Neopterin, the Cell-Mediated Immune Response Biomarker, in Inflammatory Periodontal Diseases: A Narrative Review of a More than Fifty Years Old Biomarker
Abstract
1. Introduction
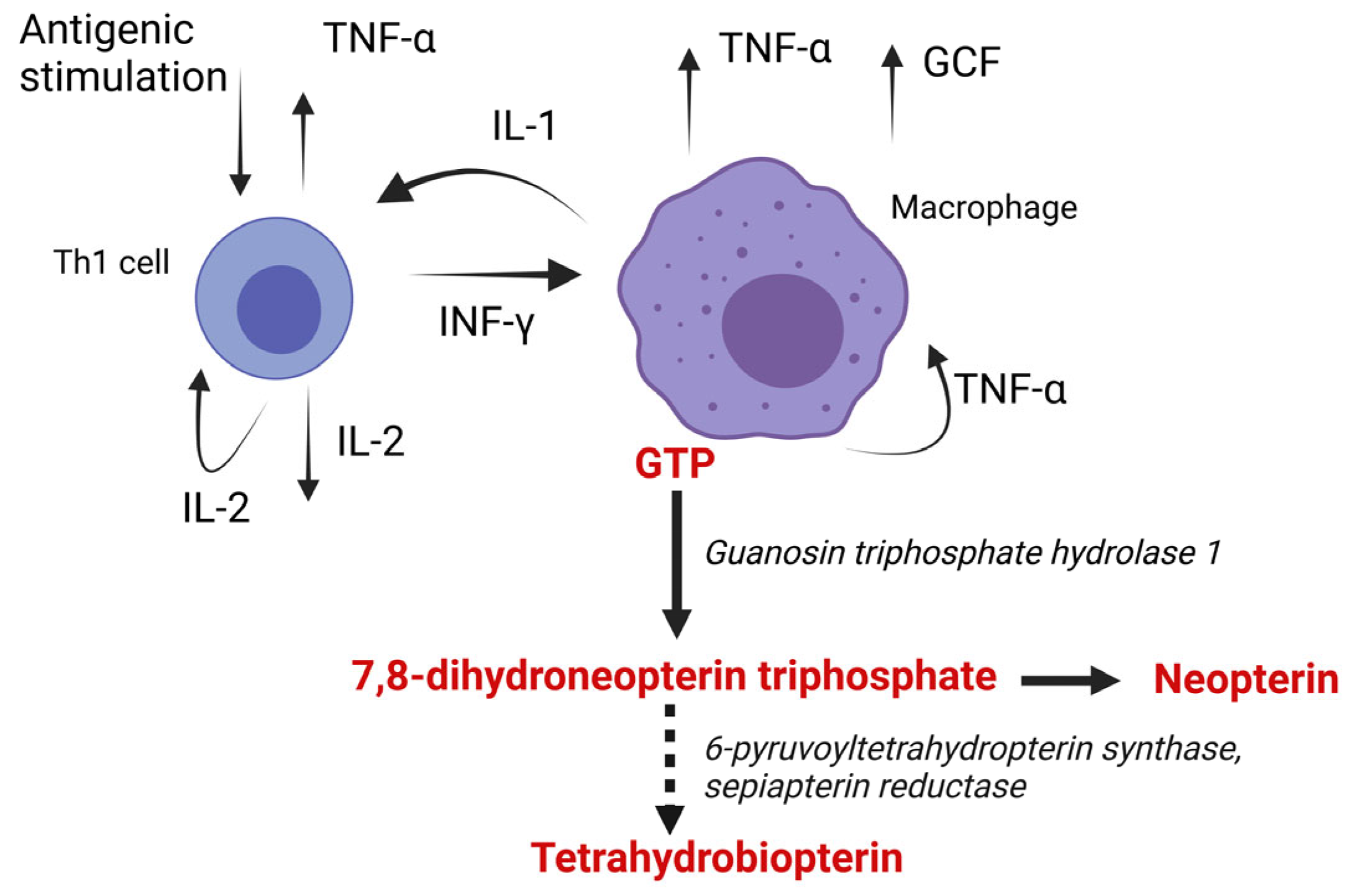
2. Synthesis of Neopterin
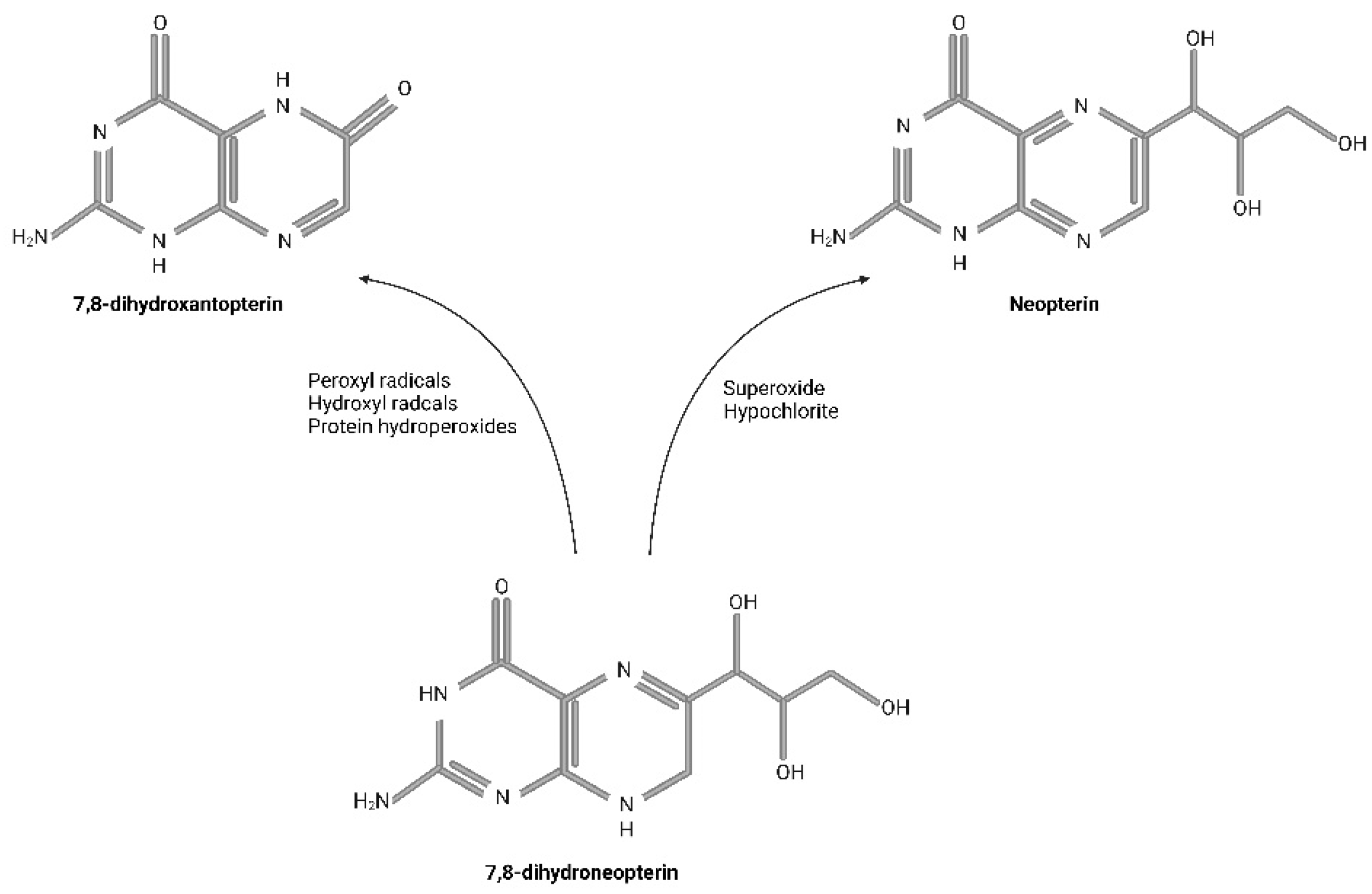
3. Biologic Impact of Neopterin
4. Neopterin as a Biomarker
5. Neopterin in Particular Diseases and Conditions
5.1. Neopterin and Cardiovascular System
5.2. Neopterin and Bacterial and Viral Diseases
5.3. Neopterin and Degenerative Diseases, Autoimmune Diseases, Tumours, and Other Conditions
6. Neopterin and Periodontitis
6.1. Periodontal Inflammation
6.2. Neopterin in Gingival Crevicular Fluid
6.3. Neopterin in Oral Fluid
6.4. Serum
6.5. Urine
6.6. Limitation of Neopterin Assessment in Periodontitis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muller, M.M.; Curtius, H.C.; Herold, M.; Huber, C.H. Neopterin in Clinical Practice. Clin. Chim. Acta 1991, 201, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, D.; Hausen, A.; Reibnegger, G.; Werner, E.R.; Dierich, M.P.; Wachter, H. Neopterin as a Marker for Activated Cell-Mediated Immunity: Application in HIV Infection. Immunol. Today 1988, 9, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Murr, C.; Widner, B.; Wirleitner, B.; Fuchs, D. Neopterin as a Marker for Immune System Activation. Curr. Drug Metab. 2002, 3, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Rembold, H.; Buschmann, L. Struktur und Synthese des Neopterins. Chem. Ber. 1963, 96, 1406–1410. [Google Scholar] [CrossRef]
- Hamerlinck, F.F. Neopterin: A Review. Exp. Dermatol. 1999, 8, 167–176. [Google Scholar] [CrossRef]
- Rembold, H.; Buschmann, L. Untersuchungen Über Die Pteridine Der Bienenpuppe (Apis Mellifica). Justus Liebigs Ann. Der Chem. 1963, 662, 72–82. [Google Scholar] [CrossRef]
- Sakurai, A.; Goto, M. Neopterin: Isolation from Human Urine. J. Biochem. 1967, 61, 142–145. [Google Scholar] [CrossRef]
- Schneider-Crease, I.A.; Feder, J.A.; Baniel, A.; McCann, C.; Haile, A.A.; Abebe, B.; Fitzgerald, L.; Gomery, M.A.; Simberloff, R.A.; Petrie, Z.L.; et al. Urinary Neopterin Reflects Immunological Variation Associated with Age, Helminth Parasitism, and the Microbiome in a Wild Primate. Sci. Rep. 2022, 12, 21307. [Google Scholar] [CrossRef]
- Rasmi, Y.; Heidari, N.; Kübra Kırboğa, K.; Hatamkhani, S.; Tekin, B.; Alipour, S.; Naderi, R.; Farnamian, Y.; Akca, I. The Importance of Neopterin in COVID-19: The Prognostic Value and Relation with the Disease Severity. Clin. Biochem. 2022, 104, 1–12. [Google Scholar] [CrossRef]
- Heneberk, O.; Vernerova, A.; Kujovska Krcmova, L.; Wurfelova, E.; Radochova, V. Neopterin Levels in Periodontitis and after Nonsurgical Periodontal Therapy: Evaluation of Gingival Crevicular Fluid, Oral Fluid, Serum and Urinary Samples—A Case-Control Study. Biomedicines 2022, 10, 3200. [Google Scholar] [CrossRef]
- Pink, R.; Melichar, B.; Tomandl, J.; Blažková, L.; Tvrdý, P.; Zapletalová, J. Salivary Neopterin Concentrations in Patients with Cancer of the Oral Cavity. Pteridines 2016, 27, 53–58. [Google Scholar] [CrossRef]
- Dogheim, G.M.; Amralla, M.T.; Werida, R.H. Role of Neopterin as an Inflammatory Biomarker in Congestive Heart Failure with Insights on Effect of Drug Therapies on Its Level. Inflammopharmacology 2022, 30, 1617–1622. [Google Scholar] [CrossRef]
- Labouret, M.; Costi, S.; Bondet, V.; Trebossen, V.; Le Roux, E.; Ntorkou, A.; Bartoli, S.; Auvin, S.; Bader-Meunier, B.; Baudouin, V.; et al. Juvenile Neuropsychiatric Systemic Lupus Erythematosus: Identification of Novel Central Neuroinflammation Biomarkers. J. Clin. Immunol. 2022, 43, 615–624. [Google Scholar] [CrossRef]
- Huber, C.; Batchelor, J.R.; Fuchs, D.; Hausen, A.; Lang, A.; Niederwieser, D.; Reibnegger, G.; Swetly, P.; Troppmair, J.; Wachter, H. Immune Response-Associated Production of Neopterin. Release from Macrophages Primarily under Control of Interferon-Gamma. J. Exp. Med. 1984, 160, 310–316. [Google Scholar] [CrossRef]
- Werner, E.R.; Werner-Felmayer, G.; Fuchs, D.; Hausen, A.; Reibnegger, G.; Yim, J.J.; Pfleiderer, W.; Wachter, H. Tetrahydrobiopterin Biosynthetic Activities in Human Macrophages, Fibroblasts, THP-1, and T 24 Cells. GTP-Cyclohydrolase I Is Stimulated by Interferon-Gamma, and 6-Pyruvoyl Tetrahydropterin Synthase and Sepiapterin Reductase Are Constitutively Present. J. Biol. Chem. 1990, 265, 3189–3192. [Google Scholar] [CrossRef]
- Schoedon, G.; Troppmair, J.; Fontana, A.; Huber, C.; Curtius, H.-C.; Niederwieser, A. Biosynthesis and Metabolism of Pterins in Peripheral Blood Mononuclear Cells and Leukemia Lines of Man and Mouse. Eur. J. Biochem. 1987, 166, 303–310. [Google Scholar] [CrossRef]
- Gieseg, S.P.; Baxter-Parker, G.; Lindsay, A. Neopterin, Inflammation, and Oxidative Stress: What Could We Be Missing? Antioxidants 2018, 7, 80. [Google Scholar] [CrossRef]
- Janmale, T.V.; Lindsay, A.; Gieseg, S.P. Nucleoside Transporters Are Critical to the Uptake and Antioxidant Activity of 7,8-Dihydroneopterin in Monocytic Cells. Free Radic. Res. 2020, 54, 341–350. [Google Scholar] [CrossRef]
- Werner-Felmayer, G.; Werner, E.R.; Fuchs, D.; Hausen, A.; Reibnegger, G.; Wachter, H. Characteristics of Interferon Induced Tryptophan Metabolism in Human Cells in Vitro. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 1989, 1012, 140–147. [Google Scholar] [CrossRef]
- Troppmair, J.; Nachbaur, K.; Herold, M.; Aulitzky, W.; Tilg, H.; Gastl, G.; Bieling, P.; Kotlan, B.; Flener, R.; Mull, B.; et al. In-Vitro and in-Vivo Studies on the Induction of Neopterin Biosynthesis by Cytokines, Alloantigens and Lipopolysaccharide (LPS). Clin. Exp. Immunol. 1988, 74, 392–397. [Google Scholar]
- Antoniades, C.; Cunnington, C.; Antonopoulos, A.; Neville, M.; Margaritis, M.; Demosthenous, M.; Bendall, J.; Hale, A.; Cerrato, R.; Tousoulis, D.; et al. Induction of Vascular GTP-Cyclohydrolase I and Endogenous Tetrahydrobiopterin Synthesis Protect Against Inflammation-Induced Endothelial Dysfunction in Human Atherosclerosis. Circulation 2011, 124, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Gieseg, S.P.; Reibnegger, G.; Wachter, H.; Esterbauer, H. 7,8 Dihydroneopterin Inhibits Low Density Lipoprotein Oxidation in Vitro. Evidence That This Macrophage Secreted Pteridine Is an Anti-Oxidant. Free Radic. Res. 1995, 23, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Gieseg, S.P.; Crone, E.M.; Flavall, E.A.; Amit, Z. Potential to Inhibit Growth of Atherosclerotic Plaque Development through Modulation of Macrophage Neopterin/7,8-Dihydroneopterin Synthesis: Effect of Neopterin/7,8-Dihydroneopterin. Br. J. Pharmacol. 2008, 153, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.; Rait, C.; Platt, A.; Gieseg, S.P. Protein and Thiol Oxidation in Cells Exposed to Peroxyl Radicals Is Inhibited by the Macrophage Synthesised Pterin 7,8-Dihydroneopterin. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2002, 1591, 139–145. [Google Scholar] [CrossRef]
- Kojima, S.; Nomura, T.; Icho, T.; Kajiwara, Y.; Kitabatake, K.; Kubota, K. Inhibitory Effect of Neopterin on NADPH-Dependent Superoxide-Generating Oxidase of Rat Peritoneal Macrophages. FEBS Lett. 1993, 329, 125–128. [Google Scholar] [CrossRef]
- Gieseg, S.P.; Maghzal, G.; Glubb, D. Protection of Erythrocytes by the Macrophage Synthesized Antioxidant 7,8 Dihydroneopterin. Free Radic. Res. 2001, 34, 123–136. [Google Scholar] [CrossRef]
- Davies, M.J. Protein Oxidation and Peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Zaloga, G.P. Narrative Review of N-3 Polyunsaturated Fatty Acid Supplementation upon Immune Functions, Resolution Molecules and Lipid Peroxidation. Nutrients 2021, 13, 662. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef]
- Gieseg, S.P.; Pearson, J.; Firth, C.A. Protein Hydroperoxides Are a Major Product of Low Density Lipoprotein Oxidation During Copper, Peroxyl Radical and Macrophage-Mediated Oxidation. Free Radic. Res. 2003, 37, 983–991. [Google Scholar] [CrossRef]
- Gieseg, S.P.; Cato, S. Inhibition of THP-1 Cell-Mediated Low-Density Lipoprotein Oxidation by the Macrophage-Synthesised Pterin, 7,8-Dihydroneopterin. Redox Rep. 2003, 8, 113–115. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Raghavamenon, A.; Garelnabi, M.O.; Santanam, N. Oxidized Low-Density Lipoprotein. In Free Radicals and Antioxidant Protocols; Uppu, R.M., Murthy, S.N., Pryor, W.A., Parinandi, N.L., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 610, pp. 403–417. ISBN 978-1-58829-710-5. [Google Scholar]
- Baxter-Parker, G.; Prebble, H.M.; Cross, S.; Steyn, N.; Shchepetkina, A.; Hock, B.D.; Cousins, A.; Gieseg, S.P. Neopterin Formation through Radical Scavenging of Superoxide by the Macrophage Synthesised Antioxidant 7,8-Dihydroneopterin. Free Radic. Biol. Med. 2020, 152, 142–151. [Google Scholar] [CrossRef]
- Ghodsian, N.; Yeandle, A.; Hock, B.D.; Gieseg, S.P. CD36 down Regulation by the Macrophage Antioxidant 7,8-Dihydroneopterin through Modulation of PPAR-γ Activity. Free Radic. Res. 2022, 56, 366–377. [Google Scholar] [CrossRef]
- Gieseg, S.P.; Whybrow, J.; Glubb, D.; Rait, C. Protection of U937 Cells from Free Radical Damage by the Macrophage Synthesized Antioxidant 7,8-Dihydroneopterin. Free Radic. Res. 2001, 35, 311–318. [Google Scholar] [CrossRef]
- Denz, H.; Fuchs, D.; Huber, H.; Nachbaur, D.; Reibnegger, G.; Thaler, J.; Werner, E.R.; Wachter, H. Correlation between Neopterin, Interferon-Gamma and Haemoglobin in Patients with Haematological Disorders. Eur. J. Haematol. 1990, 44, 186–189. [Google Scholar] [CrossRef]
- Werner, E.R.; Bichler, A.; Daxenbichler, G.; Fuchs, D.; Fuith, L.C.; Hausen, A.; Hetzel, H.; Reibnegger, G.; Wachter, H. Determination of Neopterin in Serum and Urine. Clin. Chem. 1987, 33, 62–66. [Google Scholar] [CrossRef]
- Berdowska, A.; Zwirska-Korczala, K. Neopterin Measurement in Clinical Diagnosis. J. Clin. Pharm. Ther. 2001, 26, 319–329. [Google Scholar] [CrossRef]
- Jinkawa, A.; Shimizu, M.; Nishida, K.; Kaneko, S.; Usami, M.; Sakumura, N.; Irabu, H.; Takakuwa, M.; Inoue, N.; Mizuta, M.; et al. Cytokine Profile of Macrophage Activation Syndrome Associated with Kawasaki Disease. Cytokine 2019, 119, 52–56. [Google Scholar] [CrossRef]
- Wachter, H.; Fuchs, D.; Hausen, A.; Reibnegger, G.; Werner, E.R. Neopterin as Marker for Activation of Cellular Immunity: Immunologic Basis and Clinical Application. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 1989; Volume 27, pp. 81–141. ISBN 978-0-12-010327-0. [Google Scholar]
- Widner, B.; Murr, C.; Wirleitner, B.; Mayr, C.; Spöttl, N.; Baier-Bitterlich, G.; Fuchs, D. The Importance of Neopterin as a Laboratory Diagnostic Marker of Immune Activation. Pteridines 1999, 10, 101–111. [Google Scholar] [CrossRef]
- Ozmeric, N.; Baydar, T.; Bodur, A.; Engin, A.B.; Uraz, A.; Eren, K.; Sahin, G. Level of Neopterin, a Marker of Immune Cell Activation in Gingival Crevicular Fluid, Saliva, and Urine in Patients with Aggressive Periodontitis. J. Periodontol. 2002, 73, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Plata-Nazar, K.; Jankowska, A. Clinical Usefulness of Determining the Concentration of Neopterin. Pteridines 2011, 22, 77–89. [Google Scholar] [CrossRef]
- Estelberge, W.; Weiss, G.; Petek, W.; Paletta, B.; Wächter, H.; Reibnegger, G. Determination of Renal Clearance of Neopterin by a Pharmacokinetic Approach. FEBS Lett. 1993, 329, 13–16. [Google Scholar] [CrossRef]
- Hagberg, L.; Dotevall, L.; Norkrans, G.; Larsson, M.; Wachter, H.; Fuchs, D. Cerebrospinal Fluid Neopterin Concentrations in Central Nervous System Infection. J. Infect. Dis. 1993, 168, 1285–1288. [Google Scholar] [CrossRef]
- Bogner, J.R.; Junge-Hülsing, B.; Kronawitter, U.; Sadri, I.; Matuschke, A.; Goebel, F.-D. Expansion of Neopterin and Beta2-Microglobulin in Cerebrospinal Fluid Reaches Maximum Levels Early and Late in the Course of Human Immunodeficiency Virus Infection. Clin. Investig. 1992, 70, 665–669. [Google Scholar] [CrossRef]
- Dotevall, L.; Hagberg, L.; Fuchs, D.; Reibnegger, G.; Wachter, H. Cerebrospinal Fluid and Serum Neopterin Levels in Patients with Lyme Neuroborreliosis. Infection 1990, 18, 210–214. [Google Scholar] [CrossRef]
- Andersson, L.M.; Hagberg, L.; Fuchs, D.; Svennerholm, B.; Gisslen, M. Increased Blood-Brain Barrier Permeability in Neuro-Asymptomatic HIV-1-Infected Individualscorrelation with Cerebrospinal Fluid HIV-1 RNA and Neopterin Levels. J. Neurovirol. 2001, 7, 542–547. [Google Scholar] [CrossRef]
- Krcmova, L.K.; Cervinkova, B.; Solichova, D.; Sobotka, L.; Hansmanova, L.; Melichar, B.; Solich, P. Fast and Sensitive HPLC Method for the Determination of Neopterin, Kynurenine and Tryptophan in Amniotic Fluid, Malignant Effusions and Wound Exudates. Bioanalysis 2015, 7, 2751–2762. [Google Scholar] [CrossRef]
- Vernerová, A.; Krčmová, L.K.; Heneberk, O.; Radochová, V.; Strouhal, O.; Kašparovský, A.; Melichar, B.; Švec, F. Chromatographic Method for the Determination of Inflammatory Biomarkers and Uric Acid in Human Saliva. Talanta 2021, 233, 122598. [Google Scholar] [CrossRef]
- Prasanna, J.S.; Sumadhura, C. Biochemical Analysis of Three Biological Fluids and Its Response to Non-Surgical Periodontal Therapy in Pre and Postmenopausal Women with Periodontitis. J. Menopausal Med. 2019, 25, 149. [Google Scholar] [CrossRef]
- Vernerová, A.; Krčmová, L.K.; Heneberk, O.; Radochová, V.; Švec, F. Liquid Chromatography Method with Tandem Mass Spectrometry and Fluorescence Detection for Determination of Inflammatory Biomarkers in Gingival Crevicular Fluid as a Tool for Diagnosis of Periodontal Disease. J. Pharm. Biomed. Anal. 2022, 212, 114644. [Google Scholar] [CrossRef]
- Solarino, G.; Bizzoca, D.; Moretti, L.; Vicenti, G.; Piazzolla, A.; Moretti, B. What’s New in the Diagnosis of Periprosthetic Joint Infections: Focus on Synovial Fluid Biomarkers. Trop. Med. Infect. Dis. 2022, 7, 355. [Google Scholar] [CrossRef]
- Melichar, B.; Solichova, D.; Freedman, R.S. Neopterin as an Indicator of Immune Activation and Prognosis in Patients with Gynecological Malignancies. Int. J. Gynecol. Cancer 2006, 16, 240–252. [Google Scholar] [CrossRef]
- Dhondt, J.-L.; Tilmont, P.; Ringel, J.; Farriaux, J.-P. Pterins Analysis in Amniotic Fluid for the Prenatal Diagnosis of GTP Cyclohydrolase Deficiency. J. Inher. Metab. Dis. 1990, 13, 879–882. [Google Scholar] [CrossRef]
- Firth, C.A.; Laing, A.D.; Baird, S.K.; Pearson, J.; Gieseg, S.P. Inflammatory Sites as a Source of Plasma Neopterin: Measurement of High Levels of Neopterin and Markers of Oxidative Stress in Pus Drained from Human Abscesses. Clin. Biochem. 2008, 41, 1078–1083. [Google Scholar] [CrossRef]
- Reibnegger, G.; Huber, L.A.; Jürgens, G.; Schönitzer, D.; Werner, E.R.; Wachter, H.; Wick, G.; Traill, K.N. Approach to Define “Normal Aging” in Man. Immune Function, Serum Lipids, Lipoproteins and Neopterin Levels. Mech. Ageing Dev. 1988, 46, 67–82. [Google Scholar] [CrossRef]
- Sack, U.; Burkhardt, U.; Borte, M.; Schädlich, H.; Berg, K.; Emmrich, F. Age-Dependent Levels of Select Immunological Mediators in Sera of Healthy Children. Clin. Diagn. Lab. Immunol. 1998, 5, 28–32. [Google Scholar] [CrossRef]
- Parrak, V.; Secnik, P.; Skrakova, M. Neopterin Screening of Blood Donations. Pteridines 2006, 17, 105–106. [Google Scholar] [CrossRef]
- Frick, B.; Schroecksnadel, K.; Neurauter, G.; Leblhuber, F.; Fuchs, D. Increasing Production of Homocysteine and Neopterin and Degradation of Tryptophan with Older Age. Clin. Biochem. 2004, 37, 684–687. [Google Scholar] [CrossRef]
- Radunovic, N.; Kuczynski, E.; Rebarber, A.; Nastic, D.; Lockwood, C.J. Neopterin Concentrations in Fetal and Maternal Blood: A Marker of Cell-Mediated Immune Activation. Am. J. Obstet. Gynecol. 1999, 181, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, K.; Sumi-Ichinose, C.; Suganuma, Y.; Kano, T.; Ihira, N.; Nagatsu, T.; Kondo, K. Salivary Neopterin and Related Pterins: Their Comparison to Those in Plasma and Changes in Individuals. J. Biochem. 2021, 170, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Dántola, M.L.; Vignoni, M.; Capparelli, A.L.; Lorente, C.; Thomas, A.H. Stability of 7,8-Dihydropterins in Air-Equilibrated Aqueous Solutions. Helv. Chim. Acta 2008, 91, 411–425. [Google Scholar] [CrossRef]
- Flavall, E.A.; Crone, E.M.; Moore, G.A.; Gieseg, S.P. Dissociation of Neopterin and 7,8-Dihydroneopterin from Plasma Components before HPLC Analysis. J. Chromatogr. B 2008, 863, 167–171. [Google Scholar] [CrossRef]
- Jiménez Girón, A.; Martín-Tornero, E.; Hurtado Sánchez, M.C.; Durán Merás, I.; Espinosa Mansilla, A. A Simple HPLC-ESI-MS Method for the Direct Determination of Ten Pteridinic Biomarkers in Human Urine. Talanta 2012, 101, 465–472. [Google Scholar] [CrossRef]
- Moutereau, S.; Ech Chad, N.; Devanlay, M.; Esmilaire, L.; Loric, S. Improved Neopterin ELISA Kit: A Good Compromise between HPLC Results and Clinical Practice. Clin. Chem. Lab. Med. 2011, 49, 99–112. [Google Scholar] [CrossRef]
- Rokos, H.; Bienhaus, G.; Gadow, A.; Rokos, K. Determination of neopterin and reduced neopterins by radioimmunoassay. In February 23–March 2, 1985, St. Christoph, Arlberg, Austria; Wachter, H., Curtius, H.C., Pfleiderer, W., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 1985; pp. 73–84. ISBN 978-3-11-086056-6. [Google Scholar]
- Hammerer-Lercher, A.; Moser, C.; Leichtfried, V.; Schobersberger, W.; Griesmacher, A.; Fuchs, D. Comparison of a Commercial Urinary Neopterin Radioimmunoassay with High Performance Liquid Chromatography. Clin. Chem. Lab. Med. 2012, 50, 1075–1078. [Google Scholar] [CrossRef]
- Makoto, S.; Tokio, Y.; Takashi, S.; Sadao, M.; Toshiharu, N. Polarization Fluoroimmunoassay of Biopterin and Neopterin in Human Urine. Clin. Chim. Acta 1984, 138, 275–282. [Google Scholar] [CrossRef]
- Vrecko, K.; Staedtler, P.; Mischak, I.; Maresch, L.; Reibnegger, G. Periodontitis and Concentrations of the Cellular Immune Activation Marker Neopterin in Saliva and Urine. Clin. Chim. Acta 1997, 268, 31–40. [Google Scholar] [CrossRef]
- Bodur, A.; Baydar, T.; Ozmeric, N.; Engin, A.; Uraz, A.; Eren, K.; Sahin, G. Neopterin Profile to Evaluate the Effectiveness of Treatment in Aggressive Periodontitis. Pteridines 2003, 14, 77–81. [Google Scholar] [CrossRef]
- Prasanna, J.S.; Sumadhura, C.; Karunakar, P.; Rohini, N. Comparative Evaluation of Salivary Neopterin Levels and Its Effects to Periodontal Therapy in Pre- and Post-Menopausal Women. J. Menopausal Med. 2017, 23, 32–41. [Google Scholar] [CrossRef]
- Mahendra, L.; Mahendra, J.; Borra, S.K.; Nagarajan, A. Estimation of Salivary Neopterin in Chronic Periodontitis. Indian J. Dent. Res. 2014, 25, 794–796. [Google Scholar] [CrossRef]
- Adachi, T.; Naruko, T.; Itoh, A.; Komatsu, R.; Abe, Y.; Shirai, N.; Yamashita, H.; Ehara, S.; Nakagawa, M.; Kitabayashi, C.; et al. Neopterin Is Associated with Plaque Inflammation and Destabilisation in Human Coronary Atherosclerotic Lesions. Heart 2006, 93, 1537–1541. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Dziubek, W.; Tylutka, A.; Wacka, E.; Morawin, B.; Bulinska, K.; Stefanska, M.; Wozniewski, M.; Szuba, A. Assessment of Serum Neopterin as a Biomarker in Peripheral Artery Disease. Diagnostics 2021, 11, 1911. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Anzaldi, M.; Fiore, V.; Candido, S.; Di Marco, R.; Mangano, K.; Quattrocchi, C.; Neri, S. Neopterin: A Potential Marker in Chronic Peripheral Arterial Disease. Mol. Med. Rep. 2013, 7, 1855–1858. [Google Scholar] [CrossRef]
- McDermott, M.M.; Guralnik, J.M.; Corsi, A.; Albay, M.; Macchi, C.; Bandinelli, S.; Ferrucci, L. Patterns of Inflammation Associated with Peripheral Arterial Disease: The InCHIANTI Study. Am. Heart J. 2005, 150, 276–281. [Google Scholar] [CrossRef]
- Sugioka, K.; Naruko, T.; Hozumi, T.; Nakagawa, M.; Kitabayashi, C.; Ikura, Y.; Shirai, N.; Matsumura, Y.; Ehara, S.; Ujino, K.; et al. Elevated Levels of Neopterin Are Associated with Carotid Plaques with Complex Morphology in Patients with Stable Angina Pectoris. Atherosclerosis 2010, 208, 524–530. [Google Scholar] [CrossRef]
- Avanzas, P. Markers of Inflammation and Multiple Complex Stenoses (Pancoronary Plaque Vulnerability) in Patients with Non-ST Segment Elevation Acute Coronary Syndromes. Heart 2004, 90, 847–852. [Google Scholar] [CrossRef]
- Ren, J.; Chen, Y.-B.; Zhang, Y.-Y.; Zhou, Q.-B.; Chen, S.; Yang, J.-Y.; Tao, J. Decreased Circulating Neopterin Is Associated with Increased Arterial Elasticity: A Beneficial Role of Periodontal Treatment. Aust. Dent. J. 2016, 61, 76–83. [Google Scholar] [CrossRef]
- Parenica, J.; Kala, P.; Mebazaa, A.; Littnerova, S.; Benesova, K.; Tomandl, J.; Goldbergová Pavkova, M.; Jarkovský, J.; Spinar, J.; Tomandlova, M.; et al. Activation of the Nitric Oxide Pathway and Acute Myocardial Infarction Complicated by Acute Kidney Injury. Cardiorenal Med. 2020, 10, 85–96. [Google Scholar] [CrossRef]
- Grammer, T.B.; Fuchs, D.; Boehm, B.O.; Winkelmann, B.R.; Maerz, W. Neopterin as a Predictor of Total and Cardiovascular Mortality in Individuals Undergoing Angiography in the Ludwigshafen Risk and Cardiovascular Health Study. Clin. Chem. 2009, 55, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Nechita, V.-I.; Hajjar, N.A.; Drugan, C.; Cătană, C.-S.; Moiş, E.; Nechita, M.-A.; Graur, F. Chitotriosidase and Neopterin as Potential Biomarkers for the Evaluation of Complicated Cholecystitis—A Pilot Study. JCM 2023, 12, 1641. [Google Scholar] [CrossRef] [PubMed]
- Kamal, Z.B.; Naji, R.E.; Ali, H.A. Comparative Study between Neopterin and Alvarado Score in the Diagnosis of Acute Appendicitis and Its Severity. Maced. J. Med. Sci. 2021, 9, 42–47. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Rezaei, N. Elevated Neopterin in Tuberculosis and Co-Infection with HIV and the Effect of Treatment: A Systematic Review, Meta-Analysis, and Meta-Regression. Int. Immunopharmacol. 2022, 111, 109147. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.L.; Chan, J.; Triebold, K.J.; Dalton, D.K.; Stewart, T.A.; Bloom, B.R. An Essential Role for Interferon Gamma in Resistance to Mycobacterium Tuberculosis Infection. J. Exp. Med. 1993, 178, 2249–2254. [Google Scholar] [CrossRef]
- Kaneko, S.; Hatasaki, K.; Ueno, K.; Fujita, S.; Igarashi, N.; Kuroda, M.; Wada, T. One-Year-Old Boy with Refractory Listeria Monocytogenes Meningitis Due to Persistent Hypercytokinemia. J. Infect. Chemother. 2022, 28, 1682–1686. [Google Scholar] [CrossRef]
- Kip, A.E.; Wasunna, M.; Alves, F.; Schellens, J.H.M.; Beijnen, J.H.; Musa, A.M.; Khalil, E.A.G.; Dorlo, T.P.C. Macrophage Activation Marker Neopterin: A Candidate Biomarker for Treatment Response and Relapse in Visceral Leishmaniasis. Front. Cell Infect. Microbiol. 2018, 8, 181. [Google Scholar] [CrossRef]
- Wirleitner, B.; Rudzite, V.; Neurauter, G.; Murr, C.; Kalnins, U.; Erglis, A.; Trusinskis, K.; Fuchs, D. Immune Activation and Degradation of Tryptophan in Coronary Heart Disease. Eur. J. Clin. Investig. 2003, 33, 550–554. [Google Scholar] [CrossRef]
- Mildvan, D.; Spritzler, J.; Grossberg, S.E.; Fahey, J.L.; Johnston, D.M.; Schock, B.R.; Kagan, J. Serum Neopterin, an Immune Activation Marker, Independently Predicts Disease Progression in Advanced HIV-1 Infection. Clin. Infect. Dis. 2005, 40, 853–858. [Google Scholar] [CrossRef]
- Williams, M.E.; Stein, D.J.; Joska, J.A.; Naudé, P.J.W. Cerebrospinal Fluid Immune Markers and HIV-Associated Neurocognitive Impairments: A Systematic Review. J. Neuroimmunol. 2021, 358, 577649. [Google Scholar] [CrossRef]
- Ozger, H.S.; Dizbay, M.; Corbacioglu, S.K.; Aysert, P.; Demirbas, Z.; Tunccan, O.G.; Hizel, K.; Bozdayi, G.; Caglar, K. The Prognostic Role of Neopterin in COVID-19 Patients. J. Med. Virol. 2021, 93, 1520–1525. [Google Scholar] [CrossRef]
- Robertson, J.; Gostner, J.M.; Nilsson, S.; Andersson, L.-M.; Fuchs, D.; Gisslen, M. Serum Neopterin Levels in Relation to Mild and Severe COVID-19. BMC Infect. Dis. 2020, 20, 942. [Google Scholar] [CrossRef]
- Zheng, B.; Cao, K.-Y.; Chan, C.P.Y.; Choi, J.W.Y.; Leung, W.; Leung, M.; Duan, Z.-H.; Gao, Y.; Wang, M.; Di, B.; et al. Serum Neopterin for Early Assessment of Severity of Severe Acute Respiratory Syndrome. Clin. Immunol. 2005, 116, 18–26. [Google Scholar] [CrossRef]
- Gulcan, E.M.; Tirit, I.; Anil, A.; Adal, E.; Ozbay, G. Serum Neopterin Levels in Children with Hepatitis-B-Related Chronic Liver Disease and Its Relationship to Disease Severity. WJG 2008, 14, 6840. [Google Scholar] [CrossRef]
- Leonardi, S.; Avola, E.; Sciacca, A.; Gregorio, F.D.; Musumeci, S. Neopterin as a Marker of C Hepatitis in Thalassaemia Major. J. Pediatr. Gastroenterol. Nutr. 1991, 12, 315–318. [Google Scholar] [CrossRef]
- Reibnegger, G.; Auhuber, I.; Fuchs, D.; Hausen, A.; Judmaier, G.; Prior, C.; Werner, E.R.; Wachter, H. Urinary Neopterin Levels in Acute Viral Hepatitis. Hepatology 1988, 8, 771–774. [Google Scholar] [CrossRef]
- Miyaue, N.; Hosokawa, Y.; Yamanishi, Y.; Tada, S.; Ando, R.; Nagai, M. Clinical Use of CSF Neopterin Levels in CNS Demyelinating Diseases. J. Neurol. Sci. 2022, 441, 120385. [Google Scholar] [CrossRef] [PubMed]
- Widner, B.; Leblhuber, F.; Fuchs, D. Increased Neopterin Production and Tryptophan Degradation in Advanced Parkinson’s Disease. J. Neural Transm. 2002, 109, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Henríquez, G.; Lugo-Marín, J.; Gisbert, L.; Setién-Ramos, I.; Martínez-Gallo, M.; Pujol-Borrell, R.; Ramos-Quiroga, J.A. Activation of the Monocyte/Macrophage System and Abnormal Blood Levels of Lymphocyte Subpopulations in Individuals with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 14329. [Google Scholar] [CrossRef] [PubMed]
- Endres, D.; Frye, B.C.; Schlump, A.; Kuzior, H.; Feige, B.; Nickel, K.; Urbach, H.; Schiele, M.A.; Domschke, K.; Berger, B.; et al. Sarcoidosis and Obsessive-Compulsive Symptoms. J. Neuroimmunol. 2022, 373, 577989. [Google Scholar] [CrossRef] [PubMed]
- Bahrehmand, F.; Vaisi-Raygani, A.; Kiani, A.; Rahimi, Z.; Tavilani, H.; Ardalan, M.; Vaisi-Raygani, H.; Shakiba, E.; Pourmotabbed, T. Matrix Metalloproteinase 9 Polymorphisms and Systemic Lupus Erythematosus: Correlation with Systemic Inflammatory Markers and Oxidative Stress. Lupus 2015, 24, 597–605. [Google Scholar] [CrossRef]
- Abdel-Haq, A.; Kusnierz-Cabala, B.; Darczuk, D.; Sobuta, E.; Dumnicka, P.; Wojas-Pelc, A.; Chomyszyn-Gajewska, M. Interleukin-6 and Neopterin Levels in the Serum and Saliva of Patients with Lichen Planus and Oral Lichen Planus. J. Oral Pathol. Med. 2014, 43, 734–739. [Google Scholar] [CrossRef]
- Peng, Q.-L.; Zhang, Y.-M.; Liang, L.; Liu, X.; Ye, L.-F.; Yang, H.-B.; Zhang, L.; Shu, X.-M.; Lu, X.; Wang, G.-C. A High Level of Serum Neopterin Is Associated with Rapidly Progressive Interstitial Lung Disease and Reduced Survival in Dermatomyositis. Clin. Exp. Immunol. 2020, 199, 314–325. [Google Scholar] [CrossRef]
- El-Lebedy, D.; Hussein, J.; Ashmawy, I.; Mohammed, A.M. Serum Level of Neopterin Is Not a Marker of Disease Activity in Treated Rheumatoid Arthritis Patients. Clin. Rheumatol. 2017, 36, 1975–1979. [Google Scholar] [CrossRef]
- Videm, V.; Houge, I.S.; Liff, M.H.; Hoff, M. Inflammation Mediates Approximately One Quarter of Excess Relative All-Cause Mortality in Persons with Rheumatoid Arthritis: The Trøndelag Health Study. Sci. Rep. 2022, 12, 18599. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Melichar, B.; Spisarová, M.; Bartoušková, M.; Krčmová, L.K.; Javorská, L.; Študentová, H. Neopterin as a Biomarker of Immune Response in Cancer Patients. Ann. Transl. Med. 2017, 5, 280. [Google Scholar] [CrossRef]
- Ciocan, A.; Ciocan, R.A.; Al Hajjar, N.; Benea, A.M.; Pandrea, S.L.; Cătană, C.S.; Drugan, C.; Oprea, V.C.; Dîrzu, D.S.; Bolboacă, S.D. Exploratory Evaluation of Neopterin and Chitotriosidase as Potential Circulating Biomarkers for Colorectal Cancer. Biomedicines 2023, 11, 894. [Google Scholar] [CrossRef]
- Berchtold, J.; Murr, C.; Norer, B.; Waldhart, E.; Wachter, H.; Fuchs, D. Urinary Neopterin Excretion in Patients with Squamous Carcinoma of the Oral Cavity. Cancer Lett. 1995, 95, 227–232. [Google Scholar] [CrossRef]
- Murr, C.; Berchtold, J.; Norer, B.; Waldhart, E.; Wachter, H.; Fuchs, D. Neopterin as a Prognostic Parameter in Patients with Squamous-Cell Carcinomas of the Oral Cavity. Int. J. Cancer 1998, 79, 476–480. [Google Scholar] [CrossRef]
- Weinlich, G.; Murr, C.; Richardsen, L.; Winkler, C.; Fuchs, D. Decreased Serum Tryptophan Concentration Predicts Poor Prognosis in Malignant Melanoma Patients. Dermatology 2007, 214, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hausen, A.; Fuchs, D.; Grünewald, K.; Huber, H.; König, K.; Wechter, H. Urinary Neopterine as Marker for Haematological Neoplasias. Clin. Chim. Acta 1981, 117, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Aulitzky, W.; Frick, J.; Fuchs, D.; Hausen, A.; Reibnegger, G.; Wachter, H. Significance of Urinary Neopterin in Patients with Malignant Tumors of the Genitourinary Tract. Cancer 1985, 55, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Manes, G.; Spada, O.A.; Rabitti, P.G.; Feola, B.; Misso, S.; Minerva, A.; Uomo, G. Neopterin Serum Levels in Pancreatic Adenocarcinoma. IJGC 1999, 25, 31–38. [Google Scholar] [CrossRef]
- Zuo, H.; Tell, G.S.; Vollset, S.E.; Ueland, P.M.; Nygård, O.; Midttun, Ø.; Meyer, K.; Ulvik, A.; Eussen, S.J.P.M. Interferon-γ-Induced Inflammatory Markers and the Risk of Cancer: The Hordaland Health Study. Cancer 2014, 120, 3370–3377. [Google Scholar] [CrossRef]
- Bizjak, D.A.; Schulz, S.V.W.; John, L.; Schellenberg, J.; Bizjak, R.; Witzel, J.; Valder, S.; Kostov, T.; Schalla, J.; Steinacker, J.M.; et al. Running for Your Life: Metabolic Effects of a 160.9/230 Km Non-Stop Ultramarathon Race on Body Composition, Inflammation, Heart Function, and Nutritional Parameters. Metabolites 2022, 12, 1138. [Google Scholar] [CrossRef]
- Dantas de Lucas, R.; Caputo, F.; Mendes de Souza, K.; Sigwalt, A.R.; Ghisoni, K.; Lock Silveira, P.C.; Remor, A.P.; da Luz Scheffer, D.; Antonacci Guglielmo, L.G.; Latini, A. Increased Platelet Oxidative Metabolism, Blood Oxidative Stress and Neopterin Levels after Ultra-Endurance Exercise. J. Sport. Sci. 2014, 32, 22–30. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus Report of Workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions: Classification and Case Definitions for Periodontitis. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef]
- Newman, M.G.; Takei, H.H.; Klokkevold, P.R.; Carranza, F.A. (Eds.) Carranza’s Clinical Periodontology, 20th ed.; Elsevier Saunders: St. Louis, MO, USA, 2015; ISBN 978-0-323-22799-5. [Google Scholar]
- Meyle, J.; Chapple, I. Molecular Aspects of the Pathogenesis of Periodontitis. Periodontology 2000 2015, 69, 7–17. [Google Scholar] [CrossRef]
- Grossi, S.G.; Zambon, J.J.; Ho, A.W.; Koch, G.; Dunford, R.G.; Machtei, E.E.; Norderyd, O.M.; Genco, R.J. Assessment of Risk for Periodontal Disease. I. Risk Indicators for Attachment Loss. J. Periodontol. 1994, 65, 260–267. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, Fallacies and the Future. Periodontology 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, Y.; Duan, D.; Wang, P.; Xin, Y.; Bai, L.; Liu, Y.; Xu, Y. Enhanced Activity of Macrophage M1/M2 Phenotypes in Periodontitis. Arch. Oral Biol. 2018, 96, 234–242. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Zhou, L.; Bi, C.; Gao, L.; An, Y.; Chen, F.; Chen, F. Macrophage Polarization in Human Gingival Tissue in Response to Periodontal Disease. Oral Dis. 2019, 25, 265–273. [Google Scholar] [CrossRef]
- Arjunkumar, R.; Sudhakar, U.; Jayakumar, P.; Arunachalam, L.; Suresh, S.; Virupapuram, P. Comparative Analysis of Gingival Crevicular Fluid Neopterin Levels in Health and Periodontal Disease: A Biochemical Study. Indian J. Dent. Res. 2013, 24, 582–586. [Google Scholar] [CrossRef]
- Fenol, A.; Swetha, V.R.; Krishnan, S.; Perayil, J.; Vyloppillil, R.; Bhaskar, A.; Shereef, M.; Balakrishnan, B.; Puzhankara, L. Correlation of Salivary Neopterin and Plasma Fibrinogen Levels in Patients with Chronic Periodontitis and/or Type 2 Diabetes Mellitus. Pteridines 2017, 28, 177–183. [Google Scholar] [CrossRef]
- Patil, A.; Ranganath, V.; Naresh Kumar, C.; Naik, R.; John, A.; Pharande, S. Evaluation of Salivary Biomarkers of Periodontitis among Smokers and Nonsmokers: A Novel Study. J. Fam. Med. Prim. Care 2020, 9, 1136. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Kumar, M.S.; Ramachandraprasad, M.V.; Shikha, C. Gingival Crevicular Fluid Levels of Neopterin in Healthy Subjects and in Patients with Different Periodontal Diseases. J. Periodontol. 2007, 78, 1962–1967. [Google Scholar] [CrossRef]
- Prasanna, J.S.; Sumadhura, C.; Karunakar, P.; Rekharani, K.; Himabindu, G.; Manasa, A. Correlative Analysis of Plasma and Urine Neopterin Levels in the Pre- and Post-Menopausal Women with Periodontitis, Following Nonsurgical Periodontal Therapy. J. Indian Soc. Periodontol. 2017, 21, 276–284. [Google Scholar] [CrossRef]
- Turgut Cankaya, Z.; Bodur, A.; Tacoy, G.; Erguder, I.; Aktuna, D.; Cengel, A. The Effect of Periodontal Therapy on Neopterin and Vascular Cell Adhesion Molecule-1 Levels in Chronic Periodontitis Patients with and without Acute Myocardial Infarction: A Case-Control Study. J. Appl. Oral Sci. 2018, 26, e20170199. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, E.; Lamster, I.B. Analysis of Saliva for Periodontal Diagnosis: A Review. J. Clin. Periodontol. 2000, 27, 453–465. [Google Scholar] [CrossRef] [PubMed]

| Article | Disease or Condition | Comment |
|---|---|---|
| Zembron-Lacny et al. [77] | Atherosclerosis | Np played a crucial role in the atheromatous process and is useful in monitoring disease severity. |
| Avanzas et al. [81] | Acute coronary syndrome | Np was a sensitive biomarker for the prediction of the disruption of complex coronary artery lesion. |
| Nechita et al. [85] | Cholecystitis | Np was found to predict complicated cholecystitis. |
| Solarino et al. [55] | Periprosthetic joint infections | Np was a promising biomarker for the diagnosis of periprosthetic joint infection. |
| Saghazared and Rezaei [87] | Tuberculosis | Np levels were found to increase in tuberculosis. |
| Kaneko et al. [89] | Listeriosis | Listeria monocytogenes meningitis was associated with increased Np levels. |
| Kip et al. [90] | Visceral leishmaniasis | Np serum levels were found to be higher. |
| Mildvan et al. [92] | HIV infection | NP predicted disease progression in advanced HIV-1 infection. |
| Ozger et al. [94] | COVID–19 | Np was proposed as an early prognostic biomarker on admission. |
| Gulcan et al. [94] | Viral hepatitis | Np was proposed as a biomarker for hepatitis-B-related chronic liver disease. |
| Miyaue et al. [100] | Demyelinating | Np levels in cerebrospinal fluid differed in different demyelinating diseases. |
| Widner et al. [101] | Parkinson disease | Np levels were increased in advanced Parkinson´s diseases. |
| Arteaga-Henríquez et al. [102] | Autism spectrum disorder | Increased Np levels were associated with autism spectrum disorder. |
| Endres et al. [103] | Sarcoidosis | Sarcoidosis was associated with increased Np levels. |
| Bahrehmand et al. [104] | Systemic lupus erythematosus | Np was proposed to evaluate the progression of systemic lupus erythematosus. |
| Peng et al. [106] | Dermatomyositis | Increased Np levels were associated with a reduced outcome in subjects with dermatomyositis. |
| Abdel-Haq et al. [105] | Lichen planus | Increased serum Np levels were reported in subjects with lichen planus. |
| El-Lebedy et al. [107] | Rheumatoid arthritis | Increased Np levels were found in subjects with rheumatoid arthritis. |
| Ciocan et al. [111] | Colorectal carcinoma | Np levels in oral fluid were increased in subjects with squamous cell carcinoma in oral cavity. |
| Pink et al. [11] | Squamous cell carcinoma | Np was proposed as a potential biomarker of colorectal carcinoma. |
| Weinlich et al. [114] | Melanoma | Np predicted a worse outcome in subjects with melanoma. |
| Hausen et al. [115] | Haematological malignancies | Urinary Np levels were correlated with tumour stage in subjects with chronic lymphocytic leukemia and with non-Hodgkin’s disease. |
| Aulitzky et al. [116] | Genitourinary tumours | Higher stages were associated with elevated Np levels in the urine. |
| Manes et al. [117] | Pancreatic adenocarcinoma | Serum Np levels were higher in subjects with pancreatic carcinoma than in subjects with pancreatitis. |
| Bizjak et al. [119] | Ultramaraton race | Higher Np levels indicated a decrease in the total antioxidant capacity after the race. |
| Study | Key Findings |
|---|---|
| Abdel-Haq et al. [105] | The purpose of the study was to evaluate Np levels in lichen planus, but serum Np levels were correlated with clinical parameters of periodontitis. |
| Arjunkumar et al. [130] | GCF Np concentrations were positively associated with periodontal disease. |
| Bodur et al. [73] | Neopterin levels in oral fluid were found to be increased in subjects with periodontitis before treatment compared to the control group. |
| Fenol et al. [131] | Oral fluid Np was significantly higher in subjects with periodontitis compared to those in the control group and decreased significantly after nonsurgical periodontal therapy. |
| Heneberk et al. [10] | Np total amount in GCF and Np to creatinine ratio in urine were higher in subjects with periodontitis. After nonsurgical periodontal therapy, Np in oral fluid increased significantly; in GCF Np concentrations were significantly higher than in the control group. |
| Mahendra et al. [75] | Np levels in oral fluid were found to be higher in subjects with periodontitis compared to subjects in the control group. |
| Ozmeric et al. [44] | The total amount of Np in GCF and the concentrations of Np in oral fluid were significantly higher in subjects with aggressive periodontitis compared to the control group. |
| Patil et al. [132] | Np levels in oral fluid were found to be higher in subjects with periodontitis compared to the control group. |
| Pink et al. [11] | Comparison of subjects with oral carcinoma with healthy control, but the study group had significantly worse periodontal parameters. |
| Pradeep et al. [133] | Np concentrations in GCF were significantly higher in subjects with periodontitis compared to the healthy control group. |
| Prasanna et al. [134] | Np levels in urine and serum decreased significantly after periodontal therapy. |
| Prasanna et al. [74] | Np levels in oral fluid decreased significantly after nonsurgical periodontal therapy. |
| Prasanna and Sumadhura [53] | Np concentrations in serum and oral fluid decreased significantly after nonsurgical periodontal therapy. |
| Ren et al. [82] | Serum Np levels were lower in subjects after nonsurgical periodontal therapy compared to those who received only supragingival calculus removal. |
| Turgut-Cankaya et al. [135] | Both Np concentrations and total amount were significantly higher in subjects with periodontitis and decreased after nonsurgical periodontal therapy. |
| Vrecko et al. [72] | Np levels in the oral fluid were significantly higher in subjects with aggressive periodontitis compared to the healthy control group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heneberk, O.; Wurfelova, E.; Radochova, V. Neopterin, the Cell-Mediated Immune Response Biomarker, in Inflammatory Periodontal Diseases: A Narrative Review of a More than Fifty Years Old Biomarker. Biomedicines 2023, 11, 1294. https://doi.org/10.3390/biomedicines11051294
Heneberk O, Wurfelova E, Radochova V. Neopterin, the Cell-Mediated Immune Response Biomarker, in Inflammatory Periodontal Diseases: A Narrative Review of a More than Fifty Years Old Biomarker. Biomedicines. 2023; 11(5):1294. https://doi.org/10.3390/biomedicines11051294
Chicago/Turabian StyleHeneberk, Ondrej, Eliska Wurfelova, and Vladimira Radochova. 2023. "Neopterin, the Cell-Mediated Immune Response Biomarker, in Inflammatory Periodontal Diseases: A Narrative Review of a More than Fifty Years Old Biomarker" Biomedicines 11, no. 5: 1294. https://doi.org/10.3390/biomedicines11051294
APA StyleHeneberk, O., Wurfelova, E., & Radochova, V. (2023). Neopterin, the Cell-Mediated Immune Response Biomarker, in Inflammatory Periodontal Diseases: A Narrative Review of a More than Fifty Years Old Biomarker. Biomedicines, 11(5), 1294. https://doi.org/10.3390/biomedicines11051294






