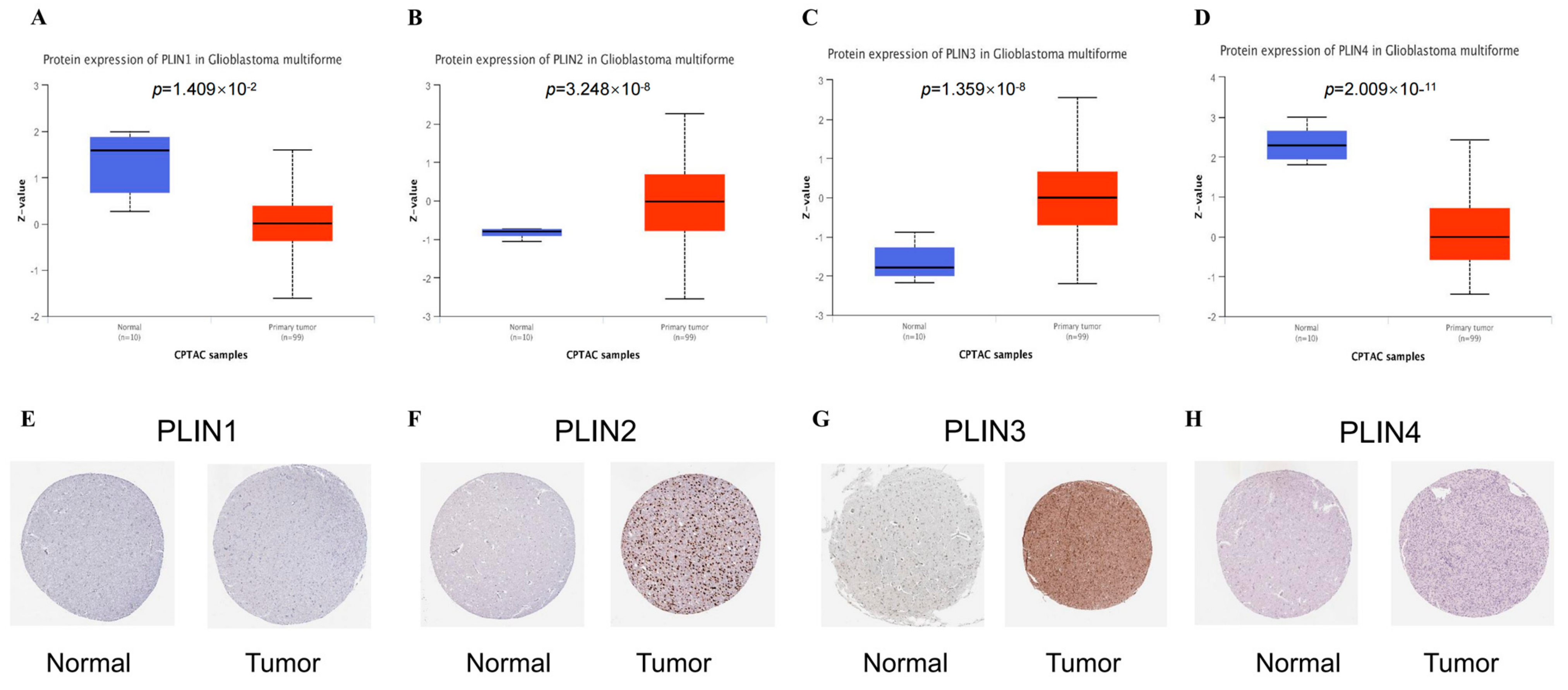
3.1. Expression Levels of PLINs in Pan-Cancer and Gliomas
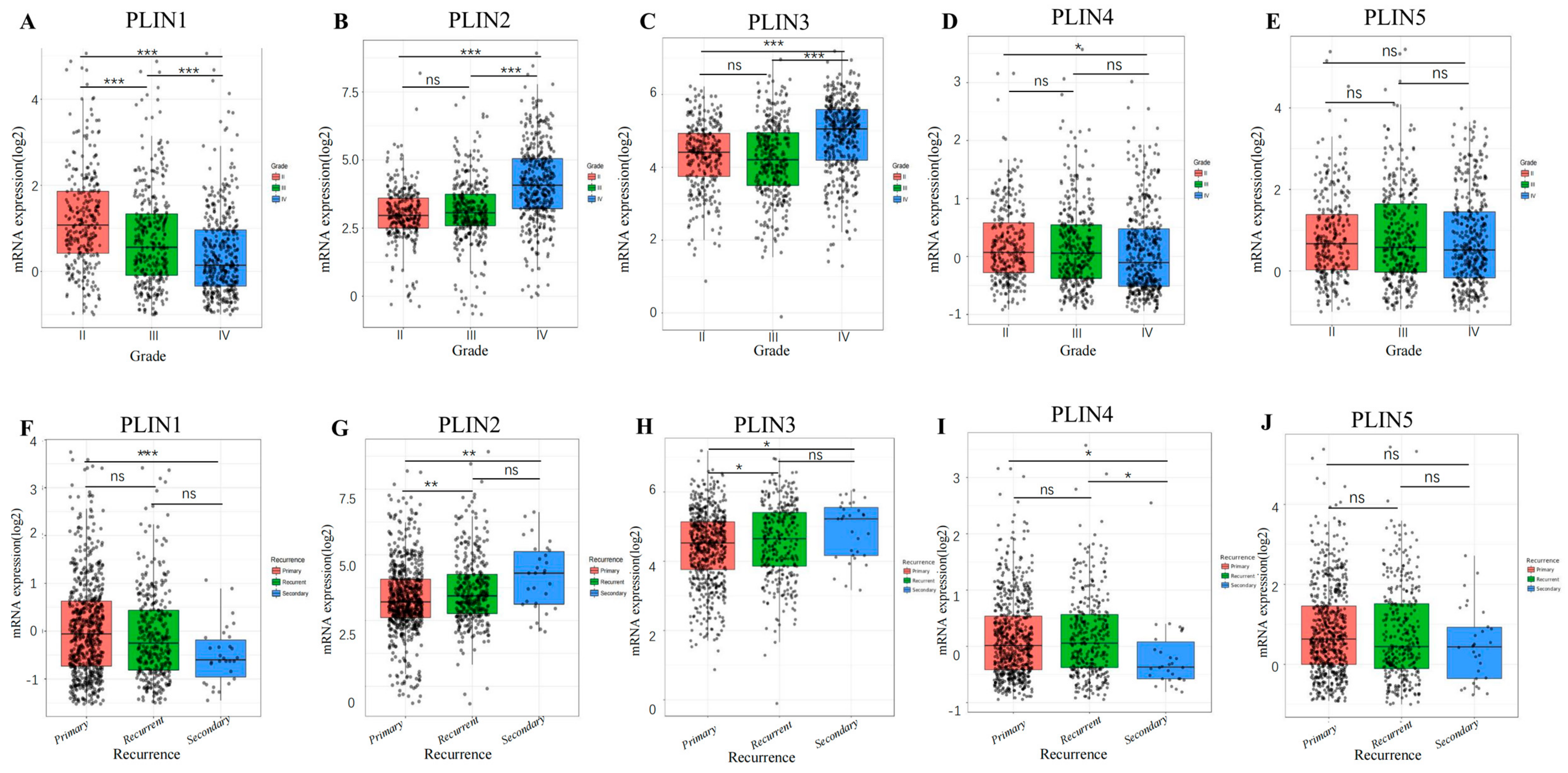
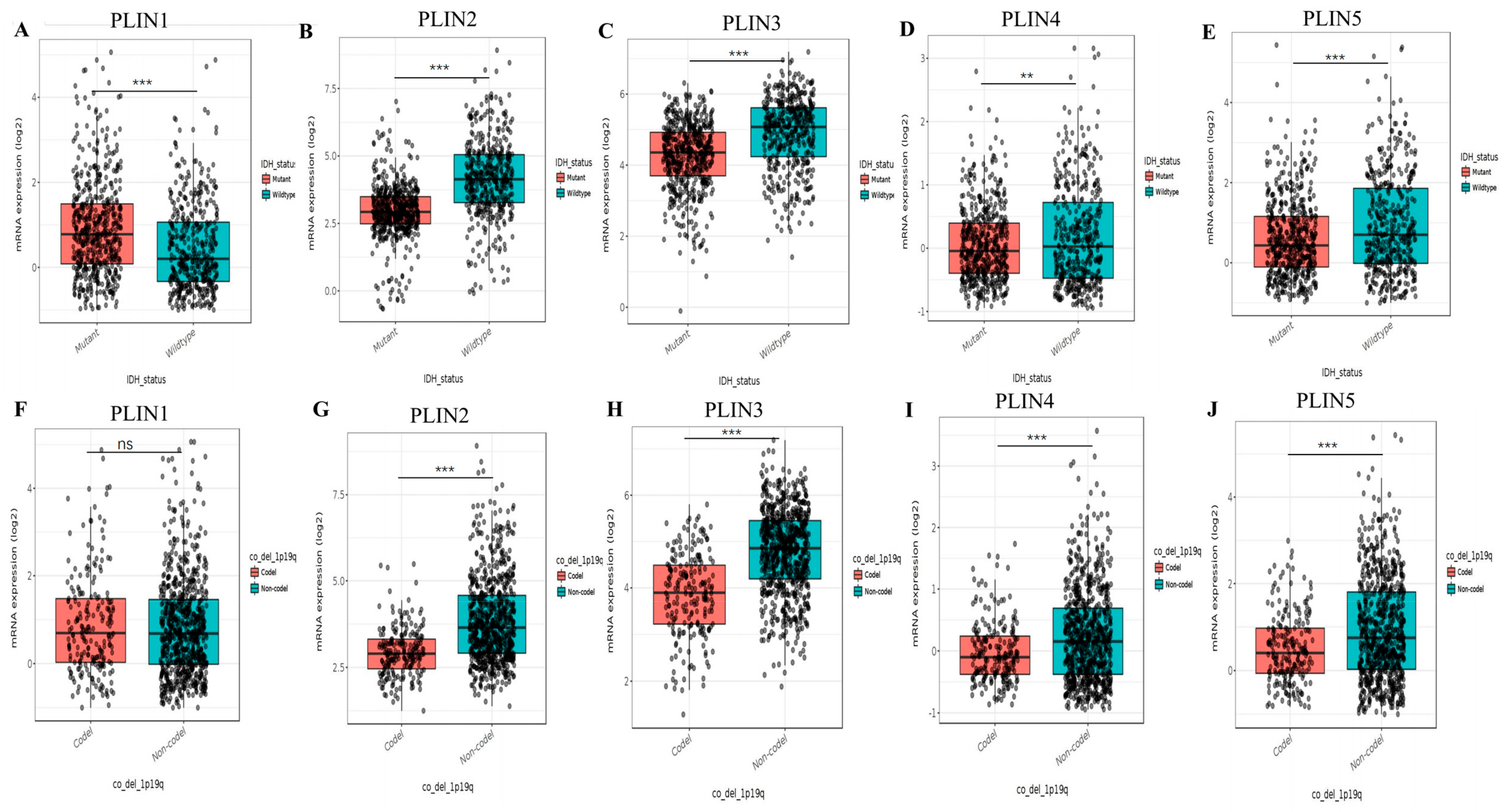
3.2. The Correlation between PLINs Family and Clinicopathological Features in Glioma Patients
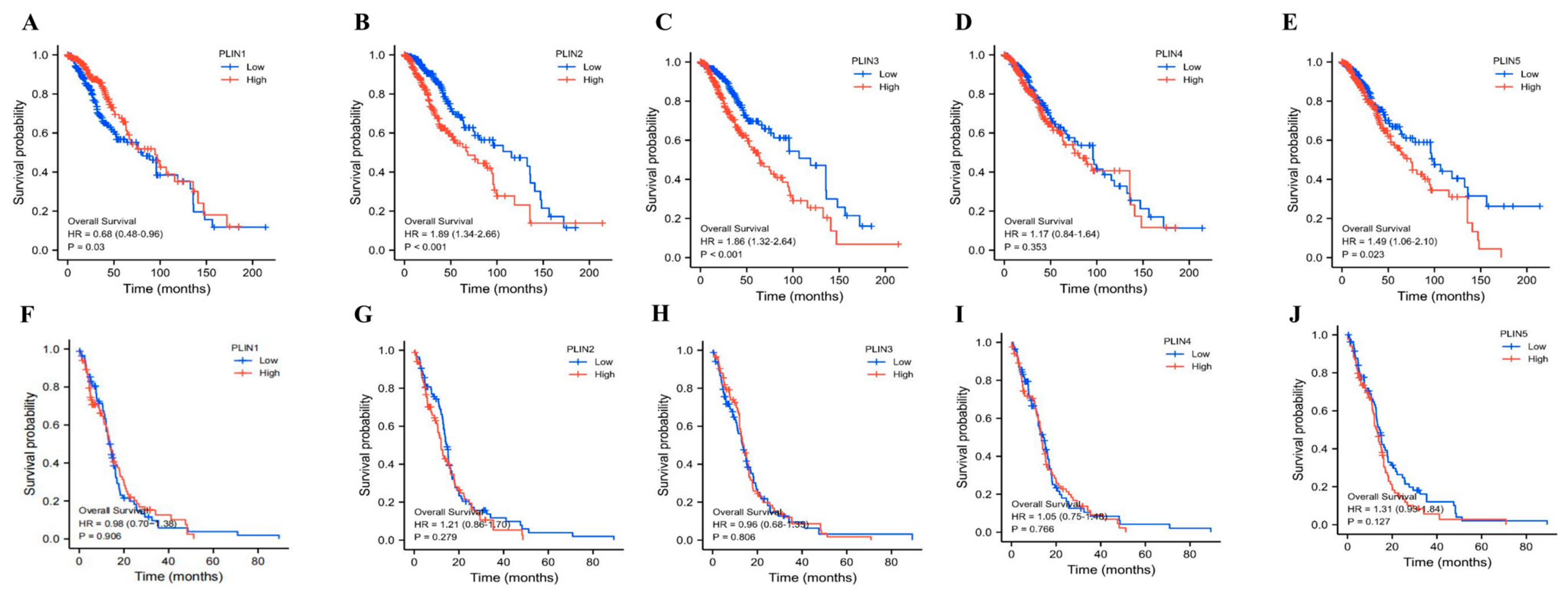
3.3. Prognostic Value of PLINs mRNA Expression in Glioma Patients
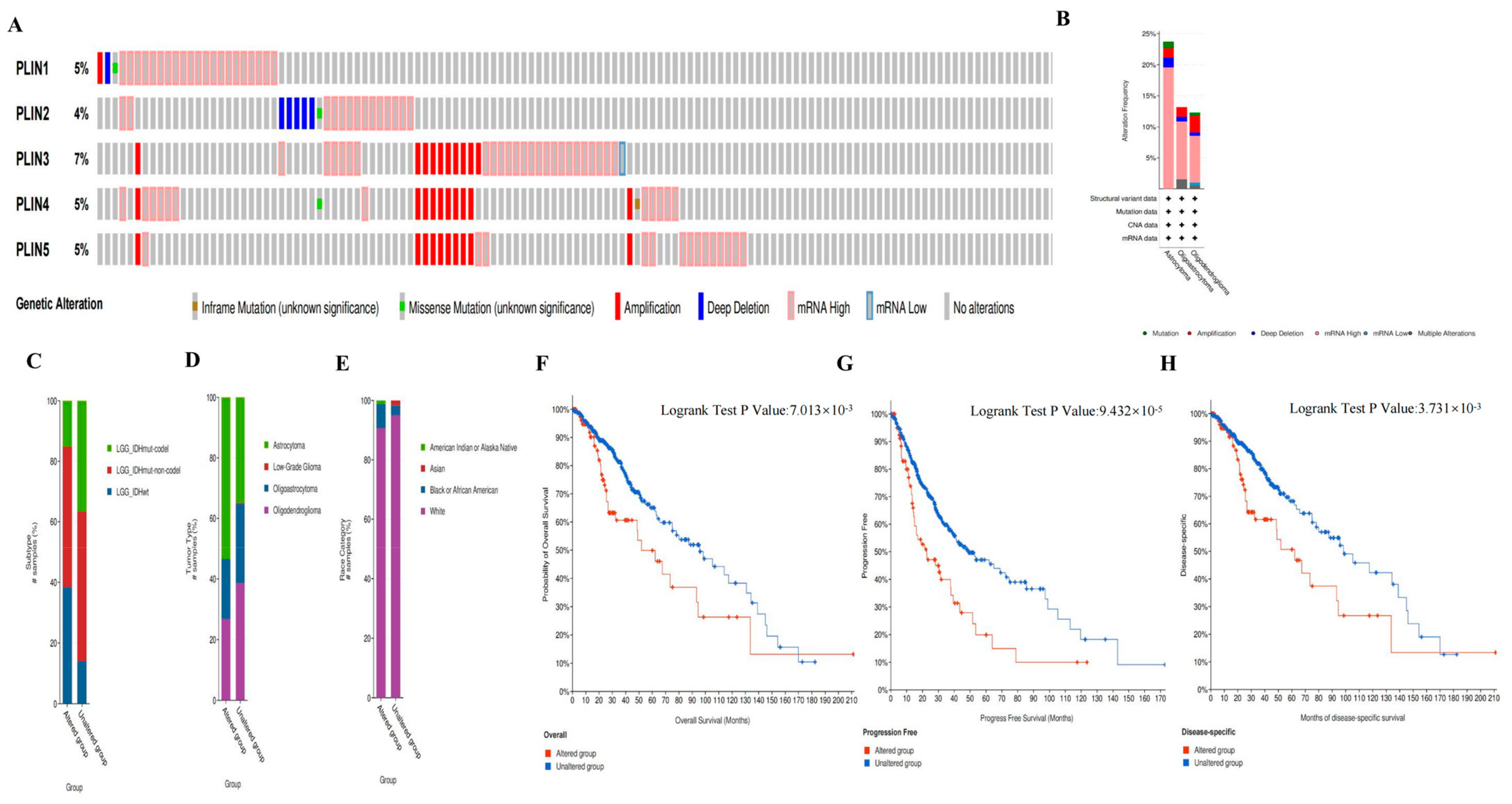
3.4. Genetic Alterations of PLINs in Glioma
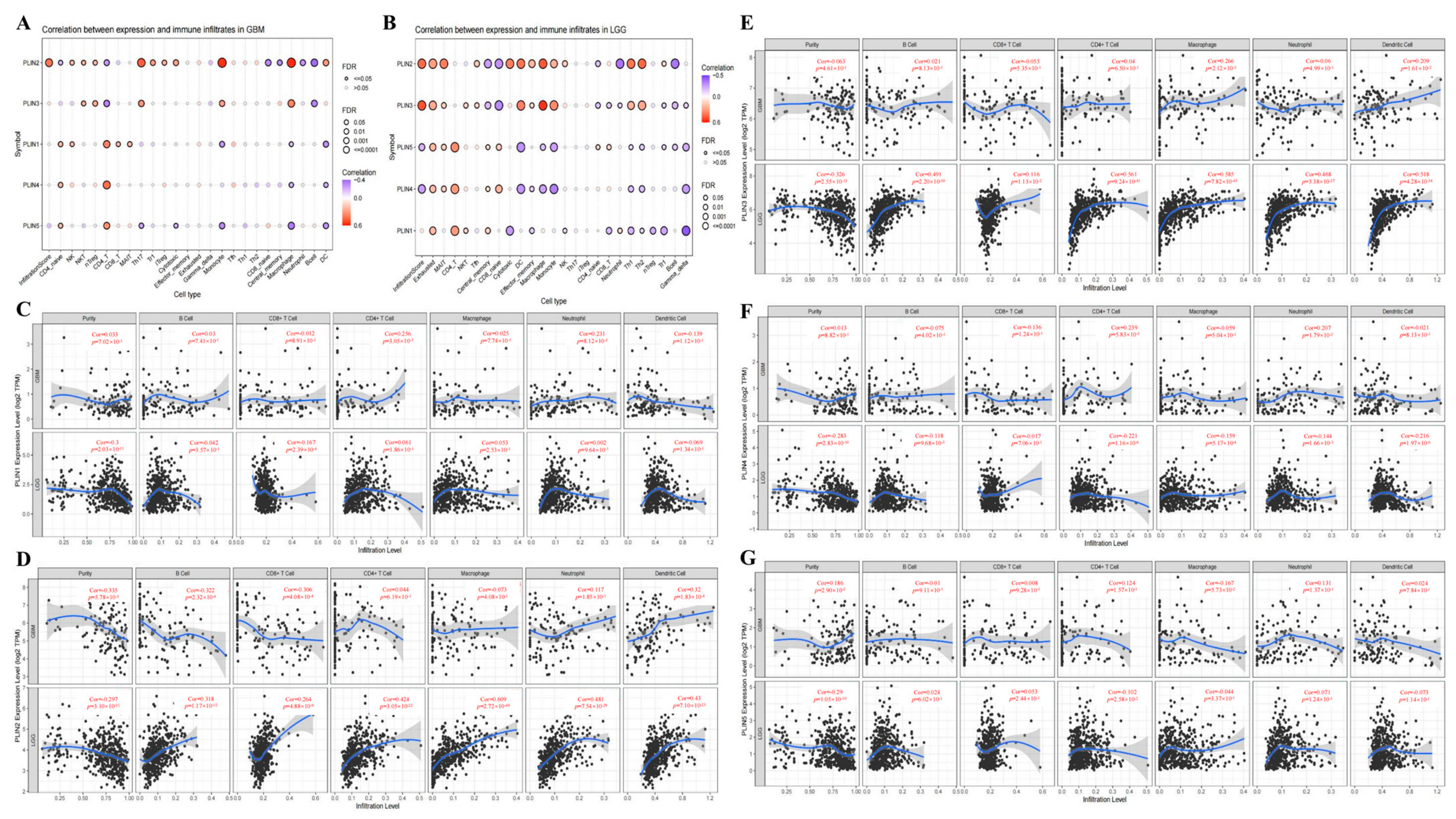
3.5. Correlations between the Immune Microenvironment of Glioma and the Expression of PLINs
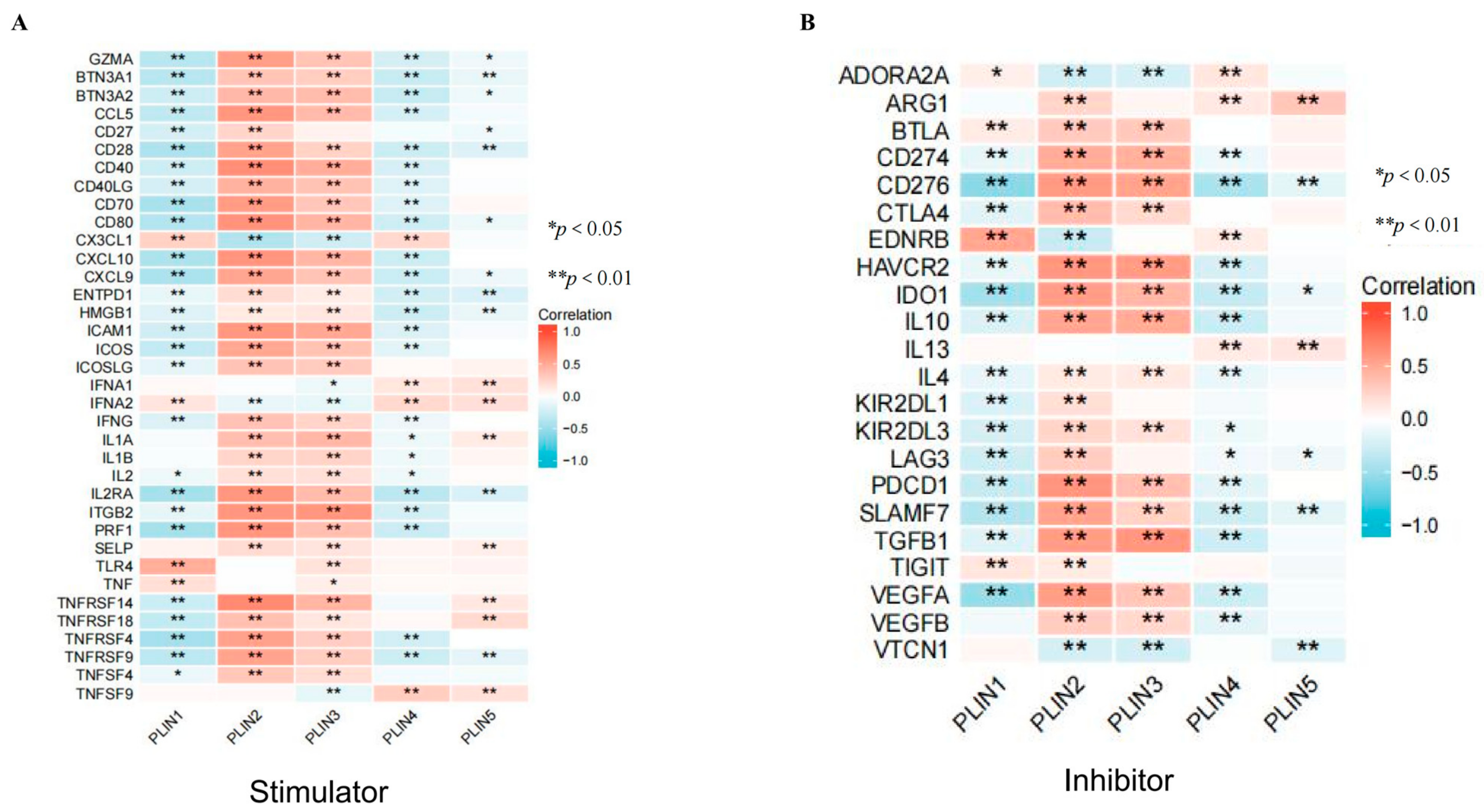
3.6. Correlation of PLINs with Immune Checkpoints and Drug Sensitivity in Gliomas
3.7. Interaction Networks and Gene Enrichment Analysis of PLINs Family Genes in Glioma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BP | Biological processes |
| CC | Cellular components |
| CI | Confidence intervals |
| CTRP | Cancer Therapeutics Response Portal |
| CGGA | Chinese Glioma Genome Atlas |
| GSCA | Gene Set Cancer Analysis |
| GDSC | Genomics of Drug Sensitivity in Cancer |
| GBM | Glioblastoma multiforme |
| GO | Gene Ontology |
| HRs | Hazard ratios |
| IDH | Isocitrate dehydrogenase |
| KM | Kaplan–Meier |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LGG | Low-grade glioma |
| LD | Lipid droplets |
| MF | Molecular functions |
| MAIT | Mucosal-associated invariant T cells |
| OS | Overall survival |
| PLIN | Perilipin |
| PFS | Progression-Free Survival |
| TCGA | The Cancer Genome Atlas |
| TIMER | Tumor Immune Estimation Resource |
| WHO | World Health Organization |
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dai, Z.; Zheng, L.; Xu, B.; Zhang, H.; Fan, F.; Zhang, X.; Liang, X.; Liu, Z.; Yang, K.; et al. Ferroptosis Activation Scoring Model Assists in Chemotherapeutic Agents’ Selection and Mediates Cross-Talk With Immunocytes in Malignant Glioblastoma. Front. Immunol. 2021, 12, 747408. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xiao, J.; Ke, C.; Ni, X.; Xiu, R.; Tian, Q.; Pan, H.; Zou, L.; Wang, F.; Ma, T.; et al. TOPK inhibits autophagy by phosphorylating ULK1 and promotes glioma resistance to TMZ. Cell Death Dis. 2019, 10, 583. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, M.; Chen, S.; Sun, Y.; Tan, R.; Huang, B. The copper-associated protein STEAP2 correlated with glioma prognosis and immune infiltration. Front. Cell. Neurosci. 2022, 16, 13. [Google Scholar] [CrossRef]
- Youssef, G.; Miller, J.J. Lower Grade Gliomas. Curr. Neurol. Neurosci. Rep. 2020, 20, 21. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.; Su, C. Role of Aberrant Lipid Metabolism of Cancer Stem Cells in Cancer Progression. Curr. Cancer Drug Targets 2021, 21, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Bartz, R.; Li, W.-H.; Venables, B.; Zehmer, J.K.; Roth, M.R.; Welti, R.; Anderson, R.G.W.; Liu, P.; Chapman, K.D. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res. 2007, 48, 837–847. [Google Scholar] [CrossRef]
- Bozza, P.T.; Viola, J.P.B. Lipid droplets in inflammation and cancer. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Den Brok, M.H.; Raaijmakers, T.K.; Collado-Camps, E.; Adema, G.J. Lipid Droplets as Immune Modulators in Myeloid Cells. Trends Immunol. 2018, 39, 380–392. [Google Scholar] [CrossRef]
- Scorletti, E.; Carr, R.M. A new perspective on NAFLD: Focusing on lipid droplets. J. Hepatol. 2022, 76, 934–945. [Google Scholar] [CrossRef]
- Bickel, P.E.; Tansey, J.T.; Welte, M.A. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2009, 1791, 419–440. [Google Scholar] [CrossRef] [PubMed]
- Tauchi-Sato, K.; Ozeki, S.; Houjou, T.; Taguchi, R.; Fujimoto, T. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J. Biol. Chem. 2002, 277, 44507–44512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, J.; Tong, Q.; Lin, L. SIRT3 Acts as a Positive Autophagy Regulator to Promote Lipid Mobilization in Adipocytes via Activating AMPK. Int. J. Mol. Sci. 2020, 21, 372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Meng, L.; Song, L.; Du, J.; Du, S.; Cui, W.; Liu, C.; Li, F. Roles of Perilipins in Diseases and Cancers. Curr. Genom. 2018, 19, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Gandotra, S.; Le Dour, C.; Bottomley, W.; Cervera, P.; Giral, P.; Reznik, Y.; Charpentier, G.; Auclair, M.; Delepine, M.; Barroso, I.; et al. Perilipin Deficiency and Autosomal Dominant Partial Lipodystrophy. N. Engl. J. Med. 2011, 364, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Langlois, D.; Forcheron, F.; Li, J.-Y.; Del Carmine, P.; Neggazi, S.; Beylot, M. Increased Atherosclerosis in Mice Deficient in Perilipin1. Lipids Health Dis. 2011, 10, 169. [Google Scholar] [CrossRef]
- Carr, R.M.; Peralta, G.; Yin, X.; Ahima, R.S. Absence of Perilipin 2 Prevents Hepatic Steatosis, Glucose Intolerance and Ceramide Accumulation in Alcohol-Fed Mice. PLoS ONE 2014, 9, e97118. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, A.; Nagao, T.; Endo, H.; Kato, N.; Hirokawa, M.; Mizobuchi, K.; Komatsu, M.; Igarashi, T.; Yokoyama, M.; Masuda, S.; et al. Sebaceous epithelial-myoepithelial carcinoma of the salivary gland: Clinicopathologic and immunohistochemical analysis of 6 cases of a new histologic variant. Am. J. Surg. Pathol. 2008, 32A, 913–923. [Google Scholar] [CrossRef]
- Straub, B.K.; Herpel, E.; Singer, S.; Zimbelmann, R.; Breuhahn, K.; Macher-Goeppinger, S.; Warth, A.; Lehmann-Koch, J.; Longerich, T.; Heid, H.; et al. Lipid droplet-associated PAT-proteins show frequent and differential expression in neoplastic steatogenesis. Mod. Pathol. 2010, 23, 480–492. [Google Scholar] [CrossRef]
- Qu, L.-W.; Zhou, B.; Wang, G.-Z.; Chen, Y.; Zhou, G.-B. Genomic variations in paired normal controls for lung adenocarcinomas. Oncotarget 2017, 8, 104113–104122. [Google Scholar] [CrossRef]
- Zhang, X.; Su, L.; Sun, K. Expression status and prognostic value of the perilipin family of genes in breast cancer. Am. J. Transl. Res. 2021, 13, 4450–4463. [Google Scholar] [PubMed]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Edwards, N.J.; Oberti, M.; Thangudu, R.R.; Cai, S.; McGarvey, P.B.; Jacob, S.; Madhavan, S.; Ketchum, K.A. The CPTAC Data Portal: A Resource for Cancer Proteomics Research. J. Proteome Res. 2015, 14, 2707–2713. [Google Scholar] [CrossRef]
- Ponten, F.; Jirstrom, K.; Uhlen, M. The Human Protein Atlas—A tool for pathology. J. Pathol. 2008, 216, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Bowman, R.L.; Wang, Q.; Carro, A.; Verhaak, R.G.W.; Squatrito, M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro-Oncology 2017, 19, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, K.; Peng, Y.; Shen, L.; Shen, L.; Zhou, Y. Comprehensive analysis of the expression profile and clinical implications of regulator of chromosome condensation 2 in pan-cancers. Aging 2022, 14, 9221–9242. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Hu, J.; Yu, A.; Othmane, B.; Qiu, D.; Li, H.; Li, C.; Liu, P.; Ren, W.; Chen, M.; Gong, G.; et al. Siglec15 shapes a non-inflamed tumor microenvironment and predicts the molecular subtype in bladder cancer. Theranostics 2021, 11, 3089–3108. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Qiu, D.; Yu, A.; Hu, J.; Deng, H.; Li, H.; Yi, Z.; Chen, J.; Zu, X. YTHDF1 Is a Potential Pan-Cancer Biomarker for Prognosis and Immunotherapy. Front. Oncol. 2021, 11, 607224. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, W.C.; Sunshine, M.; Liu, H.; Varma, S.; Kohn, K.W.; Morris, J.; Doroshow, J.; Pommier, Y. CellMiner: A Web-Based Suite of Genomic and Pharmacologic Tools to Explore Transcript and Drug Patterns in the NCI-60 Cell Line Set. Cancer Res. 2012, 72, 3499–3511. [Google Scholar] [CrossRef]
- Kuniyoshi, S.; Miki, Y.; Sasaki, A.; Iwabuchi, E.; Ono, K.; Onodera, Y.; Hirakawa, H.; Ishida, T.; Yoshimi, N.; Sasano, H. The significance of lipid accumulation in breast carcinoma cells through perilipin 2 and its clinicopathological significance. Pathol. Int. 2019, 69, 463–471. [Google Scholar] [CrossRef]
- Qiu, B.; Ackerman, D.; Sanchez, D.J.; Li, B.; Ochocki, J.D.; Grazioli, A.; Bobrovnikova-Marjon, E.; Diehl, J.A.; Keith, B.; Simon, M.C. HIF2 alpha-Dependent Lipid Storage Promotes Endoplasmic Reticulum Homeostasis in Clear-Cell Renal Cell Carcinoma. Cancer Discov. 2015, 5, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, M.; Zhou, L.; Zhang, Y.; Liu, W.; Qin, W.; He, R.; Lu, Y.; Wang, Y.; Chen, X.-Z.; et al. Prognostic significance of PLIN1 expression in human breast cancer. Oncotarget 2016, 7, 54488–54502. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Egan, J.J.; Wek, S.A.; Garty, N.B.; Blanchette-Mackie, E.J.; Londos, C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem. 1991, 266, 11341–11346. [Google Scholar] [CrossRef]
- Wolins, N.E.; Quaynor, B.K.; Skinner, J.R.; Schoenfish, M.J.; Tzekov, A.; Bickel, P.E. S3-12, Adipophilin, and TIP47 package lipid in adipocytes. J. Biol. Chem. 2005, 280, 19146–19155. [Google Scholar] [CrossRef] [PubMed]
- Bensaad, K.; Favaro, E.; Lewis, C.A.; Peck, B.; Lord, S.; Collins, J.M.; Pinnick, K.E.; Wigfield, S.; Buffa, F.M.; Li, J.-L.; et al. Fatty Acid Uptake and Lipid Storage Induced by HIF-1 alpha Contribute to Cell Growth and Survival after Hypoxia-Reoxygenation. Cell Rep. 2014, 9, 349–365. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Li, W.; Zhang, N.; Hou, Y.-L.; Niu, Z.-Q.; Zhong, Y.-J.; Zhang, Y.-P.; Yang, S.-Y. Identification of adipophilin as a potential diagnostic tumor marker for lung adenocarcinoma. Int. J. Clin. Exp. Med. 2014, 7, 1190–1196. [Google Scholar]
- Szigeti, A.; Minik, O.; Hocsak, E.; Pozsgai, E.; Boronkai, A.; Farkas, R.; Balint, A.; Bodis, J.; Sumegi, B.; Bellyei, S. Preliminary Study of TIP47 as a Possible New Biomarker of Cervical Dysplasia and Invasive Carcinoma. Anticancer Res. 2009, 29, 717–724. [Google Scholar] [PubMed]
- Zhou, L.; Song, Z.; Hu, J.; Liu, L.; Hou, Y.; Zhang, X.; Yang, X.; Chen, K. ACSS3 represses prostate cancer progression through downregulating lipid droplet-associated protein PLIN3. Theranostics 2021, 11, 841–860. [Google Scholar] [CrossRef]
- Brat, D.J.; Verhaak, R.G.W.; Aldape, K.D.; Yung, W.K.A.; Salama, S.R.; Cooper, L.A.D.; Rheinbay, E.; Miller, C.R.; Vitucci, M.; Morozova, O.; et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, Z.; Zhao, H.; Teng, C.; Liu, H.; Huang, Q.; Wanggou, S.; Li, X. Identification of the novel prognostic biomarker, MLLT11, reveals its relationship with immune checkpoint markers in glioma. Front. Oncol. 2022, 12, 889351. [Google Scholar] [CrossRef] [PubMed]
- Satish, L.; Krill-Burger, J.M.; Gallo, P.H.; Etages, S.D.; Liu, F.; Philips, B.J.; Ravuri, S.; Marra, K.G.; LaFramboise, W.A.; Kathju, S.; et al. Expression analysis of human adipose-derived stem cells during in vitro differentiation to an adipocyte lineage. BMC Med. Genom. 2015, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, P.; Li, B.; Dang, H.; Jiang, J.; Meng, L.; Zhang, H.; Zhang, Y.; Wang, X.; Li, Q.; et al. The Expression of Perilipin Family Proteins can be used as Diagnostic Markers of Liposarcoma and to Differentiate Subtypes. J. Cancer 2020, 11, 4081–4090. [Google Scholar] [CrossRef]
- Kuramoto, K.; Okamura, T.; Yamaguchi, T.; Nakamura, T.Y.; Wakabayashi, S.; Morinaga, H.; Nomura, M.; Yanase, T.; Otsu, K.; Usuda, N.; et al. Perilipin 5, a Lipid Droplet-binding Protein, Protects Heart from Oxidative Burden by Sequestering Fatty Acid from Excessive Oxidation. J. Biol. Chem. 2012, 287, 23852–23863. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulou, A.; Vucur, M.; Luedde, T.; Schneiders, S.; Kalampoka, S.; Weiss, T.S.; Weiskirchen, R. Perilipin 5 and Lipocalin 2 Expression in Hepatocellular Carcinoma. Cancers 2019, 11, 385. [Google Scholar] [CrossRef]
- Hashani, M.; Witzel, H.R.; Pawella, L.M.; Lehmann-Koch, J.; Schumacher, J.; Mechtersheimer, G.; Schnoelzer, M.; Schirmacher, P.; Roth, W.; Straub, B.K. Widespread expression of perilipin 5 in normal human tissues and in diseases is restricted to distinct lipid droplet subpopulations. Cell Tissue Res. 2018, 374, 121–136. [Google Scholar] [CrossRef]
- Ye, J.; Liu, H.; Xu, Z.-L.; Zheng, L.; Liu, R.-Y. Identification of a multidimensional transcriptome prognostic signature for lung adenocarcinoma. J. Clin. Lab. Anal. 2019, 33, e22990. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Long, G.; Tao, Y.; Zhou, L.; Tang, J. Identification and validation of a tyrosine metabolism-related prognostic prediction model and characterization of the tumor microenvironment infiltration in hepatocellular carcinoma. Front. Immunol. 2022, 13, 994259. [Google Scholar] [CrossRef]
- Gerard, C.L.; Delyon, J.; Wicky, A.; Homicsko, K.; Cuendet, M.A.; Michielin, O. Turning tumors from cold to inflamed to improve immunotherapy response. Cancer Treat. Rev. 2021, 101, 102227. [Google Scholar] [CrossRef]
- Hu, K.; Yao, L.; Yan, Y.; Zhou, L.; Li, J. Comprehensive Analysis of YTH Domain Family in Lung Adenocarcinoma: Expression Profile, Association with Prognostic Value, and Immune Infiltration. Dis. Markers 2021, 2021, 2789481. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bae, J.-S. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediat. Inflamm. 2016, 2016, 6058147. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chen, T.; Hu, R.; Zhu, R.; Li, C.; Ruan, Y.; Xie, X.; Li, Y. Next frontier in tumor immunotherapy: Macrophage-mediated immune evasion. Biomark. Res. 2021, 9, 72. [Google Scholar] [CrossRef]
- Mishra, A.K.; Banday, S.; Bharadwaj, R.; Ali, A.; Rashid, R.; Kulshreshtha, A.; Malonia, S.K. Macrophages as a Potential Immunotherapeutic Target in Solid Cancers. Vaccines 2022, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Kang, K.; Shen, L.; Shen, L.; Zhou, Y. Integrative Analysis of the Predictive Value of Perilipin Family on Clinical Significance, Prognosis and Immunotherapy of Glioma. Biomedicines 2023, 11, 1009. https://doi.org/10.3390/biomedicines11041009
Li X, Kang K, Shen L, Shen L, Zhou Y. Integrative Analysis of the Predictive Value of Perilipin Family on Clinical Significance, Prognosis and Immunotherapy of Glioma. Biomedicines. 2023; 11(4):1009. https://doi.org/10.3390/biomedicines11041009
Chicago/Turabian StyleLi, Xuanxuan, Kuo Kang, Lin Shen, Liangfang Shen, and Yangying Zhou. 2023. "Integrative Analysis of the Predictive Value of Perilipin Family on Clinical Significance, Prognosis and Immunotherapy of Glioma" Biomedicines 11, no. 4: 1009. https://doi.org/10.3390/biomedicines11041009
APA StyleLi, X., Kang, K., Shen, L., Shen, L., & Zhou, Y. (2023). Integrative Analysis of the Predictive Value of Perilipin Family on Clinical Significance, Prognosis and Immunotherapy of Glioma. Biomedicines, 11(4), 1009. https://doi.org/10.3390/biomedicines11041009





