Exploratory Evaluation of Neopterin and Chitotriosidase as Potential Circulating Biomarkers for Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
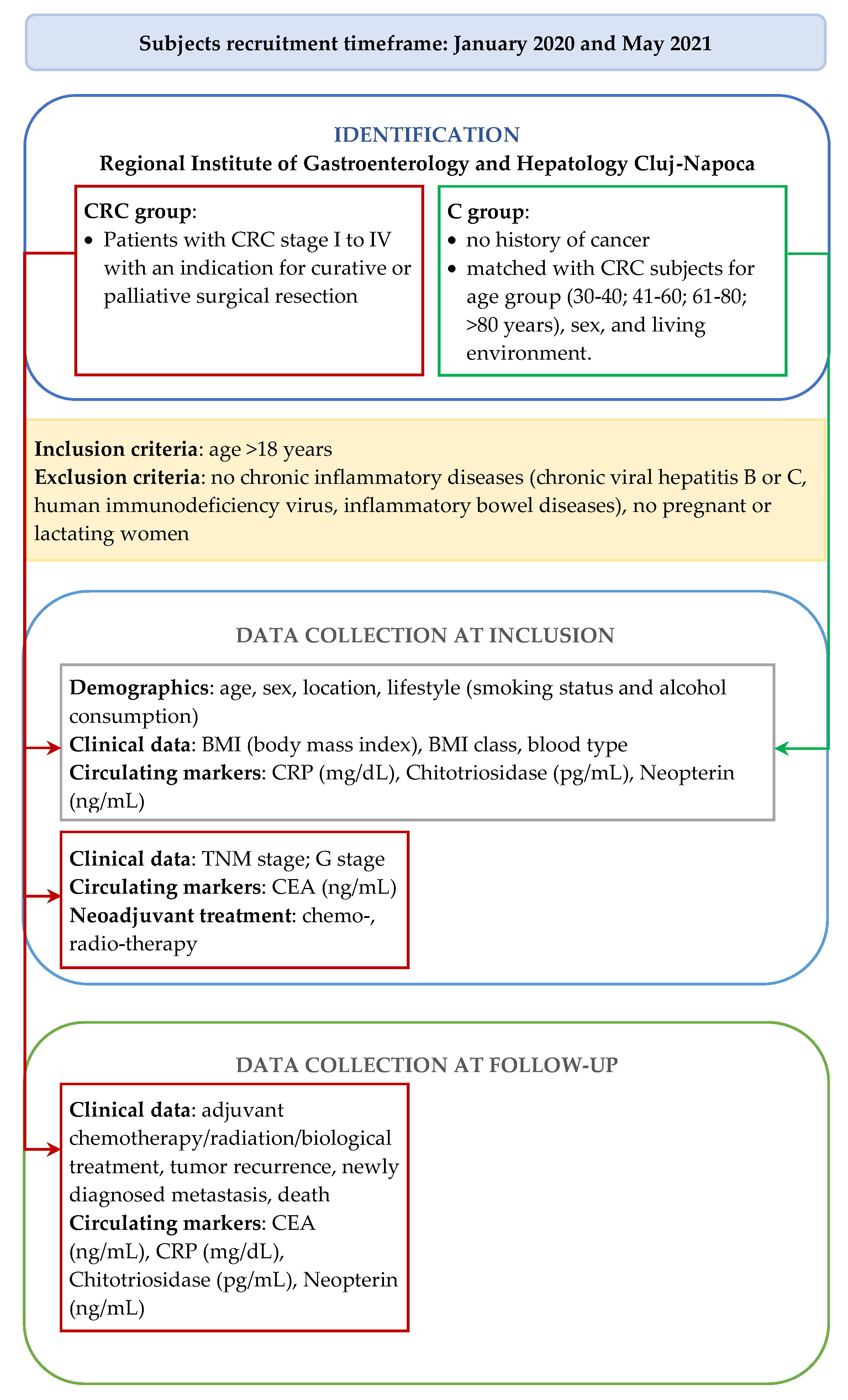
2.1. Study Design
2.2. Demographic and Clinical Characteristics
2.3. Measurement of Circulating Biomarkers
2.4. Statistical Methods
3. Results
3.1. Colorectal Cancer Group vs. Control Group
3.2. Patients with Colorectal Cancer: Pre- and Postoperative Comparison
4. Discussion
4.1. Patients with Colorectal Cancer versus Controls
4.2. Changes of Evaluate Biomarkers at 1-Year Follow-Up in Patients with Colorectal Cancer
4.3. Study Limitations and Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Cancer Information System. Colorectal Cancer Burden in EU-27 [Online]. Available online: https://ecis.jrc.ec.europa.eu/ (accessed on 27 January 2023).
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Zhang, F.; Qiao, S. Research progress on the relationship between inflammation and colorectal cancer. Ann. Gastroent. Surg. 2022, 6, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Cătană, C.S.; Pichler, M.; Giannelli, G.; Mader, R.M.; Berindan-Neagoe, I. Non-coding RNAs, the Trojan horse in two-way communication between tumor and stroma in colorectal and hepatocellular carcinoma. Oncotarget 2017, 8, 29519–29534. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Coussens, L.M. Cancer: An inflammatory link. Nature 2004, 431, 405–406. [Google Scholar] [CrossRef]
- Siveen, K.S.; Kuttan, G. Role of macrophages in tumour progression. Immunol. Lett. 2009, 123, 97–102. [Google Scholar] [CrossRef]
- Kelly, D.; Yang, L.; Pei, Z. Gut Microbiota, Fusobacteria, and Colorectal Cancer. Diseases 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Alhinai, E.A.; Walton, G.E.; Commane, D.M. The Role of the Gut Microbiota in Colorectal Cancer Causation. Int. J. Mol. Sci. 2019, 20, 5295. [Google Scholar] [CrossRef]
- Tuomisto, A.E.; Mäkinen, M.J.; Väyrynen, J.P. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. 2019, 25, 4383–4404. [Google Scholar] [CrossRef]
- Ünüvar, S.; Erge, D.; Kılıçarslan, B.; Gözükara, B.; Çatal, F.; Girgin, G.; Baydar, T. Neopterin Levels and Indoleamine 2,3-Dioxygenase Activity as Biomarkers of Immune System Activation and Childhood Allergic Diseases. Ann. Lab. Med. 2019, 39, 284–290. [Google Scholar] [CrossRef]
- Gieseg, S.P.; Baxter-Parker, G.; Lindsay, A. Neopterin, Inflammation, and Oxidative Stress: What Could We Be Missing? Antioxidants 2018, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Girgin, G.; Sabuncuoglu, S.; Ünal, A.Z.; Baydar, T. Assessment of Antioxidant and Cytotoxic Activity of Known Antioxidants Compared to Neopterin. Proceedings 2017, 1, 1071. [Google Scholar] [CrossRef]
- Kunter, I.; Najjar, M.; Subasi, Y.; Zabib, N.; Sahin, G. The Effects of Neopterin on the Viability and Motility of Different HCC Cell Lines. Proceedings 2018, 2, 1581. [Google Scholar] [CrossRef]
- Putzki, H.; Aschern, F.; Henkel, E.; Heymann, H. Neopterin: A tumor marker in colorectal carcinoma? Dis. Colon Rectum 1987, 30, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Chuang, S.C.; Boeing, H.; Zuo, H.; Tell, G.S.; Pischon, T.; Jenab, M.; Bueno-de-Mesquita, B.; Vollset, S.E.; Midttun, Ø.; et al. A prospective study of the immune system activation biomarker neopterin and colorectal cancer risk. J. Natl. Cancer Inst. 2015, 107, djv010. [Google Scholar] [CrossRef]
- Zuo, H.; Tell, G.S.; Vollset, S.E.; Ueland, P.M.; Nygård, O.; Midttun, Ø.; Meyer, K.; Ulvik, A.; Eussen, S.J. Interferon-γ-induced inflammatory markers and the risk of cancer: The Hordaland Health Study. Cancer 2014, 120, 3370–3377. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, M.; van Roomen, C.P.A.A.; Renkema, G.H.; Bussink, A.P.; Andrews, L.; Blommaart, E.F.C.; Sugar, A.; Verhoeven, A.J.; Boot, R.G.; Aerts, J.M. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int. Immunol. 2005, 17, 1505–1512. [Google Scholar] [CrossRef]
- Li, F.F.; Yan, P.; Zhao, Z.X.; Liu, Z.; Song, D.W.; Zhao, X.W.; Wang, X.S.; Wang, G.Y.; Liu, S.L. Polymorphisms in the CHIT1 gene: Associations with colorectal cancer. Oncotarget 2016, 7, 39572–39581. [Google Scholar] [CrossRef]
- Renkema, G.H.; Boot, R.G.; Muijsers, A.O.; Donker-Koopman, W.E.; Aerts, J.M. Purification and characterization of human chitotriosidase, a novel member of the chitinase family of proteins. J. Biol. Chem. 1995, 270, 2198–2202. [Google Scholar] [CrossRef]
- Thein, M.S.; Kohli, A.; Ram, R.; Ingaramo, M.C.; Jain, A.; Fedarko, N.S. Chitotriosidase, a marker of innate immunity, is elevated in patients with primary breast cancer. Cancer Biomark. 2017, 19, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Ciocan, R.A.; Drugan, C.; Gherman, C.D.; Cătană, C.S.; Ciocan, A.; Drugan, T.C.; Bolboacă, S.D. Evaluation of Chitotriosidase as a Marker of Inflammatory Status in Critical Limb Ischemia. Ann. Clin. Lab. Sci. 2017, 47, 713–719. [Google Scholar]
- Cutaș, A.; Drugan, C.; Roman, G.; Rusu, A.; Cătană, C.S.; Achimaș-Cadariu, A.; Drugan, T. Evaluation of Chitotriosidase and Neopterin as Biomarkers of Microvascular Complications in Patients with Type 1 Diabetes Mellitus. Diagnostics 2021, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; He, W.; Boer, A.M.; Wevers, R.A.; de Bruijn, A.M.; Groener, J.E.; Hollak, C.E.; Aerts, J.M.; Galjaard, H.; van Diggelen, O.P. Elevated plasma chitotriosidase activity in various lysosomal storage disorders. J. Inherit. Metab. Dis. 1995, 18, 717–722. [Google Scholar] [CrossRef]
- Țaranu, I.; Iancu, M.; Lazea, C.; Alkhzouz, C.; Răcătăianu, N.; Cătană, C.S.; Mirea, A.M.; Miclea, D.; Bolboacă, S.D.; Drugan, C. Evaluation of Circulating Chitotriosidase Activity in Children with Obesity. J. Clin. Med. 2022, 11, 3634. [Google Scholar] [CrossRef]
- Bargagli, E.; Margollicci, M.; Nikiforakis, N.; Luddi, A.; Perrone, A.; Grosso, S.; Rottoli, P. Chitotriosidase activity in the serum of patients with sarcoidosis and pulmonary tuberculosis. Respiration 2007, 74, 548–552. [Google Scholar] [CrossRef]
- Bennett, D.; Cameli, P.; Lanzarone, N.; Carobene, L.; Bianchi, N.; Fui, A.; Rizzi, L.; Bergantini, L.; Cillis, G.; d’Alessandro, M.; et al. Chitotriosidase: A biomarker of activity and severity in patients with sarcoidosis. Respir. Res. 2020, 21, 6. [Google Scholar] [CrossRef]
- Song, Z.; Chen, E.; Qian, J.; Xu, J.; Cao, G.; Zhou, W.; Wang, F.; Chen, M.; Xu, D.; Wang, X.; et al. Serum chitinase activity prognosticates metastasis of colorectal cancer. BMC Cancer 2019, 19, 629. [Google Scholar] [CrossRef] [PubMed]
- Kawada, M.; Seno, H.; Kanda, K.; Nakanishi, Y.; Akitake, R.; Komekado, H.; Kawada, K.; Sakai, Y.; Mizoguchi, E.; Chiba, T. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene 2012, 31, 3111–3123. [Google Scholar] [CrossRef]
- Hari, D.M.; Leung, A.M.; Lee, J.H.; Sim, M.S.; Vuong, B.; Chiu, C.G.; Bilchik, A.J. AJCC Cancer Staging Manual 7th edition criteria for colon cancer: Do the complex modifications improve prognostic assessment? J. Am. Coll. Surg. 2013, 217, 181–910. [Google Scholar] [CrossRef]
- Thygesen, L.C.; Grønbaek, M.; Johansen, C.; Fuchs, C.S.; Willett, W.C.; Giovannucci, E. Prospective weight change and colon cancer risk in male US health professionals. Int. J. Cancer 2008, 123, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Wolpin, B.M.; Huang, E.S.; Giovannucci, E.L.; Kraft, P.; Fuchs, C.S.; Chan, A.T. ABO blood group and risk of colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2011, 5, 1017–1020. [Google Scholar] [CrossRef]
- Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today (accessed on 28 May 2021).
- SEER*Stat Database: Incidence—SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (1973-2013 Varying)—Linked to County Attributes—Total U.S., 1969–2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch [Internet]; Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) [published November 2015]. Available online: https://seer.cancer.gov/data-software/documentation/seerstat/nov2015/ (accessed on 28 May 2022).
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Botteri, E.; Iodice, S.; Bagnardi, V.; Raimondi, S.; Lowenfels, A.B.; Maisonneuve, P. Smoking and colorectal cancer: A meta-analysis. JAMA 2008, 300, 2765–2778. [Google Scholar] [CrossRef]
- Ordóñez-Mena, J.M.; Walter, V.; Schöttker, B.; Jenab, M.; O’Doherty, M.G.; Kee, F.; Bueno-de-Mesquita, B.; Peeters, P.H.M.; Stricker, B.H.; Ruiter, R.; et al. Consortium on Health and Ageing: Network of Cohorts in Europe and the United States (CHANCES). Impact of prediagnostic smoking and smoking cessation on colorectal cancer prognosis: A meta-analysis of individual patient data from cohorts within the CHANCES consortium. Ann. Oncol. 2018, 29, 472–483. [Google Scholar] [CrossRef]
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Stryjkowska-Góra, A.; Rudzki, S. Risk Factors for the Diagnosis of Colorectal Cancer. Cancer Control 2022, 29, 10732748211056692. [Google Scholar] [CrossRef] [PubMed]
- Kahramanca, S.; Anuk, T.; Yıldırım, A.C.; Kaya, O. Blood Group Characteristics in Colorectal Cancers. Turk. J. Color. Dis. 2018, 28, 76–79. [Google Scholar] [CrossRef]
- Huang, J.Y.; Wang, R.; Gao, Y.T.; Yuan, J.M. ABO blood type and the risk of cancer—Findings from the Shanghai Cohort Study. PLoS ONE 2017, 12, e0184295. [Google Scholar] [CrossRef]
- Holm, M.; Saraswat, M.; Joenväärä, S.; Ristimäki, A.; Haglund, C.; Renkonen, R. Colorectal cancer patients with different C-reactive protein levels and 5-year survival times can be differentiated with quantitative serum proteomics. PLoS ONE 2018, 13, e0195354. [Google Scholar] [CrossRef]
- Hacisevki, A.; Baba, B.; Aslan, S.; Ozkan, Y. Neopterin: A Possible Biomarker in Gastrointestinal Cancer. J. Fac. Pharm. Ankara 2018, 42, 32–41. [Google Scholar] [CrossRef]
- Popa, S.; Mota, M.; Popa, A.; Mota, E.; Timar, R.; Serafinceanu, C.; Cheta, D.; Graur, M.; Hancu, N. Prevalence of dyslipidemia and its association with cardiometabolic factors and kidney function in the adult Romanian population: The PREDATORR study. Diabetes Metab. Syndr. 2019, 13, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.C.; Boeing, H.; Vollset, S.E.; Midttun, Ø.; Ueland, P.M.; Bueno-de-Mesquita, B.; Lajous, M.; Fagherazzi, G.; Boutron-Ruault, M.C.; Kaaks, R.; et al. Cellular immune activity biomarker neopterin is associated hyperlipidemia: Results from a large population-based study. Immun. Ageing 2016, 13, 5. [Google Scholar] [CrossRef]
- Duffy, M.J. Carcinoembryonic antigen as a marker for colorectal cancer: Is it clinically useful? Clin. Chem. 2001, 47, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Ogunwobi, O.O.; Mahmood, F.; Akingboye, A. Biomarkers in Colorectal Cancer: Current Research and Future Prospects. Int. J. Mol. Sci. 2020, 21, 5311. [Google Scholar] [CrossRef] [PubMed]


| All (n = 88) | CRC Group (n = 44) | C Group (n = 44) | p-Value | |
|---|---|---|---|---|
| Age, years a | 63 (10.6) | 63.4 (11) | 62.5 (10.3) | 0.6982 |
| Smoking, yes b | 32 (36.4) | 22 (50) | 10 (22.7) | 0.0078 |
| Alcohol, yes b | 22 (25) | 13 (29.5) | 9 (20.5) | 0.3248 |
| BMI, kg/m2 c | 25 [23.8 to 28] | 26 [22 to 29] | 25 [24 to 26.3] | 0.5259 |
| BMI class a | 0.7549 | |||
| Normal Overweight Obese | 35 (39.8) 6 (6.8) 47 (53.4) | 17 (38.6) 4 (9.1) 23 (52.3) | 18 (40.9) 2 (4.5) 24 (54.5) | |
| Blood type b | 0.0123 | |||
| 0 A B AB | 29 (33) 35 (39.8) 13 (14.8) 11 (12.5) | 14 (31.8) 24 (54.5) 3 (6.8) 3 (6.8) | 15 (34.1) 11 (25.0) 10 (22.7) 8 (18.2) |
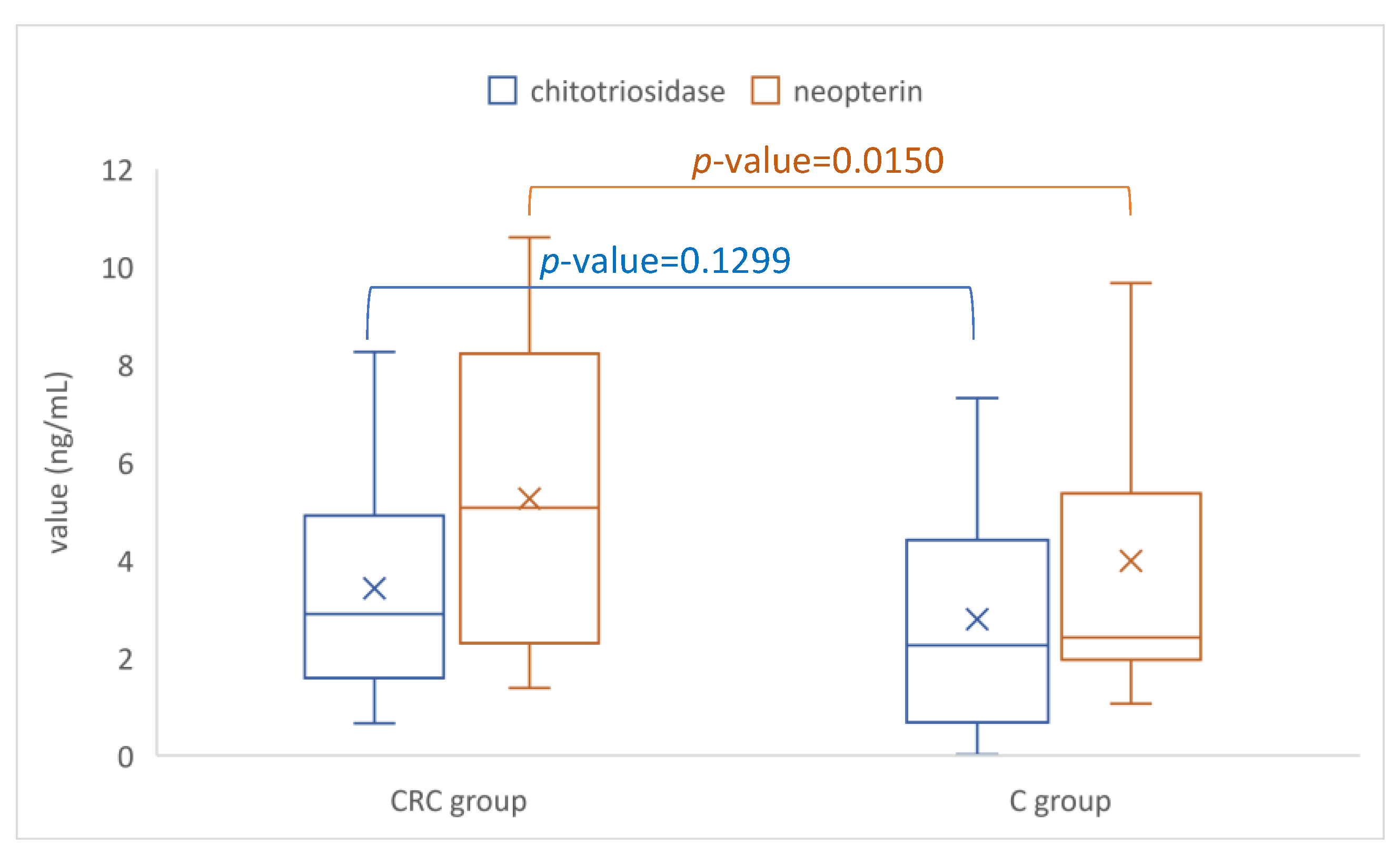
| Marker | CRC Group (n = 44) | C Group (n = 44) | p-Value |
|---|---|---|---|
| CRP, mg/dL | 1.3 [0.48 to 4.43] | 0.39 [0.2 to 0.7] | <0.0001 |
| Chitotriosidase, ng/mL | 2.9 [1.6 to 4.7] | 2.3 [0.7 to 4.3] | 0.1299 |
| Neopterin, ng/mL | 5.2 [2.3 to 8.3] | 2.4 [2 to 5.3] | 0.0150 |
| Characteristic | n (%) | Characteristic | n (%) |
|---|---|---|---|
| T stage | M stage | ||
| T1/T2 T3 T4 | 10 (22.7) 29 (65.9) 5 (11.4) | M0 M1 | 35 (79.5) 9 (20.5) |
| N stage | G grade | ||
| N0 N1 N2 | 15 (34.1) 18 (40.9) 11 (25) | G1 G2 G3 | 17 (38.6) 20 (45.5) 7 (15.9) |
| Complications, yes a | 31 (70.5) |
| Characteristic | CEA, ng/mL | CRP, mg/dL | ||||
|---|---|---|---|---|---|---|
| Baseline | 1-y Follow-Up | p-Value c | Baseline | 1-y Follow-Up | p-Value c | |
| T-stage | ||||||
| T1T2, n = 10 T3, n = 29 T4, n = 3 p-value a | 5.4 [3.7 to 10.8] 5.2 [2.7 to 6.7] 8.4 [8.2 to 8.6] 0.0147 * | 3.7 [2.2 to 4] 2.9 [1.7 to 5.6] 12 [11.7 to 35.7] 0.0553 | 0.6465 0.1059 | 1.4 [0.6 to 3.8] 1.2 [0.4 to 3.2] 2 [1.2 to 2.7] 0.5603 | 3.5 [0.9 to 5.7] 0.9 [0.4 to 2.2] 1.2 [1.1 to 2.1] 0.2122 | 0.4413 0.1494 |
| N-stage | ||||||
| N0, n = 15 N1, n = 17 N2, n = 10 p-value a | 5 [3.6 to 6.9] 5.6 [2.2 to 7.9] 5.7 [3.1 to 7.4] 0.7163 | 3.2 [2.1 to 4.1] 3.4 [1.8 to 5.8] 3.4 [1.8 to 11.6] 0.9890 | 0.2013 0.9588 0.9594 | 1.2 [0.4 to 4.7] 1 [0.5 to 2.5] 2.2 [0.7 to 12] 0.4440 | 1 [0.5 to 5.1] 0.9 [0.5 to 2.9] 0.8 [0.5 to 2.1] 0.6889 | 0.7299 0.8203 0.3329 |
| M-stage | ||||||
| M0, n = 35 M1, n = 7 p-value b | 5.6 [2.7 to 7.3] 5.2 [4 to 11.2] 0.0943 | 2.9 [1.8 to 4.1] 18 [9 to 25] 0.0098 | 0.0340 0.0630 | 1.2 [0.5 to 3.3] 1.2 [0.7 to 3.7] 0.5904 | 0.9 [0.4 to 3] 1.5 [0.8 to 4.1] 0.2877 | 0.2959 0.7532 |
| Metastasis | n.a. | n.a. | ||||
| Liver, n = 5 Others, n = 2 | 5 [3 to 8.4] 16.5 [10.8 to 22.1] | 5.8 [4.2 to 12] 16.9 [11.3 to 22.4] | 1.2 [1 to 1.4] 3 [2 to 3.9] | 1.5 [1 to 2.9] 1 [0.8 to 1.3] | ||
| G grade | ||||||
| G1, n = 17 G2, n = 18 G3, n = 7 p-value a | 5.6 [2.7 to 7] 5.5 [2.7 to 7.4] 5.2 [3.4 to 9.9] 0.7331 | 2 [1.6 to 4.2] 3.7 [2.1 to 8.6] 5.8 [2.4 to 17.1] 0.2081 | 0.0929 0.9826 0.8658 | 1.2 [0.3 to 3.4] 2 [0.6 to 3.8] 1.2 [1.1 to 2.1] 0.6443 | 0.9 [0.3 to 1] 1.7 [0.5 to 3.2] 1.5 [0.9 to 2.3] 0.3367 | 0.3088 0.5701 0.6121 |
| Complications | ||||||
| Yes, n = 28 No, n = 13 p-value b | 5.6 [3.4 to 7.1] 3.8 [2.4 to 7.4] 0.3679 | 2.9 [1.8 to 5.6] 3.9 [2.2 to 9.6] 0.4793 | 0.1648 0.9165 | 2 [0.5 to 4.9] 1 [0.5 to 1.2] 0.1728 | 0.9 [0.4 to 3] 1.6 [1 to 3] 0.0970 | 0.0912 0.1730 |
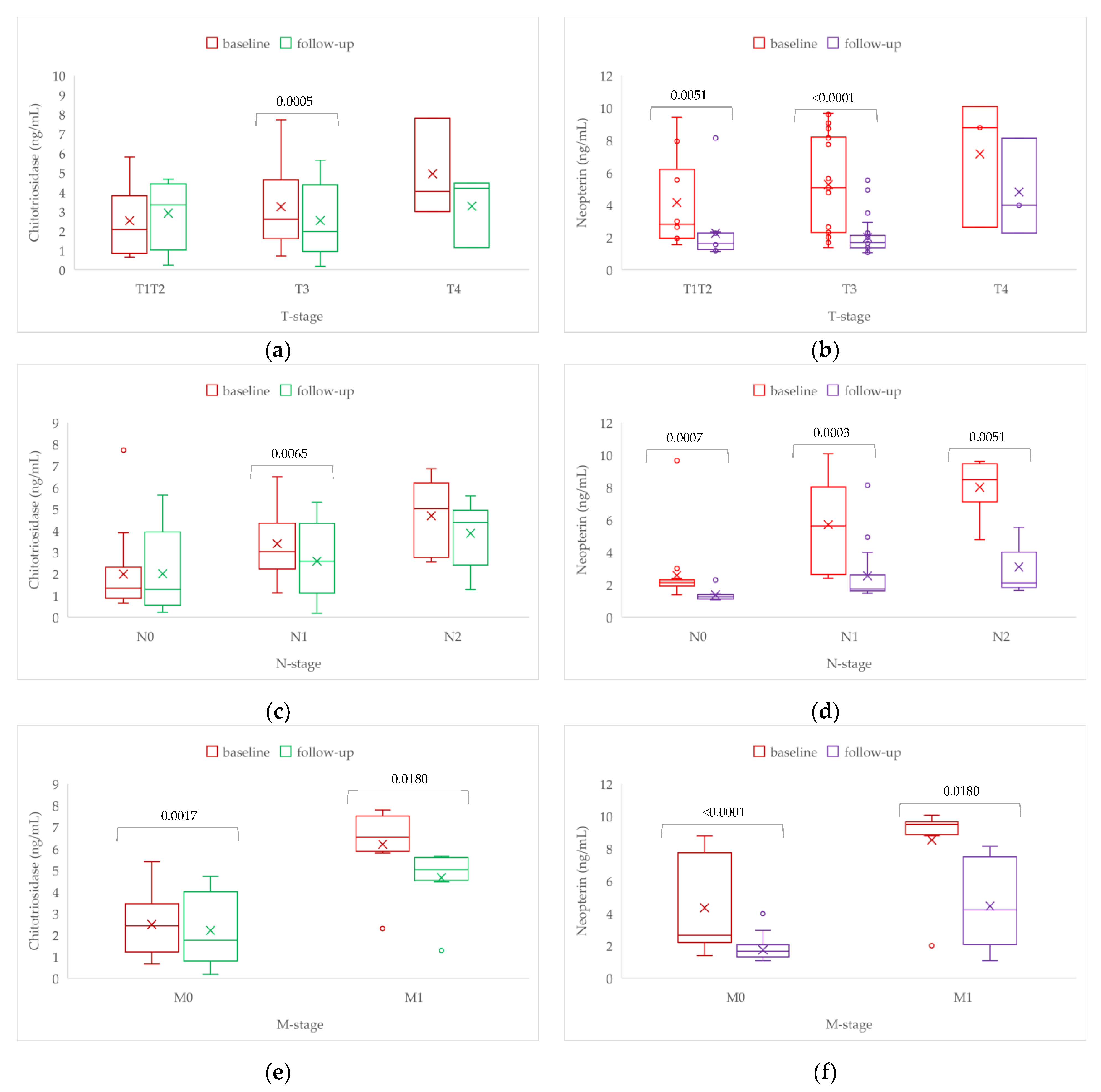
| Characteristics | Chitotriosidase (ng/mL) | Neopterin (ng/mL) | ||
|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | |
| T-stage | ||||
| T1/T2, n = 10 T3, n = 29 T4, n = 3 p-value a | 2.1 [1 to 3.4] 2.6 [1.6 to 4.6] 4 [3.5 to 5.9] 0.0209 1a | 3.3 [1.6 to 4.3] 2 [1 to 4.1] 4.2 [2.7 to 4.3] 0.8331 | 2.8 [2 to 5.6] 5.1 [2.3 to 8.2] 8.8 [5.7 to 9.4] 0.0219 1b | 1.6 [1.3 to 2.1] 1.7 [1.4 to 2.1] 4 [3.1 to 6.1] 0.0609 |
| N-stage | ||||
| N0, n = 15 N1, n = 17 N2, n = 10 p-value a | 1.3 [0.9 to 2] 3 [2.4 to 4] 5 [3 to 6] 0.0005 2a, 2d | 1.3 [0.6 to 3.3] 2.6 [1.2 to 4.2] 4.4 [3.1 to 4.7] 0.0249 2b, 2e, 2h | 2.1 [1.9 to 2.3] 5.6 [2.6 to 7.9] 8.5 [7.9 to 9.3] <0.0001 2f, 2i | 1.3 [1.2 to 1.4] 1.7 [1.6 to 2.3] 2.1 [1.9 to 3.2] 0.0001 2c,2g |
| M-stage | ||||
| M0, n = 35 M1, n = 7 p-value b | 2.4 [1.3 to 3.4] 6.6 [6.3 to 7.3] <0.0001 | 1.5 [0.9 to 4] 5.3 [4.7 to 5.6] <0.0001 | 2.6 [2.2 to 6.7] 9.6 [9.2 to 9.6] <0.0001 | 1.6 [1.3 to 2] 4.9 [2.9 to 6.8] 0.0003 |
| Metastasis | ||||
| Liver Others | 6.6 [6.1 to 7.7] 6.7 [6.6 to 6.8] | 4.7 [4.7 to 5.5] 5.5 [5.4 to 5.5] | 9.6 [9.4 to 9.7] 9.3 [9.2 to 9.4] | 5.5 [3.5 to 8.1] 3.5 [2.7 to 4.2] |
| G grade | ||||
| G1, n = 17 G2, n = 18 G3, n = 7 p-value a | 2.4 [1.2 to 3.2] 2.4 [1.4 to 4.3] 5 [4.3 to 6.3] 0.0136 3a | 1.4 [1 to 2.6] 3.3 [0.9 to 4.3] 4.7 [3.7 to 5] 0.0101 3b, 3c | 2.5 [2.1 to 7.8] 4.9 [2.4 to 7.2] 8.1 [5.1 to 8.9] 0.2628 | 1.5 [1.3 to 1.7] 1.9 [1.6 to 2.3] 2.3 [1.7 to 3.9] 0.0680 |
| Complications | ||||
| Yes, n = 29 No, n = 13 p-value b | 2.5 [1.2 to 3.8] 3.4 [2.3 to 4.6] 0.3544 | 2 [1 to 4.3] 4 [1.4 to 4.7] 0.2313 | 5.1 [2.3 to 7.9] 4.8 [2.3 to 8.2] 0.8169 | 1.7 [1.4 to 2] 2.1 [1.3 to 2.9] 0.6243 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciocan, A.; Ciocan, R.A.; Al Hajjar, N.; Benea, A.M.; Pandrea, S.L.; Cătană, C.S.; Drugan, C.; Oprea, V.C.; Dîrzu, D.S.; Bolboacă, S.D. Exploratory Evaluation of Neopterin and Chitotriosidase as Potential Circulating Biomarkers for Colorectal Cancer. Biomedicines 2023, 11, 894. https://doi.org/10.3390/biomedicines11030894
Ciocan A, Ciocan RA, Al Hajjar N, Benea AM, Pandrea SL, Cătană CS, Drugan C, Oprea VC, Dîrzu DS, Bolboacă SD. Exploratory Evaluation of Neopterin and Chitotriosidase as Potential Circulating Biomarkers for Colorectal Cancer. Biomedicines. 2023; 11(3):894. https://doi.org/10.3390/biomedicines11030894
Chicago/Turabian StyleCiocan, Andra, Răzvan A. Ciocan, Nadim Al Hajjar, Andreea M. Benea, Stanca L. Pandrea, Cristina S. Cătană, Cristina Drugan, Valentin C. Oprea, Dan S. Dîrzu, and Sorana D. Bolboacă. 2023. "Exploratory Evaluation of Neopterin and Chitotriosidase as Potential Circulating Biomarkers for Colorectal Cancer" Biomedicines 11, no. 3: 894. https://doi.org/10.3390/biomedicines11030894
APA StyleCiocan, A., Ciocan, R. A., Al Hajjar, N., Benea, A. M., Pandrea, S. L., Cătană, C. S., Drugan, C., Oprea, V. C., Dîrzu, D. S., & Bolboacă, S. D. (2023). Exploratory Evaluation of Neopterin and Chitotriosidase as Potential Circulating Biomarkers for Colorectal Cancer. Biomedicines, 11(3), 894. https://doi.org/10.3390/biomedicines11030894







