A Small Molecule That In Vitro Neutralizes Infection of SARS-CoV-2 and Its Most Infectious Variants, Delta, and Omicron
Abstract
1. Introduction
2. Methods
2.1. Molecular Modeling
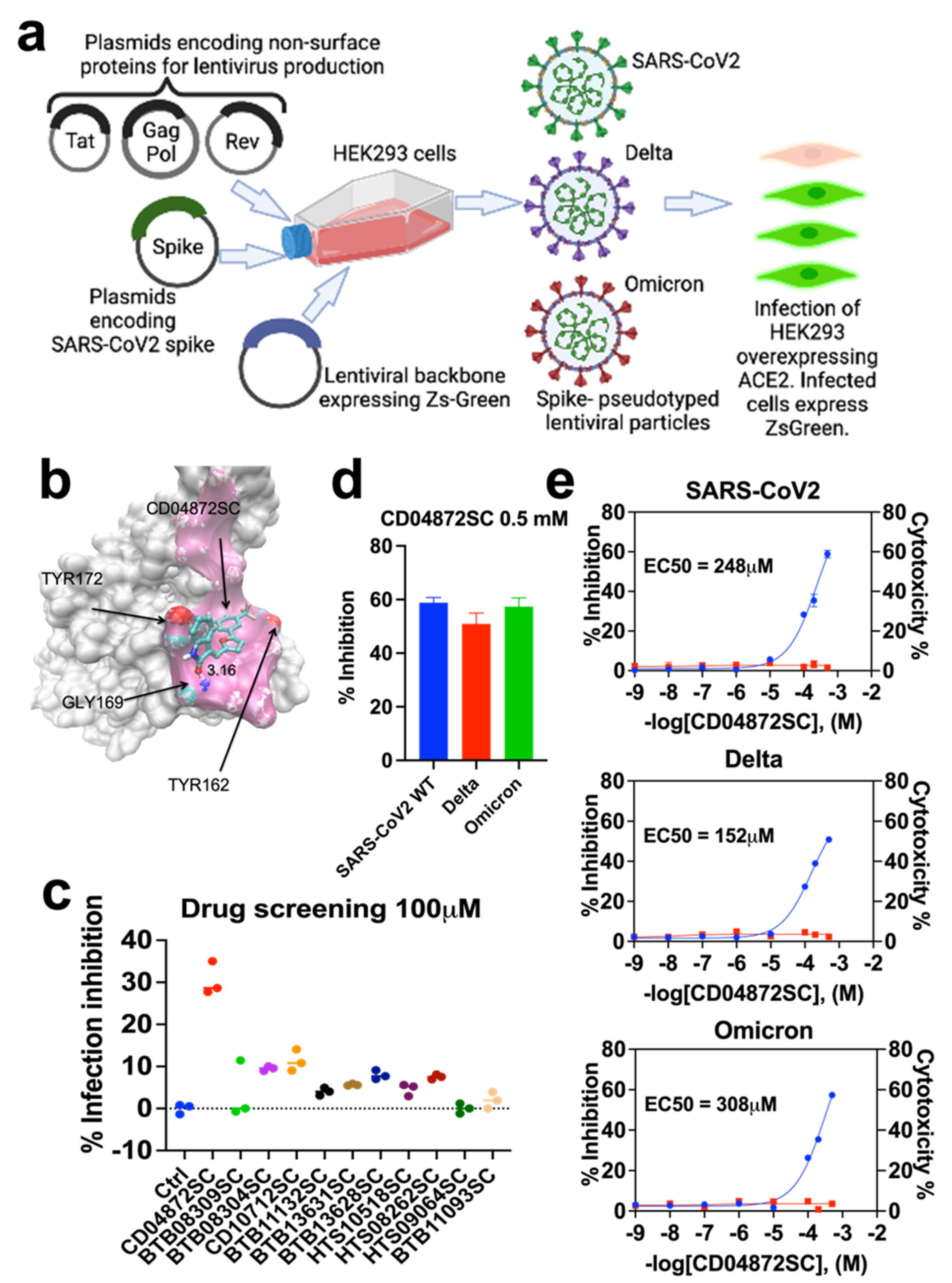
2.2. Lentivirus-Mediated Expression of the Spike Protein of SARS-CoV-2
2.3. Neutralization Assay
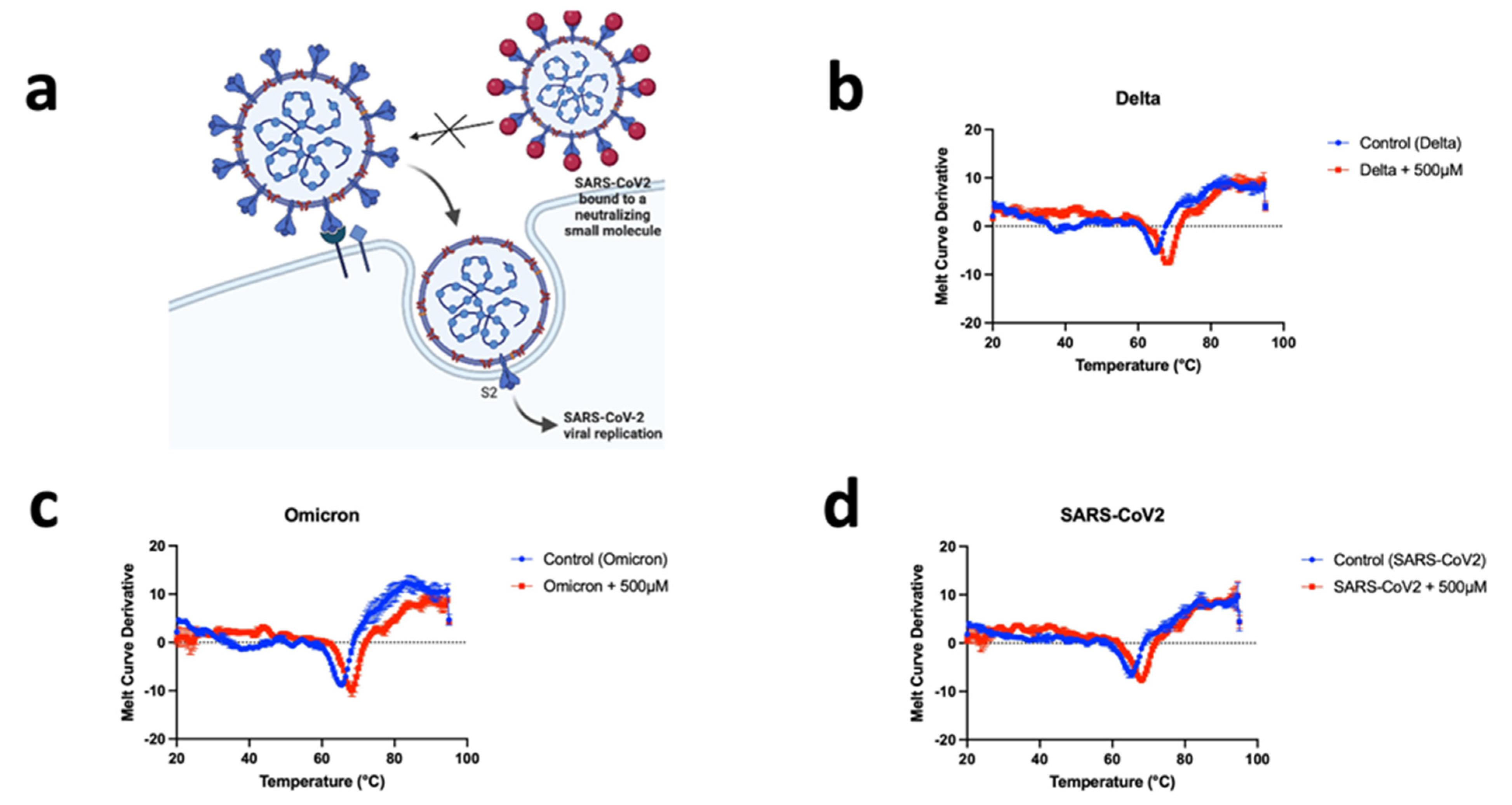
2.4. Thermal Shift Assay
2.5. Statistics and Reproducibility
3. Results
3.1. In Silico Screening
3.2. Inhibition of In Vitro Infection of SARS-CoV2, Delta, and Omicron
3.3. CD04872SC Inhibits Viral Infection by Direct Binding to the Spike Protein of SARS-CoV2, Delta, and Omicron
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. 2022. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019?adgroupsurvey={adgroupsurvey}&gclid=Cj0KCQiA6rCgBhDVARIsAK1kGPIBLi4xGuc4weRcWRdwPmvLrON8UP5H-STxfCe6E5lCf-RC7vaoM3waAvNgEALw_wcB (accessed on 3 December 2022).
- Mahase, E. COVID-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ 2021, 375, n273. [Google Scholar] [CrossRef] [PubMed]
- Collie, S.; Champion, J.; Moultrie, H.; Bekker, L.G.; Gray, G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 494–496. [Google Scholar] [CrossRef] [PubMed]
- VanBlargan, L.A.; Errico, J.M.; Halfmann, P.J.; Zost, S.J.; Crowe, J.E.; Purcell, L.A.; Kawaoka, Y.; Corti, D.; Fremont, D.H.; Diamond, M.S. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat. Med. 2022, 28, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L. Structure of the SARS-CoV-2 spike receptor- binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D.B. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 180, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Bakthavatsalam, D.; Craft, J.W.J.; Kazansky, A.; Nguyen, N.; Bae, G.; Caivano, A.R.; Gundlach, C.W.I.; Aslam, A.; Ali, S.; Gupta, S.; et al. Identification of Inhibitors of Integrin Cytoplasmic Domain Interactions With Syk. Front. Immunol. 2021, 11, 575085. [Google Scholar] [CrossRef] [PubMed]
- Cherubin, P.; Garcia, M.C.; Curtis, D.; Britt, C.B.T.; Craft, J.W.; Burress, H.; Berndt, C.; Reddy, S.; Guyette, J.; Zheng, T.; et al. Inhibition of Cholera Toxin and Other AB Toxins by Polyphenolic Compounds. PLoS ONE 2016, 11, e0166477. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.H.; Eguia, R.; Dingens, A.S.; Loes, A.N.; Malone, K.D.; Wolf, C.R.; Chu, H.Y.; Tortorici, M.A.; Veesler, D.; Murphy, M.; et al. Protocol and Reagents for Pseudotyping Lentiviral Particles with SARS-CoV-2 Spike Protein for Neutralization Assays. Viruses 2020, 12, 513. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Koshland, D.E. Application of a Theory of Enzyme Specificity to Protein Synthesis. Proc. Natl. Acad. Sci. USA 1958, 44, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Mannhold, R.; Poda, G.I.; Ostermann, C.; Tetko, I.V. Calculation of Molecular Lipophilicity: State-of-the-Art and Comparison of Log P Methods on more than 96,000 Compounds. J. Pharm. Sci. 2009, 98, 861–893. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mi, L.; Xu, J.; Yu, J.; Wang, X.; Jiang, J.; Xing, J.; Shang, P.; Qian, A.; Li, Y.; et al. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 2005, 191, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.E.A.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.-X.; Gong, L.; et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef] [PubMed]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-L.; Buck, M. Neuropilin-1 assists SARSCoV-2 infection by stimulating the separation of spike protein domains S1 and S2. Biophys. J. 2021, 120, 2828–2837. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Du, L.; Jiang, S. Learning from the past: Development of safe and effective COVID-19 vaccines. Nat. Rev. Microbiol. 2021, 19, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Investigational COVID-19 Convalescent Plasma: Guidance for Industry; United States Food and Drug Administration: Rockville, MD, USA, 2022.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Alcaraz, A.; Qasim, H.; Merlinsky, E.; Fox, G.; Islam, T.; Medina, B.; Schwartz, R.J.; Craft, J.W., Jr.; McConnell, B.K. A Small Molecule That In Vitro Neutralizes Infection of SARS-CoV-2 and Its Most Infectious Variants, Delta, and Omicron. Biomedicines 2023, 11, 916. https://doi.org/10.3390/biomedicines11030916
Reyes-Alcaraz A, Qasim H, Merlinsky E, Fox G, Islam T, Medina B, Schwartz RJ, Craft JW Jr., McConnell BK. A Small Molecule That In Vitro Neutralizes Infection of SARS-CoV-2 and Its Most Infectious Variants, Delta, and Omicron. Biomedicines. 2023; 11(3):916. https://doi.org/10.3390/biomedicines11030916
Chicago/Turabian StyleReyes-Alcaraz, Arfaxad, Hanan Qasim, Elizabeth Merlinsky, Glenn Fox, Tasneem Islam, Bryan Medina, Robert J. Schwartz, John W. Craft, Jr., and Bradley K. McConnell. 2023. "A Small Molecule That In Vitro Neutralizes Infection of SARS-CoV-2 and Its Most Infectious Variants, Delta, and Omicron" Biomedicines 11, no. 3: 916. https://doi.org/10.3390/biomedicines11030916
APA StyleReyes-Alcaraz, A., Qasim, H., Merlinsky, E., Fox, G., Islam, T., Medina, B., Schwartz, R. J., Craft, J. W., Jr., & McConnell, B. K. (2023). A Small Molecule That In Vitro Neutralizes Infection of SARS-CoV-2 and Its Most Infectious Variants, Delta, and Omicron. Biomedicines, 11(3), 916. https://doi.org/10.3390/biomedicines11030916






