Chemotherapy-Induced Molecular Changes in Skeletal Muscle
Abstract
1. Introduction
2. Materials and Methods
| Chemotherapy | Animal Model/Muscle | Tumor Data | Main Results | Ref. |
|---|---|---|---|---|
| Doxorubicin | ||||
| 15 mg/kg IP injection | Male, C57BL/6 mice, 7-week-old (unspecified muscle) | Non tumor bearing | ↑atrogin-1, MuRF-1 mRNA | [13] |
| 20 mg/kg IP injection | Adult male, NMRI mice, TNFR1 receptor-deficient (EDL) | Non tumor bearing | ↓ body weight, EDL muscle weight (20%), estimated CSA, maximal absolute force (40 ± 2%), maximal specific force (28 ± 5%), specific (59 ± 5%) | [14] |
| 20 mg/kg IP injection | Male, Sprague-Dawley rats, 8–10 weeks old (gastrocnemius) | Non tumor bearing | ↓ body weight, whole-body fat, lean mass, maximal respiratory capacity (72 h after), energy expenditure ↑ mitochondrial H2O2 emission | [15] |
| 1.5/4.5 mg/kg IP injection | Male, Sprague Dawley rats (plantaris, soleus, gastrocnemius) | Non tumor bearing | 1.5 mg/kg ↑ [NO] intra in soleus at 24 h ↑ [NO] interstitial at 24, 48, 72, 96, and 120 h 4.5 mg/kg ↓ [NO]intra in soleus at 24 h and 48 h ↓ [NO]intra in white gastrocnemius at 4 h and 48 h ↑ [NO]interstitial at 96 h | [16] |
| 15 mg/kg IP injection | Male, Wistar rats,~14 weeks old (EDL) | Non tumor bearing | ↓ skeletal muscle, CSA, IRS-1, and GSK3-b mRNA, GLUT4, AMPK α (pT172) levels, activity of mitochondrial complex 3, IL-10 levels ↑ AST, uric acid, corticosterone/testosterone ratio, insulin, glucose, FFA, activity of mitochondrial complex 1 = IL-6 and TNF-α levels | [17] |
| 12 mg/kg (3 injections of 4 mg/kg separated by two weeks) IP injection | Female, Sprague-Dawley rats, 8 weeks old ovariectomized (soleus and EDL) | Non tumor bearing | ↓ pooled-fiber CSA in the soleus, fiber CSA in the EDL, Pax7-positive satellite cell in the soleus (38 ± 4%), capillary content in the soleus (35 ± 5%), MGF mRNA in the EDL ↑ MYF5 mRNA in the soleus = Ki67+ satellite cells, capillary content of the EDL | [18] |
| 20 mg/kg IP injection | Male, C57BL/6NJ mice, 10–24 weeks old (EDL) | Non tumor bearing | ↓ absolute respiratory sensitivity, membrane potential, specific force, fatigue resistance, rate of sarcoplasmic reticulum Ca2+ uptake ↑ soleus half-relaxation time | [19] |
| Cisplatin | ||||
| 15 mg/kg (3 injections of 5 mg/kg) IV injection | Male, CD2F1 mice, 7–8 weeks old (gastrocnemius) | C26 adenocarcinoma cell-line injection | ↓ MuRF-1 mRNA | [20] |
| 12 mg/kg (4 daily injections of 3 mg/kg) IP injection | Male, C57BL/6J, mice 8–9 weeks old, 23–27 g (quadriceps) | Non tumor bearing | ↓ body weight, muscle mass, food intake, myofiber diameters, p-Akt, p-FOXO3a, IGF mRNA ↑ FOXO3, FOXO3a, MAFbx, atrogin-1, MuRF-1, p21 and Mstn mRNA, p-Smad2 = FOXO1 mRNA | [21] |
| 2.5 mg/kg (for up to 2 weeks) IP injection | Male, mice CD2F1, 8 weeks old (quadriceps) | Non tumor bearing | ↓ body weight (−6.6 g), muscle strength (−23%) ↑atrogin-1 mRNA | [22] |
| Oxaliplatin | ||||
| 20 mg/kg (4 injections of 5 mg/kg over 6 days) Or 30 mg/kg (12 injections of 2.5 mg/kg over 17 days) | Male, mice C57BL/6J, 8 weeks old (gastrocnemius and soleus) | Non tumor bearing | ↓ body weight (20 mg/kg and 30 mg/kg), muscle weights and average speed (30 mg/kg) ↑ p-STAT3 (Tyr705) (20 mg/kg) in the gastrocnemius, atrogin-1, BNIP3, and FOXO3 mRNA (30 mg/kg) = FOXO1 mRNA (30 mg/kg) | [23] |
| Docetaxel | ||||
| 20 mg/kg IV injection | Female, mice, 8–11 weeks old (EDL, soleus, and gastrocnemius) | Non tumor bearing | Repeated: ↓ body mass (1–3%), soleus muscle mass (9%), EDL muscle mass (7%), gastrocnemius muscle mass (10%), muscle cross-sectional area soleus, and EDL ↓ specific force of soleus muscles (17%) (acute) | [24] |
| Sorafenib | ||||
| 90 mg/kg intragastrically | Male, Wistar rats, 5 weeks old (gastrocnemius) | Ascites hepatoma cells | ↑ weight of Soleus (5 mg/100 g), of EDL (9 mg/100 g), of gastrocnemius (105 mg/100 g), grip strength | [25] |
| 5-fluorouracil (5-FU) | ||||
| 35 mg/kg IP injection | Male, C57BL/6 mice, 14 weeks old (quadriceps and gastrocnemius) | Non tumor bearing | ↓ body weight (2–8%), average daily food intake (20.5% g/day), IL-1β (95%) and IFNγ (75%) mRNA =IL-6, TNF-α, and MCP-1 mRNA | [26] |
| 23 mg/kg IP injection | Male, Balb/c mice, 6 weeks old (soleus and EDL) | Non tumor bearing | ↓ desmin, dystrophin, p-Akt (Ser473), ↑ p-p38, p-65 subunit of NF-κB =Ankrd2, TFAM, PGC1α, PGC1β, DRP-1, and OPA-1 protein expression | [27] |
| Combinations of chemotherapy agents | ||||
| doxorubicin (10 mg/kg) and dexamethasone (2.5 mg/kg) (every 3 weeks for a maximum of 4 cycles on days 1–5 of each cycle) IP injection | Female, C57BL/6 mice, 9 weeks old (lower limb) | Non tumor bearing | ↓ weight (23%), CS activity, Parkin levels, ratio Parkin/VDAC ↑ ROS production per unit respiration | [28] |
| gemcitabine (1000 mg/m2 per 3 days) cisplatin (75 mg/m2/week) (for 21 days) IP injection | Female, athymic nude mice (BALB/c), 7 weeks old, ~25 g (gastrocnemius and soleus) | T24 human bladder-cancer cell | ↓ body weight (28.3 ± 1.8%) ↑ ActRIIB, FOXO3, MuRF 1, atrogin-1, myostatin, and activin A mRNA, muscular proteasome activity (chymotrypsin) | [29] |
| folfiri [5-fluorouracil (50 mg/kg), leucovorin (90 mg/kg), CPT-11 (24 mg/kg)] (twice a week for five consecutive weeks) IP injection | Male, CD2F1 mice, 8 weeks old (quadriceps and gastrocnemius muscles) | C26 adenocarcinoma cells | ↓ body weight (15%), gastrocnemius mass (11%), quadriceps mass (20%), 15 calcium-binding proteins, MYOZ2 levels ↑ PSMA6, UBA1, KERA, LAMA2 protein levels | [30] |
| folfiri (5-fluorouracil, leucovorin, CPT-11) (for up to 5 consecutive weeks) IP injection | Male, CD2F1 mice (tibialis anterior, EDL, gastrocnemius and quadriceps) | Non tumor bearing | ↓ body weight (10%), quadriceps muscle (23%), muscle force (17%), size of oxidative and glycolytic fibers, p-Akt, PGC1α, and PGC1β levels, cytochrome C, SDH activity, Ucp1, Cidea1, Acot2, and Fhl3 mRNA, gastrocnemius and tibialis anterior mass, fiber size and oxidative fibers in the tibialis, mitochondria density and size ↑ Alb, Fga, Fgb, Dnah5, Fabp1, Apoa1, Apob, Apoa2, Prkcz, and Scd2 mRNA, glycolytic fibers in the tibialis, p-ERK1/2, p-p38 MAPKs, ROS levels =Atrogin-1, MuRF-1, Fbxo21, Fbxo30, and Fbxo31 mRNA | [31] |
| folfox (5-fluorouracil, leucovorin, oxaliplatin) (for up to 5 consecutive weeks) IP injection | Male, CD2F1 mice (tibialis anterior, EDL, gastrocnemius and quadriceps) | Non tumor bearing | ↓ muscle mass, PGC1α, PGC1β, cytochrome C, SDH activity, oxidative fibers in the tibialis, mitochondria density and size ↓ oxidative fibers and ↑ glycolytic fibers ↑ p-ERK1/2, p-p38 MAPKs, PGC1α, PGC1β, ROS levels = atrogin-1, MuRF-1, Fbxo21, Fbxo30, and Fbxo31 mRNA | [31] |
| folfiri [5-fluorouracil (50 mg/kg), leucovorin (90 mg/kg), and CPT-11 (24 mg/kg)] (twice a week) IP injection | Male, CD2F1 mice, 8 weeks old (gastrocnemius) | Non tumor bearing | ↓ muscle weight, muscle-grip strength, fiber CSA (40–50%), PDH levels, ATP content, creatine phosphate ↑ AMP content, ROS levels = hexokinase activity, lactate, BCAA levels | [32] |
| Male, CD2F1mice, 8 weeks old (gastrocnemius) | C26 adenocarcinoma cells | ↑ muscle weight, ROS levels ↓ muscle-grip strength, fiber CSA (40–50%), PDH and hexokinase activity, ATP and creatine phosphate content, acetylcarnitine = lactate, BCAA levels | [32] | |
| Authors/Year | Chemotherapy | Cohort Characterization | Main Result |
|---|---|---|---|
| Poterucha et al., 2011 [33] USA | Bevacizumab 5 or 7.5 mg/kg plus fluoropyrimidine, oxaliplatin, and leucovorin or irinotecan-based regimen or single-agent capecitabine or 5-fluorouracil and leucovorin | 57 patients with metastatic CRC (59 (26–84) yo, M: 53%); March of 2004 to 2007 (CT at L3 level) | ↓ Muscle area (3 cm2) |
| Awad et al., 2012 [34] UK | Epirubicin/cisplatin/5-fluorouracil or cisplatin/5-fluorouracil (neoadjuvant) | 47 patients with oesophagogastric cancer (63 ± 12 yo, M: 72.3%) (CT at L3 level) | ↓ SMA (9.6 cm2) |
| Ida et al., 2014 [35] Japan | Docetaxel (60 mg/m2), 5-fluorouracil (350 mg/m2), and cisplatin (6 mg/m2) (neoadjuvant) | 30 patients with EC (65 (53–75) yo, M: 83.3%) August 2010 to April 2013 (BIA) | = BMI, SkM |
| Cooper et al., 2014 [36] Hershey | Gemcitabine and cisplatin + 30 Gy x 10 concurrent gemcitabine (neoadjuvant chemoradiation; 12 weeks) | 89 with resectabel PDAC patients (63 (38–79) yo, M: 55%) (CT at L3 level) | ↓ SkM (1.2 cm2/m2) |
| Kimura et al., 2015 [37] Japan | Single-agent chemotherapy (docetaxel, gemcitabine, or vinorelbine), platinum-based chemotherapy (cisplatin + pemetrexed, carboplatin + paclitaxel ± bevacizumab, carboplatin + pemetrexed ± bevacizumab, cisplatin + docetaxel, carboplatin + S-1, nedaplatin + docetaxel, or carboplatin + gemcitabine), or molecular targeted treatment (gefitinib or elrotinib) | 134 patients with NSCLC (66 (35–86) yo, M: 59.7%) January 2010 to August 2011 (CT at L3 level) | ↓ LSMI (1.3 cm2/m2) |
| Yoon et al., 2015 [38] Japan | 5-fluorouracil (1000 mg/m2), cisplatin (60 mg/m2), plus radiotherapy (40 to 50 Gy) (neoadjuvant) | 248 patients with EC (63.5 ± 7.6 yo, M: 100%) 2005 to 2017 (CT at L3 level) | ↓ BMI (0.8 kg), SMI (4.6 cm2/m2) |
| Reisinger et al., 2015 [39] Netherlands | Cisplatin/5-fluorouracil plus radiotherapy, paclitaxel/carboplatin radiotherapy, or epirubicin/cisplatin/capecitabine radiotherapy (neoadjuvant) | 96 patients with EC (63 ± 9.2 yo, M: 83.3%) January 2008 to January 2012 (CT at L3 level) | ↓ L3 index (2.5 cm2/m2) |
| Paireder et al., 2016 [40] Austria | Taxane-based, platinum-based combination of taxane/platinum, other/unknown, or chemoradiotherapy (neoadjuvant) | 130 patients with EC (61.4 (30.8–81.0) yo, M: 81.5%) 2006 to 2013 (CT at L3 level) | ↓ Male SMI (2.3 cm2/m2) ↓ Female SMI (2.8 cm2/m2) not significative |
| Rollins et al., 2016 [41] UK | Gemcitabine-based chemotherapy (palliative) | 98 patients with pancreatic cancer or distal cholangiocarcinoma (64.8 ± 8.7 yo, M: 56.1%) 2006 to 2013 (CT at L3 level) | ↓ SMA (7.4 cm2), SMI (2.6 cm2/m2), SMD (1.0 HU) |
| Heus et al., 2016 [42] Netherlands | Capecitabine 1500 mg + 28 × 1.8 Gy (total of 50.4 Gy) (neoadjuvant chemoradiation; 5 weeks) | 74 patients with locally advanced RC (64.0 ± 10 yo, M: 53%) 2006 to 2013 (CT at L3 level) | ↑ SMA (2.2 cm2) |
| Liu et al., 2016 [43] Japan | 5-fluorouracil 800 mg/m2 and cisplatin or nedaplatin 80 mg/m2 +40 Gy × 20 (neoadjuvant chemoradiation) | 84 patients EC (63 (40–74) yo, M: 85.7%) September 2008 to January 2015 (CT at L3 level) | ↓ PMI (0.14 cm2/m2 in males and 0.32 cm2/m2 in females) |
| Mayanagi et al., 2017 [44] Japan | Platinum plus fluorouracil-based (neoadjuvant) | 66 patients with thoracic EC (63.3 ± 8.0 yo; M: 86%) May 2004 to December 2013 (CT at L3 level) | ↑ SkM (0.3 ± 3.0 cm2/m2) |
| Kakinuma et al., 2017 [45] Japan | Carboplatin + emetrexed + bevacizumab or cisplatin+ emetrexed + bevacizumab or carboplatin + gemcitabine or cisplatin + gemcitabine or carboplatin + paclitaxel or carboplatin + nab- paclitaxel or cisplatin + docetaxel or emetrexed | 65 patients with NSCLC (67.2 ± 7.7 yo; M: 70.5%) January 2012 to December 2014 (CT at L3 level) | ↓ SMI (4.4 cm2/m2), ΔSMA (11.5 cm2) |
| Levolger et al., 2017 [46] Netherlands | Capecitabine (825 mg/m2) and radiotherapy (50 Gy) (neoadjuvant) | 122 patients with RC (61.0 (53.0–66.3) yo, M: 58.2%) August 2004 to December 2012 (CT at L3 level) | = SMI |
| Miyata et al., 2017 [47] Japan | 5-fluorouracil (700 mg/m2), cisplatin (70 mg/m2), and adriamycin (70 mg/m2) or 5-fluorouracil (700 mg/m2), cisplatin (70 mg/m2), and docetaxel (70 mg/m2) (neoadjuvant) | 94 patients with EC (64.2 ± 8.8 yo, M: 80.9%) January 2013 to August 2016 (BIA) | ↓ SMM (0.1 kg) not significative ↑ Sarcopenia (47% to 53%) |
| Guinan et al., 2017 [48] Ireland | Etoposide, cisplatin, 5-fluorouracil or capecitabine or chemoradiotherapy (cisplatin/5-fluorouracil, 40 Gy or carboplatin/paclitaxel, 41.4 Gy) (neoadjuvant) | 28 patients with EC (62.8 ± 8.2 yo, M: 82%) January 2014 to October 2016 (CT at L3 level) | ↓ SMI (5.6 cm2/m2), overall HGS (4.3kg) |
| Naito et al., 2017 [49] Japan | Single-agent chemotherapy (docetaxel (60 mg/m2) or vinorelbine (25 mg/m2)) or platinum-based chemotherapy (carboplatin + paclitaxel (200 mg/m2) or cisplatin (75 mg/m2) + pemetrexed (500 mg/m2) or cisplatin (80 mg/m2) + gemcitabine (1000 mg/m2) or cisplatin (80 mg/m2) + vinorelbine (25 mg/)) or gefitinib (250 mg) | 30 patients with NSCLC (74 (70–82) yo, M: 63.3%) January 2013 to January 2014 (CT at L3 level) | ↓ L3 index (1.8 cm2/m2), HGS (0.7 kg) |
| Cho et al., 2017 [50] Korea | Gemcitabine/platinum or 5-fluorouracil/platinum chemotherapy (palliative) | 524 patients with biliary tract cancer (61 ± 9.4 yo, M: 65,6%) 2003 to 2013 (CT at L3 level) | ↓ SMI (5.35 cm2/m2), male SMI (5.72 cm2/m2), female SMI (4.61 cm2/m2) |
| Motoori et al., 2018 [51] Japan | Cisplatin (70 mg/m2), docetaxel (70 mg/m2), and 5-fluorouracil (700 mg/m2) (neoadjuvant) | 83 patients with EC (65 (45–81) yo, M: 79.5%) January 2013 to December 2015 (BIA) | 15 patients (18%) lost more than 5% of SkM |
| Lee et al., 2018 [52] Taiwan | Cisplatin (40 mg/m2) plus radiotherapy (neoadjuvant) | 245 patients with cervical cancer (63.0 ± 12.7 yo, M: 0%) March 2004 to December 2015 (CT at L3 level) | ↓ SMD (1.2 HU) ↓ SMI (0.3 cm2/m2) not significative |
| Panje et al., 2018 [53] Taiwan | Induction chemotherapy (docetaxel (75 mg/m2), cisplatin (75 mg/m2)) and radiochemotherapy (45 Gy; docetaxel (20 mg/m2) and cisplatin (25 mg/m2) with or without cetuximab (250 mg/m2)) (neoadjuvant) | 300 patients with EC (61 (36–75) yo, M: 87.7%) May 2010 to December 2013 (CT at L3 level) | ↓ L3 index (15.7 cm2/m2), SMA (14.6 cm2) |
| Järvinen et al., 2018 [54] Finland | Epirubicin–oxaliplatin–capecitabine or platin- and 5-fluorouracil-based therapy plus 45 Gy total dose of radiotherapy (neoadjuvant) | 115 patients with EC (63 ± 9 yo, M: 74.8%) 2010 to 2014 (CT at L3 level) | 50% of patients had severe SkM loss |
| Sandini et al., 2018 [55] Boston | FOLFIRINOX PAXG PEXG (neoadjuvant) | 193 patients with borderline resectable and locally advanced PC (64 ± 11 yo, M: 50.3%) January 2013 to December 2015 (CT at L3 level) | ↑ Lean mass, muscle gain (6.8 cm2) [FOLFIRINOX] SkM↓ 2.5 cm2 (no resection) ↑ 7.2 cm2 (resection) |
| Kays et al., 2018 [56] USA | FOLFIRINOX | 53 patients with locally advanced and metastatic PDAC (59.5 ± 9.9 yo, M: 62.3%) July 2010 to August 2015 (CT at L3 level) | ↓ SkM (3.5 kg), SMI (7.2%), SkM (0.7 HU) |
| Park et al., 2018 [57] Korea | Combined capecitabine and oxaliplatin (adjuvant 8 weeks) | 136 patients with GC (55.0 (20–76) yo, M: 70.6%) May 2006 to April 2009 (CT at L3 level) | SMI: 48.3 cm2/m2 surgery only SMI: 44.8 cm2/m2 adjuvant chemotherapy |
| Guigni et al., 2018 [58] Vermont | Cyclophosphamide or doxorubicin or trastuzumab (adjuvant) | 13 patients with BC (66 ± 5 yo, M: 0%) vastus lateralis | ↓ Single-muscle fiber CSA, MHC II fiber CSA, intermyofibrillar mitochondrial fractional area, average mitochondrial area, fractional content of MHC I proteins ↑ Oxidized Prx 3 = Myosin or actin protein, number of mitochondria |
| Okuno et al., 2018 [59] Houston | Oxaliplatin based, irinotecan based, bevacizumab, cetuximab/panitumumab (neoadjuvant) | 169 patients with CRLM (56.2 ± 11.7 yo, M: 57.4%) January 2009 to December 2013 (CT at L3 level) | ↓ SMI (0.52 cm2/m2) major mass loss (≥7%) was observed in all regiments |
| Lyon et al., 2019 [60] Rochester | Gemcitabine, cisplatin (neoadjuvant) | 183 patients with MIBC (65 (57–72) yo, M: 85%) 2000 to 2016 (CT at L3 level) | ↓ SMA (3.6 cm2) ↓ SMI (1.1 cm2) |
| Methotrexate, vinblastine, adriamycin , and cisplatin or cisplatin, methotrexate, and vinblastine (neoadjuvant) | ↓ SMA (7.0 cm2) ↓ SMI (2.3 cm2) | ||
| Other cisplatin-based (neoadjuvant) | ↓ SMA (3.9 cm2) ↓ SMI (1.2 cm2) | ||
| Degens et al., 2019 [61] Netherlands | Paclitaxel, carboplatin, bevacizumab, and nitroglycerin | 111 patients with NSCLC (61 (39–79) yo; M: 54%) (CT at L3 level) | ↓ SkM CSA (1.2 cm2/m2) ↓SkM CSA (2.7% cm2/m2) |
| Griffin et al., 2019 [62] Ireland | FOLFIRINOX; gemcitabine and nab-paclitaxel; gemcitabine; gemcitabine and oxaliplatin; gemcitabine and cisplatin/carboplatin; 5-fluorouracil (neoadjuvant) | 78 patients with PC (64.2 ± 7.9 yo, M: 47%) 2012 to 2015 (CT at L3 level) | ↓ SkM (8.4 cm2), LSMI (3.3 cm2/m2), MA (0.2 HU), estimated SMM (1.47 kg) |
| Matsuura et al., 2019 [63] Japan | S-1 (prodrug of 5-fluorouracil) 80 mg/m2 + cisplatin 60 mg/m2 or + cisplatin 60 mg/m2 + docetaxel 40 mg/m2 or + oxaliplatin 100 mg/m2 (neoadjuvant) | 41 patients with advanced GC (72 (48–82) yo, M: 68.3%) January 2013 to December 2016 (CT at psoas muscle level) | ↓ PMI (5.93%) |
| Rier et al., 2019 [64] Netherlands | FAC (5-fuourouracil 500 mg/m2, doxorubicin 50 mg/m2 and cyclophosphamide 500 mg/m2) or paclitaxel 80 mg/m2 (palliative) | 98 patients with metastatic BC (FAC: 57.0 (49.5–67.0) yo, M: 0%) (Paclitaxel: 56.0 (48.0–62.5) yo, M: 0%) January 2000 to March 2016 (CT at L3 level) | FAC: ↓ MA (0.7 HU), LSMI (0.5 cm2/m2) Paclitaxel: ↓ MA (0.7 HU), ↑ LSMI (0.3 cm2/m2) |
| Fukuoka et al., 2019 [65] Japan | Capecitabine + oxaliplatin (CapeOx), capecitabine + oxaliplatin + cetuximab (CapeOx + cetuximab), modified FOLFOX6 or chemoradiotherapy (1.8 or 2.0 Gy and capecitabine (825–900 mg/m2) (neoadjuvant) | 47 patients with RC (66 (27–88) yo, M: 74.5%) January 2010 to December 2016 (CT at navel level) | ↓ PMI (12.4 cm2/m2; 4.3%) |
| Nardi et al., 2019 [66] Italy | Oxaliplatin 100 mg/m2 plus 5-fluorouracil 200 mg/m2 plus a total of 41.4 Gy chemoradiotherapy (neoadjuvant) | 52 patients with RC (63 (32–79) yo, M: 65%) January 2010 to March 2014 (CT at L3 level) | 36.5% had SkM loss >2% 30.7% had SkM loss >5% ↓ SMA (0.48 cm2) not significative |
| Ozawa et al., 2019 [67] Japan | CDDP (80 mg/m2) and 5-fluorouracil (800 mg/m2/day) or chemoradiotherapy (CDDP (70 mg/m2), 5-fluorouracil (700 mg/m2/day and 30 Gy long-T) (neoadjuvant) | 82 patients with EC (63.5 ± 7.5 yo, M: 86.6%) January 2008 to December 2013 (CT at L3 level) | ↓ PMI (0.2 cm2/m2) |
| Ishida et al., 2019 [68] Japan | Docetaxel (70 mg/m2), cisplatin (70 mg/m2), and 5-fluorouracil (700 mg/m2) or adriamycin (35 mg/m2), cisplatin, (70 mg/m2), and 5-fluorouracil (700 mg/m2) (neoadjuvant) | 165 patients with EC (Low PMI: 68.3 ± 6.4 yo, M:93%) (High PMI: 65.1 ± 9.9 yo, M: 85.3%) January 2010 to December 2013 (CT at L3 level) | ↓ PMI (0.2 cm2/m2) |
| Huang et al., 2019 [69] China | Radical radiotherapy ± target therapy or concurrent chemoradiotherapy ± target therapy/adjuvant chemotherapy or induction chemotherapy + concurrent chemoradiotherapy ± target therapy/adjuvant chemotherapy or induction chemotherapy + radical radiotherapy | 394 patients with nasopharyngeal cancer (46 (18–79) yo, M: 75.6%) January 2015 to December 2017 (CT at L3 level) | ↓ SMA (13.1 cm2), SMI (4.7 cm2/m2) |
| Li et al., 2019 [70] China | 5-fluorouracil (400 mg/m2), leucovorin (200 mg/m2), and radiotherapy (45 Gy to 50.5 Gy) (adjuvant) | 153 patients with GC (52.1 (26–89) yo, M: 66.0%) January 2008 to December 2016 (CT at L3 level) | ↓ SMI (1.6 cm2/m2) |
| Lee et al., 2019 [71] Taiwan | Paclitaxel 175 mg/m2 and carboplatin AUC5 and radiotherapy (neoadjuvant) | 131 patients with endometrial cancer (54.3 ± 9.6 yo, M: 0%) 2008 to December 2016 (CT at L3 level) | ↓ SMI (0.1 cm2/m2), SMD (1.0 HU), SMG (SkM gauge) (37.2) not significative |
| Yassaie et al., 2019 [72] New Zealand | Epirubcin/cisplatin/capecitabine chemotherapy (MAGIC protocol) or carboplatin/paclitaxel or chemoradiotherapy (CROSS) protocol (neoadjuvant) | 53 patients with EC (loss of TPA ≤ 4%: 62.6 ± 6.7 yo, M: 85%) (loss of TPA >4%: 65.8 ± 8.0 yo, M: 97%) August 2008 to February 2018 (CT at L4 level) | ↓ Total psoas area (TPA) (7.3%) |
| Kawakita et al., 2020 [73] Japan | Chemoradiotherapy (40–41.4 Gy and 5-fluorouracil (800 mg/m2/day) plus cisplatin or nedaplatin (80 mg/m2/day)) (neoadjuvant) | 113 patients with EC (loss of PMI ≥ 20%: 65 (56–68) yo, M: 85.2%) (loss of PMI < 20%: 64 (59–68) yo, M: 85.9%) April 2009 to March 2017 (CT at L3 level) | ↓ PMI (5.3%) |
| den Boer et al., 2020 [74] UK | Cisplatin–capecitabine, epirubicin–cisplatin–capecitabine, or other (neoadjuvant) | 199 patients with gastro-oesophageal cancer (66.1 (28.4–80.0) yo, M: 79.4%) March 2016 to June 2019 (CT at L3 level) | ↓ SMA (7.81 cm2), SMI (2.68 cm2/m2) |
| Grün et al., 2020 [75] Japan | FLOT protocol or CROSS protocol (neoadjuvant) | 52 patients with EC (67.4 ± 12.0 yo, M: 86.5%) January 2018 to July 2019 (CT at L3 level) | ↓ SMI (5.6 cm2/m2) |
| Ishibashi et al., 2020 [76] Japan | Cisplatin (80 mg/m2) and 5-fluorouracil (800 mg/m2) (neoadjuvant) | 85 patients with EC (68.6 ± 0.9 yo, M: 89.0%) January 2009 to December 2014 (CT at L3 level) | ↓ PMI (0.26 cm2/m2) |
| Kamitani et al., 2019 [77] Japan | DCF (docetaxel 70 mg/m2, cisplatin 70 mg/m2, 5-fluorouracil 700 mg/m2); divided DCF (docetaxel 35 mg/m2, cisplatin 6 mg/m2, 5-fluorouracil 350 mg/m2); FP (cisplatin 5 mg/m2, 5-fluorouracil 250 mg/m2) (neoadjuvant) | 119 patients with EC (42 patients ≥ 66 yo and 48 patients (<66 yo, M: 86%) February 2007 to August 2018 (CT at L3 level) | ↓ SMI (68.9%) |
| Fujihata et al., 2020 [78] Japan | FP therapy (5-fluorouracil and cisplatin) DCF therapy (docetaxel, cisplatin and 5-fluorouracil) (neoadjuvant) | 99 patients with EC (68.0 (61.0–71.5) yo, M: 90%) August 2008 to June 2019 (CT at L3 level) | ↓ SMI (1.87% cm2/m2), psoas major (0.4%), side abdominal muscles (3.0%), rectus abdominis (2.7%) |
| Dolly et al., 2020 [79] France | Bevacizumab 5 mg/kg body in 5-fluorouracil based regimens 7.5 mg/kg in capecitabine-based regimens | 72 patients with metastatic CRC (64.2 ± 10.5 yo, M: 62.5%) January 2007 to December 2012 (CT at L3 level) | ↓ SMM (8.1%) |
| Yoshino et al., 2020 [80] Japan | Paclitaxel and carboplatin or docetaxel and carboplatin or irinotecan and carboplatin (neoadjuvant) | 75 patients with EOC (stage III/IV) (63.5 (43–81) yo, M: 0%) January 2010 to December 2017 | ↓ SMA (3.8 cm2) |
| Park et al., 2020 [81] South Korea | S-1/cisplatin or capecitabine and cisplatin, FOLFOX or XELOX (capecitabine and oxaliplatin), trastuzumab plus capecitabine and cisplatin, S-1 or capecitabine (palliative) | 194 patients with advanced GC (65 (31–87) yo, M: 72.1%) September 2010 to December 2019 (CT at L3 level) | ↓ SMI (4.5 cm2/m2; 11.3%) Male: ↓ SMI (4.3 cm2/m2; 10.1%) Female: ↓ SMI (4.5 cm2/m2; 12.8%) |
| Huang et al., 2020 [82] Taiwan | Platinum-based (adjuvant) | 139 patients with EOC (stage III) (54.4 ± 10.3 yo, M: 0%) 2008 to 2017 (CT at L3 to the iliac crest level) | ↓ SMI (0.8 cm2/m2), SMD (0.8 HU) |
| Kita et al., 2021 [83] Japan | -Cisplatin 70 mg/m2 -Adriamycin 35 mg/m2 -5-fluorouracil 700 mg/m2 (neoadjuvant) | 87 patients with EC (stage IIA, IIB, III or IV) (EN group: 62.5 ± 8.1 yo, M: 72.3%) (PN group: 63.2 ± 7.4 yo, M: 82.5%) (CT at L3 level) | ↓ SkM (2.1 cm2/m2) |
| Nakayama et al., 2021 [84] Japan | 5-fluorouracil (800 mg/m2) and cisplatin (80 mg/m2), docetaxel (70 mg/m2), cisplatin (70 mg/m2), and 5-fluorouracil (700 mg/m2), 5-fluorouracil (800 mg/m2), cisplatin (80 mg/m2), and radiotherapy (40 Gy) (neoadjuvant) | 63 patients with EC (66.3 ± 8.0 yo, M: 90.5%) January 2008 to December 2015 (CT at L3 level) | ↑ Male PMI (0.05 cm2/m2), female PMI (0.5 cm2/m2) not significative |
| Zhang et al., 2021 [85] China | SOX, XELOX/FOLFOX, or chemoradiotherapy (neoadjuvant) | 110 patients with GC (61.5 (53–67) yo, M: 72.7%) January 2016 to December 2018 (CT at L3 level) | = Total SkM |
| Rinninella et al., 2021 [86] Italy | FLOT docetaxel at 50 mg/mq, oxaliplatin at 85 mg/mq, leucovorin at 200 mg/mq, and 5-fluorouracil 2600 mg/mq (neoadjuvant) | 26 patients with advanced GC (63.3 ± 11.2 yo, M: 69.2%) April 2019 to January 2020 (CT at L3 level) | ↓ Weight (5 kg), BMI (1.8 kg/m2), SMA (6.5 cm2), SMI (2.22 cm2/m2) |
3. Overview of Selected Studies
3.1. Effects of Chemotherapy on Skeletal-Muscle Mass and Function
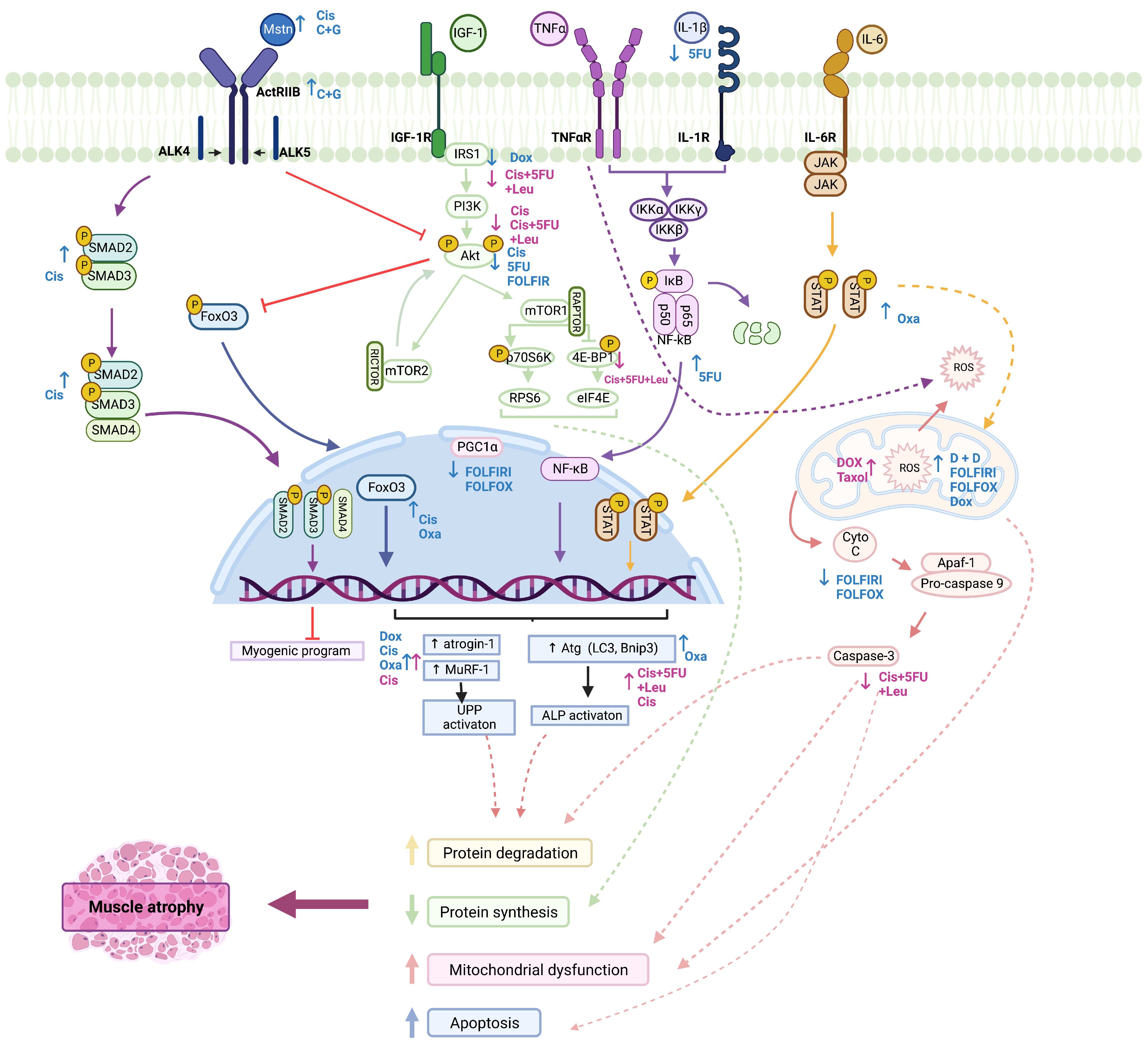
3.2. Molecular Impact of Chemotherapy on Skeletal Muscle
3.2.1. Metabolic Reprogramming
3.2.2. IGF-1/PI3K/Akt/mTOR Pathway
3.2.3. Regulation of Satellite-Cell Activation
3.2.4. Myostatin/ActRIIB Pathway
3.2.5. IL-6/JAK/STAT Pathway
3.2.6. NF-κB Pathway
3.2.7. Autophagy–Lysosome Pathway
3.2.8. Ubiquitin–Proteasome Pathway
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Chemotherapy | Sample | Main Results | Ref. |
|---|---|---|---|
| Doxorubicin 50 or 175 μM Incubated for (0–120 min) | Male CD2F1 mice, EDL muscle (ex vivo) | ↓ Maximal forces, maximal relaxation, maximal contraction velocity | [87] |
| Cisplatin 10, 50, or 100 μM Incubated for up to 72 h | C2C12 myoblasts | ↓ Size of myotubes (50%), Cav-3, myogenin, and p-Akt levels ↑ Atrogin-1, BNIP3, and LC3-II expression | [89] |
| Taxol (40 nM), doxorubicin (0.2 μM), or cisplatin (10 μM) Incubated over 3 days | C2C12 myoblasts | Taxol: ↑ Tubulin and detyrosinated tubulin expression, ROS production ↓ Myotube myosin content, mitochondrial content, Prx 3 expression | [58] |
| Doxorubicin:↑ oxidized Prx 3, ROS production ↓ Mitochondrial content, myotube diameter | |||
| Cisplatin: ↓ myotube myosin content; = ROS production | |||
| Cisplatin (20 μg/mL), 5-fluorouracil (50 μg/mL), and leucovorin (10 μg/mL) Incubated for 24 h, 48 h, or 72 h | L6 myoblasts | ↓ Peptide chain initiation in myotubes, myofibrillar, and sarcoplasmic fractions; p-AKTSer473, p-S6Ser235/236, p-S6K1Thr389, p-4EBP1Thr37/46, p-IRS1 (60%), GSSer641 (60%), IRS-1 level, caspase 3 (50%), caspase 7 (tend to), p62 abundance in myotubes (48 h), SDHA (50%-60%) and COXIV (75%) levels, and amino-acid transporter SLC38A9/SNAT1 (>50%) ↑ p-GS (50%), IRS1Ser612(70%), beclin1 (tend to), LC3BII, cleaved caspase 3 = Cleaved caspase 7 | [88] |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer: Cancer Tomorrow. Available online: https://gco.iarc.fr/ (accessed on 13 December 2021).
- Campelj, D.G.; Goodman, C.A.; Rybalka, E. Chemotherapy-Induced Myopathy: The Dark Side of the Cachexia Sphere. Cancers 2021, 13, 3615. [Google Scholar] [CrossRef] [PubMed]
- Kayl, A.E.; Meyers, C.A.; Williams, L. Side-Effects of Chemotherapy and Quality of Life in Ovarian and Breast Cancer Patients. Curr. Opin. Obstet. Gynecol. 2006, 18, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Arunachalam, S.S.; Shetty, A.P.; Panniyadi, N.; Meena, C.; Kumari, J.; Rani, B.; Das, P.; Kumari, S. Study on Knowledge of Chemotherapy’s Adverse Effects and Their Self-Care Ability to Manage—The Cancer Survivors Impact. Clin. Epidemiol. Glob. Health 2021, 11, 100765. [Google Scholar] [CrossRef]
- Jang, M.K.; Park, C.; Hong, S.; Li, H.; Rhee, E.; Doorenbos, A.Z. Skeletal Muscle Mass Change during Chemotherapy: A Systematic Review and Meta-Analysis. Anticancer Res. 2020, 40, 2409–2418. [Google Scholar] [CrossRef]
- van der Zanden, V.; van Soolingen, N.J.; Viddeleer, A.R.; Trum, J.W.; Amant, F.; Mourits, M.J.E.; Portielje, J.E.A.; Baalbergen, A.; Souwer, E.T.D.; van Munster, B.C. Loss of Skeletal Muscle Density during Neoadjuvant Chemotherapy in Older Women with Advanced Stage Ovarian Cancer Is Associated with Postoperative Complications. Eur. J. Surg. Oncol. 2021, 48, 896–902. [Google Scholar] [CrossRef]
- Berardi, E.; Madaro, L.; Lozanoska-Ochser, B.; Adamo, S.; Thorrez, L.; Bouche, M.; Coletti, D. A Pound of Flesh: What Cachexia Is and What It Is Not. Diagnostics 2021, 11, 116. [Google Scholar] [CrossRef]
- Moreira-Pais, A.; Ferreira, R.; da Costa, R.G. Platinum-Induced Muscle Wasting in Cancer Chemotherapy: Mechanisms and Potential Targets for Therapeutic Intervention. Life Sci. 2018, 208, 1–9. [Google Scholar] [CrossRef]
- Pin, F.; Couch, M.E.; Bonetto, A. Preservation of Muscle Mass as a Strategy to Reduce the Toxic Effects of Cancer Chemotherapy on Body Composition. Curr. Opin. Support. Palliat. Care 2018, 12, 420–426. [Google Scholar] [CrossRef]
- Coletti, D. Chemotherapy-Induced Muscle Wasting: An Update. Eur. J. Transl. Myol. 2018, 28, 153–157. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hoshino, Y.; Ito, T.; Nariai, T.; Mohri, T.; Obana, M.; Hayata, N.; Uozumi, Y.; Maeda, M.; Fujio, Y.; et al. Atrogin-1 Ubiquitin Ligase Is Upregulated by Doxorubicin via P38-MAP Kinase in Cardiac Myocytes. Cardiovasc. Res. 2008, 79, 89–96. [Google Scholar] [CrossRef]
- Gilliam, L.A.A.; Ferreira, L.F.; Bruton, J.D.; Moylan, J.S.; Westerblad, H.; St Clair, D.K.; Reid, M.B. Doxorubicin Acts through Tumor Necrosis Factor Receptor Subtype 1 to Cause Dysfunction of Murine Skeletal Muscle. J. Appl. Physiol. 2009, 107, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, L.A.A.; Fisher-Wellman, K.H.; Lin, C.-T.; Maples, J.M.; Cathey, B.L.; Neufer, P.D. The Anticancer Agent Doxorubicin Disrupts Mitochondrial Energy Metabolism and Redox Balance in Skeletal Muscle. Free. Radic. Biol. Med. 2013, 65, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Fabris, S.; MacLean, D.A. Doxorubicin Chemotherapy Affects Intracellular and Interstitial Nitric Oxide Concentrations in Skeletal Muscle: Effect of Doxorubicin on Intracellular and Interstitial NO in Skeletal Muscle. Cell Biol. Toxicol. 2016, 32, 121–131. [Google Scholar] [CrossRef]
- de Lima Junior, E.A.; Yamashita, A.S.; Pimentel, G.D.; de Sousa, L.G.O.; Santos, R.V.T.; Gonçalves, C.L.; Streck, E.L.; de Lira, F.S.; Neto, J.C.R. Doxorubicin Caused Severe Hyperglycaemia and Insulin Resistance, Mediated by Inhibition in AMPk Signalling in Skeletal Muscle. J. Cachexia Sarcopenia Muscle 2016, 7, 615–625. [Google Scholar] [CrossRef]
- D’Lugos, A.C.; Fry, C.S.; Ormsby, J.C.; Sweeney, K.R.; Brightwell, C.R.; Hale, T.M.; Gonzales, R.J.; Angadi, S.S.; Carroll, C.C.; Dickinson, J.M. Chronic Doxorubicin Administration Impacts Satellite Cell and Capillary Abundance in a Muscle-Specific Manner. Physiol. Rep. 2019, 7, e14052. [Google Scholar] [CrossRef] [PubMed]
- Tarpey, M.D.; Amorese, A.J.; Balestrieri, N.P.; Fisher-Wellman, K.H.; Spangenburg, E.E. Doxorubicin Causes Lesions in the Electron Transport System of Skeletal Muscle Mitochondria That Are Associated with a Loss of Contractile Function. J. Biol. Chem. 2019, 294, 19709–19722. [Google Scholar] [CrossRef]
- Damrauer, J.S.; Stadler, M.E.; Acharyya, S.; Baldwin, A.S.; Couch, M.E.; Guttridge, D.C. Chemotherapy-Induced Muscle Wasting Association with NF-κB and Cancer Cachexia. Basic Appl. Myol. 2008, 18, 158–166. [Google Scholar] [CrossRef]
- Sakai, H.; Sagara, A.; Arakawa, K.; Sugiyama, R.; Hirosaki, A.; Takase, K.; Jo, A.; Sato, K.; Chiba, Y.; Yamazaki, M.; et al. Mechanisms of Cisplatin-Induced Muscle Atrophy. Toxicol. Appl. Pharmacol. 2014, 278, 190–199. [Google Scholar] [CrossRef]
- Essex, A.L.; Pin, F.; Huot, J.R.; Bonewald, L.F.; Plotkin, L.I.; Bonetto, A. Bisphosphonate Treatment Ameliorates Chemotherapy-Induced Bone and Muscle Abnormalities in Young Mice. Front. Endocrinol. 2019, 10, 809. [Google Scholar] [CrossRef] [PubMed]
- Feather, C.E.; Lees, J.G.; Makker, P.G.S.; Goldstein, D.; Kwok, J.B.; Moalem-Taylor, G.; Polly, P. Oxaliplatin Induces Muscle Loss and Muscle-Specific Molecular Changes in Mice. Muscle Nerve 2018, 57, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Chaillou, T.; McPeek, A.; Lanner, J.T. Docetaxel Does Not Impair Skeletal Muscle Force Production in a Murine Model of Cancer Chemotherapy. Physiol. Rep. 2017, 5, e13261. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.; Penna, F.; Oliva, F.; Luque, M.; Betancourt, A.; Marmonti, E.; López-Soriano, F.J.; Argilés, J.M.; Busquets, S. A Multifactorial Anti-Cachectic Approach for Cancer Cachexia in a Rat Model Undergoing Chemotherapy. J. Cachexia Sarcopenia Muscle 2016, 7, 48–59. [Google Scholar] [CrossRef] [PubMed]
- VanderVeen, B.N.; Sougiannis, A.T.; Velazquez, K.T.; Carson, J.A.; Fan, D.; Murphy, E.A. The Acute Effects of 5 Fluorouracil on Skeletal Muscle Resident and Infiltrating Immune Cells in Mice. Front. Physiol. 2020, 11, 593468. [Google Scholar] [CrossRef]
- Campelj, D.G.; Timpani, C.A.; Cree, T.; Petersen, A.C.; Hayes, A.; Goodman, C.A.; Rybalka, E. Metronomic 5-Fluorouracil Delivery Primes Skeletal Muscle for Myopathy but Does Not Cause Cachexia. Pharmaceuticals 2021, 14, 478. [Google Scholar] [CrossRef]
- Gouspillou, G.; Scheede-Bergdahl, C.; Spendiff, S.; Vuda, M.; Meehan, B.; Mlynarski, H.; Archer-Lahlou, E.; Sgarioto, N.; Purves-Smith, F.M.; Konokhova, Y.; et al. Anthracycline-Containing Chemotherapy Causes Long-Term Impairment of Mitochondrial Respiration and Increased Reactive Oxygen Species Release in Skeletal Muscle. Sci. Rep. 2015, 5, 8717. [Google Scholar] [CrossRef]
- Chen, M.-C.; Hsu, W.-L.; Hwang, P.-A.; Chen, Y.-L.; Chou, T.-C. Combined Administration of Fucoidan Ameliorates Tumor and Chemotherapy-Induced Skeletal Muscle Atrophy in Bladder Cancer-Bearing Mice. Oncotarget 2016, 7, 51608–51618. [Google Scholar] [CrossRef]
- Barreto, R.; Mandili, G.; Witzmann, F.A.; Novelli, F.; Zimmers, T.A.; Bonetto, A. Cancer and Chemotherapy Contribute to Muscle Loss by Activating Common Signaling Pathways. Front. Physiol. 2016, 7, 472. [Google Scholar] [CrossRef]
- Barreto, R.; Waning, D.L.; Gao, H.; Liu, Y.; Zimmers, T.A.; Bonetto, A. Chemotherapy-Related Cachexia Is Associated with Mitochondrial Depletion and the Activation of ERK1/2 and P38 MAPKs. Oncotarget 2016, 7, 43442–43460. [Google Scholar] [CrossRef]
- Pin, F.; Barreto, R.; Couch, M.E.; Bonetto, A.; O’Connell, T.M. Cachexia Induced by Cancer and Chemotherapy Yield Distinct Perturbations to Energy Metabolism. J. Cachexia Sarcopenia Muscle 2019, 10, 140–154. [Google Scholar] [CrossRef]
- Poterucha, T.; Burnette, B.; Jatoi, A. A Decline in Weight and Attrition of Muscle in Colorectal Cancer Patients Receiving Chemotherapy with Bevacizumab. Med. Oncol. 2012, 29, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.; Tan, B.H.; Cui, H.; Bhalla, A.; Fearon, K.C.H.; Parsons, S.L.; Catton, J.A.; Lobo, D.N. Marked Changes in Body Composition Following Neoadjuvant Chemotherapy for Oesophagogastric Cancer. Clin. Nutr. 2012, 31, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Watanabe, M.; Karashima, R.; Imamura, Y.; Ishimoto, T.; Baba, Y.; Iwagami, S.; Sakamoto, Y.; Miyamoto, Y.; Yoshida, N.; et al. Changes in Body Composition Secondary to Neoadjuvant Chemotherapy for Advanced Esophageal Cancer Are Related to the Occurrence of Postoperative Complications after Esophagectomy. Ann. Surg. Oncol. 2014, 21, 3675–3679. [Google Scholar] [CrossRef]
- Cooper, A.B.; Slack, R.; Fogelman, D.; Holmes, H.M.; Petzel, M.; Parker, N.; Balachandran, A.; Garg, N.; Ngo-Huang, A.; Varadhachary, G.; et al. Characterization of Anthropometric Changes That Occur during Neoadjuvant Therapy for Potentially Resectable Pancreatic Cancer. Ann. Surg. Oncol. 2015, 22, 2416–2423. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Naito, T.; Kenmotsu, H.; Taira, T.; Wakuda, K.; Oyakawa, T.; Hisamatsu, Y.; Tokito, T.; Imai, H.; Akamatsu, H.; et al. Prognostic Impact of Cancer Cachexia in Patients with Advanced Non-Small Cell Lung Cancer. Support. Care Cancer 2015, 23, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.G.; Oh, D.; Ahn, Y.C.; Noh, J.M.; Pyo, H.; Cho, W.K.; Song, Y.M.; Park, M.; Hwang, N.Y.; Sun, J.M.; et al. Prognostic Impact of Sarcopenia and Skeletal Muscle Loss during Neoadjuvant Chemoradiotherapy in Esophageal Cancer. Cancers 2020, 12, 925. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, K.W.; Bosmans, J.W.A.M.; Uittenbogaart, M.; Alsoumali, A.; Poeze, M.; Sosef, M.N.; Derikx, J.P.M. Loss of Skeletal Muscle Mass during Neoadjuvant Chemoradiotherapy Predicts Postoperative Mortality in Esophageal Cancer Surgery. Ann. Surg. Oncol. 2015, 22, 4445–4452. [Google Scholar] [CrossRef]
- Paireder, M.; Asari, R.; Kristo, I.; Rieder, E.; Tamandl, D.; Ba-Ssalamah, A.; Schoppmann, S.F. Impact of Sarcopenia on Outcome in Patients with Esophageal Resection Following Neoadjuvant Chemotherapy for Esophageal Cancer. Eur. J. Surg. Oncol. 2017, 43, 478–484. [Google Scholar] [CrossRef]
- Rollins, K.E.; Tewari, N.; Ackner, A.; Awwad, A.; Madhusudan, S.; Macdonald, I.A.; Fearon, K.C.H.; Lobo, D.N. The Impact of Sarcopenia and Myosteatosis on Outcomes of Unresectable Pancreatic Cancer or Distal Cholangiocarcinoma. Clin. Nutr. 2016, 35, 1103–1109. [Google Scholar] [CrossRef]
- Heus, C.; Cakir, H.; Lak, A.; Doodeman, H.J.; Houdijk, A.P.J. Visceral Obesity, Muscle Mass and Outcome in Rectal Cancer Surgery after Neo-Adjuvant Chemo-Radiation. Int. J. Surg. 2016, 29, 159–164. [Google Scholar] [CrossRef]
- Liu, J.; Motoyama, S.; Sato, Y.; Wakita, A.; Kawakita, Y.; Saito, H.; Minamiya, Y. Decreased Skeletal Muscle Mass after Neoadjuvant Therapy Correlates with Poor Prognosis in Patients with Esophageal Cancer. Anticancer Res. 2016, 36, 6677–6685. [Google Scholar] [CrossRef] [PubMed]
- Mayanagi, S.; Tsubosa, Y.; Omae, K.; Niihara, M.; Uchida, T.; Tsushima, T.; Yokota, T.; Sato, H.; Naito, T.; Yasui, H. Negative Impact of Skeletal Muscle Wasting after Neoadjuvant Chemotherapy Followed by Surgery on Survival for Patients with Thoracic Esophageal Cancer. Ann. Surg. Oncol. 2017, 24, 3741–3747. [Google Scholar] [CrossRef] [PubMed]
- Kakinuma, K.; Tsuruoka, H.; Morikawa, K.; Furuya, N.; Inoue, T.; Miyazawa, T.; Mineshita, M. Differences in Skeletal Muscle Loss Caused by Cytotoxic Chemotherapy and Molecular Targeted Therapy in Patients with Advanced Non-Small Cell Lung Cancer. Thorac. Cancer 2018, 9, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Levolger, S.; van Vledder, M.G.; Alberda, W.J.; Verhoef, C.; de Bruin, R.W.F.; IJzermans, J.N.M.; Burger, J.W. Muscle Wasting and Survival Following Pre-Operative Chemoradiotherapy for Locally Advanced Rectal Carcinoma. Clin. Nutr. 2018, 37, 1728–1735. [Google Scholar] [CrossRef]
- Miyata, H.; Sugimura, K.; Motoori, M.; Fujiwara, Y.; Omori, T.; Yanagimoto, Y.; Ohue, M.; Yasui, M.; Miyoshi, N.; Tomokuni, A.; et al. Clinical Assessment of Sarcopenia and Changes in Body Composition during Neoadjuvant Chemotherapy for Esophageal Cancer. Anticancer Res. 2017, 37, 3053–3059. [Google Scholar] [CrossRef]
- Guinan, E.M.; Doyle, S.L.; Bennett, A.E.; O’Neill, L.; Gannon, J.; Elliott, J.A.; O’Sullivan, J.; Reynolds, J.V.; Hussey, J. Sarcopenia during Neoadjuvant Therapy for Oesophageal Cancer: Characterising the Impact on Muscle Strength and Physical Performance. Support. Care Cancer 2018, 26, 1569–1576. [Google Scholar] [CrossRef]
- Naito, T.; Okayama, T.; Aoyama, T.; Ohashi, T.; Masuda, Y.; Kimura, M.; Shiozaki, H.; Murakami, H.; Kenmotsu, H.; Taira, T.; et al. Skeletal Muscle Depletion during Chemotherapy Has a Large Impact on Physical Function in Elderly Japanese Patients with Advanced Non-Small-Cell Lung Cancer. BMC Cancer 2017, 17, 571. [Google Scholar] [CrossRef]
- Cho, K.-M.; Park, H.; Oh, D.-Y.; Kim, T.-Y.; Lee, K.H.; Han, S.-W.; Im, S.-A.; Kim, T.-Y.; Bang, Y.-J. Skeletal Muscle Depletion Predicts Survival of Patients with Advanced Biliary Tract Cancer Undergoing Palliative Chemotherapy. Oncotarget 2017, 8, 79441–79452. [Google Scholar] [CrossRef]
- Motoori, M.; Fujitani, K.; Sugimura, K.; Miyata, H.; Nakatsuka, R.; Nishizawa, Y.; Komatsu, H.; Miyazaki, S.; Komori, T.; Kashiwazaki, M.; et al. Skeletal Muscle Loss during Neoadjuvant Chemotherapy Is an Independent Risk Factor for Postoperative Infectious Complications in Patients with Advanced Esophageal Cancer. Oncology 2018, 95, 281–287. [Google Scholar] [CrossRef]
- Lee, J.; Chang, C.L.; Lin, J.-B.; Wu, M.H.; Sun, F.J.; Jan, Y.T.; Hsu, S.M.; Chen, Y.J. Skeletal Muscle Loss Is an Imaging Biomarker of Outcome after Definitive Chemoradiotherapy for Locally Advanced Cervical Cancer. Clin. Cancer Res. 2018, 24, 5028–5036. [Google Scholar] [CrossRef] [PubMed]
- Panje, C.M.; Höng, L.; Hayoz, S.; Baracos, V.E.; Herrmann, E.; Schüler, H.G.; Meier, U.R.; Henke, G.; Schacher, S.; Hawle, H.; et al. Skeletal Muscle Mass Correlates with Increased Toxicity during Neoadjuvant Radiochemotherapy in Locally Advanced Esophageal Cancer: A SAKK 75/08 Substudy. Radiat. Oncol. 2019, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.; Ilonen, I.; Kauppi, J.; Salo, J.; Räsänen, J. Loss of Skeletal Muscle Mass during Neoadjuvant Treatments Correlates with Worse Prognosis in Esophageal Cancer: A Retrospective Cohort Study. World J. Surg. Oncol. 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Sandini, M.; Patiňo, M.; Ferrone, C.R.; Alvarez-Pérez, C.A.; Honselmann, K.C.; Paiella, S.; Catania, M.; Riva, L.; Tedesco, G.; Casolino, R.; et al. Association between Changes in Body Composition and Neoadjuvant Treatment for Pancreatic Cancer. JAMA Surg. 2018, 153, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Kays, J.K.; Shahda, S.; Stanley, M.; Bell, T.M.; O’Neill, B.H.; Kohli, M.D.; Couch, M.E.; Koniaris, L.G.; Zimmers, T.A. Three Cachexia Phenotypes and the Impact of Fat-Only Loss on Survival in FOLFIRINOX Therapy for Pancreatic Cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 673–684. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, H.S.; Beom, S.H.; Rha, S.Y.; Chung, H.C.; Kim, J.H.; Chun, Y.J.; Lee, S.W.; Choe, E.A.; Heo, S.J.; et al. Marked Loss of Muscle, Visceral Fat, or Subcutaneous Fat after Gastrectomy Predicts Poor Survival in Advanced Gastric Cancer: Single-Center Study from the CLASSIC Trial. Ann. Surg. Oncol. 2018, 25, 3222–3230. [Google Scholar] [CrossRef]
- Blas, X.; Guigni, A.; Callahan, D.M.; Tourville, T.W.; Miller, M.S.; Fiske, B.; Voigt, T.; Korwin-Mihavics, B.; Anathy, V.; Dittus, K.; et al. Skeletal Muscle Atrophy and Dysfunction in Breast Cancer Patients: Role for Chemotherapy-Derived Oxidant Stress. Am. J. Physiol. Cell Physiol. 2018, 315, 744–756. [Google Scholar] [CrossRef]
- Okuno, M.; Goumard, C.; Kopetz, S.; Vega, E.A.; Joechle, K.; Mizuno, T.; Tzeng, C.W.D.; Chun, Y.S.; Lee, J.E.; Vauthey, J.N.; et al. Loss of Muscle Mass during Preoperative Chemotherapy as a Prognosticator for Poor Survival in Patients with Colorectal Liver Metastases. Surgery 2019, 165, 329–336. [Google Scholar] [CrossRef]
- Lyon, T.D.; Frank, I.; Takahashi, N.; Boorjian, S.A.; Moynagh, M.R.; Shah, P.H.; Tarrell, R.F.; Cheville, J.C.; Viers, B.R.; Tollefson, M.K. Sarcopenia and Response to Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer. Clin. Genitourin. Cancer 2019, 17, 216–222.e5. [Google Scholar] [CrossRef]
- Degens, J.H.R.J.; Sanders, K.J.C.; de Jong, E.E.C.; Groen, H.J.M.; Smit, E.F.; Aerts, J.G.; Schols, A.M.W.J.; Dingemans, A.M.C. The Prognostic Value of Early Onset, CT Derived Loss of Muscle and Adipose Tissue during Chemotherapy in Metastatic Non-Small Cell Lung Cancer. Lung Cancer 2019, 133, 130–135. [Google Scholar] [CrossRef]
- Griffin, O.M.; Duggan, S.N.; Ryan, R.; McDermott, R.; Geoghegan, J.; Conlon, K.C. Characterising the Impact of Body Composition Change during Neoadjuvant Chemotherapy for Pancreatic Cancer. Pancreatology 2019, 19, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, N.; Motoori, M.; Fujitani, K.; Nishizawa, Y.; Komatsu, H.; Miyazaki, Y.; Miyazaki, S.; Tomokuni, A.; Komori, T.; Iwase, K. Correlation between Skeletal Muscle Mass and Adverse Events of Neoadjuvant Chemotherapy in Patients with Gastric Cancer. Oncology 2020, 98, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Rier, H.N.; Jager, A.; Sleijfer, S.; van Rosmalen, J.; Kock, M.C.J.M.; Levin, M.D. Changes in Body Composition and Muscle Attenuation during Taxane-Based Chemotherapy in Patients with Metastatic Breast Cancer. Breast Cancer Res. Treat. 2018, 168, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, T.; Nagahara, H.; Shibutani, M.; Iseki, Y.; Matsutani, S.; Hirakawa, K.; Ohira, M.; Maeda, K. Change in PMI during Neoadjuvant Therapy Is a Predictive Prognostic Marker in Rectal Cancer. Anticancer Res. 2019, 39, 5157–5163. [Google Scholar] [CrossRef] [PubMed]
- De Nardi, P.; Salandini, M.; Chiari, D.; Pecorelli, N.; Cristel, G.; Damascelli, A.; Ronzoni, M.; Massimino, L.; De Cobelli, F.; Braga, M. Changes in Body Composition during Neoadjuvant Therapy Can Affect Prognosis in Rectal Cancer Patients: An Exploratory Study. Curr. Probl. Cancer 2020, 44, 100510. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Nakano, T.; Taniyama, Y.; Sakurai, T.; Onodera, Y.; Kamiya, K.; Hikage, M.; Sato, C.; Takaya, K.; Konno, T.; et al. Evaluation of the Impact of Psoas Muscle Index, a Parameter of Sarcopenia, in Patients with Esophageal Squamous Cell Carcinoma Receiving Neoadjuvant Therapy. Esophagus 2019, 16, 345–351. [Google Scholar] [CrossRef]
- Ishida, T.; Makino, T.; Yamasaki, M.; Tanaka, K.; Miyazaki, Y.; Takahashi, T.; Kurokawa, Y.; Motoori, M.; Kimura, Y.; Nakajima, K.; et al. Impact of Measurement of Skeletal Muscle Mass on Clinical Outcomes in Patients with Esophageal Cancer Undergoing Esophagectomy after Neoadjuvant Chemotherapy. Surgery 2019, 166, 1041–1047. [Google Scholar] [CrossRef]
- Huang, X.; Ma, J.; Li, L.; Zhu, X.-D. Severe Muscle Loss during Radical Chemoradiotherapy for Non-Metastatic Nasopharyngeal Carcinoma Predicts Poor Survival. Cancer Med. 2019, 8, 6604–6613. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.-B.; Jiang, H.-G.; Dai, J.; Xia, L.; Chen, J.; Xie, C.-H.; Peng, J.; Liao, Z.-K.; Gao, Y.; et al. Predictive Value of Pancreatic Dose-Volume Metrics on Sarcopenia Rate in Gastric Cancer Patients Treated with Adjuvant Chemoradiotherapy. Clin. Nutr. 2019, 38, 1713–1720. [Google Scholar] [CrossRef]
- Lee, J.; Lin, J.-B.; Wu, M.H.; Jan, Y.T.; Chang, C.L.; Huang, C.Y.; Sun, F.J.; Chen, Y.J. Muscle Radiodensity Loss during Cancer Therapy Is Predictive for Poor Survival in Advanced Endometrial Cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 814–826. [Google Scholar] [CrossRef]
- Yassaie, S.S.; Keane, C.; French, S.J.H.; Al-Herz, F.A.J.; Young, M.K.; Gordon, A.C. Decreased Total Psoas Muscle Area after Neoadjuvant Therapy Is a Predictor of Increased Mortality in Patients Undergoing Oesophageal Cancer Resection. ANZ J. Surg. 2019, 89, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, Y.; Motoyama, S.; Sato, Y.; Wakita, A.; Nagaki, Y.; Imai, K.; Minamiya, Y. Decreases in the Psoas Muscle Index Correlate More Strongly with Survival than Other Prognostic Markers in Esophageal Cancer after Neoadjuvant Chemoradiotherapy Plus Esophagectomy. World J. Surg. 2020, 44, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- den Boer, R.B.; Jones, K.I.; Ash, S.; van Boxel, G.I.; Gillies, R.S.; O’Donnell, T.; Ruurda, J.P.; Sgromo, B.; Silva, M.A.; Maynard, N.D. Impact on Postoperative Complications of Changes in Skeletal Muscle Mass during Neoadjuvant Chemotherapy for Gastro-Oesophageal Cancer. BJS Open 2020, 4, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Grün, J.; Elfinger, L.; Le, H.; Weiß, C.; Otto, M.; Reißfelder, C.; Blank, S. The Influence of Pretherapeutic and Preoperative Sarcopenia on Short-Term Outcome after Esophagectomy. Cancers 2020, 12, 3409. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Tsujimoto, H.; Hiraki, S.; Kouzu, K.; Tsuchiya, S.; Itazaki, Y.; Yaguchi, Y.; Horiguchi, H.; Nomura, S.; Ito, N.; et al. Predictive Value of Immuno-Inflammatory and Nutritional Measures Modulated by Neoadjuvant Chemotherapy on the Response of Neoadjuvant Chemotherapy and Long-Term Outcomes in Patients with Esophageal Cancer. Oncol. Lett. 2020, 19, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Kamitani, N.; Migita, K.; Matsumoto, S.; Wakatsuki, K.; Kunishige, T.; Nakade, H.; Miyao, S.; Sho, M. Association of Skeletal Muscle Loss with the Long-Term Outcomes of Esophageal Cancer Patients Treated with Neoadjuvant Chemotherapy. Surg. Today 2019, 49, 1022–1028. [Google Scholar] [CrossRef]
- Fujihata, S.; Ogawa, R.; Nakaya, S.; Hayakawa, S.; Okubo, T.; Sagawa, H.; Tanaka, T.; Takahashi, H.; Matsuo, Y.; Takiguchi, S. The Impact of Skeletal Muscle Wasting during Neoadjuvant Chemotherapy on Postoperative Anastomotic Leakage in Patients with Esophageal Cancer. Esophagus 2021, 18, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Dolly, A.; Lecomte, T.; Bouché, O.; Borg, C.; Terrebonne, E.; Douillard, J.Y.; Chautard, R.; Raoul, W.; Ternant, D.; Leger, J.; et al. Concurrent Losses of Skeletal Muscle Mass, Adipose Tissue and Bone Mineral Density during Bevacizumab/Cytotoxic Chemotherapy Treatment for Metastatic Colorectal Cancer. Clin. Nutr. 2020, 39, 3319–3330. [Google Scholar] [CrossRef]
- Yoshino, Y.; Taguchi, A.; Nakajima, Y.; Takao, M.; Kashiyama, T.; Furusawa, A.; Kino, N.; Yasugi, T. Extreme Skeletal Muscle Loss during Induction Chemotherapy Is an Independent Predictor of Poor Survival in Advanced Epithelial Ovarian Cancer Patients. J. Obstet. Gynaecol. Res. 2020, 46, 2662–2671. [Google Scholar] [CrossRef]
- Park, S.E.; Choi, J.H.; Park, J.Y.; Kim, B.J.; Kim, J.G.; Kim, J.W.; Park, J.M.; Chi, K.C.; Hwang, I.G. Loss of Skeletal Muscle Mass during Palliative Chemotherapy Is a Poor Prognostic Factor in Patients with Advanced Gastric Cancer. Sci. Rep. 2020, 10, 17683. [Google Scholar] [CrossRef]
- Huang, C.Y.; Yang, Y.C.; Chen, T.C.; Chen, J.R.; Chen, Y.J.; Wu, M.H.; Jan, Y.T.; Chang, C.L.; Lee, J. Muscle Loss during Primary Debulking Surgery and Chemotherapy Predicts Poor Survival in Advanced-Stage Ovarian Cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Kita, R.; Miyata, H.; Sugimura, K.; Tanaka, K.; Makino, T.; Yamashita, K.; Yamasaki, M.; Motoori, M.; Shiraishi, O.; Kimura, Y.; et al. Clinical Effect of Enteral Nutrition Support during Neoadjuvant Chemotherapy on the Preservation of Skeletal Muscle Mass in Patients with Esophageal Cancer. Clin. Nutr. 2021, 40, 4380–4385. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Furuya, S.; Kawaguchi, Y.; Shoda, K.; Akaike, H.; Hosomura, N.; Amemiya, H.; Kawaida, H.; Sudoh, M.; Kono, H.; et al. Prognostic Value of Preoperative Psoas Muscle Index as a Measure of Nutritional Status in Patients with Esophageal Cancer Receiving Neoadjuvant Therapy. Nutrition 2021, 90, 111232. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Jiang, L.; Xue, Z.; Ma, Z.; Kang, W.; Ye, X.; Liu, Y.; Jin, Z.; Yu, J. Impact of Body Composition on Clinical Outcomes in People with Gastric Cancer Undergoing Radical Gastrectomy after Neoadjuvant Treatment. Nutrition 2021, 85, 111135. [Google Scholar] [CrossRef]
- Rinninella, E.; Strippoli, A.; Cintoni, M.; Raoul, P.; Vivolo, R.; Di Salvatore, M.; Genco, E.; Manfredi, R.; Bria, E.; Tortora, G.; et al. Body Composition Changes in Gastric Cancer Patients during Preoperative Flot Therapy: Preliminary Results of an Italian Cohort Study. Nutrients 2021, 13, 960. [Google Scholar] [CrossRef] [PubMed]
- van Norren, K.; van Helvoort, A.; Argilés, J.M.; van Tuijl, S.; Arts, K.; Gorselink, M.; Laviano, A.; Kegler, D.; Haagsman, H.P.; van der Beek, E.M. Direct Effects of Doxorubicin on Skeletal Muscle Contribute to Fatigue. Br. J. Cancer 2009, 100, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Adegoke, O.A.J. The Effect of a Chemotherapy Drug Cocktail on Myotube Morphology, Myofibrillar Protein Abundance, and Substrate Availability. Physiol. Rep. 2021, 9, e14927. [Google Scholar] [CrossRef]
- Fanzani, A.; Zanola, A.; Rovetta, F.; Rossi, S.; Aleo, M.F. Cisplatin Triggers Atrophy of Skeletal C2C12 Myotubes via Impairment of Akt Signalling Pathway and Subsequent Increment Activity of Proteasome and Autophagy Systems. Toxicol. Appl. Pharmacol. 2011, 250, 312–321. [Google Scholar] [CrossRef]
- Hiensch, A.E.; Bolam, K.A.; Mijwel, S.; Jeneson, J.A.L.; Huitema, A.D.R.; Kranenburg, O.; van der Wall, E.; Rundqvist, H.; Wengstrom, Y.; May, A.M. Doxorubicin-Induced Skeletal Muscle Atrophy: Elucidating the Underlying Molecular Pathways. Acta Physiol. 2020, 229, e13400. [Google Scholar] [CrossRef]
- Penna, F.; Ballarò, R.; Beltrà, M.; de Lucia, S.; Castillo, L.G.; Costelli, P. The Skeletal Muscle as an Active Player against Cancer Cachexia. Front. Physiol. 2019, 10, 41. [Google Scholar] [CrossRef]
- Sera, T. Computed Tomography. In Transparency in Biology; Springer: Singapore, 2021; pp. 167–187. [Google Scholar]
- Kim, E.Y.; Jun, K.H.; Kim, S.Y.; Chin, H.M. Body Mass Index and Skeletal Muscle Index Are Useful Prognostic Factors for Overall Survival after Gastrectomy for Gastric Cancer: Retrospective Cohort Study. Medicine 2020, 99, e23363. [Google Scholar] [CrossRef]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle Type and Fiber Type Specificity in Muscle Wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Bresciani, E.; Rizzi, L.; Cappellari, O.; de Luca, A.; Torsello, A.; Liantonio, A. Cisplatin-Induced Skeletal Muscle Dysfunction: Mechanisms and Counteracting Therapeutic Strategies. Int. J. Mol. Sci. 2020, 21, 1242. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, L.A.A.; St. Clair, D.K. Chemotherapy-Induced Weakness and Fatigue in Skeletal Muscle: The Role of Oxidative Stress. Antioxid. Redox Signal. 2011, 15, 2543–2563. [Google Scholar] [CrossRef]
- Moresi, V.; Adamo, S.; Berghella, L. The JAK/STAT Pathway in Skeletal Muscle Pathophysiology. Front. Physiol. 2019, 10, 500. [Google Scholar] [CrossRef]
- Gorini, S.; de Angelis, A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxid. Med. Cell. Longev. 2018, 2018, 7582730. [Google Scholar] [CrossRef]
- Onishi, Y.; Ueha, T.; Kawamoto, T.; Hara, H.; Toda, M.; Harada, R.; Minoda, M.; Kurosaka, M.; Akisue, T. Regulation of Mitochondrial Proliferation by PGC-1α Induces Cellular Apoptosis in Musculoskeletal Malignancies. Sci. Rep. 2014, 4, 3916. [Google Scholar] [CrossRef]
- Vaidya, V.S.; Ferguson, M.A.; Bonventre, J.V. Biomarkers of Acute Kidney Injury. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 463–493. [Google Scholar] [CrossRef] [PubMed]
- O’neill, L.M.; Guo, C.A.; Ding, F.; Phang, Y.X.; Liu, Z.; Shamsuzzaman, S.; Ntambi, J.M. Stearoyl-CoA Desaturase-2 in Murine Development, Metabolism, and Disease. Int. J. Mol. Sci. 2020, 21, 8619. [Google Scholar] [CrossRef]
- Martin, A.; Freyssenet, D. Phenotypic Features of Cancer Cachexia-Related Loss of Skeletal Muscle Mass and Function: Lessons from Human and Animal Studies. J. Cachexia Sarcopenia Muscle 2021, 12, 252–273. [Google Scholar] [CrossRef]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M. Branched-Chain Amino Acid Oxidation in Skeletal Muscle—Physiological and Clinical Importance of Its Modulation by Reactant Availability. Curr. Nutr. Food Sci. 2011, 7, 50–56. [Google Scholar] [CrossRef]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-Chain Amino Acids: Catabolism in Skeletal Muscle and Implications for Muscle and Whole-Body Metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef]
- Latres, E.; Amini, A.R.; Amini, A.A.; Griffiths, J.; Martin, F.J.; Wei, Y.; Hsin, C.L.; Yancopoulos, G.D.; Glass, D.J. Insulin-like Growth Factor-1 (IGF-1) Inversely Regulates Atrophy-Induced Genes via the Phosphatidylinositol 3-Kinase/Akt/Mammalian Target of Rapamycin (PI3K/Akt/MTOR) Pathway. J. Biol. Chem. 2005, 280, 2737–2744. [Google Scholar] [CrossRef]
- Sakai, H.; Asami, M.; Naito, H.; Kitora, S.; Suzuki, Y.; Miyauchi, Y.; Tachinooka, R.; Yoshida, S.; Kon, R.; Ikarashi, N.; et al. Exogenous Insulin-like Growth Factor 1 Attenuates Cisplatin-Induced Muscle Atrophy in Mice. J. Cachexia Sarcopenia Muscle 2021, 12, 1570–1581. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Kaczmarek, M.; Ciałowicz, M.; Clemente, F.M.; Wolański, P.; Badicu, G.; Murawska-Ciałowicz, E. The Role of Satellite Cells in Skeletal Muscle Regeneration—The Effect of Exercise and Age. Biology 2021, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [CrossRef]
- Asfour, H.A.; Shaqoura, E.I.; Said, R.S.; Mustafa, A.G.; Emerald, B.S.; Allouh, M.Z. Differential Response of Oxidative and Glycolytic Skeletal Muscle Fibers to Mesterolone. Sci. Rep. 2021, 11, 12301. [Google Scholar] [CrossRef]
- Mallard, J.; Hucteau, E.; Hureau, T.J.; Pagano, A.F. Skeletal Muscle Deconditioning in Breast Cancer Patients Undergoing Chemotherapy: Current Knowledge and Insights from Other Cancers. Front. Cell Dev. Biol. 2021, 9, 719643. [Google Scholar] [CrossRef]
- Motohashi, N.; Asakura, A. Muscle Satellite Cell Heterogeneity and Self-Renewal. Front. Cell Dev. Biol. 2014, 2, 1. [Google Scholar] [CrossRef]
- von Maltzahn, J.; Jones, A.E.; Parks, R.J.; Rudnicki, M.A. Pax7 Is Critical for the Normal Function of Satellite Cells in Adult Skeletal Muscle. Proc. Natl. Acad. Sci. USA 2013, 110, 16474–16479. [Google Scholar] [CrossRef] [PubMed]
- Doles, J.D.; Olwin, B.B. The Impact of JAK-STAT Signaling on Muscle Regeneration. Nat. Med. 2014, 20, 1094–1095. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.R. Insulin-like Growth Factor in Muscle Growth and Its Potential Abuse by Athletes. West. J. Med. 2001, 175, 7–9. [Google Scholar] [CrossRef]
- Baczek, J.; Silkiewicz, M.; Wojszel, Z.B. Myostatin as a Biomarker of Muscle Wasting and Other Pathologies-State of the Art and Knowledge Gaps. Nutrients 2020, 12, 2401. [Google Scholar] [CrossRef]
- Han, H.Q.; Mitch, W.E. Targeting the Myostatin Signaling Pathway to Treat Muscle Wasting Diseases. Curr. Opin. Support. Palliat. Care 2011, 5, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The Nuclear Factor NF-κB Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Xia, Q.; Huang, X.; Huang, J.; Zheng, Y.; March, M.E.; Li, J.; Wei, Y. The Role of Autophagy in Skeletal Muscle Diseases. Front. Physiol. 2021, 12, 638983. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Yuan, M.; Fan, H.; Cai, Z. Role of AMPK in Autophagy. Front. Physiol. 2022, 13, 1015500. [Google Scholar] [CrossRef]
- Kitajima, Y.; Yoshioka, K.; Suzuki, N. The Ubiquitin-Proteasome System in Regulation of the Skeletal Muscle Homeostasis and Atrophy: From Basic Science to Disorders. J. Physiol. Sci. 2020, 70, 40. [Google Scholar] [CrossRef]
- Allen, D.D.; Caviedes, R.; Cárdenas, A.M.; Shimahara, T.; Segura-Aguilar, J.; Caviedes, P.A. Cell Lines as in Vitro Models for Drug Screening and Toxicity Studies. Drug Dev. Ind. Pharm. 2005, 31, 757–768. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrosa, M.B.; Barbosa, S.; Vitorino, R.; Ferreira, R.; Moreira-Gonçalves, D.; Santos, L.L. Chemotherapy-Induced Molecular Changes in Skeletal Muscle. Biomedicines 2023, 11, 905. https://doi.org/10.3390/biomedicines11030905
Pedrosa MB, Barbosa S, Vitorino R, Ferreira R, Moreira-Gonçalves D, Santos LL. Chemotherapy-Induced Molecular Changes in Skeletal Muscle. Biomedicines. 2023; 11(3):905. https://doi.org/10.3390/biomedicines11030905
Chicago/Turabian StylePedrosa, Mafalda Barbosa, Samuel Barbosa, Rui Vitorino, Rita Ferreira, Daniel Moreira-Gonçalves, and Lúcio Lara Santos. 2023. "Chemotherapy-Induced Molecular Changes in Skeletal Muscle" Biomedicines 11, no. 3: 905. https://doi.org/10.3390/biomedicines11030905
APA StylePedrosa, M. B., Barbosa, S., Vitorino, R., Ferreira, R., Moreira-Gonçalves, D., & Santos, L. L. (2023). Chemotherapy-Induced Molecular Changes in Skeletal Muscle. Biomedicines, 11(3), 905. https://doi.org/10.3390/biomedicines11030905









