A Preclinical Investigation on the Role of IgG Antibodies against Coagulant Components in Multiple Sclerosis
Abstract
1. Introduction
- Identification of the role of IgG antibodies against coagulation components in MS. Following a series of in vitro experiments with purified IgG antibodies, the role of such molecules will be characterized regarding the changes in the expression levels of inflammatory mediators.
- Analysis of the expression levels of inflammatory and neuroinflammatory mediators in MS patients and comparison with controls.
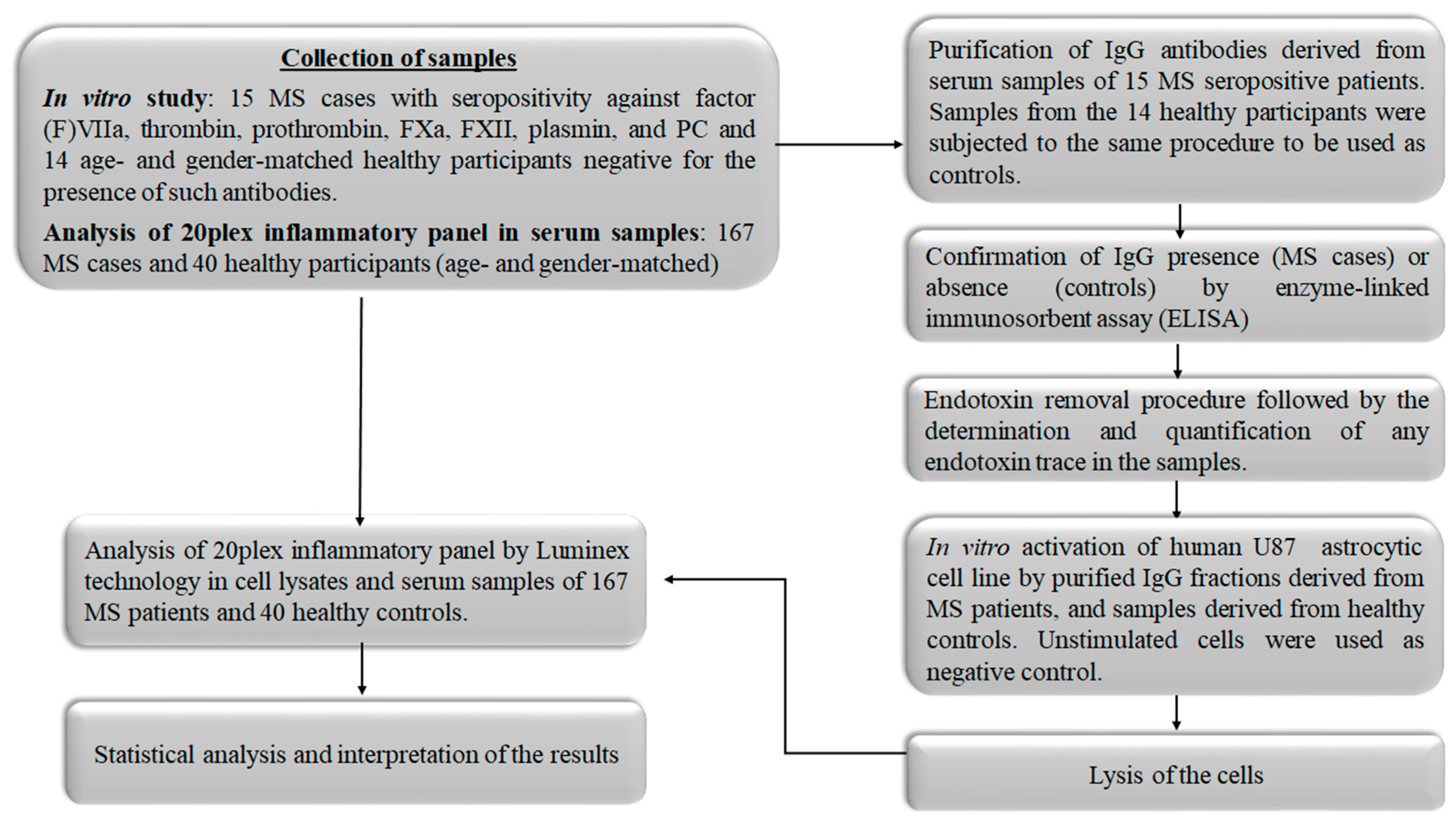
2. Materials and Methods
2.1. Study Participants
2.2. Purification of IgG Antibodies against Coagulant Components from Serum Samples Using Affinity Chromatography
2.3. Activation of Astrocytic U87 Cell Line
2.4. Lysis of Cells
2.5. Analysis of Pro- and Anti-Inflammatory Factors Performing Multiplexed Immunoassay System
2.6. Statistical Analysis
3. Results
3.1. Characterization of the Role of IgG Antibodies against Coagulation Components
3.1.1. Purified IgG Antibody Fractions
3.1.2. Activation of Intracellular Pathways upon Astrocyte Stimulation with IgG Antibodies
3.1.3. Correlation between the Concentration Levels of Pro-Inflammatory Mediators
3.2. Evaluation of Inflammatory Factors in Serum Samples of MS and HC Participants
3.2.1. Data of Study Participants
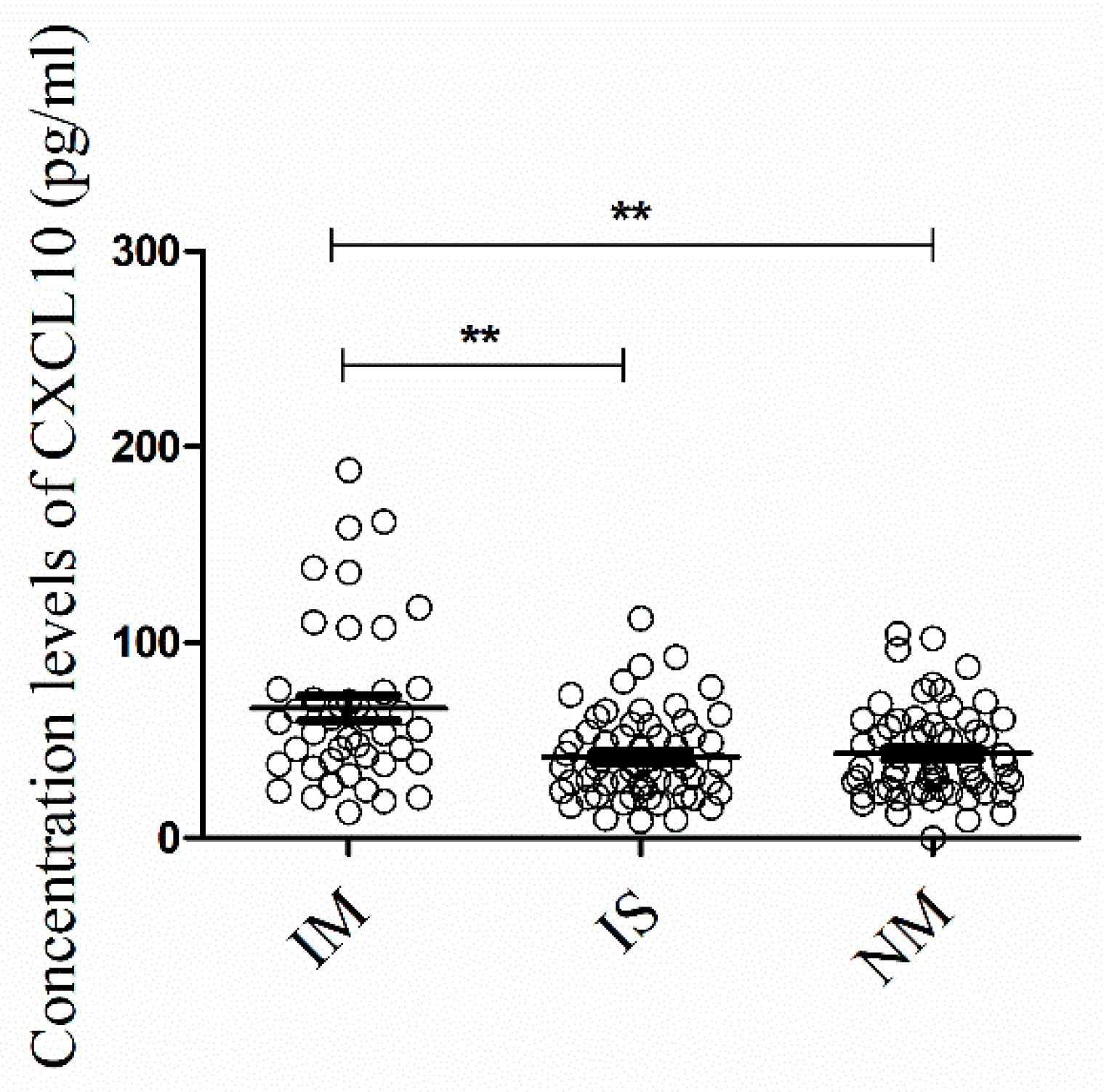
3.2.2. Detection and Quantification of Pro- and Anti-Inflammatory Mediators in Patients and HCs
3.2.3. Assessing the Pro-Inflammatory Profile of Patients Regarding Their Medication
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maghzi, A.H.; Borazanci, A.; McGee, J.; Steven Alexander, J.; Gonzalez-Toledo, E.; Minagar, A. Multiple Sclerosis: Pathophysiology, Clinical Features, Diagnosis, and Management. In Neuroinflammation; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–23. [Google Scholar]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [PubMed]
- Ziliotto, N.; Bernardi, F.; Jakimovski, D.; Zivadinov, R. Coagulation Pathways in Neurological Diseases: Multiple Sclerosis. Front. Neurol. 2019, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Koudriavtseva, T.; Zannino, S.; Filippi, M.M.; Cortese, A.; Piantadosi, C.; Lapucci, C.; Fiorelli, M.; Giannarelli, D.; Mandoj, C.; Stefanile, A.; et al. Coagulation Activation and Cerebral Hypoperfusion in Relapsing-Remitting Multiple Sclerosis. J. Neurol. Sci. 2019, 405, 197. [Google Scholar] [CrossRef]
- Göbel, K.; Pankratz, S.; Asaridou, C.M.; Herrmann, A.M.; Bittner, S.; Merker, M.; Ruck, T.; Glumm, S.; Langhauser, F.; Kraft, P.; et al. Blood Coagulation Factor XII Drives Adaptive Immunity during Neuroinflammation via CD87-Mediated Modulation of Dendritic Cells. Nat. Commun. 2016, 7, 11626. [Google Scholar] [CrossRef]
- Sárváry, A.; Szucs, S.; Balogh, I.; Becsky, Á.; Bárdos, H.; Kávai, M.; Seligsohn, U.; Egbring, R.; Lopaciuk, S.; Muszbek, L.; et al. Possible Role of Factor XIII Subunit A in Fcγ and Complement Receptor-Mediated Phagocytosis. Cell. Immunol. 2004, 228, 81–90. [Google Scholar] [CrossRef]
- Davalos, D.; Baeten, K.M.; Whitney, M.A.; Mullins, E.S.; Friedman, B.; Olson, E.S.; Ryu, J.K.; Smirnoff, D.S.; Petersen, M.A.; Bedard, C.; et al. Early Detection of Thrombin Activity in Neuroinflammatory Disease. Ann. Neurol. 2014, 75, 303–308. [Google Scholar] [CrossRef]
- De Luca, C.; Virtuoso, A.; Maggio, N.; Papa, M. Neuro-Coagulopathy: Blood Coagulation Factors in Central Nervous System Diseases. Int. J. Mol. Sci. 2017, 18, 2128. [Google Scholar] [CrossRef]
- Plantone, D.; Inglese, M.; Salvetti, M.; Koudriavtseva, T. A Perspective of Coagulation Dysfunction in Multiple Sclerosis and in Experimental Allergic Encephalomyelitis. Front. Neurol. 2019, 9, 1175. [Google Scholar] [CrossRef]
- Davalos, D.; Akassoglou, K. Fibrinogen as a Key Regulator of Inflammation in Disease. Semin. Immunopathol. 2012, 34, 43–62. [Google Scholar] [CrossRef]
- Davalos, D.; Kyu Ryu, J.; Merlini, M.; Baeten, K.M.; Le Moan, N.; Petersen, M.A.; Deerinck, T.J.; Smirnoff, D.S.; Bedard, C.; Hakozaki, H.; et al. Fibrinogen-Induced Perivascular Microglial Clustering Is Required for the Development of Axonal Damage in Neuroinflammation. Nat. Commun. 2012, 3, 1227. [Google Scholar] [CrossRef]
- Yates, R.L.; Esiri, M.M.; Palace, J.; Jacobs, B.; Perera, R.; DeLuca, G.C. Fibrin(Ogen) and Neurodegeneration in the Progressive Multiple Sclerosis Cortex. Ann. Neurol. 2017, 82, 259–270. [Google Scholar] [CrossRef]
- Wadgaonkar, R.; Somnay, K.; Garcia, J.G.N. Thrombin Induced Secretion of Macrophage Migration Inhibitory Factor (MIF) and Its Effect on Nuclear Signaling in Endothelium. J. Cell. Biochem. 2008, 105, 1279–1288. [Google Scholar] [CrossRef]
- Filippidou, N.; Krashias, G.; Pericleous, C.; Rahman, A.; Ioannou, Y.; Giles, I.; Demetriou, C.; Anatolitou, A.; Christodoulou, C.; Pantzaris, M.; et al. The Association between IgG and IgM Antibodies against Cardiolipin, Β2-Glycoprotein I and Domain I of Β2-Glycoprotein I with Disease Profile in Patients with Multiple Sclerosis. Mol. Immunol. 2016, 75, 161–167. [Google Scholar] [CrossRef]
- Artim-Esen, B.; Pericleous, C.; Mackie, I.; Ripoll, V.M.; Latchman, D.; Isenberg, D.; Rahman, A.; Ioannou, Y.; Giles, I. Anti-Factor Xa Antibodies in Patients with Antiphospholipid Syndrome and Their Effects upon Coagulation Assays. Arthritis Res. Ther. 2015, 17, 47. [Google Scholar] [CrossRef]
- Lambrianides, A.; Turner-Stokes, T.; Pericleous, C.; Ehsanullah, J.; Papadimitraki, E.; Poulton, K.; Ioannou, Y.; Lawrie, A.; MacKie, I.; Chen, P.; et al. Interactions of Human Monoclonal and Polyclonal Antiphospholipid Antibodies with Serine Proteases Involved in Hemostasis. Arthritis Rheum. 2011, 63, 3512–3521. [Google Scholar] [CrossRef]
- Hadjiagapiou, M.S.; Krashias, G.; Deeba, E.; Christodoulou, C.; Pantzaris, M.; Lambrianides, A. Antibodies to Blood Coagulation Components Are Implicated in Patients with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2022, 62, 103775. [Google Scholar] [CrossRef]
- Koudriavtseva, T. Thrombotic Processes in Multiple Sclerosis as Manifestation of Innate Immune Activation. Front. Neurol. 2014, 5, 119. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic Criteria for Multiple Sclerosis: 2010 Revisions to the McDonald Criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Le Boedec, K. Sensitivity and Specificity of Normality Tests and Consequences on Reference Interval Accuracy at Small Sample Size: A Computer-Simulation Study. Vet. Clin. Pathol. 2016, 45, 648–656. [Google Scholar] [CrossRef]
- Tai, K.Y.; Dhaliwal, J.; Balasubramaniam, V. Leveraging Mann–Whitney U Test on Large-Scale Genetic Variation Data for Analysing Malaria Genetic Markers. Malar. J. 2022, 21, 79. [Google Scholar] [CrossRef]
- Tomczak, S.K. Ratio Selection between Six Sectors in the Visegrad Group Using Parametric and Nonparametric Anova. Energies 2021, 14, 7120. [Google Scholar] [CrossRef]
- Claverie, J.-M.; Santini, S. Validation of Predicted Anonymous Proteins Simply Using Fisher’s Exact Test. Bioinform. Adv. 2021, 1, vbab034. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Phadikar, S.; Deb, N.; Sinha, N.; Das, P.; Ghaderpour, E. Automatic Eyeblink and Muscular Artifact Detection and Removal from EEG Signals Using k-Nearest Neighbour Classifier and Long Short-Term Memory Networks. IEEE Sens. J. 2023, 23, 5422–5436. [Google Scholar] [CrossRef]
- Kang, J.S.; Shin, D.H.; Baek, J.W.; Chung, K. Activity Recommendation Model Using Rank Correlation for Chronic Stress Management. Appl. Sci. 2019, 9, 4284. [Google Scholar] [CrossRef]
- Alabanza, L.M.; Bynoe, M.S. Thrombin Induces an Inflammatory Phenotype in a Human Brain Endothelial Cell Line. J. Neuroimmunol. 2012, 245, 48–55. [Google Scholar] [CrossRef]
- Ishida, Y.; Nagai, A.; Kobayashi, S.; Kim, S.U. Upregulation of Protease-Activated Receptor-1 in Astrocytes in Parkinson Disease: Astrocyte-Mediated Neuroprotection through Increased Levels of Glutathione Peroxidase. J. Neuropathol. Exp. Neurol. 2006, 65, 66–77. [Google Scholar] [CrossRef]
- Delvaeye, M.; Conway, E.M. Coagulation and Innate Immune Responses: Can We View Them Separately? Blood 2009, 114, 2367–2374. [Google Scholar] [CrossRef]
- Coughlin, S.R.; Camerer, E. PARticipation in Inflammation. J. Clin. Investig. 2003, 111, 25–27. [Google Scholar] [CrossRef]
- Foley, J.H.; Conway, E.M. Cross Talk Pathways between Coagulation and Inflammation. Circ. Res. 2016, 118, 1392–1408. [Google Scholar] [CrossRef]
- Yang, C.-D.; Hwang, K.-K.; Yan, W.; Gallagher, K.; FitzGerald, J.; Grossman, J.M.; Hahn, B.H.; Chen, P.P. Identification of Anti-Plasmin Antibodies in the Antiphospholipid Syndrome That Inhibit Degradation of Fibrin. J. Immunol. 2004, 172, 5765–5773. [Google Scholar] [CrossRef]
- Bidot, C.J.; Jy, W.; Horstman, L.L.; Huisheng, H.; Jimenez, J.J.; Yaniz, M.; Ahn, Y.S. Factor VII/VIIa: A New Antigen in the Anti-Phospholipid Antibody Syndrome. Br. J. Haematol. 2003, 120, 618–626. [Google Scholar] [CrossRef]
- Hwang, K.K.; Yang, C.-D.; Yan, W.; Grossman, J.M.; Hahn, B.H.; Chen, P.P. A Thrombin-Cross-Reactive Anticardiolipin Antibody Binds to and Inhibits the Anticoagulant Function of Activated Protein C. Arthritis Rheum. 2003, 48, 1622–1630. [Google Scholar] [CrossRef]
- Arachchillage, D.R.J.; Efthymiou, M.; Mackie, I.J.; Lawrie, A.S.; Machin, S.J.; Cohen, H. Anti-Protein C Antibodies Are Associated with Resistance to Endogenous Protein C Activation and a Severe Thrombotic Phenotype in Antiphospholipid Syndrome. J. Thromb. Haemost. 2014, 12, 1801–1809. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Hwang, K.-K.; FitzGerald, J.; Grossman, J.M.; Taylor, M.; Hahn, B.H.; Chen, P.P. Antibodies against the Activated Coagulation Factor X (FXa) in the Antiphospholipid Syndrome That Interfere with the FXa Inactivation by Antithrombin. J. Immunol. 2006, 177, 8219–8225. [Google Scholar] [CrossRef]
- McDonnell, T.; Amarnani, R.; Spicer, C.; Jbari, H.; Pericleous, C.; Spiteri, V.; Wincup, C.; Artim-Esen, B.; Mackie, I.; Botto, M.; et al. Antibodies to FXa and Thrombin in Patients with SLE Differentially Regulate C3 and C5 Cleavage. Lupus Sci. Med. 2022, 9, e000738. [Google Scholar] [CrossRef]
- Berden, A.E.; Nolan, S.L.; Morris, H.L.; Bertina, R.M.; Erasmus, D.D.; Hagen, E.C.; Hayes, D.P.; Van Tilburg, N.H.; Bruijn, J.A.; Savage, C.O.S.; et al. Anti-Plasminogen Antibodies Compromise Fibrinolysis and Associate with Renal Histology in ANCA-Associated Vasculitis. J. Am. Soc. Nephrol. 2010, 21, 2169–2179. [Google Scholar] [CrossRef]
- Suidan, G.L.; Singh, P.K.; Patel-Hett, S.; Chen, Z.L.; Volfson, D.; Yamamoto-Imoto, H.; Norris, E.H.; Bell, R.D.; Strickland, S. Abnormal Clotting of the Intrinsic/Contact Pathway in Alzheimer Disease Patients Is Related to Cognitive Ability. Blood Adv. 2018, 2, 954–963. [Google Scholar] [CrossRef]
- Zamolodchikov, D.; Renné, T.; Strickland, S. The Alzheimer’s Disease Peptide β-Amyloid Promotes Thrombin Generation through Activation of Coagulation Factor XII. J. Thromb. Haemost. 2016, 14, 995–1007. [Google Scholar] [CrossRef]
- Kallaur, A.P.; Oliveira, S.R.; Simao, A.N.C.; De Almeida, E.R.D.; Morimoto, H.K.; Lopes, J.; De Carvalho Jennings Pereira, W.L.; Andrade, R.M.; Pelegrino, L.M.; Borelli, S.D.; et al. Cytokine Profile in Relapsing-Remitting Multiple Sclerosis Patients and the Association between Progression and Activity of the Disease. Mol. Med. Rep. 2013, 7, 1010–1020. [Google Scholar] [CrossRef]
- Brucklacher-Waldert, V.; Stuerner, K.; Kolster, M.; Wolthausen, J.; Tolosa, E. Phenotypical and Functional Characterization of T Helper 17 Cells in Multiple Sclerosis. Brain 2009, 132, 3329–3341. [Google Scholar] [CrossRef]


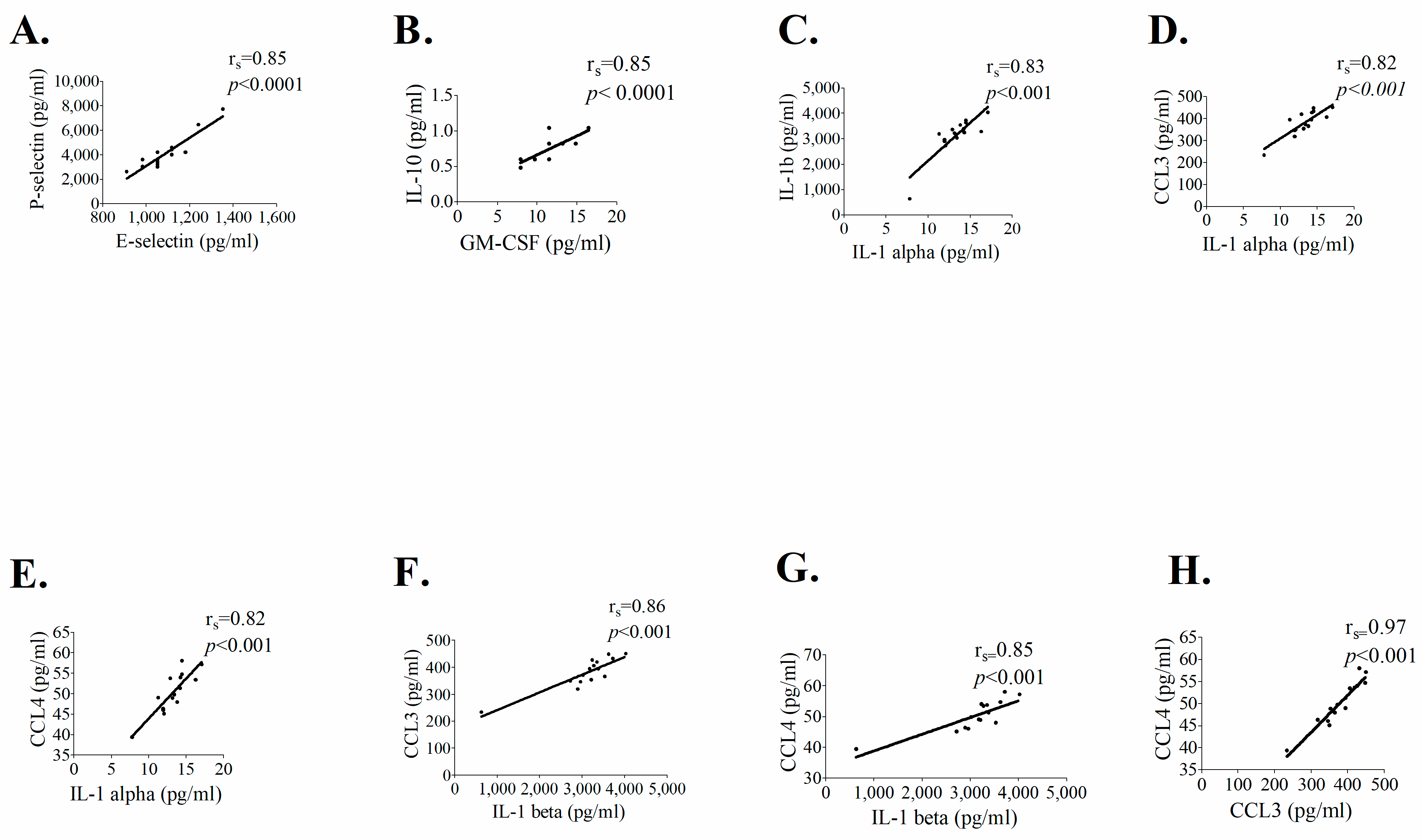


| MS Patients | Gender | Age | Disease Duration | Disease Course | EDSS | MSSS | Medication | Laboratory Findings |
|---|---|---|---|---|---|---|---|---|
| −1 | F | 41 | 23 | RRMS | 4.0 | 2.78 | Interferon | Anti-FXa |
| −2 | F | 59 | 30 | RRMS | 2.5 | 1.19 | - | Anti-FVIIa |
| −3 | M | 58 | 24 | SPMS | 6.0 | 5.03 | - | Anti-plasmin |
| −4 | F | 47 | 21 | SPMS | 4.0 | 2.97 | - | Anti-plasmin, anti-FXa, anti-FXII |
| −5 | F | 65 | 25 | RRMS | 3.0 | 1.56 | - | Anti-plasmin, anti-FXa |
| −6 | M | 39 | 12 | RRMS | 3.0 | 3.25 | Interferon | Anti-plasmin, anti-FXa |
| −7 | F | 63 | 19 | PPMS | 6.5 | 6.59 | - | Anti-plasmin, anti-FXa, antithrombin |
| −8 | M | 33 | 13 | RRMS | 3.0 | 3.05 | Fingolimand | Anti-plasmin, anti-PT, anti-FXII, anti-PC |
| −9 | M | 62 | 10 | SPMS | 7.0 | 8.92 | - | Anti-FXII |
| −10 | F | 51 | 11 | RRMS | 5.0 | 5.82 | Fingolimand | Anti-PC |
| −11 | M | 32 | 2 | RRMS | 3.5 | 7.98 | Interferon | Antithrombin |
| −12 | F | 53 | 3 | CIS | 1.0 | 1.77 | - | Anti-PT |
| −13 | M | 40 | 3 | RRMS | 3.0 | 6.81 | Fingolimand | Antithrombin |
| −14 | M | 36 | 11 | RRMS | 3.5 | 4.21 | Fingolimand | Anti-FVIIa |
| −15 | F | 28 | 1 | RRMS | 3.0 | 7.93 | Interferon | Anti-FXII |
| U87 Cells upon Stimulation with MS IgG Fractions | U87 Cells upon Stimulation with HC Samples | |||||
|---|---|---|---|---|---|---|
| Pro- or Anti-Inflammatory Molecules | Mean (pg/mL) | SEM | Mean (pg/mL) | SEM | p Value | AUC (95% CI) |
| E-selectin | 1089 | 28.12 | 702.3 | 15.90 | <0.0001 | 1.00 |
| P-selectin | 4108 | 349.5 | 7755 | 343.7 | <0.0001 | 0.95 (0.88–1.0) |
| GM-CSF | 11.69 | 0.7272 | 8.435 | 0.7792 | =0.0050 | 0.76 (0.59–0.94) |
| ICAM-1 | 10,790 | 586.4 | 9087 | 616.3 | =0.0137 | 0.77 (0.59–1.95) |
| IFN-gamma | 2.459 | 0.0835 | 2.878 | 0.1255 | =0.0059 | 0.80 (0.62–1.98) |
| IL-1 alpha | 13.30 | 0.5678 | 9.574 | 0.4023 | <0.0001 | 0.93 (0.82–1.0) |
| IL-1 beta | 3120 | 197.9 | 2706 | 115.0 | =0.0094 | 0.78 (0.60–1.96) |
| IL-4 | 12.75 | 0.8069 | 14.96 | 1.467 | =0.19 | - |
| IL-6 | 394.5 | 19.26 | 447.5 | 13.91 | =0.036 | 0.73 (0.54–0.92) |
| IL-8 | 251.1 | 11.61 | 159.6 | 4.82 | <0.0001 | 0.93 (0.80–1.0) |
| IL-10 | 0.7533 | 0.0480 | 0.7469 | 0.0717 | =0.94 | - |
| IL-12p70 | 7.781 | 0.4429 | 6.933 | 0.2768 | =0.12 | - |
| IL-13 | 2.644 | 0.1391 | 1.893 | 0.1619 | =0.0016 | 0.77 (0.58–0.96) |
| IL-17a | 2.787 | 0.0781 | 3.446 | 0.1063 | <0.0001 | 0.91 (0.79–1.0) |
| CXCL10 | 2.807 | 0.1334 | 2.001 | 0.2249 | =0.0042 | 0.78 (0.62–0.95) |
| CCL2 | 17.08 | 0.4888 | 13.13 | 0.5012 | <0.0001 | 0.89 (0.77–1.0) |
| CCL3 | 380.8 | 14.69 | 296.8 | 15.87 | =0.0013 | 0.85 (0.71–0.99) |
| CCL4 | 50.30 | 1.294 | 39.19 | 1.708 | <0.0001 | 0.91 (0.81–1.0) |
| TNF-a | 22.37 | 0.6531 | 15.77 | 0.3263 | <0.0001 | 0.98 (0.93–1.0) |
| Features | MS Patients (n = 167) | HCs (n = 40) | p Value |
|---|---|---|---|
| Gender Female/Male | 110/57 | 21/19 | 0.20 |
| Age in years Mean ± SD Min–Max | 48 ± 13 21–80 | 45 ± 10 24–63 | 0.29 |
| Disease course (CIS/RRMS/SPMS/PPMS) | 1/130/29/7 | N/A | |
| Disease Duration (years) Mean ± SD | 15.3 ± 8.9 | N/A | |
| EDSS Median (interquartile range) Mild: 0–3.0 [n (%)] Moderate: 3.5–5.5 [n (%)] Severe: 6.0–9.5 [n (%)] | 3.5 (2.5–5.5) 75 (45.0) 57 (34.0) 35 (21.0) | N/A | |
| MSSS Median (interquartile range) Benign MS: 1–2 [n (%)] Severe MS: 7–10 [n (%)] | 3.9 (2.4–5.5) 20 (12.0) 20 (12.0) | N/A | |
| Medication [n (%)] Interferon beta-1a or -1b Natalizumab Fingolimod Other * None | 40 (24.0) 18 (11.0) 26 (16.0) 24 (14.0) 59 (35.0) | N/A |
| MS Patients | HCs | ||||
|---|---|---|---|---|---|
| Pro- or Anti-Inflammatory Molecules | Mean (pg/mL) | SEM | Mean (pg/mL) | SEM | p Value |
| E-selectin | 28,320 | 912.7 | 29,120 | 2452 | 0.91 |
| P-selectin | 937,900 | 58,580 | 872,400 | 126,600 | 0.42 |
| GM-CSF | 7.33 | 2.40 | 8.74 | 2.08 | 0.0102 |
| ICAM-1 | 333,500 | 28,960 | 271,000 | 36,170 | 0.53 |
| IFN-alpha | 3.20 | 0.49 | 3.25 | 0.65 | 0.43 |
| IFN-gamma | 23.09 | 2.40 | 20.19 | 4.36 | 0.25 |
| IL-1alpha | 4.85 | 0.65 | 6.29 | 1.06 | 0.012 |
| IL-1beta | 5.79 | 1.02 | 5.52 | 1.33 | 0.68 |
| IL-4 | 34.92 | 2.98 | 31.46 | 4.88 | 0.26 |
| IL-6 | 26.28 | 5.54 | 15.90 | 2.92 | 0.64 |
| IL-8 | 55.59 | 12.34 | 84.97 | 19.21 | 0.003 |
| IL-10 | 1.95 | 0.54 | 1.22 | 0.23 | 0.32 |
| IL-12p70 | 14.84 | 2.01 | 13.30 | 1.44 | 0.89 |
| IL-13 | 10.79 | 1.37 | 9.37 | 1.14 | 0.62 |
| IL-17a | 10.31 | 1.13 | 8.26 | 1.13 | 0.47 |
| CXCL10 | 48.68 | 2.40 | 47.11 | 4.44 | 0.98 |
| CCL2 | 167.3 | 11.13 | 156.2 | 14.94 | 0.68 |
| CCL3 | 37.37 | 2.54 | 45.70 | 5.82 | 0.12 |
| CCL4 | 150.9 | 10.48 | 193.4 | 19.87 | 0.0043 |
| TNF-a | 50.27 | 2.55 | 48.89 | 4.86 | 0.96 |
| Pro-Inflammatory Factors | Mean Index (±SEM) | Kruskal-Wallis Test p Value | Dunn’s Multiple Comparisons Test | ||||
|---|---|---|---|---|---|---|---|
| RRMS | SPMS | HCs | RRMS-HCs | SPMS-HCs | RRMS-SPMS | ||
| p Value | |||||||
| GM-CSF | 8.82 (±3.17) | 3.47 (±2.54) | 8.74 (±2.08) | 0.03 | ns | ns | ns |
| IL-1a | 4.27 (±0.67) | 4.48 (±0.89) | 6.29 (±1.06) | 0.02 | * | ns | ns |
| IL-8 | 47.85 (±13.01) | 40.39 (±15.08) | 84.97 (±19.21) | 0.007 | ** | ns | ns |
| CCL4 | 139.4 (±10.18) | 152.2 (±20.66) | 193.4 (±19.85) | 0.007 | ** | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjiagapiou, M.S.; Krashias, G.; Deeba, E.; Christodoulou, C.; Pantzaris, M.; Lambrianides, A. A Preclinical Investigation on the Role of IgG Antibodies against Coagulant Components in Multiple Sclerosis. Biomedicines 2023, 11, 906. https://doi.org/10.3390/biomedicines11030906
Hadjiagapiou MS, Krashias G, Deeba E, Christodoulou C, Pantzaris M, Lambrianides A. A Preclinical Investigation on the Role of IgG Antibodies against Coagulant Components in Multiple Sclerosis. Biomedicines. 2023; 11(3):906. https://doi.org/10.3390/biomedicines11030906
Chicago/Turabian StyleHadjiagapiou, Maria S., George Krashias, Elie Deeba, Christina Christodoulou, Marios Pantzaris, and Anastasia Lambrianides. 2023. "A Preclinical Investigation on the Role of IgG Antibodies against Coagulant Components in Multiple Sclerosis" Biomedicines 11, no. 3: 906. https://doi.org/10.3390/biomedicines11030906
APA StyleHadjiagapiou, M. S., Krashias, G., Deeba, E., Christodoulou, C., Pantzaris, M., & Lambrianides, A. (2023). A Preclinical Investigation on the Role of IgG Antibodies against Coagulant Components in Multiple Sclerosis. Biomedicines, 11(3), 906. https://doi.org/10.3390/biomedicines11030906







