Recent Clinical Treatment and Basic Research on the Alveolar Bone
Abstract
1. Introduction
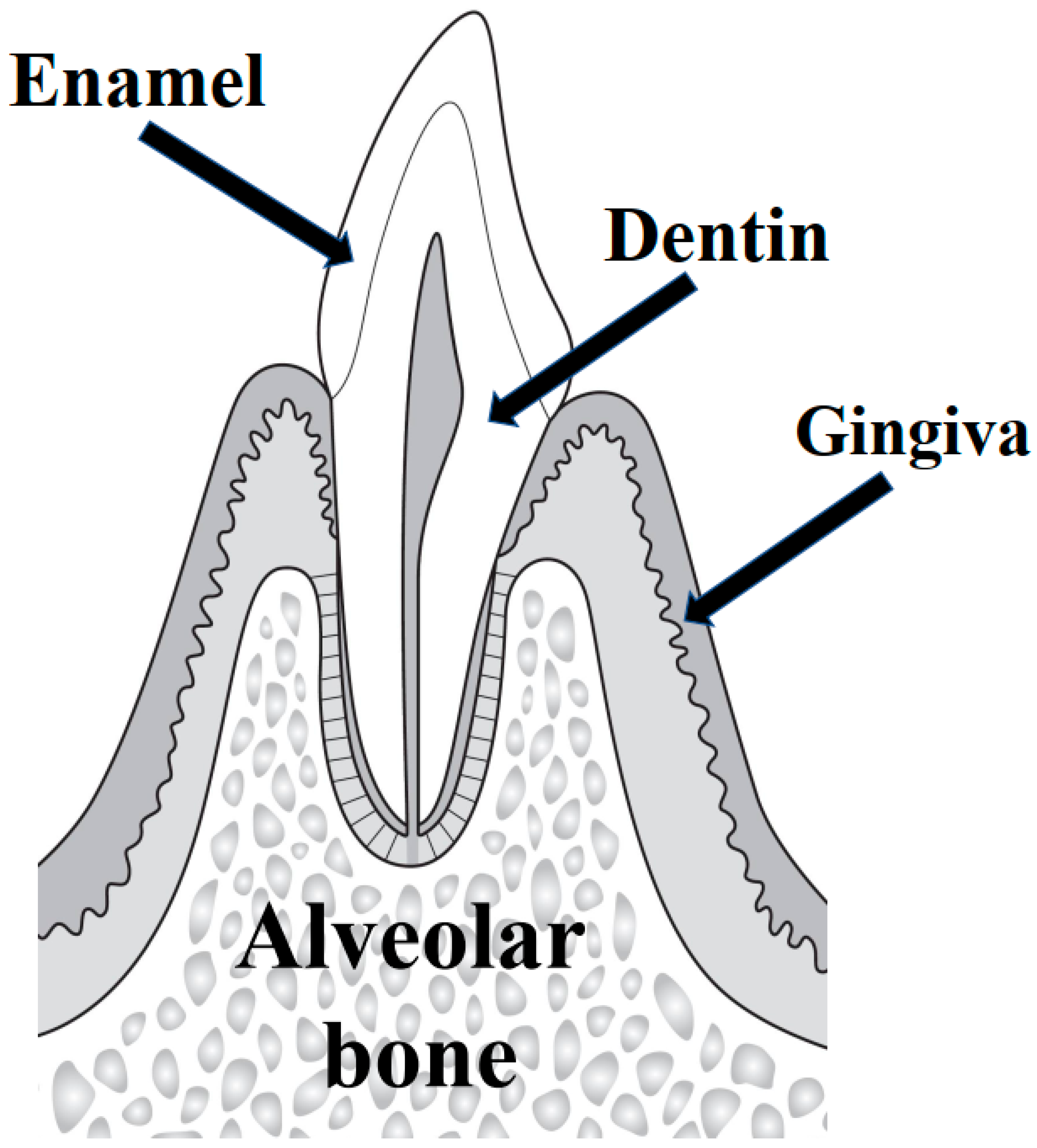
1.1. Alveolar Bone Resorption
- (1)
- Horizontal bone resorption. There is uniform horizontal resorption of the alveolar bone that occurs extensively on multiple teeth, e.g., the line connecting the cement–enamel border of the adjacent teeth is parallel to the alveolar crest.
- (2)
- Vertical resorption. There is vertical resorption of the alveolar bone from the apex, often confined to one or two teeth. The line connecting the cement–enamel border of the adjacent tooth and the alveolar crest is not parallel.
- (3)
- Mixed bone resorption. A combination of horizontal and vertical bone resorption occurs.
1.2. Alveolar Bone Surgery
1.3. Alveolar Osteotomy
2. Characteristics of Periodontal Treatment
2.1. New Classification of Periodontal Disease (2018 American Academy of Periodontology and European Federation of Periodontology)
2.2. Tissue Engineering for Periodontal Tissue Regeneration
3. Guided Bone Regeneration (GBR)
Clinical Applications of Nonabsorbable and Resorbable Bone-Replacing Materials
4. Clinical Applications of Membranes
5. Implant Therapy
6. Bone Grafting
6.1. Types and Characteristics of Bone Grafting Methods
- (1)
- Autologous bone grafting
- (2)
- Allogeneic bone grafting
- (3)
- Bone xenografting
- (4)
- Artificial bone grafts
6.2. Indications
6.3. Key Points of the Procedure
- (1)
- For preoperative preparation, thorough plaque control by the patient is important [127,128,129]. In the case of upset teeth, wound healing stability may be impaired, and the prognosis may be poor; thus, occlusal adjustment and splint therapy should be used to reduce excessive tooth movement and fremitus.
- (2)
- In bone grafting, the incision line and gingival valve should be preserved to ensure that the bone graft material is completely covered by the gingiva and retained within the bone defect [95,130]. Incisions close to or over the bone defect should be avoided. Soft tissue involvement should be carefully minimized during gingival valve dissection.
- (3)
- For receptor preparation, the root surface and bone defect, which is the graft bed, should be debrided by hand or by a rotary cutting instrument, such as an ultrasonic scaler or finishing bar. The cortical bone may be perforated using a round bar to supply blood to the defect.
- (4)
- Preparation of bone graft materials
- (5)
- Filling and suturing bone graft materials
7. New Findings from Clinical and Basic Research on the Alveolar Bone
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Balta, M.G.; Papathanasiou, E.; Blix, I.J.; Van Dyke, T.E. Host Modulation and Treatment of Periodontal Disease. J. Dent. Res. 2021, 100, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L.M.; Morford, L.A.; Nibali, L. Periodontal health and disease: The contribution of genetics. Periodontology 2000 2021, 85, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.B.; Yap, A.U.; Allen, P.F. Periodontal disease and quality of life: Umbrella review of systematic reviews. J. Periodontal Res. 2021, 56, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.X.; Kang, X.N.; Wang, Y.X.; Huang, Y.N.; Pang, C.F.; Chen, Y.X.; Kuang, Z.L.; Peng, Y. Periodontal disease and emotional disorders: A meta-analysis. J. Clin. Periodontol. 2021, 48, 180–204. [Google Scholar] [CrossRef]
- Clark, D.; Kotronia, E.; Ramsay, S.E. Frailty, aging, and periodontal disease: Basic biologic considerations. Periodontology 2000 2021, 87, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, M.; Kurihara, H. Molecular Mechanisms of Periodontal Disease. Int. J. Mol. Sci. 2021, 22, 930. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontology 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Donos, N. The periodontal pocket. Periodontology 2000 2018, 76, 7–15. [Google Scholar] [CrossRef]
- Hajishengallis, G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontology 2000 2022, 89, 9–18. [Google Scholar] [CrossRef]
- Bascones-Martínez, A.; González-Febles, J.; Sanz-Esporrín, J. Diabetes and periodontal disease. Review of the literature. Am. J. Dent. 2014, 27, 63–67. [Google Scholar]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 22, 17038. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association be-tween periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Curtis, D.A.; Lin, G.H.; Rajendran, Y.; Gessese, T.; Suryadevara, J.; Kapila, Y.L. Treatment planning considerations in the older adult with periodontal disease. Periodontology 2000 2021, 87, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Obata, Y.; Mochizuki, Y.; Kitamura, M.; Mitsunari, K.; Matsuo, T.; Ohba, K.; Mukae, H.; Nishino, T.; Yoshimura, A.; et al. Periodontal Disease in Patients Receiving Dialysis. Int. J. Mol. Sci. 2019, 20, 3805. [Google Scholar] [CrossRef]
- Kitamura, M.; Mochizuki, Y.; Miyata, Y.; Obata, Y.; Mitsunari, K.; Matsuo, T.; Ohba, K.; Mukae, H.; Yoshimura, A.; Nishino, T.; et al. Pathological Characteristics of Periodontal Disease in Patients with Chronic Kidney Disease and Kidney Trans-plantation. Int. J. Mol. Sci. 2019, 20, 3413. [Google Scholar] [CrossRef]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 2000 2018, 76, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R. Osteoimmunology: Inflammatory osteolysis and regeneration of the alveolar bone. J. Clin. Periodontol. 2019, 46, 52–69. [Google Scholar] [CrossRef]
- Hollý, D.; Klein, M.; Mazreku, M.; Zamborský, R.; Polák, Š.; Danišovič, Ľ.; Csöbönyeiová, M. Stem Cells and Their Deriva-tives-Implications for Alveolar Bone Regeneration: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 11746. [Google Scholar] [CrossRef]
- Monje, A.; Chan, H.L.; Galindo-Moreno, P.; Elnayef, B.; Suarez-Lopez del Amo, F.; Wang, F.; Wang, H.L. Alveo-lar Bone Architecture: A Systematic Review and Meta-Analysis. J. Periodontol. 2015, 86, 1231–1248. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 195–223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ling, J.; Jiang, Q. Inflammasomes in Alveolar Bone Loss. Front. Immunol. 2021, 12, 691013. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhou, X.; Liu, C.; Xu, X. Oral Osteomicrobiology: The Role of Oral Microbiota in Alveolar Bone Homeostasis. Front. Cell. Infect. Microbiol. 2021, 11, 751503. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, K.A. The osteoimmunology of alveolar bone loss. Connect. Tissue Res. 2016, 57, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Hathaway-Schrader, J.D.; Novince, C.M. Maintaining homeostatic control of periodontal bone tissue. Periodontology 2000 2021, 86, 157–187. [Google Scholar] [CrossRef] [PubMed]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Araújo, M.G.; Buser, D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontology 2000 2017, 73, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.L.; Wong, T.L.; Wong, M.C.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimen-sional changes in humans. Clin. Oral Implants Res. 2012, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Matthies, L.; Windisch, P.; Gosau, M.; Jung, R.; Brodala, N.; Stefanini, M.; Kleinheinz, J.; Payer, M.; Henningsen, A.; et al. Horizontal augmentation techniques in the mandible: A systematic review. Int. J. Implant. Dent. 2022, 8, 23. [Google Scholar] [CrossRef]
- Aloy-Prósper, A.; Carramolino-Cuéllar, E.; Peñarrocha-Oltra, D.; Soto-Peñaloza, D.; Peñarrocha-Diago, M. Intraoral onlay block bone grafts versus cortical tenting technique on alveolar ridge augmentations: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2022, 27, e181–e190. [Google Scholar] [CrossRef]
- Willenbacher, M.; Al-Nawas, B.; Berres, M.; Kämmerer, P.W.; Schiegnitz, E. The Effects of Alveolar Ridge Preservation: A Meta-Analysis. Clin. Implant Dent. Relat. Res. 2016, 18, 1248–1268. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.H.; Fu, J.H.; Qi, W.; Du, Y.; Pan, J.; Wang, H.L. Graft-Free Maxillary Sinus Floor Elevation: A Systematic Review and Meta-Analysis. J. Periodontol. 2017, 88, 550–564. [Google Scholar] [CrossRef]
- Langer, L.; Langer, B.; Salem, D. Unintentional root fragment retention in proximity to dental implants: A series of six human case reports. Int. J. Periodontics Restor. Dent. 2015, 35, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.P. Long-term effects of segmental alveolar osteotomy. Int. J. Oral Surg. 1979, 8, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, P.Y.; Dottore, A.M.; Bechara, K.; Feres, M.; Shibli, J.A. Alveolar osteotomy associated with resorbable non-ceramic hydroxylapatite or intra-oral autogenous bone for height augmentation in posterior mandibular sites: A split-mouth prospective study. Clin. Oral Implants Res. 2013, 24, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Rittersma, J. Total mandibular alveolar osteotomy. J. Oral Surg. 1981, 39, 903–906. [Google Scholar]
- MacIntosh, R.B.; Shivapuja, P.K.; Warren, E.D. Total Maxillary Alveolar Osteotomy: Surgical Technique and Review of Its Efficacy. J. Oral Maxillofac. Surg. 2018, 76, 1097. [Google Scholar] [CrossRef]
- Roccuzzo, A.; Marchese, S.; Worsaae, N.; Jensen, S.S. The sandwich osteotomy technique to treat vertical alveolar bone defects prior to implant placement: A systematic review. Clin. Oral Investig. 2020, 24, 1073–1089. [Google Scholar] [CrossRef]
- Philip, M.R. Posterior maxillary segmental osteotomy for prosthodontic rehabilitation of vertically excess maxilla-a review. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 450–455. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontology 2000 2020, 84, 14–34. [Google Scholar] [CrossRef]
- Ryder, M.I.; Armitage, G.C. Minimally invasive periodontal therapy for general practitioners. Periodontology 2000 2016, 71, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, M.G.P.; Willems, E.J.S.; Slot, D.E.; Van der Weijden, G.A.F. Success of supportive periodontal therapy in perio dontitis patients—A retrospective analysis. Int. J. Dent. Hyg. 2022, 20, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, S.K.; Kohnen, R.; Ruetters, M.; Krisam, J.; Kim, T.S. Adherence to long-term supportive periodontal therapy in groups with different periodontal risk profiles. J. Clin. Periodontol. 2020, 47, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Pardo, M.; Márquez-Arrico, C.F.; Pallarés-Serrano, A.; Silvestre, F.J. Adherence to supportive periodontal treatment in relation to patient awareness. J. Clin. Exp. Dent. 2022, 14, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral Sci. 2020, 28, e20190248. [Google Scholar] [CrossRef]
- Cobb, C.M.; Sottosanti, J.S. A re-evaluation of scaling and root planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Caffesse, R.G.; Echeverría, J.J. Treatment trends in periodontics. Periodontology 2000 2019, 79, 7–14. [Google Scholar] [CrossRef]
- Mainas, G.; Ide, M.; Rizzo, M.; Magan-Fernandez, A.; Mesa, F.; Nibali, L. Managing the Systemic Impact of Periodontitis. Medicina (Kaunas) 2022, 58, 621. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S1–S8. [Google Scholar] [CrossRef]
- Cirkel, L.L.; Jacob, L.; Smith, L.; López-Sánchez, G.F.; Konrad, M.; Kostev, K. Relationship between chronic gingivitis and subsequent depression in 13,088 patients followed in general practices. J. Psychiatr. Res. 2021, 138, 103–106. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Caggiano, M.; Schiavo, L.; Savarese, G.; Carpinelli, L.; Amato, A.; Iandolo, A. Chronic Stress and Depression in Periodontitis and Peri-Implantitis: A Narrative Review on Neurobiological, Neurobehavioral and Immune–Microbiome Interplays and Clinical Management Implications. Dent. J. 2022, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Pilipchuk, S.P.; Bartold, P.M.; Hutmacher, D.W.; Giannobile, W.V.; Ivanovski, S. Tissue Engineered Constructs for Periodontal Regeneration: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, e1800457. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, G.; Xiropaidis, A.V.; Wikesjö, U.M. Biology and principles of periodontal wound healing/regeneration. Periodontology 2000 2006, 41, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Ward, E. A Review of Tissue Engineering for Periodontal Tissue Regeneration. J. Vet. Dent. 2022, 39, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Nagata, M.; Iwasaki, K.; Akazawa, K.; Komaki, M.; Yokoyama, N.; Izumi, Y.; Morita, I. Conditioned Medium from Periodontal Ligament Stem Cells Enhances Periodontal Regeneration. Tissue Eng. Part A 2017, 23, 367–377. [Google Scholar] [CrossRef]
- Raju, R.; Oshima, M.; Inoue, M.; Morita, T.; Huijiao, Y.; Waskitho, A.; Baba, O.; Inoue, M.; Matsuka, Y. Three-dimensional periodontal tissue regeneration using a bone-ligament complex cell sheet. Sci. Rep. 2020, 10, 1656. [Google Scholar] [CrossRef]
- Carter, S.D.; Costa, P.F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Malda, J. Additive Biomanufacturing: An Advanced Approach for Periodontal Tissue Regeneration. Ann. Biomed. Eng. 2017, 45, 12–22. [Google Scholar] [CrossRef]
- Andrei, M.; Dinischiotu, A.; Didilescu, A.C.; Ionita, D.; Demetrescu, I. Periodontal materials and cell biology for guid-ed tissue and bone regeneration. Ann. Anat. 2018, 216, 164–169. [Google Scholar] [CrossRef]
- Tomokiyo, A.; Wada, N.; Maeda, H. Periodontal Ligament Stem Cells: Regenerative Potency in Periodontium. Stem Cells Dev. 2019, 28, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Sun, H.H.; Lu, H.; Yu, Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012, 33, 6320–6344. [Google Scholar] [CrossRef]
- Bartold, P.M.; Gronthos, S.; Ivanovski, S.; Fisher, A.; Hutmacher, D.W. Tissue engineered periodontal products. J. Periodontal Res. 2016, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Guo, W.; Chen, M.; Zheng, Y.; Zhou, J.; Kim, S.G.; Embree, M.C.; Songhee Song, K.; Marao, H.F.; Mao, J.J. Perio-dontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement. Front. Oral Biol. 2016, 18, 1–8. [Google Scholar]
- Pitzurra, L.; Jansen, I.D.C.; de Vries, T.J.; Hoogenkamp, M.A.; Loos, B.G. Effects of L-PRF and A-PRF+ on periodontal fibroblasts in in vitro wound healing experiments. J. Periodontal Res. 2020, 55, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Sokos, D.; Everts, V.; de Vries, T.J. Role of periodontal ligament fibroblasts in osteoclastogenesis: A review. J. Periodontal Res. 2015, 50, 152–159. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, W.; Wang, H.; Xue, C.; Feng, J.; He, Y.; Wang, P.; Li, S.; Bai, D.; Shu, R. Periodontal ligament fibroblasts regulate osteoblasts by exosome secretion induced by inflammatory stimuli. Arch. Oral Biol. 2019, 105, 27–34. [Google Scholar] [CrossRef]
- Symmank, J.; Zimmermann, S.; Goldschmitt, J.; Schiegnitz, E.; Wolf, M.; Wehrbein, H.; Jacobs, C. Mechanically-induced GDF15 Secretion by Periodontal Ligament Fibroblasts Regulates Osteogenic Transcription. Sci. Rep. 2019, 9, 11516. [Google Scholar] [CrossRef]
- Yu, M.; Wang, L.; Ba, P.; Li, L.; Sun, L.; Duan, X.; Yang, P.; Yang, C.; Sun, Q. Osteoblast Progenitors Enhance Osteogenic Differentiation of Periodontal Ligament Stem Cells. J. Periodontol. 2017, 88, e159–e168. [Google Scholar] [CrossRef]
- Devecioğlu, D.; Tözüm, T.F.; Sengün, D.; Nohutcu, R.M. Biomaterials in periodontal regenerative surgery: Effects of cryo-preserved bone, commercially available coral, demineralized freeze-dried dentin, and cementum on periodontal ligament fibroblasts and osteoblasts. J. Biomater. Appl. 2004, 19, 107–120. [Google Scholar] [CrossRef]
- Bosshardt, D.D. Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 87–105. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, R.; Scantlebury, V.T.; Sanchez, R.; Whitely, N.; Ambruster, J. Membrane design criteria for guided bone regeneration of the alveolar ridge. In Guided Bone Regeneration in Implant Dentistry; Buser, D., Dahlin, C., Schenk, R.K., Eds.; Quin-tessence: Chicago, IL, USA, 1994; pp. 101–136. [Google Scholar]
- McAllister, B.S.; Haghighat, K. Bone augmentation techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Gotfredsen, K.; Warrer, K.; Hjörting-Hansen, E.; Karring, T. Effect of membranes and porous hydroxyapatite on healing in bone defects around titanium dental implants. An experimental study in monkeys. Clin. Oral. Implants Res. 1991, 2, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Yeo, A.; Dard, M.; Flunziker, E.; Schenk, R.; Buser, D. Evaluation of a novel biphasic calcium phosphate in stand-ardized bone defects: A histologic and histomorphometric study in the mandibles of minipigs. Clin. Oral Implants Res. 2007, 18, 752–760. [Google Scholar] [CrossRef]
- Yukna, C.N.; Yukna, R.A. Multi-center evaluation of bioabsorbable collagen membrane for guided tissue regeneration in human Class II furcations. J. Periodontol. 1996, 67, 650–657. [Google Scholar] [CrossRef]
- Bartee, B.K. The use of high-density polytetrafluoroethylene membrane to treat osseous defects: Clinical reports. Implant Dent. 1995, 4, 21–26. [Google Scholar] [CrossRef]
- Hoffmann, O.; Bartee, B.K.; Beaumont, C.; Kasaj, A.; Deli, G.; Zafiropoulos, G.G. Alveolar bone preservation in extraction sockets using non-resorbable dPTFE membranes: A retrospective non-randomized study. J. Periodontol. 2008, 79, 1355–1369. [Google Scholar] [CrossRef]
- Schopper, C.; Goriwoda, W.; Moser, D.; Spassova, E.; Watzinger, F.; Ewers, R. Long-term results after guided bone regeneration with resorbable and microporous titanium membranes. Oral Maxillofac. Surg. Clin. N. Am. 2001, 13, 449–458. [Google Scholar] [CrossRef]
- Pitaru, S.; Noff, M.; Grosskopf, A.; Moses, O.; Tal, H.; Savion, N. Heparan sulfate and fibronectin improve the capacity of col-lagen barriers to prevent apical migration of the junctional epithelium. J. Periodontol. 1991, 62, 598–601. [Google Scholar] [CrossRef]
- Urban, I.A.; Nagursky, H.; Lozada, J.L.; Nagy, K. Horizontal ridge augmentation with a collagen membrane and a combination of particulated autogenous bone and anorganic bovine bone-derived mineral: A prospective case series in 25 patients. Int. J. Periodontics Restor. Dent. 2013, 33, 299–307. [Google Scholar] [CrossRef]
- Judy, K.W. The impact of implants on dental practice. A review of the 1988 NIH Consensus Development Conference. N. Y. State Dent. J. 1989, 55, 24–27. [Google Scholar]
- Meffert, R.M.; Langer, B.; Fritz, M.E. Dental implants: A review. J. Periodontol. 1992, 63, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999-2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Seyssens, L.; De Lat, L.; Cosyn, J. Immediate implant placement with or without connective tissue graft: A systematic review and meta-analysis. J. Clin. Periodontol. 2021, 48, 284–301. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Bartold, P.M.; Huang, Y.S. The role of foreign body response in peri-implantitis: What is the evidence? Periodontology 2000 2022, 90, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Chrcanovic, B.; Östman, P.O.; Sennerby, L. Initial and long-term crestal bone responses to modern den-tal implants. Periodontology 2000 2017, 73, 41–50. [Google Scholar] [CrossRef]
- Yamamoto, R.; Amano, K.; Takahashi, S.W.; To, M.; Takahashi, S.; Matsuo, M. Changes in the microcirculation in periodontal tissue due to experimental peri-implantitis. J. Oral Biosci. 2021, 63, 153–160. [Google Scholar] [CrossRef]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Gatto, R.; Monaco, A. Periodontitis, implant loss and peri-implantitis. A meta-analysis. Clin. Oral Implants Res. 2015, 26, e8–e16. [Google Scholar] [CrossRef]
- Stacchi, C.; Berton, F.; Perinetti, G.; Frassetto, A.; Lombardi, T.; Khoury, A.; Andolsek, F.; Di Lenarda, R. Risk Factors for Peri-Implantitis: Effect of History of Periodontal Disease and Smoking Habits. A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Res. 2016, 7, e3. [Google Scholar]
- Mazel, A.; Belkacemi, S.; Tavitian, P.; Stéphan, G.; Tardivo, D.; Catherine, J.H.; Aboudharam, G. Peri-implantitis risk factors: A prospective evaluation. J. Investig. Clin. Dent. 2019, 10, e12398. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Olmedo, D.G. Peri-implantitis is not periodontitis: Scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontology 2000 2021, 86, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Manoil, D. Microbial Community-Driven Etiopathogenesis of Peri-Implantitis. J. Dent. Res. 2021, 100, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Grischke, J.; Szafrański, S.P.; Muthukumarasamy, U.; Haeussler, S.; Stiesch, M. Removable denture is a risk indicator for peri-implantitis and facilitates expansion of specific periodontopathogens: A cross-sectional study. BMC Oral Health 2021, 21, 173. [Google Scholar] [CrossRef] [PubMed]
- Adam, M. ‘Combined endo-perio lesions’—What is the best treatment? Evid. Based. Dent. 2021, 22, 158–159. [Google Scholar] [CrossRef]
- Reynolds, M.A.; Kao, R.T.; Camargo, P.M.; Caton, J.G.; Clem, D.S.; Fiorellini, J.P.; Geisinger, M.L.; Mills, M.P.; Nares, S.; Nevins, M.L. Periodontal regeneration—Intrabony defects: A consensus report from the AAP Regeneration Workshop. J. Periodontol. 2015, 86 (Suppl. 2), S105–S107. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef]
- Nibali, L.; Koidou, V.P.; Nieri, M.; Barbato, L.; Pagliaro, U.; Cairo, F. Regenerative surgery versus access flap for the treatment of intra-bony periodontal defects: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 320–351. [Google Scholar] [CrossRef]
- Goyal, S.; Masood, M.; Le, C.; Rajendran, Y.; Nanjapa, S.; Vaderhobli, R. Comparative Bone Graft Evaluation for Den-tal Implant Success: An Evidence-Based Review. J. Long Term Eff. Med. Implants 2021, 31, 33–44. [Google Scholar] [CrossRef]
- Chavda, S.; Levin, L. Human Studies of Vertical and Horizontal Alveolar Ridge Augmentation Comparing Different Types of Bone Graft Materials: A Systematic Review. J. Oral Implantol. 2018, 44, 74–84. [Google Scholar] [CrossRef]
- Yang, S.; Wang, N.; Ma, Y.; Guo, S.; Guo, S.; Sun, H. Immunomodulatory effects and mechanisms of distraction osteogenesis. Int. J. Oral Sci. 2022, 14, 4. [Google Scholar] [CrossRef]
- Kaneda-Ikeda, E.; Iwata, T.; Mizuno, N.; Nagahara, T.; Kajiya, M.; Ouhara, K.; Yoshioka, M.; Ishida, S.; Kawaguchi, H.; Ku-rihara, H. Regulation of osteogenesis via miR-101-3p in mesenchymal stem cells by human gingival fibroblasts. J. Bone Miner Metab. 2020, 38, 442–455. [Google Scholar] [CrossRef]
- Tjempakasari, A.; Suroto, H.; Santoso, D. Mesenchymal Stem Cell Senescence and Osteogenesis. Medicina (Kaunas) 2021, 58, 61. [Google Scholar] [CrossRef]
- Sampath, T.K.; Reddi, A.H. Discovery of bone morphogenetic proteins—A historical perspective. Bone 2020, 140, 115548. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Tu, H.L.; Duan, Y.J.; Chen, X. Comparison of bone morphogenetic protein and autologous grafting in the treatment of limb long bone nonunion: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2020, 15, 288. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Sánchez, R.M.; Yáñez-Vico, R.M.; Fernández-Olavarría, A.; Mosquera-Pérez, R.; Iglesias-Linares, A.; Torres-Lagares, D. Current Approaches of Bone Morphogenetic Proteins in Dentistry. J. Oral. Implantol. 2015, 41, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Körner, D.; Gonser, C.E.; Döbele, S.; Konrads, C.; Springer, F.; Keller, G. Matrix-associated autologous chondrocyte implanta-tion with autologous bone grafting of osteochondral lesions of the talus in adolescents: Patient-reported outcomes with a me-dian follow-up of 6 years. J. Orthop. Surg. Res. 2021, 16, 243. [Google Scholar] [CrossRef]
- Liu, B.; Yin, N.B.; Xiao, R.; Li, B.H.; Li, H.D.; Chen, S.X.; Li, S.L.; Wang, Y.Q. Evaluating the efficacy of recombinant human bone morphogenic protein-2 in the treatment of alveolar clefts with autologous bone grafting using computer-aided engineering techniques. Br. J. Oral Maxillofac. Surg. 2021, 59, 757–762. [Google Scholar] [CrossRef]
- Shirakata, Y.; Taniyama, K.; Yoshimoto, T.; Takeuchi, N.; Noguchi, K. Effect of bone swaging with calcium phos-phate bone cement on periodontal regeneration in dogs. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 35–42. [Google Scholar] [CrossRef]
- Shirakata, Y.; Yoshimoto, T.; Takeuchi, N.; Taniyama, K.; Noguchi, K. Effects of EMD in combination with bone swaging and calcium phosphate bone cement on periodontal regeneration in one-wall intrabony defects in dogs. J. Periodontal Res. 2013, 48, 37–43. [Google Scholar] [CrossRef]
- Kodama, T.; Minabe, M.; Sugiyama, T.; Mitarai, E.; Fushimi, H.; Kitsugi, D.; Tsutsumi, K.; Katsuki, M. Guided tissue regener-ation using a collagen barrier and bone swaging technique in noncontained infrabony defects. Int. J. Periodontics Restor. Dent. 2013, 33, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Moraschini, V.; de Almeida, D.C.F.; Kischinhevsky, M.D.; de Almeida, I.C.C.; Louro, R.S.; Granjeiro, J.M. Immunological response of allogeneic bone grafting: A systematic review of prospective studies. J. Oral Pathol. Med. 2020, 49, 395–403. [Google Scholar] [CrossRef]
- McCrary, H.; Skirko, J.R. Bone Grafting of Alveolar Clefts. Oral Maxillofac. Surg. Clin. N. Am. 2021, 33, 231–238. [Google Scholar] [CrossRef]
- Spin-Neto, R.; Stavropoulos, A.; de Freitas, R.M.; Pereira, L.A.; Carlos, I.Z.; Marcantonio, E., Jr. Immunological aspects of fresh-frozen allogeneic bone grafting for lateral ridge augmentation. Clin. Oral Implants Res. 2013, 24, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Solakoglu, Ö.; Götz, W.; Heydecke, G.; Schwarzenbach, H. Histological and immunohistochemical comparison of two differ-ent allogeneic bone grafting materials for alveolar ridge reconstruction: A prospective randomized trial in humans. Clin. Implant Dent. Relat. Res. 2019, 21, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Bracey, D.N.; Cignetti, N.E.; Jinnah, A.H.; Stone, A.V.; Gyr, B.M.; Whitlock, P.W.; Scott, A.T. Bone xenotransplantation: A review of the history, orthopedic clinical literature, and a single-center case series. Xenotransplantation 2020, 27, e12600. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef]
- Taschieri, S.; Moses, O.; Dellavia, C.; Canciani, E.; Nemcovsky, C.; Francetti, L.; Corbella, S. Comparative Study of Depro-teinized Bovine Bone Mineral and Bovine Bone Mineral Enriched with a Polymer and Gelatin in Maxillary Sinus Floor Elevation Procedures. Int. J. Periodontics Restor. Dent. 2021, 41, 579–586. [Google Scholar] [CrossRef]
- Meloni, S.M.; Jovanovic, S.A.; Lolli, F.M.; Cassisa, C.; De Riu, G.; Pisano, M.; Lumbau, A.; Lugliè, P.F.; Tullio, A. Grafting after sinus lift with anorganic bovine bone alone compared with 50:50 anorganic bovine bone and autologous bone: Results of a pilot randomised trial at one year. Br. J. Oral Maxillofac. Surg. 2015, 53, 436–441. [Google Scholar] [CrossRef]
- Lee, J.H.; Yi, G.S.; Lee, J.W.; Kim, D.J. Physicochemical characterization of porcine bone-derived grafting material and com-parison with bovine xenografts for dental applications. J. Periodontal Implant Sci. 2017, 47, 388–401. [Google Scholar] [CrossRef]
- Maiorana, C.; Poli, P.P.; Deflorian, M.; Testori, T.; Mandelli, F.; Nagursky, H.; Vinci, R. Alveolar socket preservation with demineralised bovine bone mineral and a collagen matrix. J. Periodontal Implant Sci. 2017, 47, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Barausse, C.; Barone, A.; Zucchelli, G.; Piattelli, M.; Pistilli, R.; Ippolito, D.R.; Simion, M. Interpositional Augmentation Technique in the Treatment of Posterior Mandibular Atrophies: A Retrospective Study Comparing 129 Autogenous and Heterologous Bone Blocks with 2 to 7 Years Follow-Up. Int. J. Periodontics Restor. Dent. 2017, 37, 469–480. [Google Scholar] [CrossRef]
- Perasso, R.; Imelio, M.; Schiaffino, M. Orthodontic movement into a bone defect augmented with heterologous graft. J. Clin. Orthod. 2022, 55, 401–408. [Google Scholar] [PubMed]
- Papageorgiou, S.N.; Papageorgiou, P.N.; Deschner, J.; Götz, W. Comparative effectiveness of natural and synthetic bone grafts in oral and maxillofacial surgery prior to insertion of dental implants: Systematic review and network meta-analysis of parallel and cluster randomized controlled trials. J. Dent. 2016, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tomas, M.; Čandrlić, M.; Juzbašić, M.; Ivanišević, Z.; Matijević, N.; Včev, A.; Cvijanović Peloza, O.; Matijević, M.; Perić Kačarević, Ž. Synthetic Injectable Biomaterials for Alveolar Bone Regeneration in Animal and Human Studies. Materials 2021, 14, 2858. [Google Scholar] [CrossRef]
- Burkhardt, R.; Lang, N.P. Fundamental principles in periodontal plastic surgery and mucosal augmentation—A narrative re-view. J. Clin. Periodontol. 2014, 41, S98–S107. [Google Scholar] [CrossRef]
- Ruiz Núñez, M.D.R.; da Luz Raulino, M.; Goulart Castro, R.; Schaefer Ferreira de Mello, A.L. Dental plaque control strategies for the elderly population: A scoping review. Int. J. Dent. Hyg. 2022, 20, 167–181. [Google Scholar] [CrossRef]
- Wadia, R. Role of plaque control in gingival oral lichen planus. Br. Dent. J. 2019, 227, 37. [Google Scholar] [CrossRef]
- Kofina, V.; Demirer, M.; Erdal, B.S.; Eubank, T.D.; Yildiz, V.O.; Tatakis, D.N.; Leblebicioglu, B. Bone grafting history affects soft tissue healing following implant placement. J. Periodontol. 2021, 92, 234–243. [Google Scholar] [CrossRef]
- Nkenke, E.; Neukam, F.W. Autogenous bone harvesting and grafting in advanced jaw resorption: Morbidity, resorption and implant survival. Eur. J. Oral Implantol. 2014, 7, S203–S217. [Google Scholar]
- Sittitavornwong, S.; Gutta, R. Bone graft harvesting from regional sites. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 317–330. [Google Scholar] [CrossRef]
- Miron, R.J.; Gruber, R.; Hedbom, E.; Saulacic, N.; Zhang, Y.; Sculean, A.; Bosshardt, D.D.; Buser, D. Impact of bone harvesting techniques on cell viability and the release of growth factors of autografts. Clin. Implant Dent. Relat. Res. 2013, 15, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, Y.; Li, H.J. Advances in mesenchymal stem cell exosomes: A review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in transla-tional medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef] [PubMed]
- Mianehsaz, E.; Mirzaei, H.R.; Mahjoubin-Tehran, M.; Rezaee, A.; Sahebnasagh, R.; Pourhanifeh, M.H.; Mirzaei, H.; Hamblin, M.R. Mesenchymal stem cell-derived exosomes: A new therapeutic approach to osteoarthritis? Stem Cell Res. Ther. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Li, M.; Lin, T.; Zhou, H.; Wang, F.; Su, X. Treatment of inflammatory bone loss in periodontitis by stem cell-derived exosomes. Acta Biomater. 2022, 141, 333–343. [Google Scholar] [CrossRef]
- Al-Sosowa, A.A.; Alhajj, M.N.; Abdulghani, E.A.; Al-Moraissi, E.A.; Zheng, H.; Pang, Y.; Wang, J. Three-dimensional Analysis of Alveolar Bone With and Without Periodontitis. Int. Dent. J. 2022, 24, S0020–S6539. [Google Scholar] [CrossRef]
- Takedachi, M.; Sawada, K.; Sakura, K.; Morimoto, C.; Hirai, A.; Iwayama, T.; Shimomura, J.; Kawasaki, K.; Fujihara, C.; Kashiwagi, Y.; et al. Periodontal tissue regeneration by transplantation of autologous adipose tissue-derived multi-lineage progenitor cells. Sci. Rep. 2022, 12, 8126. [Google Scholar] [CrossRef]
- Li, M.; Xing, X.; Huang, H.; Liang, C.; Gao, X.; Tang, Q.; Xu, X.; Yang, J.; Liao, L.; Tian, W. BMSC-Derived ApoEVs Promote Craniofacial Bone Repair via ROS/JNK Signaling. J. Dent. Res. 2022, 101, 714–723. [Google Scholar] [CrossRef]
- Yun, S.M.; Lee, J.H.; Jung, K.H.; Lee, H.; Lee, S.; Hong, S.; Hong, S.S. Induction of apoptosis and suppression of angiogenesis of hepatocellular carcinoma by HS-159, a novel phosphatidylinositol 3-kinase inhibitor. Int. J. Oncol. 2013, 43, 201–209. [Google Scholar] [CrossRef]
- Takahashi, H.; Rokudai, S.; Kawabata-Iwakawa, R.; Sakakura, K.; Oyama, T.; Nishiyama, M.; Chikamatsu, K. AKT3 is a key regulator of head and neck squamous cell carcinoma. Cancer Sci. 2021, 112, 2325–2334. [Google Scholar] [CrossRef]
- Abdel Ghafar, M.T.; Soliman, N.A. Metadherin (AEG-1/MTDH/LYRIC) expression: Significance in malignancy and crucial role in colorectal cancer. Adv. Clin. Chem. 2022, 106, 235–280. [Google Scholar] [PubMed]
- Wang, L.; Dong, M.; Shi, D.; Yang, C.; Liu, S.; Gao, L.; Niu, W. Role of PI3K in the bone resorption of apical periodontitis. BMC Oral Health 2022, 22, 345. [Google Scholar] [CrossRef]
- Kanda, M.; Tanaka, C.; Kobayashi, D.; Tanaka, H.; Shimizu, D.; Shibata, M.; Takami, H.; Hayashi, M.; Iwata, N.; Niwa, Y.; et al. Epigenetic suppression of the immunoregulator MZB1 is asso-ciated with the malignant phenotype of gastric cancer. Int. J. Cancer 2016, 139, 2290–2298. [Google Scholar] [CrossRef]
- Miyake, K.; Mori, R.; Homma, Y.; Matsuyama, R.; Okayama, A.; Murakami, T.; Hirano, H.; Endo, I. MZB1 in borderline re-sectable pancreatic cancer resected after neoadjuvant chemoradiotherapy. J. Surg. Res. 2017, 220, 391–401. [Google Scholar] [CrossRef]
- Wei, H.; Wang, J.Y. Role of Polymeric Immunoglobulin Receptor in IgA and IgM Transcytosis. Int. J. Mol. Sci. 2021, 22, 2284. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Y.; Zhang, L.; Shi, L.; Deng, L.; Ding, Z.; Ai, R.; Zhang, X.; He, Y. MZB1 targeted by miR-185-5p inhibits the mi-gration of human periodontal ligament cells through NF-κB signaling and promotes alveolar bone loss. J. Periodontal Res. 2022, 57, 811–823. [Google Scholar] [CrossRef]
- Hou, L.; Ye, Y.; Gou, H.; Tang, H.; Zhou, Y.; Xu, X.; Xu, Y. A20 inhibits periodontal one resorption and NLRP3-mediated M1 macrophage polarization. Exp. Cell Res. 2022, 418, 113264. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Dong, H.; Luo, Y.; Shao, B. Th17 Cells in Periodontitis and Its Regulation by A20. Front. Immunol. 2021, 12, 742925. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Wu, C.; Ye, Y.; Li, L.; Wang, X.; He, W.; Ren, S.; Xu, Y. A20 inhibits osteoclastogenesis via TRAF6-dependent autophagy in human periodontal ligament cells under hypoxia. Cell Prolif. 2020, 53, e12778. [Google Scholar] [CrossRef]
- Li, Y.; Mooney, E.C.; Holden, S.E.; Xia, X.J.; Cohen, D.J.; Walsh, S.W.; Ma, A.; Sahingur, S.E. A20 Orchestrates Inflammatory Response in the Oral Mucosa through Restraining NF-κB Activity. J. Immunol. 2019, 202, 2044–2056. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chua, S., Jr. Leptin Function and Regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar] [PubMed]
- Paz-Filho, G.; Mastronardi, C.A.; Licinio, J. Leptin treatment: Facts and expectations. Metabolism 2015, 64, 146–156. [Google Scholar] [CrossRef]
- Lawler, K.; Huang-Doran, I.; Sonoyama, T.; Collet, T.H.; Keogh, J.M.; Henning, E.; O’Rahilly, S.; Bottolo, L.; Farooqi, I.S. Lep-tin-Mediated Changes in the Human Metabolome. J. Clin. Endocrinol. Metab. 2020, 105, 2541–2552. [Google Scholar] [CrossRef]
- Zhao, S.; Kusminski, C.M.; Elmquist, J.K.; Scherer, P.E. Leptin: Less Is More. Diabetes 2020, 69, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, Y.; Gao, P.; Yang, Q.; Jia, L.; Zheng, Y.; Li, W. Leptin Aggravates Periodontitis by Promoting M1 Polarization via NLRP3. J. Dent. Res. 2022, 101, 675–685. [Google Scholar] [CrossRef]
- Nemoto, E.; Darveau, R.P.; Foster, B.L.; Nogueira-Filho, G.R.; Somerman, M.J. Regulation of cementoblast function by P. gin-givalis lipopolysaccharide via TLR2. J. Dent. Res. 2006, 85, 733–738. [Google Scholar] [CrossRef]
- Kuo, C.H.; Zhang, B.H.; Huang, S.E.; Hsu, J.H.; Wang, Y.H.; Nguyen, T.T.N.; Lai, C.H.; Yeh, J.L. Xanthine Derivative KMUP-1 Attenuates Experimental Periodontitis by Reducing Osteoclast Differentiation and Inflammation. Front. Pharmacol. 2022, 13, 821492. [Google Scholar] [CrossRef]
- Akkaoui, J.; Yamada, C.; Duarte, C.; Ho, A.; Vardar-Sengul, S.; Kawai, T.; Movila, A. Contribution of Porphyromonas gingivalis lipopolysaccharide to experimental periodontitis in relation to aging. Geroscience 2021, 43, 367–376. [Google Scholar] [CrossRef]
- Li, X.; Yu, C.; Hu, Y.; Xia, X.; Liao, Y.; Zhang, J.; Chen, H.; Lu, W.; Zhou, W.; Song, Z. New Application of Psoralen and Angelicin on Periodontitis With Anti-bacterial, Anti-inflammatory, and Osteogenesis Effects. Front. Cell Infect. Microbiol. 2018, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Lai, C.H.; Kuo, C.H.; Chang, B.I.; Wu, H.L.; Cheng, T.L. Recombinant thrombomodulin lectin-like domain at-tenuates Porphyromonas gingivalis lipopolysaccharide-induced osteoclastogenesis and periodontal bone resorption. J. Periodontol. 2021, 92, 1622–1634. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Okita, K.; Kayashima, H.; Yu, G.; Fukuyasu, S.; Saeki, M.; Matsumoto, T.; Yamanaka, S.; Yatani, H. Gingival fibroblasts as a promising source of induced pluripotent stem cells. PLoS ONE 2010, 5, e12743. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Okawa, H.; Okita, K.; Kamano, Y.; Wang, F.; Saeki, M.; Yatani, H.; Egusa, H. Gingival Fibroblasts as Autologous Feeders for Induced Pluripotent Stem Cells. J. Dent. Res. 2016, 95, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Kayashima, H.; Miura, J.; Uraguchi, S.; Wang, F.; Okawa, H.; Sasaki, J.; Saeki, M.; Matsumoto, T.; Yatani, H. Comparative analysis of mouse-induced pluripotent stem cells and mesenchymal stem cells during osteogenic differentiation in vitro. Stem Cells Dev. 2014, 23, 2156–2169. [Google Scholar] [CrossRef] [PubMed]
- Okawa, H.; Kayashima, H.; Sasaki, J.; Miura, J.; Kamano, Y.; Kosaka, Y.; Imazato, S.; Yatani, H.; Matsumoto, T.; Egusa, H. Scaffold-Free Fabrication of Osteoinductive Cellular Constructs Using Mouse Gingiva-Derived Induced Pluripotent Stem Cells. Stem Cells Int. 2016, 2016, 6240794. [Google Scholar] [CrossRef] [PubMed]
- Takagi, R.; Ishimaru, J.; Sugawara, A.; Toyoshima, K.E.; Ishida, K.; Ogawa, M.; Sakakibara, K.; Asakawa, K.; Kashiwakura, A.; Oshima, M.; et al. Bioengineering a 3D integumentary organ system from iPS cells using an in vivo transplantation model. Sci. Adv. 2016, 2, e1500887. [Google Scholar] [CrossRef]
- Kawai, S.; Yoshitomi, H.; Sunaga, J.; Alev, C.; Nagata, S.; Nishio, M.; Hada, M.; Koyama, Y.; Uemura, M.; Sekiguchi, K.; et al. In vitro bone-like nodules generated from patient-derived iPSCs recapitulate pathological bone phenotypes. Nat. Biomed. Eng. 2019, 3, 558–570. [Google Scholar] [CrossRef]
- Thurzo, A.; Gálfiová, P.; Nováková, Z.V.; Polák, Š.; Varga, I.; Strunga, M.; Urban, R.; Surovková, J.; Leško, Ľ.; Hajdúchová, Z.; et al. Fabrication and In Vitro Characterization of Novel Hydroxyapatite Scaffolds 3D Printed Using Polyvinyl Alcohol as a Thermoplastic Binder. Int. J. Mol. Sci. 2022, 23, 14870. [Google Scholar] [CrossRef]
| Type | Summary | Application | Characteristics |
|---|---|---|---|
| Sinus lift | Sinus lift is a technique used to place implants in the back teeth of the upper jaw after the bone thickness has been supplemented. | Sinus lift is an alveolar bone regeneration technique performed when the maxillary bone is not thick enough. This technique is performed to build a bone by filling the space above the alveolar bone, called the maxillary sinus, with a bone filler or other materials. | Advantages: Sinus lift is a treatment method that allows extensive bone creation so that longer implants can be used. It is a treatment method known worldwide for its long-lasting effects, and many articles have been written about it. Additionally, because the treated area is visible, the risk is relatively low. Disadvantages: Extensive osteogenesis means a larger surgical area, and the treatment period is longer if the sinus lift and implant placement are done separately as a two-stage procedure. The use of expensive bone replacement materials also places a burden on the patient’s body and finances. |
| Socket lift | Socket lift is a type of osteogenic surgery applied to maxillary implant treatment. The bottom of the maxillary sinus is surgically lifted to create a space, and a bone replacement material (either your own bone or artificial bone) is grafted into the space to regenerate the alveolar bone (the bone that supports the teeth). When an implant is placed in the upper jaw, if the alveolar bone is still thin, it is impossible to place and settle the implant because of the weak foundation. In such cases, the socket lift regenerates the alveolar bone through regenerative surgery, allowing the implant body to be placed in the maxillary region. | Like sinus lift, socket lift is an alveolar bone regeneration technique performed when the maxillary bone is not thick enough. This technique is performed to build a bone by filling the space above the alveolar bone, called the maxillary sinus, with a bone filler and other materials. It is performed when the thickness of the maxillary bone is generally 5 mm or more. | Advantages: It is a type of bone regeneration surgery that involves the grafting of bone augmentation material, but the scope of the surgical procedure can be reduced. The surgical procedure is less stressful on the body. The procedures for placing the implant body can be performed at the same time. Therefore, the overall treatment time can be shortened. Disadvantages: It is assumed that a certain amount of alveolar bone thickness (3–5 mm) remains. If the alveolar bone is not more than 5 mm, the treatment may not be possible. Furthermore, the scope of the surgical procedure is narrow. It can reduce the burden on the body, but the range of bone that can be regenerated is also narrower. The condition of the maxillary sinus cannot be visually confirmed during the surgery. Therefore, there is a risk of damage to the maxillary sinus, resulting in sinusitis or sinusitis. |
| GBR (guided bone regeneration) | GBR is a method of regenerating the alveolar bone when the bone thickness or height is insufficient for implant treatment. Once the alveolar bone is lost due to bone resorption caused by severe periodontal disease or bone loss after tooth extraction, it becomes difficult to place an implant in that area. In such bone-deficient areas, “fibroblasts”, which do not form bone, tend to proliferate more easily than “osteoblasts”, which form bone. Therefore, in GBR, the area to be treated is covered with a “membrane” to prevent fibroblasts from invading the area and filling it with autologous bone or an artificial bone replacement material to promote the proliferation of osteoblasts. | While sinus lift and socket lift are performed to build bone inside the alveolar bone (jaw bone), GBR is performed to build bone outside the alveolar bone (i.e., the surface where the bone meets the gums). It is performed when the alveolar bone is not wide or high enough. | Advantages: By increasing the amount of bone, it is possible to “secure the amount of bone” necessary for implant placement and to place the implant in the appropriate position, which results in “high stability”. Disadvantages: When using autologous bone, “bone harvesting surgery” is required separately from implant placement, “treatment time is long”, and “it is not suitable for smokers and diabetics”. |
| Socket preservation | Socket preservation is an alveolar bone preservation technique used to minimize bone resorption by placing a bone filler in the extraction socket and sealing the gingiva at the time of tooth extraction. This technique is effective in cases of significant alveolar bone resorption or when a tooth is to be extracted, but an implant is desired in the future. | Socket preservation is a prophylactic technique to prevent “bone resorption” after tooth extraction. This technique is performed in conjunction with tooth extraction. | Advantages: The purpose of extraction is to prevent bone resorption after tooth extraction and to preserve the alveolar crest morphology rather than to perform an osteogenic procedure. If the alveolar crest is preserved, it can be used to support later dental implants or bridges. Disadvantages: This technique is not applicable if bone resorption is severe during tooth extraction. |
| Product Name | Summary | Characteristics |
|---|---|---|
| REGROTH® (Active ingredient: Trafermin (genetical recombination), 0.3% basic Fibroblast Growth Factor: FGF-2, Kaken Pharmaceutical Co., LTD., Tokyo, Japan) | An artificially purified basic fibroblast growth factor, which is expected to regenerate lost alveolar bone and periodontal ligament by increasing the proliferation of periodontal tissue cells. | It can regenerate bone, cementum, and periodontal ligament of a tooth that is about to fall out. It has a high ability to regenerate the alveolar bone. |
| Emdogain® Gel (Straumann, Basel, Switzerland) | A periodontal tissue regeneration material containing enamel matrix protein, which plays a vital role in tooth development. This protein may be useful for periodontal tissue regeneration. | Because of the large number of cases treated, there is a wealth of evidence (e.g., papers) and a relatively high degree of certainty. It is compatible with various bone replacement materials used as bone substitutes and can be easily used in combination with bone replacement materials. |
| Geistlich Bio-Oss® (Geistlich Pharma AG, Wolhusen, Switzerland) | A bone grafting material, which is expected to promote bone regeneration when applied to bone defects caused by periodontal disease. | It is a natural bovine-derived porous bone filler with excellent osteoconductivity. It promotes growth and provides the bone volume necessary for implant placement in alveolar bone regeneration and augmentation. It is also highly compatible with the human body. It reduces the risk of infection. |
| Cytrans® Granules (Carbonated apatite granules, GC Corporation, Tokyo, Japan). | A bone grafting material composed primarily of carbonate apatite, which is expected to promote bone regeneration when applied to bone defects caused by periodontal disease. | The main component, carbonate apatite, has the same composition as the bone, so it is efficiently replaced by the patient’s own bone, achieving strong osseointegration while maintaining the target bone surface height. |
| Cytrans® Elashield (GC Corporation, Tokyo, Japan). | An absorbable membrane prepared from a chemically synthesized heavy polymeric material, which is expected to promote bone regeneration when applied to alveolar bone defects along with bone grafting materials. | The raw material is a fully synthetic polymer with a long absorption period suitable for GBR. It is designed to be easy to use and prevent postoperative gingival fissures because of its moderate firmness, flexibility, and ability to follow the shape of the bone. It can be used not only with autologous bone but also with bone replacement materials such as Cystrans Granules. |
| Geistlich Bio-Gide® (Geistlich Pharma AG, Wolhusen, Switzerland) | An absorbable collagen membrane, which is expected to promote bone regeneration when applied to alveolar bone defects along with bone graft materials. | It has excellent tissue integration and strongly promotes bone regeneration. Due to its natural collagen structure, it is less prone to rupture than other membranes, resulting in fewer problems. The procedure is simple because membrane removal is not required. The burden on the patient is greatly reduced. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuchida, S.; Nakayama, T. Recent Clinical Treatment and Basic Research on the Alveolar Bone. Biomedicines 2023, 11, 843. https://doi.org/10.3390/biomedicines11030843
Tsuchida S, Nakayama T. Recent Clinical Treatment and Basic Research on the Alveolar Bone. Biomedicines. 2023; 11(3):843. https://doi.org/10.3390/biomedicines11030843
Chicago/Turabian StyleTsuchida, Sachio, and Tomohiro Nakayama. 2023. "Recent Clinical Treatment and Basic Research on the Alveolar Bone" Biomedicines 11, no. 3: 843. https://doi.org/10.3390/biomedicines11030843
APA StyleTsuchida, S., & Nakayama, T. (2023). Recent Clinical Treatment and Basic Research on the Alveolar Bone. Biomedicines, 11(3), 843. https://doi.org/10.3390/biomedicines11030843







