Effect of Ouabain on Glutamate Transport in the Hippocampus of Rats with LPS-Induced Neuroinflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment of Animals with OUA and LPS
- (1)
- CTR (control group): Animals received only an i.p. injection of saline solution and, after 20 min, another injection of saline solution (the second injection of saline is a control for the LPS).
- (2)
- OUA-treated group: Animals received the first injection of OUA (i.p.) at a concentration of 1.8 µg/kg (concentration at nanomolar levels) and, after 20 min, an injection of saline solution (i.p.).
- (3)
- LPS-treated group: Animals received an injection of saline solution (i.p.) first and, after 20 min, an injection of LPS at a concentration of 200 µg/kg (i.p.).
- (4)
- OUA + LPS group: Animals received the first injection of OUA at a concentration of 1.8 µg/kg (i.p.) and, after 20 min, an injection of LPS at a concentration of 200 µg/kg (i.p.).
2.2. Preparation of Samples
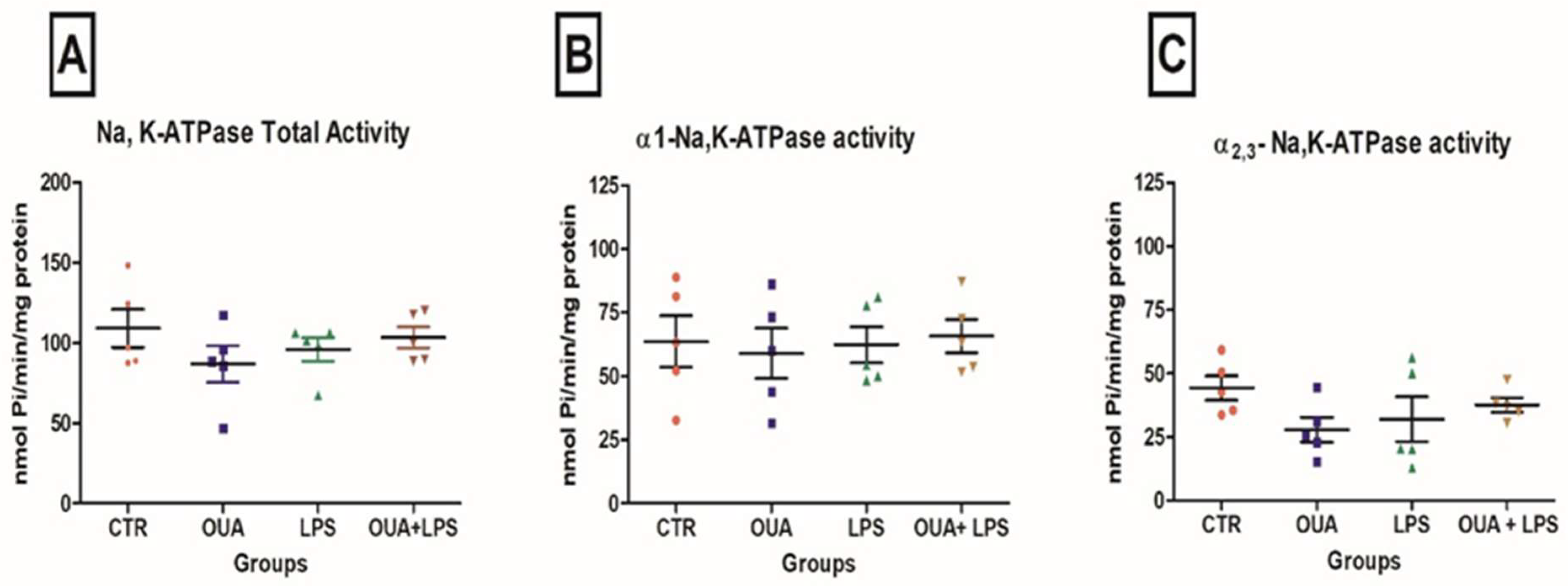
2.3. Determination of Na,K-ATPase Activity
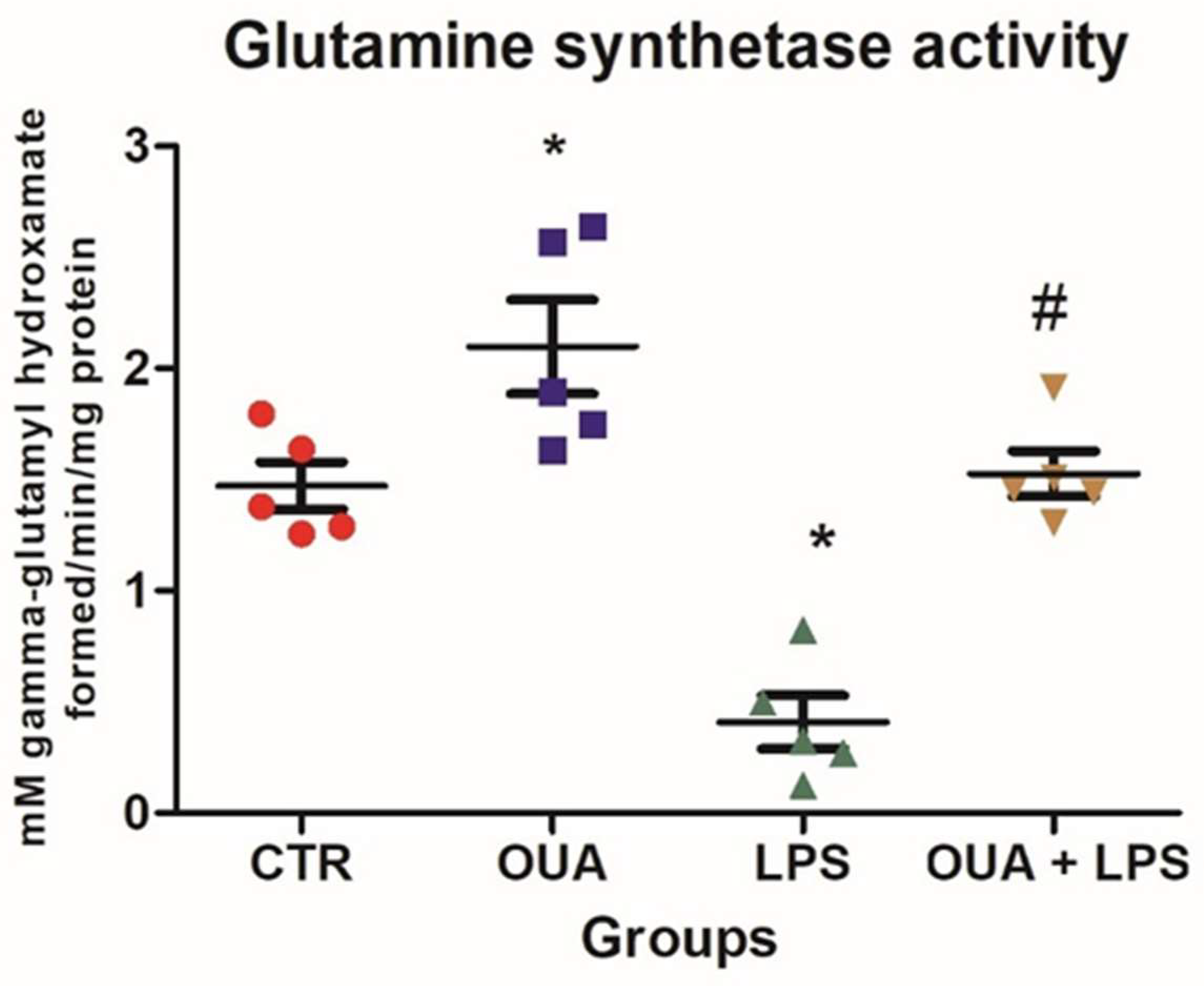
2.4. Determination of Glutamine Synthetase (GS) Activity
2.5. Anti-FXYD2 Immunoprecipitation
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
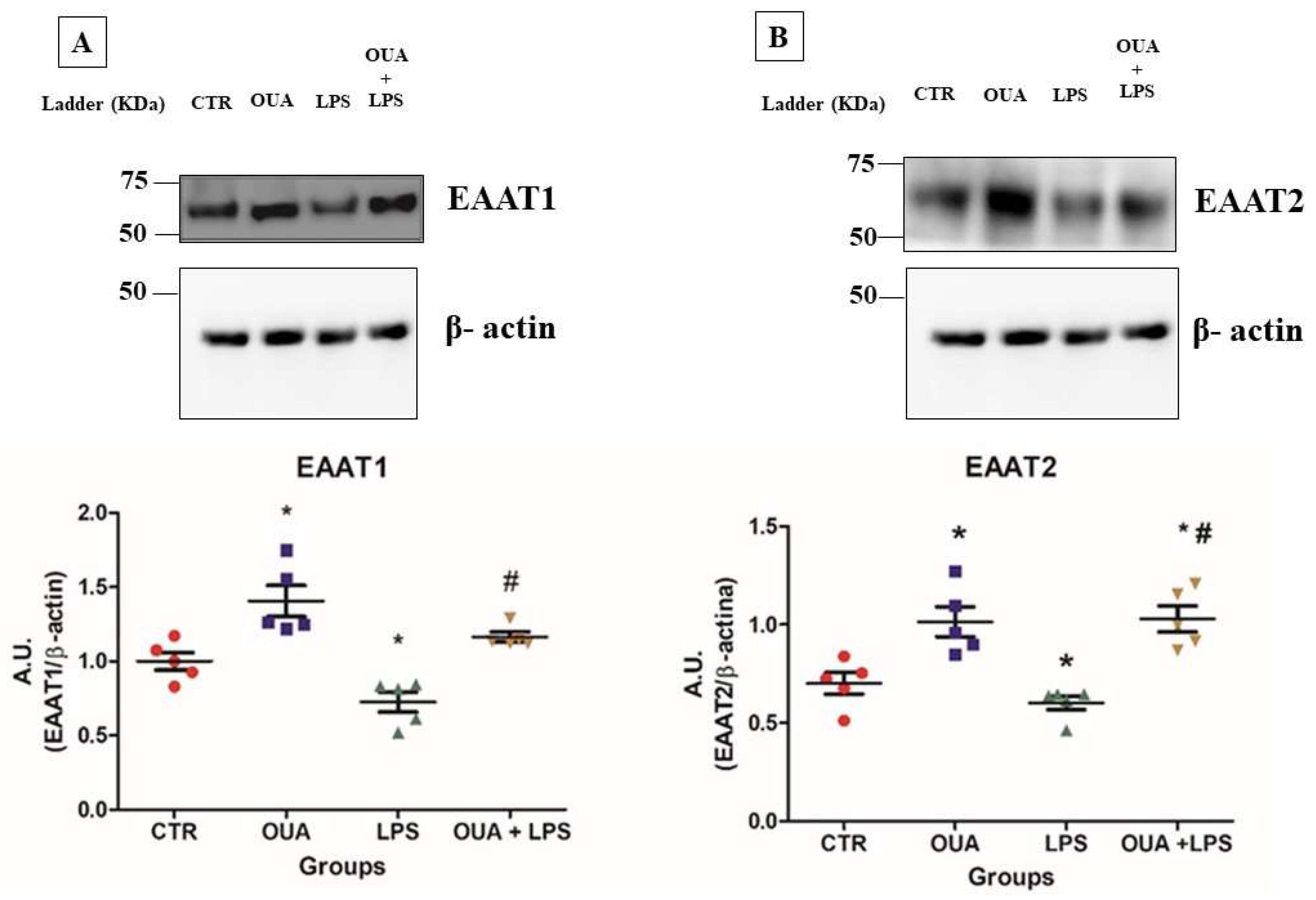
3.1. OUA Attenuates the Decrease in α2 Isoform Expression in LPS-Treated Rat Hippocampus
3.2. OUA Restores Levels of Glutamate Transporters (EAAT1 and EAAT2) in Rat Hippocampus Treated with LPS
3.3. OUA Modulates the Interaction between FXYD2, α2 and Glutamate Transporters in Hippocampus of LPS-Treated Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, G.C.; Neher, J.J. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol. 2010, 41, 242–247. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Arias-Salvatierra, D.; Silbergeld, E.K.; Acosta-Saavedra, L.C.; Calderon-Aranda, E.S. Role of nitric oxide produced by iNOS through NF-κB pathway in migration of cerebellar granule neurons induced by Lipopolysaccharide. Cell. Signal. 2011, 23, 425–435. [Google Scholar] [CrossRef]
- Leow-Dyke, S.; Allen, C.; Denes, A.; Nilsson, O.; Maysami, S.; Bowie, A.G.; Rothwell, N.J.; Pinteaux, E. Neuronal Toll-like receptor 4 signaling induces brain endothelial activation and neutrophil transmigration in vitro. J. Neuroinflamm. 2012, 9, 230. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Pivovarov, A.S.; Calahorro, F. Na(+)/K(+)-pump and neurotransmitter membrane receptors. Invertebr. Neurosci. 2018, 19, 1. [Google Scholar] [CrossRef]
- Bagrov, A.Y.; Shapiro, J.I.; Fedorova, O.V. Endogenous cardiotonic steroids: Physiology, pharmacology, and novel therapeutic targets. Pharmacol. Rev. 2009, 61, 9–38. [Google Scholar] [CrossRef]
- Blanco, G.; Mercer, R.W. Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function. Am. J. Physiol. 1998, 275, F633–F650. [Google Scholar] [CrossRef]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef]
- Bottger, P.; Doganli, C.; Lykke-Hartmann, K. Migraine- and dystonia-related disease-mutations of Na+/K+-ATPases: Relevance of behavioral studies in mice to disease symptoms and neurological manifestations in humans. Neurosci. Biobehav. Rev. 2012, 36, 855–871. [Google Scholar] [CrossRef]
- McGrail, K.M.; Phillips, J.M.; Sweadner, K.J. Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system:Both neurons and glia can express more than one Na,K-ATPase. J. Neurosci. Off. J. Soc. Neurosci. 1991, 11, 381–391. [Google Scholar] [CrossRef]
- Murata, K.; Kinoshita, T.; Ishikawa, T.; Kuroda, K.; Hoshi, M.; Fukazawa, Y. Region- and neuronal-subtype-specific expression of Na,K-ATPase alpha and beta subunit isoforms in the mouse brain. J. Comp. Neurol. 2020, 528, 2654–2678. [Google Scholar] [CrossRef]
- Gegelashvili, M.; Rodriguez-Kern, A.; Sung, L.; Shimamoto, K.; Gegelashvili, G. Glutamate transporter GLAST/EAAT1 directs cell surface expression of FXYD2/gamma subunit of Na, K-ATPase in human fetal astrocytes. Neurochem. Int. 2007, 50, 916–920. [Google Scholar] [CrossRef]
- Floyd, R.V.; Wray, S.; Martín-Vasallo, P.; Mobasheri, A. Differential cellular expression of FXYD1 (phospholemman) and FXYD2 (gamma subunit of Na, K-ATPase) in normal human tissues: A study using high density human tissue microarrays. Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2010, 192, 7–16. [Google Scholar] [CrossRef]
- Larsen, B.R.; Holm, R.; Vilsen, B.; MacAulay, N. Glutamate transporter activity promotes enhanced Na(+) /K(+) -ATPase-mediated extracellular K(+) management during neuronal activity. J. Physiol. 2016, 594, 6627–6641. [Google Scholar] [CrossRef]
- Kinoshita, P.F.; Leite, J.A.; Orellana, A.M.M.; Vasconcelos, A.R.; Quintas, L.E.M.; Kawamoto, E.M.; Scavone, C. The Influence of Na(+), K(+)-ATPase on Glutamate Signaling in Neurodegenerative Diseases and Senescence. Front. Physiol. 2016, 7, 195. [Google Scholar] [CrossRef]
- Beart, P.M.; O’Shea, R.D. Transporters for L-glutamate: An update on their molecular pharmacology and pathological involvement. Br. J. Pharmacol. 2007, 150, 5–17. [Google Scholar] [CrossRef]
- Rowe, W.B.; Ronzio, R.A.; Meister, A. Inhibition of glutamine synthetase by methionine sulfoximine. Studies on methionine sulfoximine phosphate. Biochemistry 1969, 8, 2674–2680. [Google Scholar] [CrossRef]
- Roberts, R.C.; Roche, J.K.; McCullumsmith, R.E. Localization of excitatory amino acid transporters EAAT1 and EAAT2 in human postmortem cortex: A light and electron microscopic study. Neuroscience 2014, 277, 522–540. [Google Scholar] [CrossRef]
- Zhang, X.H.; Jia, N.; Zhao, X.Y.; Tang, G.K.; Guan, L.X.; Wang, D.; Sun, H.L.; Li, H.; Zhu, Z.L. Involvement of pGluR1, EAAT2 and EAAT3 in offspring depression induced by prenatal stress. Neuroscience 2013, 250, 333–341. [Google Scholar] [CrossRef]
- Takaki, J.; Fujimori, K.; Miura, M.; Suzuki, T.; Sekino, Y.; Sato, K. L-glutamate released from activated microglia downregulates astrocytic L-glutamate transporter expression in neuroinflammation: The ‘collusion’ hypothesis for increased extracellular L-glutamate concentration in neuroinflammation. J. Neuroinflamm. 2012, 9, 275. [Google Scholar] [CrossRef]
- Garcia, I.J.; Kinoshita, P.F.; Scavone, C.; Mignaco, J.A.; Barbosa, L.A.; Santos Hde, L. Ouabain Modulates the Lipid Composition of Hippocampal Plasma Membranes from Rats with LPS-induced Neuroinflammation. J. Membr. Biol. 2015, 248, 1191–1198. [Google Scholar] [CrossRef]
- Garcia, I.J.P.; Kinoshita, P.F.; de Oliveira Braga, I.; Parreira, G.M.; Mignaco, J.A.; Scavone, C.; Barbosa, L.A.; de Lima Santos, H. Ouabain attenuates the oxidative stress induced by lipopolysaccharides in the cerebellum of rats. J. Cell Biochem. 2018, 119, 2156–2167. [Google Scholar] [CrossRef]
- Garcia, I.J.P.; Kinoshita, P.F.; Silva, L. Ouabain attenuates oxidative stress and modulates lipid composition in hippocampus of rats in lipopolysaccharide-induced hypocampal neuroinflammation in rats. J. Cell. Biochem. 2019, 120, 4081–4091. [Google Scholar] [CrossRef]
- Blaustein, M.P. Physiological effects of endogenous ouabain: Control of intracellular Ca2+ stores and cell responsiveness. Am. J. Physiol. 1993, 264, C1367–C1387. [Google Scholar] [CrossRef]
- Laursen, M.; Gregersen, J.L.; Yatime, L.; Nissen, P.; Fedosova, N.U. Structures and characterization of digoxin- and bufalin-bound Na+,K+-ATPase compared with the ouabain-bound complex. Proc. Natl. Acad. Sci. USA 2015, 112, 1755–1760. [Google Scholar] [CrossRef]
- Kinoshita, P.F.; Yshii, L.M.; Vasconcelos, A.R.; Orellana, A.M.M.; Lima, L.d.S.; Davel, A.P.C.; Rossoni, L.V.; Kawamoto, E.M.; Scavone, C. Signaling function of Na,K-ATPase induced by ouabain against LPS as an inflammation model in hippocampus. J. Neuroinflamm. 2014, 11, 218. [Google Scholar] [CrossRef]
- Song, H.L.; Demirev, A.V.; Kim, N.Y.; Kim, D.H.; Yoon, S.Y. Ouabain activates transcription factor EB and exerts neuroprotection in models of Alzheimer’s disease. Mol. Cell. Neurosci. 2019, 95, 13–24. [Google Scholar] [CrossRef]
- Orellana, A.M.; Leite, J.A.; Kinoshita, P.F.; Vasconcelos, A.R.; Andreotti, D.Z.; de Sá Lima, L.; Xavier, G.F.; Kawamoto, E.M.; Scavone, C. Ouabain increases neuronal branching in hippocampus and improves spatial memory. Neuropharmacology 2018, 140, 260–274. [Google Scholar] [CrossRef]
- Parreira, G.M.; Resende, M.D.A.; Garcia, I.J.P.; Sartori, D.B.; Umeoka, E.H.L.; Godoy, L.D.; Garcia-Cairasco, N.; Barbosa, L.A.; Santos, H.L.; Tilelli, C.Q. Oxidative stress and Na,K-ATPase activity differential regulation in brainstem and forebrain of Wistar Audiogenic rats may lead to increased seizure susceptibility. Brain Res. 2018, 1679, 171–178. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fiske, C.H.; Subbarow, Y. The Colorimetric Determination of Phosphorus. J. Biol. Chem. 1925, 66, 375. [Google Scholar] [CrossRef]
- Loreaux, E.L.; Kaul, B.; Lorenz, J.N.; Lingrel, J.B. Ouabain-Sensitive alpha1 Na,K-ATPase enhances natriuretic response to saline load. J. Am. Soc. Nephrol. 2008, 19, 1947–1954. [Google Scholar] [CrossRef]
- Ogawa, H.; Shinoda, T.; Cornelius, F.; Toyoshima, C. Crystal structure of the sodium-potassium pump (Na+,K+-ATPase) with bound potassium and ouabain. Proc. Natl. Acad. Sci. USA 2009, 106, 13742–13747. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Geng, B.; Xie, Q.; Li, W.; Deng, Y.; Shi, W.; Pan, Y.; Kang, X.; Wang, J. 2-arachidonyl glycerol modulates astrocytic glutamine synthetase via p38 and ERK1/2 pathways. J. Neuroinflamm. 2018, 15, 220. [Google Scholar] [CrossRef]
- Shin, J.W.; Nguyen, K.T.; Pow, D.V.; Knight, T.; Buljan, V.; Bennett, M.R.; Balcar, V.J. Distribution of glutamate transporter GLAST in membranes of cultured astrocytes in the presence of glutamate transport substrates and ATP. Neurochem. Res. 2009, 34, 1758–1766. [Google Scholar] [CrossRef]
- Wang, F.; Smith, N.A.; Xu, Q.; Goldman, S.; Peng, W.; Huang, J.H.; Takano, T.; Nedergaard, M. Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 17404–17412. [Google Scholar] [CrossRef]
- Vercellino, M.; Merola, A.; Piacentino, C.; Votta, B.; Capello, E.; Mancardi, G.L.; Mutani, R.; Giordana, M.T.; Cavalla, P. Altered glutamate reuptake in relapsing-remitting and secondary progressive multiple sclerosis cortex: Correlation with microglia infiltration, demyelination, and neuronal and synaptic damage. J. Neuropathol. Exp. Neurol. 2007, 66, 732–739. [Google Scholar] [CrossRef]
- Ohgoh, M.; Hanada, T.; Smith, T.; Hashimoto, T.; Ueno, M.; Yamanishi, Y.; Watanabe, M.; Nishizawa, Y. Altered expression of glutamate transporters in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2002, 125, 170–178. [Google Scholar] [CrossRef]
- Götz, J.; Ittner, L.M. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat. Rev. Neurosci. 2008, 9, 532–544. [Google Scholar] [CrossRef]
- Smith, M.E. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: A meta-analysis of structural MRI studies. Hippocampus 2005, 15, 798–807. [Google Scholar] [CrossRef]
- Colasanti, A.; Guo, Q.; Giannetti, P.; Wall, M.B.; Newbould, R.D.; Bishop, C.; Onega, M.; Nicholas, R.; Ciccarelli, O.; Muraro, P.A.; et al. Hippocampal Neuroinflammation, Functional Connectivity, and Depressive Symptoms in Multiple Sclerosis. Biol. Psychiatry 2016, 80, 62–72. [Google Scholar] [CrossRef]
- Morgan, E.E.; Li, Z.; Stebal, C.; Belliard, A.; Tennyson, G.; Salari, B.; Garlid, K.D.; Pierre, S.V. Preconditioning by subinotropic doses of ouabain in the Langendorff perfused rabbit heart. J. Cardiovasc. Pharmacol. 2010, 55, 234–239. [Google Scholar] [CrossRef]
- Pierre, S.V.; Yang, C.; Yuan, Z.; Seminerio, J.; Mouas, C.; Garlid, K.D.; Dos-Santos, P.; Xie, Z. Ouabain triggers preconditioning through activation of the Na+,K+-ATPase signaling cascade in rat hearts. Cardiovasc. Res. 2007, 73, 488–496. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Vasconcelos, A.R.; Kinoshita, P.F.; Yshii, L.M.; Marques Orellana, A.M.; Bohmer, A.E.; de Sa Lima, L.; Alves, R.; Andreotti, D.Z.; Marcourakis, T.; Scavone, C.; et al. Effects of intermittent fasting on age-related changes on Na,K-ATPase activity and oxidative status induced by lipopolysaccharide in rat hippocampus. Neurobiol. Aging 2015, 36, 1914–1923. [Google Scholar] [CrossRef]
- Rodrigues, F.S.; Souza, M.A.; Magni, D.V.; Ferreira, A.P.; Mota, B.C.; Cardoso, A.M.; Paim, M.; Xavier, L.L.; Ferreira, J.; Schetinger, M.R.; et al. N-acetylcysteine prevents spatial memory impairment induced by chronic early postnatal glutaric acid and lipopolysaccharide in rat pups. PloS ONE 2013, 8, e78332. [Google Scholar] [CrossRef]
- El-Mallakh, R.S.; El-Masri, M.A.; Huff, M.O.; Li, X.P.; Decker, S.; Levy, R.S. Intracerebroventricular administration of ouabain as a model of mania in rats. Bipolar Disord. 2003, 5, 362–365. [Google Scholar] [CrossRef]
- Palmieri, E.M.; Menga, A.; Lebrun, A.; Hooper, D.C.; Butterfield, D.A.; Mazzone, M.; Castegna, A. Blockade of Glutamine Synthetase Enhances Inflammatory Response in Microglial Cells. Antioxid Redox Signal 2017, 26, 351–363. [Google Scholar] [CrossRef]
- Palmieri, E.M.; Spera, I.; Menga, A.; Infantino, V.; Iacobazzi, V.; Castegna, A. Glutamine synthetase desensitizes differentiated adipocytes to proinflammatory stimuli by raising intracellular glutamine levels. FEBS Lett. 2014, 588, 4807–4814. [Google Scholar] [CrossRef]
- Sharma, A.; Patro, N.; Patro, I.K. Lipopolysaccharide-Induced Apoptosis of Astrocytes: Therapeutic Intervention by Minocycline. Cell. Mol. Neurobiol. 2016, 36, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Castegna, A.; Menga, A. Glutamine Synthetase: Localization Dictates Outcome. Genes 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Castegna, A.; Aksenov, M.; Aksenova, M.; Thongboonkerd, V.; Klein, J.B.; Pierce, W.M.; Booze, R.; Markesbery, W.R.; Butterfield, D.A. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part I: Creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free. Radic. Biol. Med. 2002, 33, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Castegna, A.; Palmieri, L.; Spera, I.; Porcelli, V.; Palmieri, F.; Fabis-Pedrini, M.J.; Kean, R.B.; Barkhouse, D.A.; Curtis, M.T.; Hooper, D.C. Oxidative stress and reduced glutamine synthetase activity in the absence of inflammation in the cortex of mice with experimental allergic encephalomyelitis. Neuroscience 2011, 185, 97–105. [Google Scholar] [CrossRef]
- O’Shea, R.D.; Lau, C.L.; Farso, M.C.; Diwakarla, S.; Zagami, C.J.; Svendsen, B.B.; Feeney, S.J.; Callaway, J.K.; Jones, N.M.; Pow, D.V.; et al. Effects of lipopolysaccharide on glial phenotype and activity of glutamate transporters: Evidence for delayed up-regulation and redistribution of GLT-1. Neurochem. Int. 2006, 48, 604–610. [Google Scholar] [CrossRef]
- Ghiani, C.A.; Mattan, N.S.; Nobuta, H.; Malvar, J.S.; Boles, J.; Ross, M.G.; Waschek, J.A.; Carpenter, E.M.; Fisher, R.S.; de Vellis, J. Early effects of lipopolysaccharide-induced inflammation on foetal brain development in rat. ASN Neuro 2011, 3. [Google Scholar] [CrossRef]
- Ivanova, M.A.; Kokorina, A.D.; Timofeeva, P.D.; Karelina, T.V.; Abushik, P.A.; Stepanenko, J.D.; Sibarov, D.A.; Antonov, S.M. Calcium Export from Neurons and Multi-Kinase Signaling Cascades Contribute to Ouabain Neuroprotection in Hyperhomocysteinemia. Biomolecules 2020, 10. [Google Scholar] [CrossRef]
- Rose, E.M.; Koo, J.C.P.; Antflick, J.E.; Ahmed, S.M.; Angers, S.; Hampson, D.R. Glutamate Transporter Coupling to Na,K-ATPase. J. Neurosci. 2009, 29, 8143–8155. [Google Scholar] [CrossRef]
- Cholet, N.; Pellerin, L.; Magistretti, P.J.; Hamel, E. Similar perisynaptic glial localization for the Na+,K+-ATPase alpha 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb. Cortex 2002, 12, 515–525. [Google Scholar] [CrossRef]



| Antibody | Code | Dilution for Membrane Fraction | Dilution for Immunoprecipitation | Molecular Weight (KDa) |
|---|---|---|---|---|
| FXYD2 (Mouse) | sc-81876 | - | 1:250 | 7 |
| β-actin (Mouse) | sc-81178 | 1:500 | - | 45 |
| α1 (Mouse) | sc-21712 | 1:500 | 1:250 | 110 |
| α2 (Rabbit) | CSB-PA020068 | 1:500 | 1:200 | 110 |
| α3 (Mouse) | MA3-915 | 1:1000 | 1:250 | 110 |
| EAAT1 (Rabbit) | sc-515839 | 1:500 | 1:200 | 65 |
| EAAT2 (Rabbit) | sc-365634 | 1:500 | 1:200 | 65 |
| Antibody | Code | Dilution for Membrane Fraction | Dilution for Immunoprecipitation |
|---|---|---|---|
| Mouse | sc-516102 | 1:2000 | 1:1000 |
| Rabbit | sc-2357 | 1:7000 | 1:2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, I.J.P.; Kinoshita, P.F.; Valadares, J.M.d.M.; Carvalho, L.E.D.d.; Cortes, V.F.; Barbosa, L.A.; Scavone, C.; Santos, H.d.L. Effect of Ouabain on Glutamate Transport in the Hippocampus of Rats with LPS-Induced Neuroinflammation. Biomedicines 2023, 11, 920. https://doi.org/10.3390/biomedicines11030920
Garcia IJP, Kinoshita PF, Valadares JMdM, Carvalho LEDd, Cortes VF, Barbosa LA, Scavone C, Santos HdL. Effect of Ouabain on Glutamate Transport in the Hippocampus of Rats with LPS-Induced Neuroinflammation. Biomedicines. 2023; 11(3):920. https://doi.org/10.3390/biomedicines11030920
Chicago/Turabian StyleGarcia, Israel José Pereira, Paula Fernanda Kinoshita, Jéssica Martins de Moura Valadares, Luciana Estefani Drumond de Carvalho, Vanessa Faria Cortes, Leandro Augusto Barbosa, Cristoforo Scavone, and Hérica de Lima Santos. 2023. "Effect of Ouabain on Glutamate Transport in the Hippocampus of Rats with LPS-Induced Neuroinflammation" Biomedicines 11, no. 3: 920. https://doi.org/10.3390/biomedicines11030920
APA StyleGarcia, I. J. P., Kinoshita, P. F., Valadares, J. M. d. M., Carvalho, L. E. D. d., Cortes, V. F., Barbosa, L. A., Scavone, C., & Santos, H. d. L. (2023). Effect of Ouabain on Glutamate Transport in the Hippocampus of Rats with LPS-Induced Neuroinflammation. Biomedicines, 11(3), 920. https://doi.org/10.3390/biomedicines11030920






