Pituitary Apoplexy in Patients with Pituitary Neuroendocrine Tumors (PitNET)
Abstract
1. Introduction
Aim
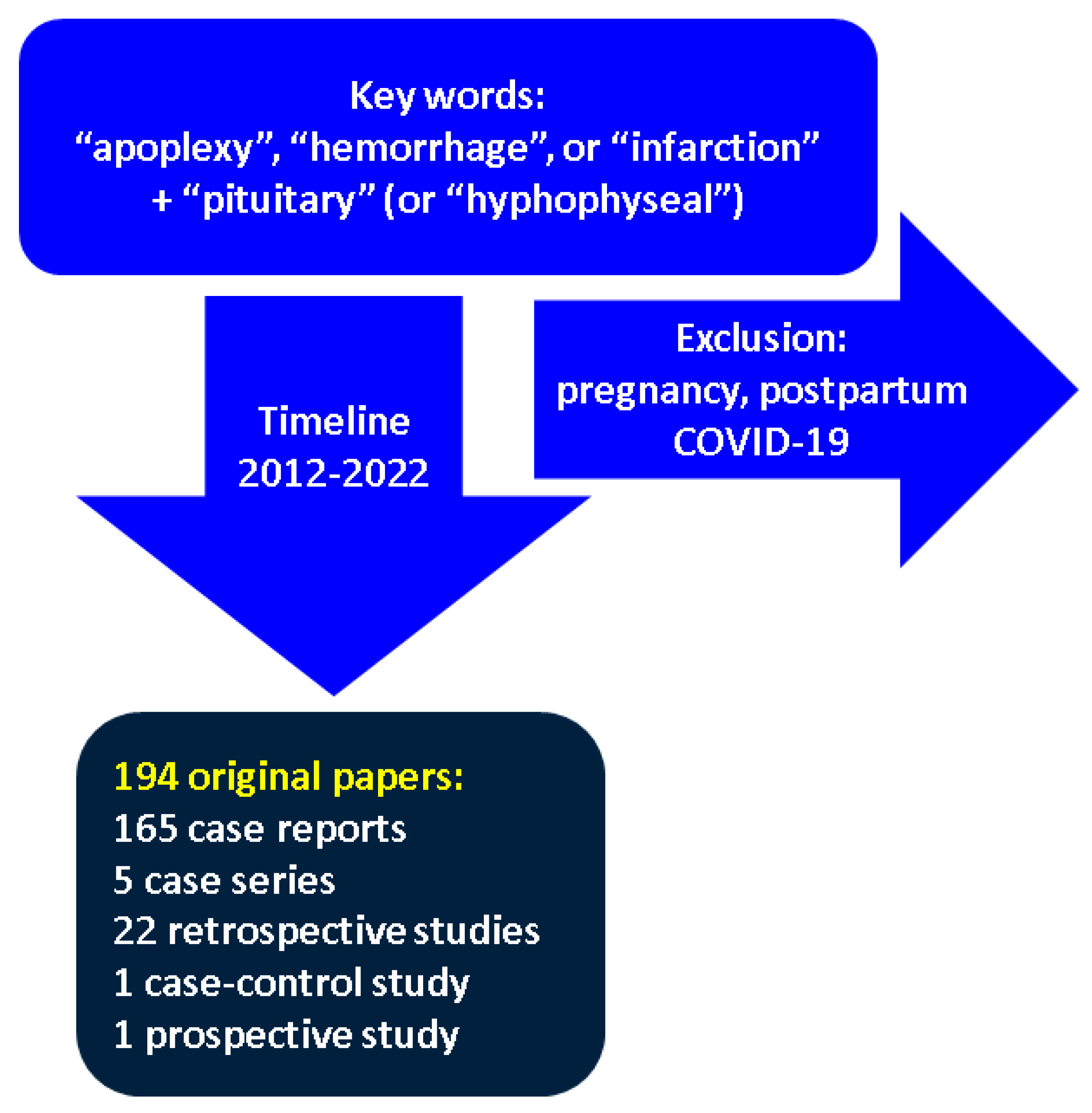
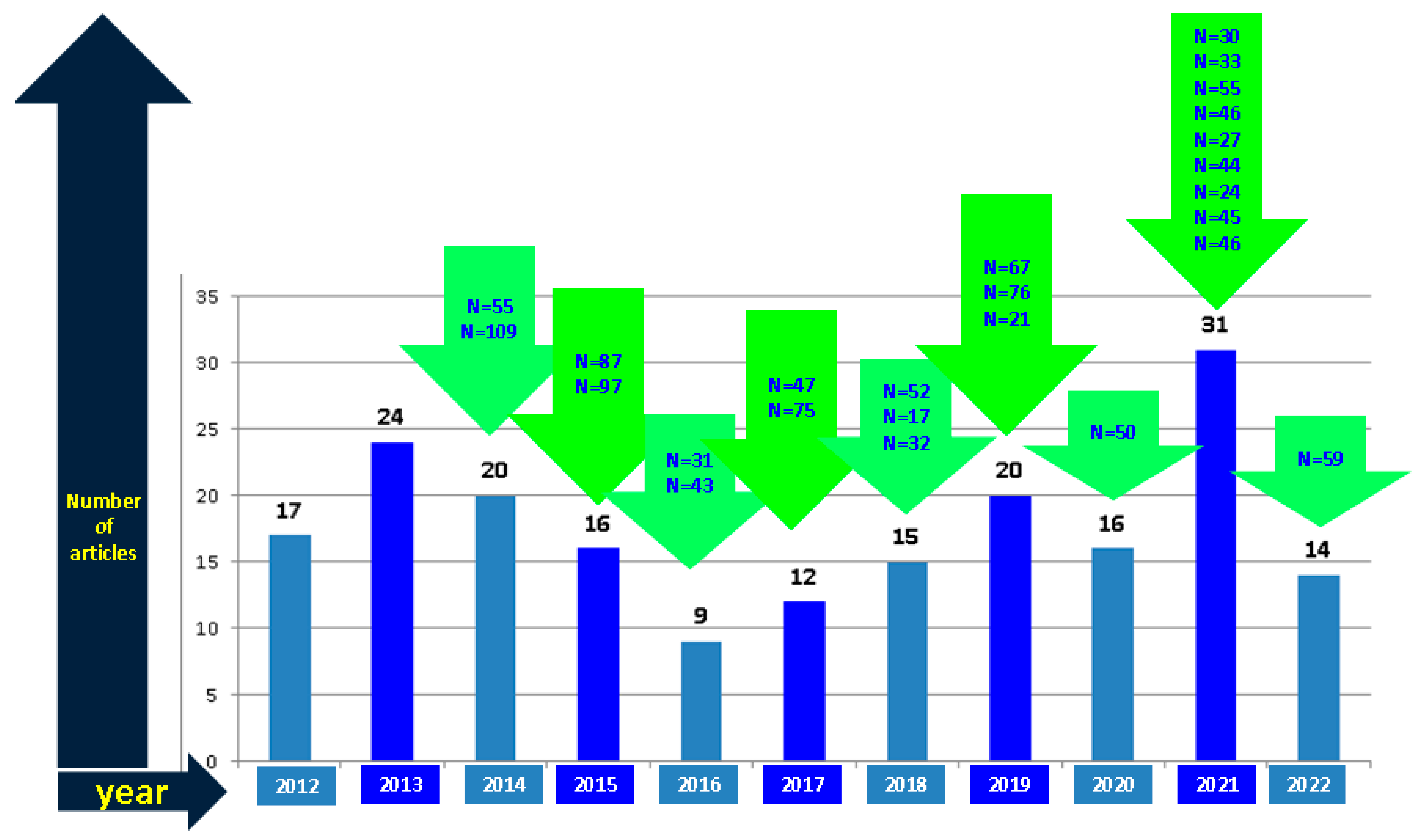
2. Method
3. PitNET Complicated with PA
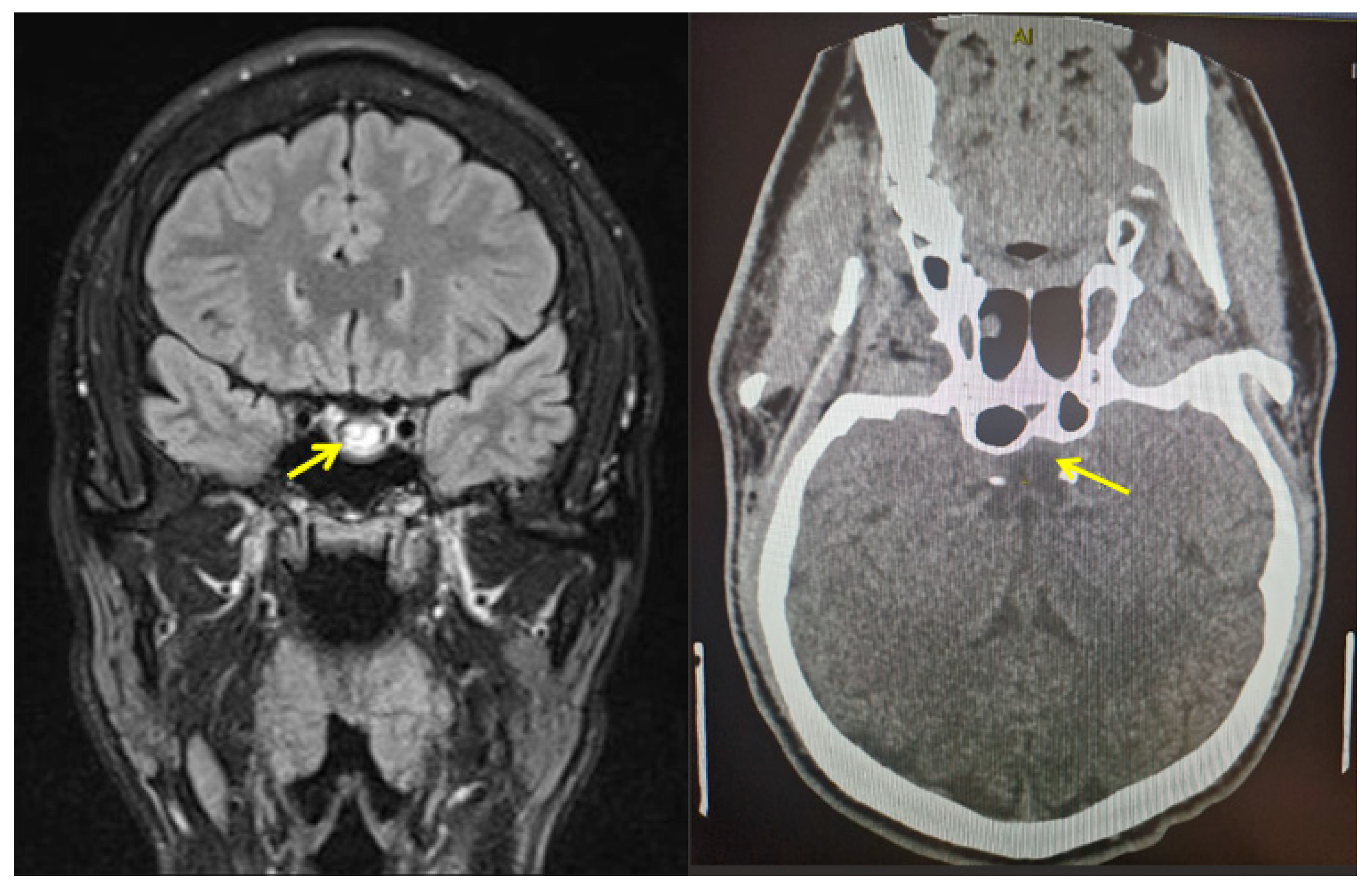
3.1. Clinical Presentation in Cases with PA: Neurologic and Ophthalmic Elements
3.2. PA and Hormonal Imbalance at First Diagnostic
3.3. Potential Triggers and Circumstantial Events of PA
3.4. PA Management
3.5. PA-Related Outcome
4. Discussion
4.1. Subentities concerning PA
4.2. Controversies in PA Domain
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| CSF | cerebrospinal fluid |
| HIF-1α | hypoxia-inducing factor |
| HMGB1 | high-mobility group box 1 |
| HR | hormonal replacement |
| GH | growth hormone |
| IGF-1 | insulin-like growth factor |
| MMP | matrix metalloproteinase |
| MRI | magnetic resonance imaging |
| PA | pituitary apoplexy |
| PAS | pituitary apoplexy score |
| PitNET | pituitary neuroendocrine tumor |
| PDE | phosphodiesterase |
| PTTG | pituitary tumor-transforming gene |
| TNF-α | tumor necrosis factor-α |
| TSS | trans-sphenoidal surgery |
| VEGF | vascular endothelial growth factor |
References
- Pearce, J.M. On the Origins of Pituitary Apoplexy. Eur. Neurol. 2015, 74, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Dunn, I.F.; Laws, E.R. Pituitary apoplexy. Endocrine 2015, 48, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Castro, M.; Berrocal, V.R.; Pascual-Corrales, E. Pituitary tumors: Epidemiology and clinical presentation spectrum. Hormones 2020, 19, 145–155. [Google Scholar] [CrossRef]
- Post, K.D. Pituitary apoplexy: Is it one entity? World Neurosurg. 2014, 82, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Barkhoudarian, G.; Kelly, D.F. Pituitary Apoplexy. Neurosurg. Clin. N. Am. 2019, 30, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.; Lecumberri, B.; Gálvez, M. Clinical practice guideline for the diagnosis and treatment of pituitary apoplexy. Endocrinol. Nutr. 2013, 60, 582.e1–582.e12. [Google Scholar] [CrossRef]
- Jankowski, P.P.; Crawford, J.R.; Khanna, P.; Malicki, D.M.; Ciacci, J.D.; Levy, M.L. Pituitary tumor apoplexy in adolescents. World Neurosurg. 2015, 83, 644–651. [Google Scholar] [CrossRef]
- Glezer, A.; Bronstein, M.D. Pituitary apoplexy: Pathophysiology, diagnosis and management. Arq. Bras. Endocrinol. Metabol. 2015, 59, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Dutta, P. Landscape of Molecular Events in Pituitary Apoplexy. Front. Endocrinol. 2018, 9, 107. [Google Scholar] [CrossRef]
- Shan, B.; Gerez, J.; Haedo, M.; Fuertes, M.; Theodoropoulou, M.; Buchfelder, M.; Losa, M.; Stalla, G.K.; Arzt, E.; Renner, U. RSUME is implicated in HIF-1-induced VEGF-A production in pituitary tumour cells. Endocr.-Relat. Cancer 2012, 19, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Sangtian, J.; Ruanpeng, D.; Tummala, R.; Clark, B.; Burmeister, L.; Peterson, D.; Venteicher, A.S.; Kawakami, Y. Acute elevation of interleukin 6 and matrix metalloproteinase 9 during the onset of pituitary apoplexy in Cushing’s disease. Pituitary 2021, 24, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Fujita, M.; Kato, A. Significance of Elevated HMGB1 Expression in Pituitary Apoplexy. Anticancer Res. 2019, 39, 4491–4494. [Google Scholar] [CrossRef] [PubMed]
- Kreitschmann-Andermahr, I.; Siegel, S.; Carneiro, R.W.; Maubach, J.M.; Harbeck, B.; Brabant, G. Headache and pituitary disease: A systematic review. Clin. Endocrinol. 2013, 79, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Suri, H.; Dougherty, C. Presentation and Management of Headache in Pituitary Apoplexy. Curr. Pain Headache Rep. 2019, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Hage, R.; Eshraghi, S.R.; Oyesiku, N.M.; Ioachimescu, A.G.; Newman, N.J.; Biousse, V.; Bruce, B.B. Third, Fourth, and Sixth Cranial Nerve Palsies in Pituitary Apoplexy. World Neurosurg. 2016, 94, 447–452. [Google Scholar] [CrossRef]
- Mavridis, I.; Meliou, M.; Pyrgelis, E.-S. Presenting Symptoms of Pituitary Apoplexy. J. Neurol. Surg. Part A Central Eur. Neurosurg. 2018, 79, 52–59. [Google Scholar] [CrossRef]
- Sarwar, K.N.; Huda, M.S.B.; Van De Velde, V.; Hopkins, L.; Luck, S.; Preston, R.; McGowan, B.; Carroll, P.V.; Powrie, J.K. The Prevalence and Natural History of Pituitary Hemorrhage in Prolactinoma. J. Clin. Endocrinol. Metab. 2013, 98, 2362–2367. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Y.; Qiao, Y.; Chen, X.; Xu, J.; Zhang, C.; Wang, W.; Li, J.; Deng, X. Risk factors for the incidence of apoplexy in pituitary adenoma: A single-center study from southwestern China. Chin. Neurosurg. J. 2020, 6, 20. [Google Scholar] [CrossRef]
- Cinar, N.; Tekinel, Y.; Dagdelen, S.; Oruckaptan, H.; Soylemezoglu, F.; Erbas, T. Cavernous sinus invasion might be a risk factor for apoplexy. Pituitary 2013, 16, 483–489. [Google Scholar] [CrossRef]
- Jahangiri, A.; Clark, A.J.; Han, S.J.; Kunwar, S.; Blevins, L.S.; Aghi, M.K. Socioeconomic factors associated with pituitary apoplexy: Clinical article. J. Neurosurg. 2013, 119, 1432–1436. [Google Scholar] [CrossRef]
- Goyal, P.; Utz, M.; Gupta, N.; Kumar, Y.; Mangla, M.; Gupta, S.; Mangla, R. Clinical and imaging features of pituitary apoplexy and role of imaging in differentiation of clinical mimics. Quant. Imaging Med. Surg. 2018, 8, 219–231. [Google Scholar] [CrossRef]
- Boellis, A.; Di Napoli, A.; Romano, A.; Bozzao, A. Pituitary apoplexy: An update on clinical and imaging features. Insights Imaging 2014, 5, 753–762. [Google Scholar] [CrossRef]
- Vaphiades, M.S. Pituitary Ring Sign Plus Sphenoid Sinus Mucosal Thickening: Neuroimaging Signs of Pituitary Apoplexy. Neuro-Ophthalmology 2017, 41, 306–309. [Google Scholar] [CrossRef]
- E Baldeweg, S.; Vanderpump, M.; Drake, W.; Reddy, N.; Markey, A.; Plant, G.T.; Powell, M.; Sinha, S.; Wass, J. Society for endocrinology endocrine emergency guidance: Emergency management of pituitary apoplexy in adult patients. Endocr. Connect. 2016, 5, G12–G15. [Google Scholar] [CrossRef]
- Seaman, S.C.; Dougherty, M.C.; Zanaty, M.; Bruch, L.A.; Graham, S.M.; Greenlee, J.D.W. Visual and Hormone Outcomes in Pituitary Apoplexy: Results of a Single Surgeon, Single Institution 15-Year Retrospective Review and Pooled Data Analysis. J. Neurol. Surg. Part B Skull Base 2021, 82, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Lu, Q.; Zhu, P.; Zheng, W. Surgical versus non-surgical treatment for pituitary apoplexy: A systematic review and meta-analysis. J. Neurol. Sci. 2016, 370, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Goshtasbi, K.; Abiri, A.; Sahyouni, R.; Mahboubi, H.; Raefsky, S.; Kuan, E.C.; Hsu, F.P.; Cadena, G. Visual and Endocrine Recovery Following Conservative and Surgical Treatment of Pituitary Apoplexy: A Meta-Analysis. World Neurosurg. 2019, 132, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sahyouni, R.; Goshtasbi, K.; Choi, E.; Mahboubi, H.; Le, R.; Khahera, A.S.; Hanna, G.K.; Hatefi, D.; Hsu, F.P.; Bhandarkar, N.D.; et al. Vision Outcomes in Early versus Late Surgical Intervention of Pituitary Apoplexy: Meta-Analysis. World Neurosurg. 2019, 127, 52–57. [Google Scholar] [CrossRef]
- Brar, K.S.; Garg, M.K. High altitude-induced pituitary apoplexy. Singap. Med. J. 2012, 53, e117–e119. [Google Scholar]
- Cagnin, A.; Marcante, A.; Orvieto, E.; Manara, R. Pituitary tumor apoplexy presenting as infective meningoencephalitis. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2012, 33, 147–149. [Google Scholar] [CrossRef]
- Chan, D.; Rong, T.C.; Dalan, R. Cushing’s disease presenting with pituitary apoplexy. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2012, 19, 1586–1589. [Google Scholar] [CrossRef] [PubMed]
- Chentli, F.; Bey, A.; Belhimer, F.; Azzoug, S. Spontaneous resolution of pituitary apoplexy in a giant boy under 10 years old. J. Pediatr. Endocrinol. Metab. 2012, 25, 1177–1179. [Google Scholar] [CrossRef]
- Choudhry, O.J.; Choudhry, A.J.; Nunez, E.A.; Eloy, J.A.; Couldwell, W.T.; Ciric, I.S.; Liu, J.K. Pituitary tumor apoplexy in patients with Cushing’s disease: Endocrinologic and visual outcomes after transsphenoidal surgery. Pituitary 2012, 15, 428–435. [Google Scholar] [CrossRef]
- Komurcu, H.F.; Ayberk, G.; Ozveren, M.F.; Anlar, O. Pituitary Adenoma Apoplexy Presenting with Bilateral Third Nerve Palsy and Bilateral Proptosis: A Case Report. Med. Princ. Pract. 2012, 21, 285–287. [Google Scholar] [CrossRef]
- Kruljac, I.; Čerina, V.; Pećina, H.I.; Pažanin, L.; Matić, T.; Božikov, V.; Vrkljan, M. Pituitary Metastasis Presenting as Ischemic Pituitary Apoplexy Following Heparin-induced Thrombocytopenia. Endocr. Pathol. 2012, 23, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, K.; Kawabori, M.; Niiya, Y.; Ohta, Y.; Mabuchi, S.; Houkin, K. Pituitary Apoplexy Manifesting as Massive Intracerebral Hemorrhage. Neurol. Med.-Chir. 2012, 52, 587–590. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Wang, X.; Liu, Y.-H.; Mao, Q. Spontaneous disappearance of the pituitary macroadenoma after apoplexy: A case report and review of the literature. Neurol. India 2012, 60, 530. [Google Scholar] [CrossRef]
- Mohindra, S.; Savardekar, A.; Tripathi, M.; Garg, R. Pituitary apoplexy presenting with pure third ventricular bleed: A neurosurgical image. Neurol. India 2012, 60, 314. [Google Scholar] [CrossRef]
- Paisley, A.N.; Syed, A.A. Pituitary apoplexy masquerading as bacterial meningitis. Can. Med. Assoc. J. 2012, 184, 1812. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tedd, H.; Tuckett, J.; Arun, C.; Dhar, A. An unusual case of sudden onset headache due to pituitary apoplexy: A case report and review of the new UK guidelines. J. R. Coll. Physicians Edinb. 2012, 42, 119–123. [Google Scholar] [CrossRef]
- Verma, R.; Singh, S.; Patil, T.B. Thalamic infarction in pituitary apolplexy syndrome. BMJ Case Rep. 2012, 2012, bcr2012006993. [Google Scholar] [CrossRef] [PubMed]
- Wildemberg, L.E.A.; Neto, L.V.; Niemeyer, P.; Gasparetto, E.L.; Chimelli, L.; Gadelha, M.R. Association of dengue hemorrhagic fever with multiple risk factors for pituitary apoplexy. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2012, 18, e97–e101. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yano, S.; Kuroda, J.-I.; Hasegawa, Y.; Hide, T.; Kuratsu, J.-I. Pituitary Apoplexy Associated with Endocrine Stimulation Test: Endocrine Stimulation Test, Treatment, and Outcome. Case Rep. Endocrinol. 2012, 2012, 826901. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zoli, M.; Mazzatenta, D.; Pasquini, E.; Ambrosetto, P.; Frank, G. Cavernous sinus apoplexy presenting isolated sixth cranial nerve palsy: Case report. Pituitary 2012, 15 (Suppl. 1), S37–S40. [Google Scholar] [CrossRef]
- Chou, H.-W.; Chang, H.-A.; Huang, S.-Y.; Tzeng, N.-S. Complex visual illusions in a patient with pituitary apoplexy. Gen. Hosp. Psychiatry 2013, 35, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- Cinar, N.U.; Metin, Y.; Dagdelen, S.; Ziyal, I.; Soylemezoglu, F.; Erbas, T. Spontaneous remission of acromegaly after infarctive apoplexy with a possible relation to MRI and diabetes mellitus. Neuro Endocrinol. Lett. 2013, 34, 339–342. [Google Scholar] [PubMed]
- Delgado-Alvarado, M.; Riancho, J.; Riancho-Zarrabeitia, L.; Sedano, M.; Polo, J.; Berciano, J. Oftalmoplejía completa unilateral sin pérdida de visión como forma de presentación de una apoplejía pituitaria. Rev. Clin. Esp. 2013, 213, e67–e70. [Google Scholar] [CrossRef]
- Deshwal, R. Pituitary Apoplexy Masquerading as Acute Mountain Sickness. Wilderness Environ. Med. 2013, 24, 88–89. [Google Scholar] [CrossRef]
- Fanous, A.A.; Quigley, E.P.; Chin, S.S.; Couldwell, W.T. Giant necrotic pituitary apoplexy. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2013, 20, 1462–1464. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.S.; Rao, P.J. A 64-year-old woman with dilated right pupil, nausea, and headache. Digit. J. Ophthalmol. 2013, 19, 13–17. [Google Scholar] [CrossRef]
- Hojo, M.; Goto, M.; Miyamoto, S. Chronic expanding pituitary hematoma without rebleeding after pituitary apoplexy. Surg. Neurol. Int. 2013, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-Y.; Lin, J.-P.; Lieu, A.-S.; Chen, Y.-T.; Chen, H.-S.; Jang, M.-Y.; Shen, J.-T.; Wu, W.-J.; Huang, S.-P.; Juan, Y.-S. Pituitary apoplexy induced by Gonadotropin-releasing hormone agonists for treating prostate cancer-report of first Asian case. World J. Surg. Oncol. 2013, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-J.; Hung, W.-W.; Hsiao, P.-J. A case of acromegaly complicated with diabetic ketoacidosis, pituitary apoplexy, and lymphoma. Kaohsiung J. Med. Sci. 2013, 29, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Miyashita, K.; Tamanaha, T.; Kobayashi, N.; Iihara, K.; Nagatsuka, K. Pituitary ischemic apoplexy in a young woman using oral contraceptives: A case report. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2013, 22, e643–e644. [Google Scholar] [CrossRef]
- Masui, K.; Yonezawa, T.; Shinji, Y.; Nakano, R.; Miyamae, S. Pituitary Apoplexy Caused by Hemorrhage From Pituitary Metastatic Melanoma: Case Report. Neurol. Medico-Chirurgica 2013, 53, 695–698. [Google Scholar] [CrossRef]
- Mir, S.A.; Masoodi, S.R.; Bashir, M.I.; Wani, A.I.; Farooqui, K.J.; Kanth, B.; Bhat, A.R. Dissociated hypopituitarism after spontaneous pituitary apoplexy in acromegaly. Indian J. Endocrinol. Metab. 2013, 17 (Suppl. 1), S102–S104. [Google Scholar]
- Mohamed, A.H.; Rodrigues, J.; Bradley, M.D.; Nelson, R.J. Retroclival subdural haematoma secondary to pituitary apoplexy. Br. J. Neurosurg. 2013, 27, 845–846. [Google Scholar] [CrossRef]
- Ní Chróinín, D.; Lambert, J. Sudden headache, third nerve palsy and visual deficit: Thinking outside the subarachnoid haemorrhage box. Age Ageing 2013, 42, 810–812. [Google Scholar] [CrossRef][Green Version]
- Oh, K.; Kim, J.-H.; Choi, J.-W.; Kang, J.-K.; Kim, S.-H. Pituitary Apoplexy Mimicking Meningitis. Brain Tumor Res. Treat. 2013, 1, 111–115. [Google Scholar] [CrossRef][Green Version]
- Radhiana, H.; O Syazarina, S.; Azura, A.M.S.; Azizi, A.B. Pituitary apoplexy: A rare cause of middle cerebral artery infarction. Med. J. Malays. 2013, 68, 264–266. [Google Scholar]
- Tutanc, M.; Altas, M.; Yengil, E.; Ustun, I.; Dolapcioglu, K.S.; Balci, A.; Sefil, F.; Gokce, C. Pituitary apoplexy due to thyroxine therapy in a patient with congenital hypothyroidism. Acta Med. Indones. 2013, 45, 306–311. [Google Scholar]
- Witczak, J.K.; Davies, R.; Okosieme, O.E. An unusual case of pituitary apoplexy. QJM Mon. J. Assoc. Physicians 2013, 106, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Das, K.; Javadpour, M. Pituitary apoplexy initially mistaken for bacterial meningitis. BMJ Case Rep. 2013, 2013, bcr2013009223. [Google Scholar] [CrossRef]
- Zieliński, G.; Witek, P.; Koziarski, A.; Podgórski, J. Spontaneous regression of non-functioning pituitary adenoma due to pituitary apoplexy following anticoagulation treatment—A case report and review of the literature. Endokrynol. Polska 2013, 64, 54–58. [Google Scholar]
- Berkenstock, M.; Szeles, A.; Ackert, J. Encephalopathy, Chiasmal Compression, Ophthalmoplegia, and Diabetes Insipidus in Pituitary Apoplexy. Neuro-Ophthalmology 2014, 38, 286–289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bujawansa, S.; Thondam, S.K.; Steele, C.; Cuthbertson, D.; Gilkes, C.E.; Noonan, C.; Bleaney, C.W.; Macfarlane, I.A.; Javadpour, M.; Daousi, C. Presentation, management and outcomes in acute pituitary apoplexy: A large single-centre experience from the United Kingdom. Clin. Endocrinol. 2014, 80, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.C.; Lin, C.J. Pituitary apoplexy in a teenager—Case report. Pediatr. Neurol. 2014, 50, 648–651. [Google Scholar] [CrossRef]
- Cho, T.H.; Rheims, S.; Ritzenthaler, T.; Berthezene, Y.; Nighoghossian, N. Stroke and pituitary apoplexy revealing an internal carotid artery dissection. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2014, 23, e473–e474. [Google Scholar] [CrossRef]
- Gupta, V.; Patil, S.; Raval, D.; Gopani, P. Pituitary apoplexy presenting as myocardial infarction. Indian J. Endocrinol. Metab. 2014, 18, 232–233. [Google Scholar] [CrossRef]
- Jho, D.H.; Biller, B.M.; Agarwalla, P.K.; Swearingen, B. Pituitary Apoplexy: Large Surgical Series with Grading System. World Neurosurg. 2014, 82, 781–790. [Google Scholar] [CrossRef]
- Lee, K.A.; Park, T.S.; Baek, H.S.; Jin, H.Y. Pituitary apoplexy in T3 thyrotoxicosis. Endocrine 2014, 45, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Maltby, V.E.; Crock, P.A.; Lüdecke, D.K. A rare case of pituitary infarction leading to spontaneous tumour resolution and CSF-sella syndrome in an 11-year-old girl and a review of the pediatric literature. J. Pediatr. Endocrinol. Metab. 2014, 27, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Man, B.L.; Fu, Y.P. Pituitary apoplexy in a patient with suspected metastatic bronchogenic carcinoma. BMJ Case Rep. 2014, 2014, bcr2013202803. [Google Scholar] [CrossRef]
- Panigrahi, S.; Das, S.; Mishra, S. Dengue hemorrhagic fever: A rare cause of pituitary apoplexy. Neurol. India 2014, 62, 92–93. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Bonnet, J.; Anda, J.J.M.; Balderrama-Soto, A.; Pérez-Reyes, S.P.; Pérez-Neri, I.; Portocarrero-Ortiz, L. Stroke associated with pituitary apoplexy in a giant prolactinoma: A case report. Clin. Neurol. Neurosurg. 2014, 116, 101–103. [Google Scholar] [CrossRef]
- Roerink, S.; Marsman, D.; Van Bon, A.; Netea-Maier, R. A Missed Diagnosis of Acromegaly During a Female-to-Male Gender Transition. Arch. Sex. Behav. 2014, 43, 1199–1201. [Google Scholar] [CrossRef]
- Tan, S.K.; Seow, C.J.; Tan, E.; Chau, Y.P.; Dalan, R. Pituitary apoplexy secondary to thrombocytopenia due to dengue hemorrhagic fever: A case report and review of the literature. Endocrine Practice: Official J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2014, 20, e58–e64. [Google Scholar] [CrossRef]
- Villar-Taibo, R.; Ballesteros-Pomar, M.D.; Vidal-Casariego, A.; Alvarez-San Martín, R.M.; Kyriakos, G.; Cano-Rodríguez, I. Spontaneous remission of acromegaly: Apoplexy mimicking meningitis or meningitis as a cause of apoplexy? Arq. Bras. Endocrinol. Metabol. 2014, 58, 76–80. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, F.; Zhu, Y.; Wang, R.; Xing, B. Cerebral infarction caused by pituitary apoplexy: Case report and review of literature. Turk. Neurosurg. 2014, 24, 782–787. [Google Scholar] [CrossRef]
- Akakın, A.; Yılmaz, B.; Ekşi, M.; Kılıç, T. A case of pituitary apoplexy following posterior lumbar fusion surgery. J. Neurosurg. Spine 2015, 23, 598–601. [Google Scholar] [CrossRef]
- Asaithambi, G. Carotid artery compression from pituitary apoplexy. QJM Int. J. Med. 2015, 108, 159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banerjee, C.; Snelling, B.; Hanft, S.; Komotar, R.J. Bilateral cerebral infarction in the setting of pituitary apoplexy: A case presentation and literature review. Pituitary 2015, 18, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Fountas, A.; Andrikoula, M.; Tsatsoulis, A. A 45 year old patient with headache, fever, and hyponatraemia. BMJ 2015, 350, h962. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, S.W.; Son, D.W.; Cha, S.H. Pituitary Apoplexy Following Mitral Valvuloplasty. J. Korean Neurosurg. Soc. 2015, 57, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Man, B.L.; Fu, Y.P. Pituitary apoplexy presenting with bilateral oculomotor nerve palsy. BMJ Case Rep. 2015, 2015, bcr2015212049. [Google Scholar] [CrossRef] [PubMed]
- Roerink, S.H.P.P.; Van Lindert, E.J.; Van De Ven, A.C. Spontaneous remission of acromegaly and Cushing’s disease following pituitary apoplexy: Two case reports. Neth. J. Med. 2015, 73, 242–246. [Google Scholar]
- Saberifard, J.; Yektanezhad, T.; Assadi, M. An Interesting Case of a Spontaneous Resolution of Pituitary Adenoma after Apoplexy. J. Belg. Soc. Radiol. 2015, 99, 101–102. [Google Scholar] [CrossRef][Green Version]
- Sasagawa, Y.; Tachibana, O.; Nakagawa, A.; Koya, D.; Iizuka, H. Pituitary apoplexy following gonadotropin-releasing hormone agonist administration with gonadotropin-secreting pituitary adenoma. J. Clin. Neurosci. 2015, 22, 601–603. [Google Scholar] [CrossRef]
- Sasaki, Y.; Nakata, K.; Suzuki, K.; Ando, Y. Pituitary apoplexy presenting with anorexia and hyponatraemia. BMJ Case Rep. 2015, 2015, bcr2014209120. [Google Scholar] [CrossRef]
- Singh, T.D.; Valizadeh, N.; Meyer, F.B.; Atkinson, J.L.D.; Erickson, D.; Rabinstein, A.A. Management and outcomes of pituitary apoplexy. J. Neurosurg. 2015, 122, 1450–1457. [Google Scholar] [CrossRef]
- Teasdale, S.; Hashem, F.; Olson, S.; Ong, B.; Inder, W.J. Recurrent pituitary apoplexy due to two successive neoplasms presenting with ocular paresis and epistaxis. Endocrinol. Diabetes Metab. Case Rep. 2015, 2015, 140088. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, X.; Wang, Y.; Zhao, X.; Jiang, C.; Zhang, Q.; Jiang, W.; Wang, Y.; Chen, H.; Shou, X.; Zhao, Y.; et al. Incidence of Pituitary Apoplexy and Its Risk Factors in Chinese People: A Database Study of Patients with Pituitary Adenoma. PLoS ONE 2015, 10, e0139088. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, C.; Sun, B.; Chen, C.; Xiong, W.; Che, C.; Huang, H. Surgical treatment of pituitary apoplexy in association with hemispheric infarction. J. Clin. Neurosci. 2015, 22, 1550–1554. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.; Eligar, V.; DeLloyd, A.; Davies, J.S. A case of pituitary apoplexy masquerading as subarachnoid hemorrhage. Clin. Case Rep. 2016, 4, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Doglietto, F.; Costi, E.; Villaret, A.B.; Mardighian, D.; Fontanella, M.; Giustina, A. New oral anticoagulants and pituitary apoplexy. Pituitary 2016, 19, 232–234. [Google Scholar] [CrossRef]
- Gambaracci, G.; Rondoni, V.; Guercini, G.; Floridi, P. Pituitary apoplexy complicated by vasospasm and bilateral cerebral infarction. BMJ Case Rep. 2016, 2016, bcr2016216186. [Google Scholar] [CrossRef]
- Giammattei, L.; Mantovani, G.; Carrabba, G.; Ferrero, S.; Di Cristofori, A.; Verrua, E.; Guastella, C.; Pignataro, L.; Rampini, P.; Minichiello, M.; et al. Pituitary apoplexy: Considerations on a single center experience and review of the literature. J. Endocrinol. Investig. 2016, 39, 739–746. [Google Scholar] [CrossRef]
- Giritharan, S.; Gnanalingham, K.; Kearney, T. Pituitary apoplexy—Bespoke patient management allows good clinical outcome. Clin. Endocrinol. 2016, 85, 415–422. [Google Scholar] [CrossRef]
- Keane, F.; Egan, A.M.; Navin, P.; Brett, F.; Dennedy, M. Gonadotropin-releasing hormone agonist-induced pituitary apoplexy. Endocrinol. Diabetes Metab. Case Rep. 2016, 2016, 160021. [Google Scholar] [CrossRef][Green Version]
- Ogawa, Y.; Niizuma, K.; Mugikura, S.; Tominaga, T. Ischemic pituitary adenoma apoplexy—Clinical appearance and prognosis after surgical intervention. Clin. Neurol. Neurosurg. 2016, 148, 142–146. [Google Scholar] [CrossRef]
- A Paschou, S.; Tzioras, K.; Trianti, V.; Lyra, S.; Lioutas, V.-A.; Seretis, A.; Vryonidou, A. Young adult patient with headache, fever and blurred vision. Hormones 2016, 15, 548–550. [Google Scholar] [CrossRef]
- Sussman, E.S.; Ho, A.L.; Pendharkar, A.V.; Achrol, A.S.; Harsh, G.R. Pituitary Apoplexy Associated with Carotid Compression and a Large Ischemic Penumbra. World Neurosurg. 2016, 92, 581.e7–581.e13. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, A.; Rao, S.B.; Savardekar, A.; Nandeesh, B. Management dilemmas in a rare case of pituitary apoplexy in the setting of dengue hemorrhagic fever. Surg. Neurol. Int. 2017, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Grangeon, L.; Moscatelli, L.; Zanin, A.; Guegan-Massardier, E.; Rouille, A.; Maltete, D. Indomethacin-Responsive Paroxysmal Hemicrania in an Elderly Man: An Unusual Presentation of Pituitary Apoplexy. Headache 2017, 57, 1624–1626. [Google Scholar] [CrossRef]
- Humphreys, G.; Waqar, M.; McBain, A.; Gnanalingham, K.K. Sphenoid sinus microbiota in pituitary apoplexy: A preliminary study. Pituitary 2017, 20, 619–623. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Law-Ye, B.; Pyatigorskaya, N.; Leclercq, D. Pituitary Apoplexy Mimicking Bacterial Meningitis with Intracranial Hypertension. World Neurosurg. 2017, 97, 748.e3–748.e5. [Google Scholar] [CrossRef]
- Pasha, S.A.; Ranganthan, L.N.; Setty, V.K.; Reddy, R.; Ponnuru, D.A. Acute Ischaemic Stroke as a Manifestation of Pituitary Apoplexy in a Young Lady. J. Clin. Diagn. Res. 2017, 11, OD03–OD05. [Google Scholar] [CrossRef]
- Patra, S.; Biswas, S.N.; Datta, J.; Chakraborty, P.P. Hypersomatotropism induced secondary polycythaemia leading to spontaneous pituitary apoplexy resulting in cure of acromegaly and remission of polycythaemia: ‘The virtuous circle’. BMJ Case Rep. 2017, 2017, bcr2017222669. [Google Scholar] [CrossRef]
- Simsek Bagir, G.; Civi, S.; Kardes, O.; Kayaselcuk, F.; Ertorer, M.E. Stubborn hiccups as a sign of massive apoplexy in a naive acromegaly patient with pituitary macroadenoma. Endocrinol. Diabetes Metab. Case Rep. 2017, 2017, 17–0044. [Google Scholar] [CrossRef]
- Souteiro, P.; Belo, S.; Carvalho, D. A rare case of spontaneous Cushing disease remission induced by pituitary apoplexy. J. Endocrinol. Investig. 2017, 40, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Waqar, M.; McCreary, R.; Kearney, T.; Karabatsou, K.; Gnanalingham, K.K. Sphenoid sinus mucosal thickening in the acute phase of pituitary apoplexy. Pituitary 2017, 20, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Milanese, L.; Faustini-Fustini, M.; Guaraldi, F.; Asioli, S.; Zenesini, C.; Righi, A.; Frank, G.; Foschini, M.P.; Sturiale, C.; et al. Endoscopic Endonasal Surgery for Pituitary Apoplexy: Evidence On a 75-Case Series From a Tertiary Care Center. World Neurosurg. 2017, 106, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Abbara, A.; Clarke, S.; Eng, P.C.; Milburn, J.; Joshi, D.; Comninos, A.N.; Ramli, R.; Mehta, A.; Jones, B.; Wernig, F.; et al. Clinical and biochemical characteristics of patients presenting with pituitary apoplexy. Endocr. Connect. 2018, 7, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Bettag, C.; Strasilla, C.; Steinbrecher, A.; Gerlach, R. Unilateral Tuberothalamic Artery Ischemia Caused by Pituitary Apoplexy. J. Neurol. Surg. Part A Central Eur. Neurosurg. 2018, 79, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Bao, X.; Wang, R. Conservative treatment cures an elderly pituitary apoplexy patient with oculomotor paralysis and optic nerve compression: A case report and systematic review of the literature. Clin. Interv. Aging 2018, 13, 1981–1985. [Google Scholar] [CrossRef]
- Joo, C.; Ha, G.; Jang, Y. Pituitary apoplexy following lumbar fusion surgery in prone position: A case report. Medicine 2018, 97, e0676. [Google Scholar] [CrossRef]
- Komshian, S.R.; Saket, R.; Bakhadirov, K. Pituitary Apoplexy With Bilateral Oculomotor Nerve Palsy. Neurohospitalist 2018, 8, NP4–NP5. [Google Scholar] [CrossRef]
- Kuzu, F.; Unal, M.; Gul, S.; Bayraktaroglu, T. Pituitary Apoplexy due to the Diagnostic Test in a Cushing’s Disease Patient. Turk. Neurosurg. 2018, 28, 323–325. [Google Scholar]
- Myla, M.; Lewis, J.; Beach, A.; Sylejmani, G.; Burge, M.R. A Perplexing Case of Pituitary Apoplexy Masquerading as Recurrent Meningitis. J. Investig. Med. High Impact Case Rep. 2018, 6, 2324709618811370. [Google Scholar] [CrossRef]
- Ricciuti, R.; Nocchi, N.; Arnaldi, G.; Polonara, G.; Luzi, M. Pituitary adenoma apoplexy: Review of personal series. Asian J. Neurosurg. 2018, 13, 560–564. [Google Scholar] [CrossRef]
- Rutkowski, M.J.; Kunwar, S.; Blevins, L.; Aghi, M.K. Surgical intervention for pituitary apoplexy: An analysis of functional outcomes. J. Neurosurg. 2018, 129, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Yamada, D.; Fujikawa, T. Pituitary apoplexy. Can. Med. Assoc. J. 2018, 190, E1419. [Google Scholar] [CrossRef]
- Almeida, J.P.; Sanchez, M.M.; Karekezi, C.; Warsi, N.; Fernández-Gajardo, R.; Panwar, J.; Mansouri, A.; Suppiah, S.; Nassiri, F.; Nejad, R.; et al. Pituitary Apoplexy: Results of Surgical and Conservative Management Clinical Series and Review of the Literature. World Neurosurg. 2019, 130, e988–e999. [Google Scholar] [CrossRef] [PubMed]
- Crisman, C.; Ward, M.; Majmundar, N.; Damodara, N.; Hsueh, W.D.; Eloy, J.A.; Liu, J.K. Pituitary Apoplexy Following Endoscopic Retrograde Cholangiopancreatography. World Neurosurg. 2019, 121, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Dupont, G.; Lachkar, S.; Iwanaga, J.; Tubbs, R.S.; Ishak, B. Sudden Headache and Blindness Due to Pituitary (Adenoma) Infarction: A Case Report. Cureus 2019, 11, e4059. [Google Scholar] [CrossRef] [PubMed]
- Ghalaenovi, H.; Azar, M.; Fattahi, A. Spontaneous regression of nonfunctioning pituitary adenoma. Br. J. Neurosurg. 2019, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Harju, T.; Alanko, J.; Numminen, J. Pituitary apoplexy following endoscopic nasal surgery: A case report. SAGE Open Med. Case Rep. 2019, 7, 2050313X19855867. [Google Scholar] [CrossRef]
- Hosmann, A.; Micko, A.; Frischer, J.M.; Roetzer, T.; Vila, G.; Wolfsberger, S.; Knosp, E. Multiple Pituitary Apoplexy—Cavernous Sinus Invasion as Major Risk Factor for Recurrent Hemorrhage. World Neurosurg. 2019, 126, e723–e730. [Google Scholar] [CrossRef]
- Kirigin Biloš, L.S.; Kruljac, I.; Radošević, J.M.; Ćaćić, M.; Škoro, I.; Čerina, V.; Pećina, I.H.; Vrkljan, M. Empty Sella in the Making. World Neurosurg. 2019, 128, 366–370. [Google Scholar] [CrossRef]
- Krug, R.G.; Chang, A.Y.; Raghunathan, A.; Van Gompel, J.J. Apoplectic Silent Crooke Cell Adenoma with Adjacent Pseudoaneurysms: Causation or Bystander? World Neurosurg. 2019, 122, 480–484. [Google Scholar] [CrossRef]
- Mittal, A.; Mishra, S.; Yadav, K.; Rajput, R. Uncontrolled diabetes as a rare presenting cause of pituitary apoplexy. BMJ Case Rep. 2019, 12, e228161. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Mori, J.; Tazoe, J.; Tomida, A.; Yagyu, S.; Nakajima, H.; Iehara, T.; Tatsuzawa, K.; Mukai, T.; Hosoi, H. Pituitary apoplexy after cardiac surgery in a 14-year-old girl with Carney complex: A case report. Endocr. J. 2019, 66, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Nioi, M.; Napoli, P.E.; Ferreli, F. Fatal Iatrogenic Pituitary Apoplexy after Surgery for Neuroophthalmological Disorder. Anesthesiology 2019, 130, 822. [Google Scholar] [CrossRef]
- Pedro, B.; Patrícia, T.; Aldomiro, F. Pituitary Apoplexy May Be Mistaken for Temporal Arteritis. Eur. J. Case Rep. Intern. Med. 2019, 6, 001261. [Google Scholar] [CrossRef]
- dos Santos, A.R.M.; Bello, C.T.; Sousa, A.; Duarte, J.S.; Campos, L.B. Pituitary Apoplexy Following Systemic Anticoagulation. Eur. J. Case Rep. Intern. Med. 2019, 6, 001254. [Google Scholar] [CrossRef]
- Sanz-Sapera, E.; Sarria-Estrada, S.; Arikan, F.; Biagetti, B. Acromegaly remission, SIADH and pituitary function recovery after macroadenoma apoplexy. Endocrinol. Diabetes Metab. Case Rep. 2019, 2019, 19–0057. [Google Scholar] [CrossRef]
- Singhal, A.; Gohlke, P.R.; Chapman, P.R. Spontaneous “pneumo-apoplexy” as a presentation of pituitary adenoma. Clin. Imaging 2019, 58, 152–155. [Google Scholar] [CrossRef]
- Swaid, B.; Kalaba, F.; Bachuwa, G.; Sullivan, S.E. Heparin-Induced Pituitary Apoplexy Presenting as Isolated Unilateral Oculomotor Nerve Palsy: A Case Report and Literature Review. Case Rep. Endocrinol. 2019, 2019, 5043925. [Google Scholar] [CrossRef]
- Thomas, M.; Robert, A.; Rajole, P.; Robert, P. A Rare Case of Pituitary Apoplexy Secondary to Dengue Fever-induced Thrombocytopenia. Cureus 2019, 11, e5323. [Google Scholar] [CrossRef]
- Uneda, A.; Hirashita, K.; Yunoki, M.; Yoshino, K.; Date, I. Pituitary adenoma apoplexy associated with vardenafil intake. Acta Neurochir. 2019, 161, 129–131. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, L.; Wang, W.; Guo, X.; Feng, C.; Lian, W.; Li, Y.; Xing, B. Coagulative necrotic pituitary adenoma apoplexy: A retrospective study of 21 cases from a large pituitary center in China. Pituitary 2019, 22, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Waqar, M.; Karabatsou, K.; Kearney, T.; Roncaroli, F.; Gnanalingham, K.K. Classical pituitary apoplexy. Br. J. Hosp. Med. 2019, 80, 114. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.V.; Post, K.D.; Cheesman, K.C. Recurrent Pituitary Apoplexy In An Adenoma With Switching Phenotypes. AACE Clin. Case Rep. 2020, 6, e221–e224. [Google Scholar] [CrossRef] [PubMed]
- Catarino, D.; Ribeiro, C.; Gomes, L.; Paiva, I. Corticotroph adenoma and pituitary fungal infection: A rare association. Endocrinol. Diabetes Metab. Case Rep. 2020, 2020, EDM200010. [Google Scholar] [CrossRef] [PubMed]
- Eichberg, D.G.; Di, L.; Shah, A.H.; Kaye, W.A.; Komotar, R.J. Spontaneous preoperative pituitary adenoma resolution following apoplexy: A case presentation and literature review. Br. J. Neurosurg. 2018, 34, 502–507. [Google Scholar] [CrossRef]
- Elarjani, T.; Chen, S.; Cajigas, I.; Saway, B.; Sur, S.; Morcos, J.J. Pituitary Apoplexy and Cerebral Infarction: Case Report and Literature Review. World Neurosurg. 2020, 141, 73–80. [Google Scholar] [CrossRef]
- Franzese, I.; Giambruno, V.; Tropea, I.; Linardi, D.; Petrilli, G.; Faggian, G. Urgent Surgery for Pituitary Adenoma Bleeding After Coronary Bypass Surgery. Ann. Thorac. Surg. 2020, 110, e19–e21. [Google Scholar] [CrossRef] [PubMed]
- Klimko, A.; Capatina, C. Pituitary Macroadenoma Presenting as Acromegaly and Subacute Pituitary Apoplexy: Case Report and Literature Review. Cureus 2020, 12, e9612. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Kim, H.K.; Ahn, D.J. Concurrent pituitary apoplexy and posterior reversible encephalopathy syndrome in a patient with end-stage renal disease on hemodialysis: A case report. Medicine 2020, 99, e18987. [Google Scholar] [CrossRef]
- Marzoughi, S.; Ganesh, A.; Qaddoura, A.; Motazedian, P.; Bal, S.S. Pearls & Oy-sters: Isolated oculomotor nerve palsy due to pituitary apoplexy missed on CT scan. Neurology 2020, 94, e1774–e1777. [Google Scholar] [CrossRef]
- Pangal, D.J.; Chesney, K.; Memel, Z.; Bonney, P.A.; Strickland, B.A.; Carmichael, J.; Shiroishi, M.; Liu, C.-S.J.; Zada, G. Pituitary Apoplexy Case Series: Outcomes After Endoscopic Endonasal Transsphenoidal Surgery at a Single Tertiary Center. World Neurosurg. 2020, 137, e366–e372. [Google Scholar] [CrossRef]
- Patel, A.; Mobley, B.C.; Jagasia, M.; Adetola, K.; Byrne, M.; Dholaria, B. Pituitary Apoplexy During Hematopoietic Cell Transplantation. Clin. Lymphoma Myeloma Leuk. 2020, 20, e691–e693. [Google Scholar] [CrossRef]
- Shetty, S.; Gnanaraj, J.; Roshan, S.J.; El Accaoui, R. Pituitary apoplexy after regadenoson myocardial perfusion scan. J. Nucl. Cardiol. 2020, 27, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Siwakoti, K.; Omay, S.B.; Inzucchi, S.E. Spontaneous Resolution of Primary Hypercortisolism of Cushing Disease After Pituitary Hemorrhage. AACE Clin. Case Rep. 2020, 6, e23–e29. [Google Scholar] [CrossRef]
- van Boven, E.; Massolt, E.T.; van Rossum, E.F.C.; Kiewiet-Kemper, R.M. Spontaneous remission of unidentified Cushing’s disease revealed by hair cortisol analysis. Neth. J. Med. 2020, 78, 297–299. [Google Scholar]
- Alam, S.; Kubihal, S.; Goyal, A.; Jyotsna, V.P. Spontaneous Remission of Acromegaly After Pituitary Apoplexy in a Middle-Aged Male. Ochsner J. 2021, 21, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Aljabri, B.; Lilleby, W.; Switlyk, M.D.; Tafjord, G. Restart of androgen deprivation therapy after goserelin induced pituitary apoplexy in a patient with disseminated prostate cancer a case report and five-years follow-up. Urol. Case Rep. 2021, 37, 101648. [Google Scholar] [CrossRef]
- Ambrose, C.; Sarma, S.; Banerjee, R.; Myers, S. Pituitary apoplexy and associated cranial nerve palsies secondary to bleeding caused by immune thrombocytopaenia in a patient with known pituitary macroadenoma. BMJ Case Rep. 2021, 14, e240105. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, K.; Sharma, V.; Gupta, S.; Motazedi, A. The snowman sign in a patient with pituitary tumor apoplexy. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 416–417. [Google Scholar] [CrossRef]
- Cavalli, A.; Martin, A.; Connolly, D.J.; Mirza, S.; Sinha, S. Pituitary apoplexy: How to define safe boundaries of conservative management? Early and long-term outcomes from a single UK tertiary neurosurgical unit. Br. J. Neurosurg. 2021, 35, 334–340. [Google Scholar] [CrossRef]
- de Silva, N.L.; Somasundaram, N.; Constantine, R.; Kularatna, H. Apoplexy of Crooke cell tumour leading to the diagnosis of severe Cushing disease; a case report. BMC Endocr. Disord. 2021, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Falhammar, H.; Tornvall, S.; Höybye, C. Pituitary Apoplexy: A Retrospective Study of 33 Cases From a Single Center. Front. Endocrinol. 2021, 12, 656950. [Google Scholar] [CrossRef] [PubMed]
- Gohil, J.; Gowda, A.; George, T.; Easwer, H.V.; George, A.; Nair, P. Pituitary apoplexy and panhypopituitarism following acute leptospirosis. Pituitary 2021, 24, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Hanna, V.; Mednick, Z.; Micieli, J. Rapid resolution of a third nerve palsy from pituitary apoplexy. BMJ Case Rep. 2021, 14, e241850. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jiang, S.; Yang, C.; Deng, K.; Wang, R.; Bao, X. Surgical treatment of a 72-year-old patient with headache, hyponatremia and oculomotor nerve palsy: A case report and literature review. Gland. Surg. 2021, 10, 364–370. [Google Scholar] [CrossRef]
- Iqbal, F.; Adams, W.; Dimitropoulos, I.; Muquit, S.; Flanagan, D. Pituitary hemorrhage and infarction: The spectrum of disease. Endocr. Connect. 2021, 10, 171–179. [Google Scholar] [CrossRef]
- Komić, L.; Kruljac, I.; Mirošević, G.; Gaćina, P.; Pećina, H.I.; Čerina, V.; Gajski, D.; Blaslov, K.; Rotim, K.; Vrkljan, M. Spontaneous Resolution of a Nonfunctioning Pituitary Adenoma over One-Month Period: A Case Report. Acta Clin. Croat. 2021, 60, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Marx, C.; Rabilloud, M.; Borson Chazot, F.; Tilikete, C.; Jouanneau, E.; Raverot, G. A key role for conservative treatment in the management of pituitary apoplexy. Endocrine 2012, 71, 168–177. [Google Scholar] [CrossRef]
- Nakhleh, A.; Assaliya Naffa, M.; Sviri, G.; Shehadeh, N.; Hochberg, I. Outcomes of pituitary apoplexy: A comparison of microadenomas and macroadenomas. Pituitary 2021, 24, 492–498. [Google Scholar] [CrossRef]
- Oudghiri, M.D.; Motaib, I.; Elamari, S.; Laidi, S.; Chadli, A. Pituitary Apoplexy in Geriatric Patients: A Report of Four Cases. Cureus 2021, 13, e20318. [Google Scholar] [CrossRef]
- Pan, J.; Yang, X.; Zhu, W. Domino effect of pituitary growth hormone tumor complicated by diabetic ketoacidosis and pituitary apoplexy: A case report. BMC Endocr. Disord. 2021, 21, 109. [Google Scholar] [CrossRef] [PubMed]
- Pattankar, S.; Chauhan, P.; Kapadia, F.; Sankhe, M. Pituitary apoplexy following severe diabetic ketoacidosis, with two uncommon complications of supraventricular tachycardia and acute limb ischemia, in a patient with neglected pituitary adenoma and undiagnosed diabetes mellitus: A rare clinical association. Asian J. Neurosurg. 2021, 16, 890–894. [Google Scholar] [CrossRef]
- Rosso, M.; Ramaswamy, S.; Sucharew, H.; Vagal, A.; Anziska, Y.; Levine, S.R. Isolated Third Cranial Nerve Palsy in Pituitary Apoplexy: Case Report and Systematic Review. J. Stroke Cerebrovasc. Dis. 2021, 30, 105969. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Cai, X.; Li, Y.; Shao, D.; Jiang, Z. Endoscopic Endonasal Transsphenoidal Approach for the Surgical Treatment of Pituitary Apoplexy and Clinical Outcomes. Technol. Cancer Res. Treat. 2021, 20, 15330338211043032. [Google Scholar] [CrossRef]
- Teramoto, S.; Tahara, S.; Kondo, A.; Morita, A. Key Factors Related to Internal Carotid Artery Stenosis Associated with Pituitary Apoplexy. World Neurosurg. 2021, 149, e447–e454. [Google Scholar] [CrossRef]
- Tumyan, G.; Mantha, Y.; Gill, R.; Feldman, M. Acute Sterile Meningitis as a Primary Manifestation of Pituitary Apoplexy. AACE Clin. Case Rep. 2021, 7, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Van Dong, H.; Tran, D.; Chu, H.T.; Pham, A.H.; Nguyen, X.T.; Duong, H.D. Emergency endoscopic surgery for pituitary apoplexy presenting as cerebral infarction in a limited resources condition: A case report. Int. J. Surg. Case Rep. 2021, 83, 106015. [Google Scholar] [CrossRef]
- Vargas, A.; Testai, F.D. Pituitary Apoplexy Causing Bilateral Internal Carotid Artery Ischemia. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2021, 50, 147–148. [Google Scholar] [CrossRef]
- Yoshida, M.; Hiu, T.; Baba, S.; Morikawa, M.; Horie, N.; Ujifuku, K.; Yoshida, K.; Matsunaga, Y.; Niino, D.; Xie, A.; et al. Ruptured aneurysm–induced pituitary apoplexy: Illustrative case. J. Neurosurg. Case Lessons 2021, 1, CASE21169. [Google Scholar] [CrossRef]
- Zhu, J.-D.; Xie, S.; Xu, L.; Xie, M.-X.; Xiao, S.-W. The surgical management of pituitary apoplexy with occluded internal carotid artery and hidden intracranial aneurysm: Illustrative case. J. Neurosurg. Case Lessons 2021, 2, CASE20115. [Google Scholar] [CrossRef]
- Zhu, Q.; Liang, Y.; Fan, Z.; Liu, Y.; Zhou, C.; Zhang, H.; Li, T.; Zhou, Y.; Yang, J.; Wang, Y.; et al. Ischemic Infarction of Pituitary Apoplexy: A Retrospective Study of 46 Cases From a Single Tertiary Center. Front. Neurosci. 2022, 15, 808111. [Google Scholar] [CrossRef]
- Cross, K.A.; Desai, R.; Vellimana, A.; Liu, Y.; Rich, K.; Zipfel, G.; Dacey, R.; Chicoine, M.; Klatt-Cromwell, C.; McJunkin, J.; et al. Surgery for Pituitary Tumor Apoplexy Is Associated with Rapid Headache and Cranial Nerve Improvement. Curr. Oncol. 2022, 29, 390. [Google Scholar] [CrossRef]
- Geyik, A.M.; Durmaz, M.O.; Dogan, A.; Ugur, B.K.; Geyik, S.; Erkutlu, I.; Yasar, S.; Kırık, A.; Kose, G.; Nehir, A. Pituitary Apoplexy: An Emergent and Potential Life-Threatening Complication of Pituitary Adenomas. Turk. J. Trauma Emerg. Surg. 2022, 28, 483–489. [Google Scholar] [CrossRef]
- Hamrick, F.A.; Findlay, M.C.; Rennert, R.C.; Budohoski, K.P.; Couldwell, W.T. Pituitary Apoplexy Precipitated by Systemic Chemotherapy. Cureus 2022, 14, e23004. [Google Scholar] [CrossRef]
- Hsu, C.C.; Lin, H.D.; Huang, C.Y.; Chiang, Y.L. Unusual manifestations of adrenal insufficiency: A case report of hypopituitarism and Well’s syndrome after apoplexy of a silent pituitary gonadotropic adenoma. Medicine 2022, 101, e29274. [Google Scholar] [CrossRef]
- Liu, T.; Rossiter, J.P.; Houlden, R.L.; Awad, S. Sparsely Granulated Corticotroph Pituitary Macroadenoma Presenting With Pituitary Apoplexy Resulting in Remission of Hypercortisolism. AACE Clin. Case Rep. 2022, 8, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.T.; Wharton, S.B.; Connolly, D.J.; Mirza, S.; Sinha, S. Pituitary apoplexy secondary to metastatic breast carcinoma into a gonadotroph cell adenoma of the pituitary. Br. J. Neurosurg. 2022, 36, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, E.H.; Merrill, M.J. Apoplexy of pituitary adenomas: The perfect storm. J. Neurosurg. 2015, 122, 1444–1449. [Google Scholar] [CrossRef]
- Puglisi, V.; Morini, E.; Biasini, F.; Vinciguerra, L.; Lanza, G.; Bramanti, P. Neurological Presentation of Giant Pituitary Tumour Apoplexy: Case Report and Literature Review of a Rare but Life-Threatening Condition. J. Clin. Med. 2022, 11, 1581. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.S.; Gelnick, S.; Pomeranz, H.; Verma, R. Recovery of Complete Blindness and Internal Ophthalmoplegia After Transsphenoidal Decompression of Pituitary Apoplexy. Cureus 2022, 14, e28681. [Google Scholar] [CrossRef]
- Shrestha, R.; Bishokarma, S.; Rayamajhi, S.; Shrestha, S.; Lamichhane, S.; Shrestha, P.; Thulung, S. Pituitary apoplexy presenting as isolated third cranial nerve palsy: Case series. J. Surg. Case Rep. 2022, 2022, rjac386. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Khurana, M.; Pal, H.; Azad, S.; Sihag, R.K.; Kumar, B. Bilateral sixth cranial nerve palsy, the first presenting feature of hemorrhagic apoplexy of pituitary macroadenoma: A case report. Int. J. Surg. Case Rep. 2022, 98, 107522. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Holmes, R. Visual recovery following surgical intervention for pituitary apoplexy correlated with preoperative optical coherence tomography. N. Z. Med. J. 2022, 135, 122–129. [Google Scholar]
- Syed, S.B.; Mourra, A.A.; Chatterjee, T. Isolated Unilateral Abducens Nerve Palsy Manifesting as a Rare Complication of Idiopathic Pituitary Apoplexy: A Case Report. Cureus 2022, 14, e22408. [Google Scholar] [CrossRef] [PubMed]
- Viola, N.; Urbani, C.; Cosottini, M.; Abruzzese, A.; Manetti, L.; Cosentino, G.; Marconcini, G.; Marcocci, C.; Bogazzi, F.; Lupi, I. An altered state of consciousness while using anticoagulants and the incidental discovery of a pituitary lesion: Considering pituitary apoplexy. Endocrinol. Diabetes Metab. Case Rep. 2022, 2022, 21-0204. [Google Scholar] [CrossRef]
- Enatsu, R.; Asahi, M.; Matsumoto, M.; Hirai, O. Pituitary Apoplexy Presenting Atypical Time Course of Ophthalmic Symptoms. Tohoku J. Exp. Med. 2012, 227, 59–61. [Google Scholar] [CrossRef][Green Version]
- Garg, M.K.; Pathak, H.C.; Singh, G. Subclinical pituitary apoplexy with preserved pituitary functions. Indian J. Endocrinol. Metab. 2014, 18, 122–123. [Google Scholar] [CrossRef]
- Mura, P.; Cossu, A.P.; Musu, M.; De Giudici, L.M.; Corda, L.; Zucca, R.; Finco, G. Pituitary apoplexy after laparoscopic surgery: A case report. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3524–3527. [Google Scholar]
- Rebeiz, T.; Cueva, W.; Ardelt, A. Unusual Case of Bilateral Caudate Infarcts Following Pituitary Apoplexy. JAMA Neurol. 2014, 71, 226–227. [Google Scholar] [CrossRef]
- Yoshida, M.; Murakami, M.; Ueda, H.; Miyata, M.; Takahashi, N.; Oiso, Y. An unusual case of hypopituitarism and transient thyrotoxicosis following asymptomatic pituitary apoplexy. Neuro Endocrinol. Lett. 2014, 35, 342–346. [Google Scholar]
- Yoshino, M.; Sekine, Y.; Koh, E.; Hata, A.; Hashimoto, N. Pituitary Apoplexy After Surgical Treatment of Lung Cancer. Ann. Thorac. Surg. 2014, 98, 1830–1832. [Google Scholar] [CrossRef] [PubMed]
- Kasl, R.A.; Hughes, J.; Burrows, A.M.; Meyer, F.B. Pediatric ischemic stroke from an apoplectic prolactinoma. Child’s Nerv. Syst. 2015, 31, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Kistka, H.M.; Turner, J.H.; Devin, J.K.; Chambless, L.B.; Kasl, R.A. Pituitary Apoplexy After Intravitreal Injection of Vascular Endothelial Growth Factor Inhibitor: A Novel Complication. J. Neurol. Surg. Rep. 2015, 76, e205–e210. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, T.; Kitano, Y.; Nishikawa, H.; Mouri, G.; Shimizu, S.; Miya, F.; Suzuki, H. Delayed Onset of Isolated Unilateral Oculomotor Nerve Palsy Caused by Post-Traumatic Pituitary Apoplexy: A Case Report. Clin. Med. Insights Case Rep. 2017, 10, 1179547617731299. [Google Scholar] [CrossRef] [PubMed]
- Rais, N.C.; Merchant, R.A.; Seetharaman, S.K. Pituitary apoplexy masquerading as functional decline in an older person. Age Ageing 2017, 46, 335–336. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hodgson, N.M.; Campbell, A.A.; Chang, J.R.; Vizcaino, A.; Eberhart, C.; Pearl, M.S.; McCulley, T.J. Pituitary Adenoma Apoplexy of the Orbit, Diagnosis, and Management With Presurgical Embolization. Ophthalmic Plast. Reconstr. Surg. 2018, 34, e196–e197. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Ko, Y.S.; Hong, E.K.; Gwak, H.-S. Extensive Pituitary Apoplexy after Chemotherapy in a Patient with Metastatic Breast Cancer. Brain Tumor Res. Treat. 2018, 6, 43–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raj, H.; Kamalanathan, S.; Sahoo, J.P.; Kadhiravan, T. Varicella causing remission of Cushing’s disease. BMJ Case Rep. 2018, 2018, bcr2018225867. [Google Scholar] [CrossRef]
- Salehi, N.; Firek, A.; Munir, I. Pituitary Apoplexy Presenting as Ophthalmoplegia and Altered Level of Consciousness without Headache. Case Rep. Endocrinol. 2018, 2018, 7124364. [Google Scholar] [CrossRef]
- Ward, M.; Kamal, N.; Majmundar, N.; de Leon, A.B.; Eloy, J.A.; Liu, J.K. Post-Traumatic Pituitary Tumor Apoplexy After Closed Head Injury: Case Report and Review of the Literature. World Neurosurg. 2018, 120, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-M.; Oh, H.-J.; Oh, J.-S.; Yoon, S.-M. Pituitary apoplexy causing acute ischemic stroke: Which treatment should be given priority. Surg. Neurol. Int. 2020, 11, 113. [Google Scholar] [CrossRef]
- Romano, A.; Ganau, M.; Zaed, I.; Scibilia, A.; Oretti, G.; Chibbaro, S. Primary Endoscopic Management of Apoplexy in a Giant Pituitary Adenoma. World Neurosurg. 2020, 142, 312–313. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Han, X.; Du, Y.; Ma, A.-Q. Takotsubo cardiomyopathy and pituitary apoplexy: A case report. BMC Cardiovasc. Disord. 2020, 20, 236. [Google Scholar] [CrossRef] [PubMed]
- Alkhaibary, A.; Alsubaie, N.; Alharbi, A.; Alghanim, N.; Khairy, S.; Almuntashri, M.; Alwohaibi, M.; Alarifi, A.; Aloraidi, A.; Alkhani, A. Oculomotor nerve palsy following coronary artery bypass graft surgery: Can pituitary apoplexy complicate the post-operative course of cardiac surgery? J. Surg. Case Rep. 2021, 2021, rjab312. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, S.; Patel, N.; Mawa, K.; Ramu, V.; Paul, T. A Rare Case of Myxedema Coma Presenting as Bradycardia and Hypotension Secondary to Pituitary Apoplexy. Cureus 2021, 13, e15196. [Google Scholar] [CrossRef]
- Elsehety, M.A.; Zeineddine, H.A.; Barreto, A.D.; Blackburn, S.L. Failed endovascular therapy for acute internal carotid artery occlusion from pituitary apoplexy: Illustrative case. J. Neurosurgery: Case Lessons 2021, 2, CASE21370. [Google Scholar] [CrossRef]
- Pokhrel, B.; Khanal, S.; Chapagain, P.; Sedain, G. Pituitary Apoplexy Complicated by Cerebral Infarction: A Case Report. J. Nepal Med. Assoc. 2021, 59, 723. [Google Scholar] [CrossRef]
- Steinberg, J.; Cohen, J.E.; Gomori, J.M.; Fraifeld, S.; Moscovici, S.; Rosenthal, G.; Shoshan, Y.; Itshayek, E. Superficial siderosis of the central nervous system due to chronic hemorrhage from a giant invasive prolactinoma. J. Clin. Neurosci. 2013, 20, 1032–1034. [Google Scholar] [CrossRef]
- Uemura, M.; Miyashita, F.; Shimomura, R.; Fujinami, J.; Toyoda, K. Pituitary apoplexy during treatment with dabigatran. Neurol. Clin. Neurosci. 2013, 1, 82–83. [Google Scholar] [CrossRef]
- Machado, M.C.; Gadelha, P.S.; Bronstein, M.D.; Fragoso, M. Spontaneous remission of hypercortisolism presumed due to asymptomatic tumor apoplexy in ACTH-producing pituitary macroadenoma. Arq. Bras. Endocrinol. Metabol. 2013, 57, 486–489. [Google Scholar] [CrossRef][Green Version]
- Sun, T.; Liu, L.; Sunnassee, A.; Zhuo, L.; Zhu, S. Sudden death in custody due to pituitary apoplexy during long restriction in a sitting position: A case report and review of the literature. J. Forensic Leg. Med. 2013, 20, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Tominaga, A.; Usui, S.; Arita, K.; Sugiyama, K.; Kurisu, K. Impact of subclinical haemorrhage on the pituitary gland in patients with pituitary adenomas. Clin. Endocrinol. 2014, 80, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.-Y.; Chen, J.; Wang, J.-W.; Liu, Y.-C.; Shu, K.; Lei, T. Overview of the 2022 WHO Classification of Pituitary Adenomas/Pituitary Neuroendocrine Tumors: Clinical Practices, Controversies, and Perspectives. Curr. Med. Sci. 2022, 42, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Mete, O.; Wenig, B.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Overview of the 2022 WHO Classification of Head and Neck Neuroendocrine Neoplasms. Head Neck Pathol. 2022, 16, 123–142. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. WHO Classification of Endocrine and Neuroendocrine Tumours; IARC: Lyon, France, 2022. [Google Scholar]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef]


| Author Reference Number/ Year of Publication | Type of Study | Population | Clinical Presentation at Admission for PA | Potential Triggers/Predisposing Factors | Underlying Pituitary Condition * |
|---|---|---|---|---|---|
| Brar [29] 2012 | Case report | 29-year-old male | Headache, nausea, non-projectile vomiting, dizziness, hypotension (systolic BP = 86–90 mmHg, diastolic BP = 56–62 mmHg) | High altitude (4572 m) | * |
| Cagnin [30] 2012 | Case report | 80-year-old male | Headache, nausea, vomiting, drowsiness, neck rigidity, fever | Head trauma hypertension | * |
| Chan [31] 2012 | Case report | 30-year-old male | Headache, decrease in visual acuity, 3rd cranial nerve palsy Clinically manifested Cushing disease | Corticotroph PitNET | |
| Chentli [32] 2012 | Case report | 9-year-old boy | Retro-orbital headache, decrease in visual acuity, diplopia, papillary edema Gigantism (+5SD) | Lactosomatotroph PitNET | |
| Choudhry [33] 2012 | Case series | 4 female patients (mean age: 41.75 years) | Headache (n = 4), nausea and vomiting (n = 3), decreased visual acuity (n = 4), bitemporal hemianopia (n = 4), 3rd cranial nerve palsy (n = 2), facial numbness/pain (n = 1) Clinically manifested Cushing disease (n = 4) | DM, hypertension | Corticotroph PitNET |
| Enatsu [196] 2012 | Case report | 65-year-old female | Left 3rd cranial nerve palsy, decrease in visual acuity | Nonfunctioning pituitary tumor | |
| Komurcu [34] 2012 | Case report | 45-year-old male | Headache, bilateral 3rd cranial nerve palsy, proptosis, diplopia, loss of vision | Coronary artery bypass surgery | Null cell pituitary adenoma |
| Kruljac [35] 2012 | Case report | 77-year-old female | Headache, decrease in vision, ptosis, diplopia | Heparin-induced thrombocytopenia, 2DM, hypertension | Squamous cell carcinoma metastasis in the pituitary |
| Kurisu [36] 2012 | Case report | 68-year-old male | Headache, nausea, vomiting, 3rd cranial nerve palsy, coma | Hypertension | Nonfunctioning pituitary tumor |
| Liu [37] 2012 | Case report | 66-year-old male | Headache, vomiting, decreased visual acuity, right 3rd cranial nerve palsy, meningism | Pituitary tumor with hypopituitarism | |
| Mohindra [38] 2012 | Case report | 40-year-old female | Headache, vision loss, coma Acromegalic features | Somatotroph PitNET | |
| Paisley [39] 2012 | Case report | 67-year-old female | Headache, vomiting, partial loss of vision, left temporal hemianopia, light sensitivity | Hypertension | * |
| Tedd [40] 2012 | Case report | 37-year-old male | Headache, nausea, vomiting, photophobia, neck stiffness | Pituitary tumor | |
| Verma [41] 2012 | Case report | 36-year-old male | Headache, fever, loss of vision, 3rd cranial nerve palsy | Pituitary tumor | |
| Wildemberg [42] 2012 | Case report | 40-year-old male | Headache, vomiting | Dengue hemorrhagic fever, thrombocytopenia | Somatotroph PitNET |
| Yamamoto [43] 2012 | Two case reports | 56-year-old female | Headache, vomiting, visual disturbance, left 3rd cranial nerve palsy | Endocrine stimulation tests | Pituitary tumor |
| 73-year-old male | Progressive visual disturbance | Endocrine stimulation tests | Pituitary tumor | ||
| Zoli [44] 2012 | Case report | 59-year-old female | Headache, 6th cranial nerve palsy | Pituitary tumor | |
| Chou [45] 2013 | Case report | 64-year-old female | Headache, nausea, vomiting, fever, high BP, visual illusions | Pituitary tumor | |
| Cinar [46] 2013 | Case report | 38-year-old male | Headache, nausea, vomiting | DM, hypertension | Somatotroph PitNET |
| Delgado-Alvarado [47] 2013 | Case report | 70-year-old male | Headache, right total ophthalmoplegia | * | |
| Deshwal 2013 [48] | Case report | 29-year-old male | Headache, nausea, loss of appetite, fatigue, difficulty sleeping, hypotension | High altitude (5200 m) | No underlying condition |
| Fanous [49] 2013 | Case report | 39-year-old male | Headache, diplopia | Pituitary macroadenoma | |
| Haider [50] 2013 | Case report | 64-year-old woman | Headache, nausea, diplopia, right ptosis | Enoxaparin | Pituitary macroadenoma |
| Hojo [51] 2013 | Case report | 29-year-old male | Headache, vomiting | Lactotroph PitNET | |
| Huang [52] 2013 | Case report | 77-year-old male | Headache, nausea, vomiting, ophthalmoplegia, visual field deficit | GnRH agonist for prostate cancer | Pituitary adenoma |
| Jiang [53] 2013 | Case report | 49-year-old male | Headache | Diabetic ketoacidosis | Somatotroph PitNET |
| Kobayashi [54] 2013 | Case report | 33-year-old female | Headache, nausea, malaise | Oral contraceptives | Nonfunctioning pituitary adenoma |
| Machado [220] 2013 | Case report | 36-year-old female | Spontaneous resolution of cushingoid features under no treatment | Hypertension | Corticotroph PitNET |
| Masui [55] 2013 | Case report | 68-year-old male | Headache, fatigue, anorexia, bitemporal hemianopia | Pituitary melanoma metastasis | |
| Mir [56] 2013 | Case report | 55-year-old male | Headache, vomiting, altered consciousness | 2DM | Somatotroph PitNET |
| Mohamed [57] 2013 | Case report | 37-year-old male | Headache, nausea, vomiting, neck pain, 3rd cranial nerve palsy, left temporal field defect, loss of visual acuity | Pituitary adenoma | |
| Ní Chróinín [58] 2013 | Case report | 75-year-old female | Headache, vomiting, diplopia, 3rd cranial nerve palsy | Hypertension, DM | Pituitary adenoma |
| Oh [59] 2013 | Case report | 42-year-old man | Headache, vomiting, fever, neck stiffness, left eye ptosis, hemianopia | Hypertension, chronic renal failure | Pituitary adenoma |
| Radhiana [60] 2013 | Case report | 44-year-old male | Headache, vomiting, coma | Pituitary adenoma | |
| Steinberg [218] 2013 | Case report | 43-year-old male | Visual loss, loss of consciousness | DM, hypertension | Lactotroph PitNET |
| Sun [221] 2013 | Case report | 49-year-old male | Exitus | Long restrain in sitting position | Gonadotroph PitNET |
| Tutanc [61] 2013 | Case report | 27-year-old female | Headache, vomiting, palpitation, sleep disturbance | Thyroxine therapy | Thyrotrophic PitNET |
| Uemura [219] 2013 | Case report | 84-year-old male | Retro-orbital pain, 3rd cranial nerve palsy | DM, dabigatran etexilate | Nonfunctioning pituitary adenoma |
| Witczak [62] 2013 | Case report | 67-year-old female | Panhypopituitarism, headache, 3rd cranial nerve palsy | Pituitary metastases from breast cancer | |
| Wong [63] 2013 | Case report | 62-year-old male | Headache, diplopia, 3rd, 4th, and 5th cranial nerve palsy | Pituitary adenoma | |
| Zieliński [64] 2013 | Case report | 59-year-old female | Headache, vomiting, nausea, altered consciousness, visual disturbances | Anticoagulation | Nonfunctioning pituitary adenoma |
| Berkenstock [65] 2014 | Case report | 50-year-old male | Headache, nausea, vomiting, unilateral loss of vision, diarrhea, polydipsia, polyuria | Hypertension | Pituitary adenoma |
| Bujawansa [66] 2014 | Retrospective analysis | 55 patients (35 males and 20 females) Mean age: 58.4 years | Acute headache (n = 48) Cranial nerve palsy (n = 26): 3rd cranial nerve palsy (n = 16), 6th cranial nerve palsy (n = 50), multiple palsies (n = 5) Diplopia (n = 21) Visual field defect (n = 20) Vomiting (n = 14) Photophobia (n = 10) Nausea (n = 9) Facial pain/trigeminal neuralgia (n = 8) Collapse (n = 2) | hypertension (n = 11), anticoagulation with warfarin (n = 3), aspirin (n = 2), coronary artery bypass grafting (n = 2), major orthopedic surgery (n = 3), clomiphene (n = 1) | Nonfunctioning pituitary adenomas in 45 cases (82%), lactotroph PitNETs in 6 cases (11.5%), somatotroph PitNETs in 4 cases (7.2%), multiple endocrine neoplasia syndrome in 2 cases |
| Chao [67] 2014 | Case report | 14-year-old female | Headache, nausea, blurred vision | Pituitary adenoma | |
| Cho [68] 2014 | Case report | 40-year-old female | Headache, diplopia, hemiparesis | Hypertension | * |
| Garg [197] 2014 | Case report | 20-year-old male | Visual impairment, bitemporal hemianopia | Pituitary adenoma | |
| Gupta [69] 2014 | Case report | 62-year-old male | Headache, 3rd cranial nerve palsy, visual field defects Acute coronary syndrome, hours after admission to hospital | DM | Pituitary macroadenoma |
| Jho [70] 2014 | Retrospective study | 109 patients (69 males and 40 females) mean age: 51 years | Headache Visual acuity/field deficits Cranial nerve palsies Vomiting Altered consciousness Meningism Fever | Anticoagulation (n = 9) | PitNET (n = 98) Rathke’s cyst (n = 8) Primitive neuroectodermal tumor (n = 1) Craniopharyngioma (n = 1) Metastatic lung carcinoma (n = 1) |
| Lee [71] 2014 | Case report | 58-year-old male | Headache, visual disturbances, visual field defect, decreased sexual activity | T3 thyrotoxicosis | Gonadotroph PitNET |
| Maltby [72] 2014 | Case report | 11-year-old female | Headache, vomiting, lethargy, weight loss Tall stature, central obesity | Possible gonadotroph PitNET | |
| Man [73] 2014 | Case report | 52-year-old male | Headache, left sided weakness | Bronchogenic carcinoma metastases | |
| Mishra [74] 2014 | Case report | 43-year-old male | Headache, vomiting, loss of vision, bitemporal hemianopia, fever | Dengue hemorrhagic fever, thrombocytopenia | Pituitary adenoma |
| Mura [198] 2014 | Case report | 85-year-old male patient | 3rd cranial nerve palsy | Laparoscopic surgery, anticoagulation (dabigatran), hypertension | Nonfunctioning pituitary adenoma |
| Navarro-Bonnet [75] 2014 | Case report | 30-year-old male | Headache, decreased visual acuity, diplopia, right ptosis Within 24 h: confusion, left hemiplegia | Giant lactotroph PitNET | |
| Rebeiz [199] 2014 | Case report | 81-year-old female | Stupor, hypotension, bilateral blindness | Hypertension | Pituitary adenoma |
| Roerink [76] 2014 | Case report | 46-year-old transgender male | Headache, nausea, vomiting, blurred vision | DM during testosterone therapy | Somatotroph PitNET |
| Tan [77] 2014 | Case report | 53-year-old male | Headache, vomiting, left eye ptosis, right 6th cranial nerve palsy, right hemianopia, fever | Dengue hemorrhagic fever, thrombocytopenia | Lactotroph and gonadotroph PitNET |
| Villar-Taibo [78] 2014 | Case report | 51-year-old female | Headache, nausea, vomiting, photophobia, photophobia meningeal signs, fever | Meningitis | Somatotroph PitNET |
| Yoshida [200] 2014 | Case report | 74-year-old female | Asymptomatic apoplexy, anterior hypopituitarism, hyperthyroidism | Pituitary adenoma | |
| Yoshino [201] 2014 | Case report | 78-year-old male | Fever, respiratory distress, polyuria | Thoracic surgery | Pituitary adenoma |
| Zhang [79] 2014 | Case report | 42-year-old male | Headache, fever, loss of consciousness, visual disturbance | Pituitary adenoma | |
| Akakın [80] 2015 | Case report | 58-year-old male | Headache, blurred vision, bitemporal hemianopia, lethargy | Posterior lumbar fusion surgery | Pituitary adenoma |
| Asaithambi [81] 2015 | Case report | 54-year-old male | Headache, visual loss, partial 3rd, 4th, and 6th cranial nerve palsies | Pituitary adenoma | |
| Banerjee [82] 2015 | Case report | 56-year-old female | Headache, visual loss, followed by neurological deterioration (unresponsive, fixed and dilated right pupil, decerebrate response to stimuli) | Fall from standing | Pituitary macroadenoma |
| Fountas [83] 2015 | Case report | 45-year-old male | Headache, fever, photophobia, hypotension, confusion | Pituitary adenoma | |
| Kasl [202] 2015 | Case report | 14-year-old female | Upper limb weakness, mental status changes | Lactotroph PitNET | |
| Kasl [203] 2015 | Case report | 74-year-old female | Unilateral vision loss, 3rd cranial nerve palsy | Intravitreal injection of vascular endothelial growth factor inhibitor | Gonadotroph PitNET |
| Kim [84] 2015 | Case report | 69-year-old male | Headache, visual field defect, diplopia, 3rd, 4th, and 6th cranial nerve palsy | Mitral valvuloplasty | Pituitary adenoma |
| Man [85] 2015 | Case report | 82-year-old male | Headache, diplopia, bilateral 3rd and 6th cranial nerve palsies | Pituitary adenoma | |
| Roerink [86] 2015 | Two case reports | 41-year-old male | Neck pain, acromegalic features | DM | Somatotroph PitNET |
| 47-year-old female | Headache, a second episode of headache and visual impairment, Cushingoid features | DM, hypertension | Corticotroph PitNET | ||
| Saberifard [87] 2015 | Case report | 50-year-old female | Headache, vomiting, visual field defect | Pituitary adenoma | |
| Sasagawa [88] 2015 | Case report | 62-year-old male | Headache, 3rd cranial nerve palsy | GnRH agonist for prostate cancer | Gonadotroph PitNET |
| Sasaki [89] 2015 | Case report | 65-year-old male | Headache, visual impairment, symptoms of adrenal insufficiency | Pituitary adenoma | |
| Singh [90] 2015 | Retrospective analysis | 87 patients (55 males and 30 females) mean age: 50.9 years | Headache (n = 78, 89.7%) Cranial nerve palsy (n = 34, 39%) Visual field defects (n = 30, 34.1%) | Hypertension (n = 34, 39%) DM (n = 11, 12.6%) cardiothoracic surgery (n = 2, 2.3%) anticoagulant therapy (n = 9, 10.3%): heparin (n = 2, 22.2%) or warfarin (n = 7, 77.8%) antiplatelet drugs (n = 17, 19.5%) | Null cell (n = 18) Lactotroph PitNET (n = 8) |
| Teasdale [91] 2015 | Case report | 72-year-old male | Headache, nausea, vomiting, visual disturbances 5 years after the initial presentation: headache, vomiting, visual disturbances, diplopia, 3rd and 6th cranial nerve palsy, epistaxis | Thyreotroph, gonadotroph PitNET 5 years after the initial presentation: malignant spindle and round-cell tumor | |
| Zhu [92] 2015 | Case-control study | 2021 patients with pituitary tumors, out of which: 97 cases with PA (70 males and 27 females) mean age: 50.1 ± 13.9 years (PA), randomly matched with 194 controls | Headache (n = 84, 86.6%) Visual deterioration (n = 60, 61.86%) Vomiting (n = 39, 40.21%) Ptosis (n = 25, 25.77%) Diplopia (n = 6, 6.18%) | Hypertension DM (differences between cases and controls were not statistically significant) | Null (non-functional) PitNET (n = 63) Lactotroph PitNET (n = 7) Somatotroph PitNET (n = 8) Corticotroph PitNET (n = 1) Thyrotroph PitNET (n = 2) Gonadotroph PitNET (n = 10) Others (n = 4) Multiple staining (n = 2) |
| Zou [93] 2015 | Case report | 23-year-old male | Headache, nausea, decreased visual acuity, loss of consciousness, fever | Somatotroph PitNET | |
| Choudhury [94] 2016 | Case report | 75-year-old male | Headache, nausea, vomiting, photophobia | Antiplatelet drug | Pituitary adenoma |
| Doglietto [95] 2016 | Case report | 76-year-old female | Headache, visual disturbances, ophthalmoplegia, 3rd cranial nerve palsy | Anticoagulant therapy (dabigatran) | Nonfunctioning pituitary adenoma |
| Gambaracci [96] 2016 | Case report | 55-year-old female | Headache, decreased visual acuity, fever | Pituitary adenoma | |
| Giammattei [97] 2016 | Case series | 8 male patients mean age: 70 years | Headache (n = 7) Nausea and vomiting (n = 4) Decreased visual acuity (n = 2) Ophthalmoplegia (n = 6) Altered consciousness (n = 1) Photophobia (n = 1) | Anticoagulant (n = 3) Antiplatelet (n = 1) Hypertension (n = 5) DM (n = 2) Autoimmune hemolytic anemia with thrombocytopenia (n = 1) | Pituitary adenoma |
| Giritharan [98] 2016 | Case series | 31 patients (19 males, 12 females), mean age: 55 years | Headache (n = 31, 100%) Nausea/vomiting (n = 17, 55%) Visual field defect (n = 18, 58%) Decrease in visual acuity (n = 7, 23%) Ocular paresis (n = 12, 39%): 3rd cranial nerve (n = 8, 26%), 6th cranial nerve (n = 6, 19%) | Hypertension (n = 5, 16%) Oral anticoagulation (n = 3, 10%) Heparin therapy (n = 1, 3%) Pregnancy (n = 1, 3%) Previously known adenoma (n = 1, 3%) | Nonfunctioning adenomas (n = 21, 67.74%) Somatotroph PitNET (n = 2, 6.45%) Lactotroph PitNET (n = 1, 3.22% ) Gonadotroph PitNET (n = 5, 16.12%) Corticotroph PitNET (n = 2, 6.45%) |
| Keane [99] 2016 | Case report | 67-year-old male | Headache, 3rd cranial nerve palsy | GnRH agonist for prostate cancer | Gonadotroph PitNET |
| Ogawa [100] 2016 | Retrospective study | 43 patients (30 males and 13 females) mean age: 56.67 years | Headache Cranial nerve palsies Aseptic meningitis Altered consciousness | Nonfunctioning pituitary adenoma (n = 29) Lactosomatotroph PitNET (n = 5) Lactotroph PitNET (n = 4) Thyrotroph PitNET (n = 3) Somatotroph PitNET (n = 1) Corticotroph PitNET (n = 1) | |
| Paschou [101] 2016 | Case report | 37-year-old male | Headache, nausea, fever, visual acuity decrease, 3rd cranial nerve palsy, neck stiffness, confusion | Gonadotroph PitNET | |
| Sussman [102] 2016 | Case report | 46-year-old male | Headache, dizziness, decrease in visual acuity, syncope, 3rd and 4th cranial nerve palsy, hemiparesis | Anti-hypertensive drugs | Pituitary adenoma |
| Balaparameswara Rao [103] 2017 | Case report | 45-year-old male | Headache, vomiting, altered consciousness | Dengue hemorrhagic fever | Corticotrophic PitNET |
| Grangeon [104] 2017 | Case report | 83-year-old male | Headache (hemicrania) | DM | Pituitary adenoma |
| Humphreys [105] 2017 | Prospective study | 10 patients, out of which 5 patients with PA (2 males and 3 females) mean age: 46 years | Headache (n = 3) Visual field defect (n = 1) Decreased visual acuity (n = 1) Irregular menses (n = 1) Hyponatremia (n = 1) Altered consciousness (n = 1) | Gonadotroph PitNET (n = 3) Thyrotroph PitNET (n = 1) Null cell (n = 1) | |
| Ishigaki [204] 2017 | Case report | 66-year-old male | Delayed 3rd cranial nerve palsy | Head trauma, hypertension, DM | Nonfunctioning pituitary adenoma |
| Law-Ye [106] 2017 | Case report | 29-year-old male | Headache, progression to coma | Bodybuilding exercises | Pituitary adenoma |
| Pasha [107] 2017 | Case report | 35-year-old female | Headache, vomiting, 3rd cranial nerve palsy, decrease in vision, acute right side motor deficits and speech impairment | Pituitary adenoma | |
| Patra [108] 2017 | Case report | 36-year-old male | Headache | Polycythemia | Somatotroph PitNET |
| Rais [205] 2017 | Case report | 86-year-old female | Functional decline | Hypertension, DM | Pituitary adenoma |
| Simsek Bagir [109] 2017 | Case report | 32-year-old male | Headache, nausea, vomiting, hiccups, acromegalic features | Lactosomatotroph PitNET | |
| Souteiro [110] 2017 | Case report | 77-year-old female | Headache, nausea, vomiting, psychomotor impairment, visual acuity loss | Hypertension, 2DM | Corticotroph PitNET |
| Waqar [111] 2017 | Retrospective study | 47 patients with pituitary apoplexy (33 males and 14 females) mean age: 54 ± 15 years - patients were compared with 50 surgically treated patients with nonfunctioning pituitary adenomas | Headache (n = 42) Nausea/vomiting (n = 25) Visual field defect (n = 26) Visual acuity defect (n = 18) Cranial nerve palsy (n = 19) Altered consciousness (n = 4) | Hypertension (n = 11) Anticoagulation-antiplatelet therapy (n = 4) | Pituitary adenoma |
| Zoli [112] 2017 | Retrospective study | 75 patients (45 males and 30 females) mean age: 52.4 ± 16.2 years | Headache (n = 75, 100%) Anterior hypopituitarism (n = 51, 68%) Visual disturbances (n = 55, 73.4%) Ophthalmoplegia (n = 38, 50.7%) Altered consciousness (n = 2, 2.6%) | Pituitary adenoma | |
| Abbara [113] 2018 | Retrospective study | 52 patients (25 males and 27 females) mean age: 46.7 years | Headache (n = 40/43) Vomiting (n = 22/43) 3rd cranial nerve palsy only (n = 12/35) 6th cranial nerve palsy only (n = 8/35) 3rd and 6th palsy (n = 3/35) Decreased visual acuity (n = 14/35) Visual fields defects (n = 13/35) | Hypertension (n = 17) Intrapartum/puerperal (n = 7) DM (n = 4) Antiplatelet or anticoagulant (n = 3) Dopamine agonists (n = 2) Radiotherapy (n = 2) None (n = 24) | Nonfunctioning adenoma or gonadotroph PitNET (n = 47) Lactotroph PitNET (n = 5) |
| Bettag [114] 2018 | Case report | 75-year-old female | Headache, diplopia, decreased consciousness | Gonadotroph PitNET | |
| Fan [115] 2018 | Case report | 79-year-old male | Headache, decreased vision, diplopia, 3rd cranial nerve palsy | Pituitary adenoma | |
| Hodgson [206] 2018 | Case report | 71-year-old female | Proptosis | Lactotroph PitNET | |
| Jang [207] 2018 | Case report | 41-year-old female | Diplopia, visual disturbances, 6th cranial nerve palsy | Chemotherapy (doxorubicin and cyclophosphamide) | Pituitary macroadenoma |
| Joo [116] 2018 | Case report | 73-year-old male | Headache, 3rd cranial nerve palsy | Lumbar fusion surgery in prone position | Pituitary macroadenoma |
| Komshian [117] 2018 | Case report | 56-year-old male | Headache, diplopia, 3rd cranial nerve palsy | Nonfunctioning pituitary adenoma | |
| Kuzu [118] 2018 | Case report | 30-year-old male | Headache, 3rd cranial nerve palsy | Dexamethasone suppression test | Corticotroph PitNET |
| Myla [119] 2018 | Case report | 59-year-old male | Headache, stiff neck, nausea | Hypertension | Nonfunctioning pituitary macroadenoma |
| Raj [208] 2018 | Case report | 18-year-old male | Vomiting, adrenal insufficiency | Varicella infection, thrombocytopenia | Corticotroph PitNET |
| Ricciuti [120] 2018 | Case series | 17 patients (12 males and 5 females) mean age: 58.76 years | Headache (n = 5) Vomiting (n = 4) 3rd cranial nerve palsy (n = 10) 6th cranial nerve palsy (n = 4) Visual acuity deficit (n = 4) Neck stiffness (n = 1) | Hypertension (n = 3) Previous radiation therapy (n = 1) | |
| Rutkowski [121] 2018 | Retrospective study | 32 patients (21 males and 11 females) mean age: 49 years | Headache (n = 32, 100%) Nausea/vomiting (n = 10, 31%) Encephalopathy (n = 6, 19%) Nuchal rigidity (n = 4, 12%) Hypopituitarism (n = 28, 88%) Decrease in visual acuity (n = 31, 97%) Cranial nerve palsy (3rd, 4th and/or 6th) (n = 16) | Nonfunctional adenoma (70%) Clinically hypersecreting adenoma (15%) Lactotroph PitNET Somatotroph PitNET Corticotroph PitNET | |
| Salehi [209] 2018 | Case report | 78-year-old male | Ophthalmoplegia, 3rd and 4th cranial nerves palsy, altered consciousness | Pituitary adenoma | |
| Ward [210] 2018 | Case report | 63-year-old male | Fever, hypotension, tachycardia, altered consciousness | Closed head injury | Nonfunctioning pituitary adenoma |
| Yamada [122] 2018 | Case report | 50-year-old male | Headache, visual impairments, loss of consciousness | * | |
| Almeida [123] 2019 | Retrospective analysis | 67 patients (41 males and 26 females) mean age: 57.4 +/− 16.2 years | Headache (n = 60) Visual deficit (n = 44) Hypopituitarism (n = 40) Cranial nerve palsy (n = 32): 3rd (n = 17), 4th (n = 8), 6th (n = 8) Altered levels of consciousness (n = 10) | Pituitary adenoma | |
| Crisman [124] 2019 | Case report | 43-year-old male | Headache, cranial nerve palsies (3rd, 4th, and 6th) | Endoscopic Retrograde Cholangiopancreatography | Pituitary adenoma |
| Dupont [125] 2019 | Case report | 83-year-old female | Headache, bilateral vision loss | Dual anti-aggregation | Pituitary adenoma |
| Ghalaenovi [126] 2019 | Case report | 28-year-old male | Resolution of initial symptoms (headache, nausea, photophobia, bitemporal hemianopia at diagnosis of a pituitary macroadenoma) | Lactotroph PitNET | |
| Harju [127] 2019 | Case report | 48-year-old male | Headache, diplopia, 3rd cranial nerve palsy, visual field defect, fever | Endoscopic endonasal surgery | Non-functional macroadenoma |
| Hosmann [128] 2019 | Retrospective analysis | 76 patients (53 males and 23 females) mean age: 53.7 +/−14.3 years | Headache (n = 63, 82.9%) Nausea/vomiting (n = 26, 34.2%) Decrease in visual acuity (n = 42, 54.9%) Visual field deficit (n = 48, 63.3%) Cranial nerve palsy: 3rd (n = 35, 46.1%), 6th (n = 22, 28.9%) Altered levels of consciousness (n = 12, 15.8%) | Hypertension (n = 23, 30.3%) Oral anticoagulation (n = 14, 18.4%) DM (n = 9, 11.8%) Extracranial surgery within 24 h before apoplexy (n = 4, 5.3%) | Clinically nonfunctioning PitNETs (81%): gonadotroph PitNET (37.9%), null-cell (29.3%), plurihormonal (8.6%), corticotroph (3.5%), somatotroph (1.7%) Clinically functioning PitNETs: lactotroph (10.4%), corticotroph (6.9%), somatotroph (1.7%) |
| Kirigin Biloš [129] 2019 | Case report | 74-year-old male | Headache, nausea, vomiting, vertigo, 3rd cranial nerve palsy | Pituitary adenoma | |
| Krug [130] 2019 | Case report | 45-year-old male | Headache, diplopia | Crooke cell adenoma | |
| Mittal [131] 2019 | Case report | 38-year-old male | Headache, nausea, 3rd cranial nerve palsy, visual field deficits | DM | Nonfunctioning pituitary adenoma |
| Naito [132] 2019 | Case report | 14-year-old female | Headache, visual impairment | Cardiac surgery | Pituitary adenoma |
| Nioi [133] 2019 | Case report | 50-year-old female | Headache, visual impairment, vertigo Hemodynamic collapse after placement of nasogastric tube | TSS, incorrect placement of nasogastric tube | Pituitary adenoma |
| Pedro [134] 2019 | Case report | 79-year-old male | Headache, photophobia, vomiting | Pituitary adenoma | |
| Santos [135] 2019 | Case report | 74-year-old female | Headache, vomiting, decrease in visual acuity, bitemporal hemianopia | Systemic anticoagulation, DM | Pituitary adenoma |
| Sanz-Sapera [136] 2019 | Case report | 50-year-old male | Headache, acromegalic features | Somatotroph PitNET | |
| Singhal [137] 2019 | Case report | 65-year-old female | Headache, vision loss, rhinorrhea | Corticotroph PitNET | |
| Swaid [138] 2019 | Case report | 65-year-old female | Headache, 3rd cranial nerve palsy | Coronary angiography, anticoagulation (heparin), 2DM, hypertension | Pituitary adenoma |
| Thomas [139] 2019 | Case report | 85-year-old male | Headache, 3rd cranial nerve palsy | Dengue fever-induced thrombocytopenia | Pituitary adenoma |
| Uneda [140] 2019 | Case report | 51-year-old male | Headache, 3rd cranial nerve palsies (ophthalmoplegia) | Vardenafil therapy | Pituitary adenoma |
| Wang [141] 2019 | Case report | 21 patients (15 males and 6 females), with a mean age of 50.7 ± 15.0 years | Headache (n = 21) Nausea (n = 15) Vomiting (n = 14) Visual disturbances: visual field defects (n = 17) decreased visual acuity (n = 17) Cranial nerves palsies (n = 10) Electrolyte disturbances (n = 12) Menstrual disturbances and galactorrhea (n = 4/6) Fever (n = 3) Altered consciousness (n = 3) | Hypertension (n = 9) Coagulation disturbances (n = 9) Diabetes mellitus (n = 4) Antiplatelet therapy (n = 1) Chronic renal insufficiency (n = 1) Atrial fibrillation (n = 1) Old myocardial infarction (n = 1) | Nonfunctioning pituitary adenoma (n = 18) Lactotroph PitNET (n = 2) Somatotroph PitNET (n = 1) |
| Waqar [142] 2019 | Case report | 51-year-old male | Headache, vomiting, diplopia, 6th cranial nerve palsy | Nonfunctioning pituitary adenoma | |
| Ahn [211] 2020 | Case report | 78-year-old male | Stupor, hemiparesis | Hypertension | Pituitary adenoma |
| Brown [143] 2020 | Case report | 56-year-old male | 2 episodes of acute-onset headache, vomiting, and a cranial nerve palsy | Switching phenotypes | |
| Catarino [144] 2020 | Case report | 55-year-old female | Headache, 3rd cranial nerve palsy | DM, Pituitary fungal infection | Corticotroph PitNET |
| Eichberg [145] 2020 | Case report | 46-year-old female | Headache, nausea, vomiting, blurred vision | Nonfunctioning pituitary adenoma | |
| Elarjani [146] 2020 | Case report | 31-year-old male | Headache, hemiparesis, unilateral decreased visual acuity | Pituitary adenoma | |
| Franzese [147] 2020 | Case report | 60-year-old male | Headache, nausea, weakness, diplopia, 3rd cranial nerve palsy | Coronary artery bypass grafting | Null cell adenoma |
| Klimko [148] 2020 | Case report | 41-year-old male | Headache, fatigue, weight loss | Somatotroph PitNET | |
| Lee [149] 2020 | Case report | 75-year-old male | Headache, vomiting, dizziness, 3rd, 4th, and 5th nerve palsies | Hypertension, DM, hemodialysis | Nonfunctioning pituitary adenoma |
| Marzoughi [150] 2020 | Case report | 70-year-old male | Headache, diplopia, 3rd cranial nerve palsy | Hypertension, DM, anticoagulant | Pituitary adenoma |
| Pangal [151] 2020 | Retrospective study | 50 patients (31 males, 19 females) mean age: 53 years | Headache (86%) Vision loss (62%) Cranial nerve palsy (40%) Decrease in consciousness (14%) | Pituitary adenoma | |
| Patel [152] 2020 | Case report | 60-year-old male | Headache, nausea, vomiting, monocular vision loss | Autologous hematopoietic cell transplantation | Somatotroph PitNET |
| Romano [212] 2020 | Case report | 65-year-old male | Blindness, hemiparesis, decreased alertness | Pituitary macroadenoma | |
| Shetty [153] 2020 | Case report | 49-year-old female | Headache, palpitations, nausea, vomiting, neck stiffness | Regadenoson myocardial perfusion scan | Nonfunctioning pituitary adenoma |
| Siwakoti [154] 2020 | Case report | 59-year-old female | Headache, nausea, dizziness | Corticotroph PitNET | |
| van Boven [155] 2020 | Case report | 31-year-old female | Headache, nausea, vomiting | 2DM | Corticotroph PitNET |
| Yang [213] 2020 | Case report | 70-year-old female | Confusion, hypotension, fever, chills, cough | Pituitary microadenoma | |
| Alam [156] 2021 | Case report | 40-year-old male | Headache acromegalic feats | Somatotroph PitNET | |
| Aljabri [157] 2021 | Case report | 74-year-old male | Headache and vomiting 2 h after goserelin injection, dizziness, diplopia | Androgen deprivation therapy (goserelin) for prostate cancer | Pituitary macroadenoma |
| Alkhaibary [214] 2021 | Case report | 65-year-old male | Diplopia, 3rd cranial nerve palsy | Coronary bypass graft surgery | Pituitary adenoma |
| Ambrose [158] 2021 | Case report | 84-year-old male | Headache, bruising 5 days after admission: bilateral ptosis, ophthalmoplegia | Immune thrombocytopenia | Pituitary macroadenoma |
| Bhogal [215] 2021 | Case report | 63-year-old male | Myxedema coma | Pituitary adenoma | |
| Bukhari [159] 2021 | Case report | 59-year-old male | Headache, loss of vision, 3rd cranial nerve palsy | Hypertension | Pituitary adenoma |
| Cavalli [160] 2021 | Retrospective study | 30 patients (22 males, 8 females) mean age: 54 years | Visual disturbance (86.7%) Headache (96%) Nausea and vomiting (33.3%) | Hypertension (53.8%) Invasive procedures (30.7%) Anticoagulation (23%) Trauma (7.7%) Antiplatelet therapy (7.7%) | PitNET |
| de Silva [161] 2021 | Case report | 35-year-old female | Headache, visual loss Cushingoid features | Crooke cell tumor | |
| Elsehety [216] 2021 | Case report | 60-year-old female | Vision loss, vomiting, coma | Nonfunctioning pituitary adenoma | |
| Falhammar [162] 2021 | Retrospective study | 33 patients (18 male and 15 female) mean age: 46.5 years | Headache (82%) Visual disturbances (36%) Nausea (36%) | Antithrombotic therapy n = 7 (21%) | PitNET |
| Gohil [163] 2021 | Case report | 56-year-old male | Headache, diplopia, 3rd cranial nerve palsy during hospitalization for leptospirosis | Leptospirosis | Pituitary adenoma |
| Hanna [164] 2021 | Case report | 49-year-old male | Headache, diplopia, 3rd cranial nerve palsy | Nonfunctioning pituitary adenoma | |
| Huang [165] 2021 | Case report | 72-year-old male | Headache, nausea, vomiting, 3rd cranial nerve palsy | Nonfunctioning pituitary adenoma | |
| Iqbal [166] 2021 | Retrospective study | 55 patients (26 males and 29 females) mean age: 50 years | Headache Confusion Visual field defect Cranial nerve palsy | Hypertension Anticoagulation Antiplatelet Previous stroke Radiotherapy | Somatotroph PitNET Lactotroph PitNET |
| Komić [167] 2021 | Case report | 54-year-old male | Headache, vomiting, photophobia | Pituitary adenoma | |
| Marx [168] 2021 | Retrospective study | 46 patients (29 males and 17 females) mean age: 47.3 years | Meningism (n = 20) Headache (n = 38) Visual acuity decrease (n = 11) Visual field defect unilateral impairment bitemporal impairment (n = 20) Cranial nerve palsies: 3rd (n = 19), 4th (n = 6), 6th (n = 13) Impaired Glasgow Coma Scale ( < 15) (n = 2) | GnRH analogs (n = 2) Anticoagulant (n = 1) Cabergoline (n = 2) Recent major surgery (n = 3) Recent rugby play without head trauma (n = 1) Pregnancy (n = 3) | Nonfunctioning adenoma (n = 31) Corticotroph PitNET (n = 1) Lactotroph PitNET (n = 12) Somatotroph PitNET (n = 1) |
| Nakhleh [169] 2021 | Retrospective study | 27 patients (14 males and 13 females) mean age: 40.7 ± 12.5 years | Headache (n = 25) Visual field defect (n = 9) Cranial nerve palsy (n = 3) Altered consciousness (n = 2) | Hypertension (n = 5) DM (n = 3) Antiplatelet therapy (n = 3) | Nonfunctioning pituitary adenoma (n = 21) Lactotroph PitNET (n = 5) Somatotroph PitNET (n = 1) |
| Oudghiri [170] 2021 | Case series | 4 patients (2 males and 2 females) mean age: 78.25 years | Headache (n = 2) Vomiting (n = 2) Visual disturbance (n = 1) Cranial nerve palsy (n = 3) | Anticoagulant (n = 2) Hypertension (n = 1) DM (n = 1) | Nonfunctioning pituitary adenoma |
| Pan [171] 2021 | Case report | 44-year-old male | Headache, vomiting, dizziness | Diabetic ketoacidosis Acute pancreatitis Hypertension | Somatotroph PitNET |
| Pattankar [172] 2021 | Case report | 20-year-old female | Headache, lethargy, altered sensorium Progressive neurological deterioration: hemiparesis, unilateral ophthalmoplegia | Diabetic ketoacidosis | Nonfunctioning pituitary adenoma |
| Pokhrel [217] 2021 | Case report | 26-year-old male | Vision reduction, retro-orbital pain, dizziness, vomiting, right limbs weakness Acromegalic features | No apparent trigger | Somatotroph PitNET |
| Rosso [173] 2021 | Case report | 76-year-old female | Headache, 3rd cranial nerve palsy | 2DM apixaban | Pituitary adenoma |
| Seaman [25] 2021 | Retrospective study | 44 patients (24 males and 20 females) median age: 55 years | Headache (n = 40) Visual disturbances (n = 30): visual field disturbances (n = 16), decreased visual acuity (n = 9), cranial nerve palsies: 3rd (n = 18), 4th (n = 6), 5th (n = 1), 6th (n = 6) Nausea/vomiting (n = 7) Altered mental status (n = 7) Hormone-related complains (n = 4) | Hypertension (n = 22) DM (n = 13) Anticoagulation (n = 4) | Nonfunctioning adenoma (n = 38) Functioning PitNET (n = 6) |
| Sun Z [174] 2021 | Retrospective study | 24 patients (13 males and 11 females) mean age: 46.46 ± 14.95 years | Headache (n = 20, 83.33%) Nausea and vomiting (n = 17, 70.83%) Loss of vision (n = 18, 75.00%) Visual field defects (n = 8, 33.33%) Ophthalmoplegia attributed mainly to 3rd and 6th cranial nerve palsies (n = 7, 29.17%) Decreased libido (n = 2, 8.33%) Amenorrhea (n = 2, 8.33%) Loss of consciousness (n = 1) | Nonfunctioning adenomas (n = 7) Lactotroph PitNET (n = 3) Corticotroph PitNET (n = 1) Gonadotroph PitNET (n = 1) Lactocorticotroph PitNET (n = 1) | |
| Teramoto [175] 2021 | Retrospective study | 45 patients (33 males and 12 females) mean age: 56 years | Headache (n = 40) Visual impairment (n = 18) Ophthalmoplegia (n = 19) Hypopituitarism (n = 23) | Hypertension (n = 12) DM (n = 10) Antithrombotic therapy (n = 7) | Nonfunctioning adenoma (n = 37) Somatotroph PitNET (n = 5) Lactotroph PitNET (n = 3) |
| Tumyan [176] 2021 | Case report | 67-year-old male | Headache, nausea, peripheral vision defect, decreased consciousness within 48 h; 3rd cranial nerve palsy, progressive confusion | DM | Pituitary adenoma |
| Van Dong [177] 2021 | Case report | 38-year-old female | Headache, decreased visual acuity, aphasia, hemiparesis, decreased consciousness | Pituitary adenoma | |
| Vargas [178] 2021 | Case report | 53-year-old female | Headache, vomiting, 3rd cranial nerve palsy, progressive neurological deterioration | Pituitary macroadenoma | |
| Yoshida [179] 2021 | Case report | 78-year-old male | Headache, nausea, hemianopia, collapse | Gonadotroph PitNET | |
| Zhu JD [180] 2021 | Case report | 48-year-old male | Headache, vomiting, 3rd cranial nerve palsy, decreased vision unilaterally within 48 h: consciousness disturbance, hemiplegia, hyperthermia | Nonfunctioning pituitary adenoma | |
| Zhu Q [181] 2021 | Retrospective study | 46 patients (35 males and 11 females) mean age: 46.78 ± 12.32 | Headache (n = 44) Visual disturbance (n = 41) Nausea/vomiting (n = 27) Hypogonadism (n = 39) Asymptomatic apoplexy (n = 1) | PitNET | |
| Cross [182] 2022 | Retrospective study | 59 patients (40 males and 19 females) median age: 54 years | Headache (n = 59) Cranial nerve deficits (n = 46): 3rd cranial nerve palsy (n = 26, 44%), 4th cranial nerve palsy (n = 1), 6th cranial nerve paly (n = 12, 20%) Vision loss (n = 5) | PitNET | |
| Geyik [183] 2022 | Retrospective study | 143 patients with pituitary adenoma, out of which 8 patients with PA (4 males and 4 females, mean age: 26.75 years) | Headache (n = 7) Visual disturbances (n = 4) Amenorrhea and galactorrhea (n = 4) | Lactotroph PitNET (n = 5) Nonfunctioning adenoma (n = 3) | |
| Hamrick [184] 2022 | Case report | 31-year-old male | Headache, nausea, vision decrease, | Systemic chemotherapy (bleomycin, etoposide, cisplatin) | Somato- and corticotroph PitNET |
| Hsu [185] 2022 | Case report | 53-year-old male | Headache, fever, pruritic skin rash, abdominal pain, fatigue | Gonadotroph PitNET | |
| Liu [186] 2022 | Case report | 33-year-old male | Headache, nausea, vomiting, bitemporal hemianopia, diplopia, 3rd cranial nerve palsy, Cushingoid features | Corticotroph PitNET | |
| Mills [187] 2022 | Case report | 65-year-old female | Headache, reduced visual acuity, bitemporal hemianopia | Metastatic breast carcinoma into gonadotroph PitNET | |
| Oldfield [188] 2022 | Case report | 57-year-old male | Headache, thirst, polydipsia, weakness | Pituitary adenoma | |
| Puglisi [189] 2022 | Case report | 81-year-old female | Headache, bilateral vision loss, altered consciousness, hematuria | Anticoagulant (acenocoumarol), hypertension | PitNET |
| Rai [190] 2022 | Case report | 64-year-old male | Headache, nausea, vomiting, decreased visual acuity, ophthalmoplegia | Hypertension, DM, polysubstance abuse | Pituitary macroadenoma |
| Shrestha [191] 2022 | 2 case reports | 56-year-old male | Headache, 3rd cranial nerve palsy, diplopia, decrease in vision | Hypertension | PitNET |
| 50-year-old male | 3rd cranial nerve palsy (ptosis) | ||||
| Singh A. [192] 2022 | Case report | 36-year-old male | Chronic headache, sudden diplopia, 6th cranial nerve palsy | Somatotroph PitNET | |
| Singh V. [193] 2022 | Case report | 52-year-old female | Headache, visual field changes | Nonfunctioning pituitary adenoma | |
| Syed [194] 2022 | Case report | 36-year-old male | Headache, diplopia, abducens nerve palsy | Pituitary adenoma | |
| Viola [195] 2022 | Case report | 63-year-old male | Headache, asthenia, diplopia | Pituitary adenoma |
| Author (Reference) | Management | Outcome |
|---|---|---|
| Brar [29] | Transfer to a lower altitude glucocorticoid replacement | Adrenal insufficiency |
| Cagnin [30] | C | Resolution of neurological symptoms, hypopituitarism |
| Chan [31] | TSS Hormonal replacement (postoperative hypopituitarism) | 3 years follow-up: remission of the pituitary tumor, regression of Cushingoid features, permanent anterior hypopituitarism and central diabetes insipidus |
| Chentli [32] | C | 2 months follow-up: visual acuity normalized, spontaneous reduction in pituitary tumor (MRI) |
| Choudhry [33] | TSS (n = 4) Hormonal replacement (postoperative hypopituitarism) | Postoperatively: vision recovery and biochemical remission of Cushing’s disease (n = 4) 6 months follow-up: regression of cushingoid features (n = 4), weight-loss (n = 4), hypertension remission (n = 3), DM resolution (n = 3), DM improvement (n = 1) 40 months follow-up: biochemical remission and postoperative anterior hypopituitarism (n = 4) |
| Enatsu [196] | TSS | 3rd cranial nerve palsy improved Complete removal of the mass (MRI) Endocrine profile recovery |
| Komurcu [34] | TSS | 4 months follow-up: complete visual recovery 6 months follow-up: complete tumor removal, no recurrence (CT) |
| Kruljac [35] | TSS | Exitus 1 month later |
| Kurisu [36] | Craniotomy, evacuation of the hemorrhage, tumor resection through anterior interhemispheric approach | Exitus within 1 month (PA causing intracerebral hemorrhage) |
| Liu [37] | C | 3 months follow-up: normal pituitary hormones secretion, complete disappearance of pituitary mass (MRI) |
| Mohindra [38] | Ventriculo-peritoneal shunt | Exitus within 48 h |
| Paisley [39] | TSS Glucocorticoid replacement | Visual recovery, partial hypopituitarism |
| Tedd [40] | Glucocorticoid replacement | |
| Verma [41] | TSS Glucocorticoid replacement | Clinical improvement |
| Wildemberg [42] | TSS | |
| Yamamoto [43] | TSS Transient desmopressin replacement | Symptoms resolved postoperatively and vision returned |
| TSS | Visual disturbances resolved + No hormonal deficits | |
| Zoli [44] | TSS | 3 months follow-up: radical tumor removal, resolution of neurological symptoms |
| Chou [45] | TSS | Resolution of visual disturbances |
| Cinar [46] | TSS | Remission of acromegaly |
| Delgado-Alvarado [47] | TSS | Resolution of ophthalmoplegia |
| Deshwal [48] | Transfer to a lower altitude + C | Full recovery |
| Fanous [49] | TSS | Near complete resolution of visual disturbances |
| Haider [50] | C (+ Dexamethasone) | Complete recovery of 3rd cranial nerve function |
| Hojo [51] | Initial C → TSS following hematoma expansion | No visual disturbances |
| Huang [52] | TSS | Vision improvement |
| Jiang [53] | C | Patient refused further treatment |
| Kobayashi [54] | C | Recovery without sequela |
| Machado [220] | C | 28 months follow-up: clinical remission |
| Masui [55] | TSS | Anterior pituitary hormonal deficits, diabetes insipidus |
| Mir [56] | TSS Glucocorticoid replacement | Remission of DM; normalization of GH levels Central hypothyroidism, hypogonadism, and hypocortisolism |
| Mohamed [57] | TSS | Postoperatively: partial improvement of vision and 3rd cranial nerve palsy |
| Ní Chróinín [58] | C | Clinical improvement |
| Oh [59] | TSS | Clinical improvement |
| Radhiana [60] | Craniotomy, decompression, tumor excision | Exitus |
| Steinberg [218] | Patient refused surgery C (Cabergoline) | Tumor size decreased, necrotic zone in the pituitary (MRI) |
| Uemura [219] | C (Glucocorticoid, levothyroxine replacement) | Resolution of signs and symptoms |
| Witczak [62] | TSS | Exitus after 3 months (due to breast cancer) |
| Wong [63] | TSS | Recovery of nerve palsies |
| Zieliński [64] | C (Glucocorticoid, levothyroxine replacement) | Tumor regression (MRI); resolution of ophthalmic and neurological symptoms |
| Berkenstock [65] | TSS | Resolution of nerve palsy, persistent visual field defects, panhypopituitarism |
| Bujawansa [66] | Pituitary surgery (n = 33, 55%): early emergency surgery (n = 23), delayed elective surgery (n = 18) conservative (n = 22, 40%) | Complete resolution of visual field defects (65%) Partial improvement of visual field defects (85%) Complete resolution of cranial nerve palsies (n = 23) Partial resolution of cranial nerve palsies (n = 26) Anterior pituitary deficits (n = 47, 85.5%) Central adrenal insufficiency (n = 40, 72.7%) Central hypothyroidism (29 patients, 52.7%) Severe growth hormone deficiency (n = 21, 38.2%) Hypogonadism (n = 27 male patients) Diabetes insipidus (n = 2) |
| Chao [67] | TSS | Visual field improvement |
| Cho [68] | C | Partial recovery, no endocrine dysfunctions |
| Garg [197] | TSS | |
| Gupta [69] | Coronary artery bypass → TSS | Resolution of nerve palsy, persistent visual field defects |
| Jho [70] | TSS (n = 101) C (n = 8) | Resolution of symptoms (n = 48) Improvement of symptoms (n = 12) Stationary (n = 1) |
| Lee [71] | TSS Glucocorticoid replacement, anti-thyroid drugs | |
| Maltby [72] | C | Panhypopituitarism, empty sella |
| Man [73] | Palliative care, dexamethasone, brain radiotherapy | |
| Mishra [74] | TSS | Visual improvement |
| Mura [198] | Conservative | Hypogonadotrophic hypogonadism |
| Navarro-Bonnet [75] | Fronto-temporoparietal craniectomy | Postoperatively: hemiplegia, hormonal substitution (prednisone, levothyroxine), cabergoline treatment 6 months follow-up: amelioration of paralysis, normalized prolactin levels, small residual tumor |
| Rebeiz [199] | TSS | No improvement |
| Roerink [76] | C (Hydrocortisone, levothyroxine, lanreotide, scheduled TSS) | 3 months follow-up: normalized IGF-1 levels |
| Tan [77] | C (Cabergoline, dexamethasone) → TSS due to visual deterioration | Hypopituitarism, persistent visual defects, residual tumor |
| Villar-Taibo [78] | C (Hormonal replacement) | Resolution of symptoms, improvement of acromegaly signs and symptoms, hypopituitarism, diabetes insipidus |
| Yoshida [200] | TSS Hormonal replacement | Hypopituitarism, resolution of hyperthyroidism, with subsequent hypothyroidism |
| Yoshino [201] | C (Glucocorticoid replacement) | Resolution of pituitary hemorrhage |
| Zhang [79] | TSS Hormonal replacement | Symptoms improvement |
| Akakın [80] | TSS Hormonal replacement | Visual defects improvement, no residual tumor |
| Asaithambi [81] | TSS | Postoperatively: bitemporal hemianopia, left and right partial 3rd cranial nerve palsy |
| Banerjee [82] | TSS | No improvement |
| Fountas [83] | TSS Hormonal replacement | Hypopituitarism |
| Kasl [202] | TSS | Improvement of neurological symptoms |
| Kasl [203] | TSS | Recovery of symptoms |
| Kim [84] | TSS | Complete recovery |
| Man [85] | TSS | Partial improvement of 3rd cranial nerve palsy |
| Roerink [86] | C | |
| C → elective TSS | ||
| Saberifard [87] | C | Empty sella |
| Sasagawa [88] | TSS | Complete resolution of nerve palsy |
| Sasaki [89] | C | |
| Singh [90] | Surgery during acute stage (n = 61, 70.1%) Delayed surgery (n = 8, 9.2%) Conservative (n = 18, 20.7%) | Improvement/complete resolution of visual defects in all survivors HRT with levothyroxine (62.7%) or cortisol (60%) Diabetes insipidus at 3-month follow-up (23%) Tumor regrowth (n = 7, 8.6%) Exitus (n = 4, 4.6%) |
| Teasdale [91] | TSS 5 years after the initial presentation: sphenoidotomy, radiotherapy | Visual improvement, panhypopituitarism 5 years after the initial presentation: exitus |
| Zhu [92] | TSS (n = 93, 95.88%) Transcranial surgery (n = 4, 4.12%) | Electrolyte disturbance (n = 18, 18.56%) Diabetes insipidus (n = 69, 71.13%) Cerebrospinal rhinorrhea (n = 2, 2.06%) Postoperative hemorrhage (n = 1, 1.03%) Postoperative infection (n = 1, 1.03%) Exitus (n = 1, 1.03%) |
| Zou [93] | Surgery | Improvement of symptoms and radiological findings |
| Choudhury [94] | C | Considerable reduction in pituitary tumor |
| Doglietto [95] | TSS | Recovery of nerve palsy, complete removal of pituitary adenoma (MRI) |
| Gambaracci [96] | TSS | |
| Giammattei [97] | TSS (n = 8) Hormonal replacement | Hypopituitarism |
| Giritharan [98] | C (n = 11, 35%) Surgery (n = 20, 65%) | Hormone deficiency (n = 26, 84%) Resolution of visual disturbances (n = 18, 72%) Improvement of visual symptoms (n = 6, 24%) |
| Keane [99] | TSS | Complete resolution of symptoms |
| Ogawa [100] | TSS (n = 43) | Resolution of neurological symptoms (n = 38) Persistence of nerve palsy (n = 3) Prolonged visual problems (n = 1) Exitus due to leukemia (n = 1) |
| Paschou [101] | TSS | Resolution of symptoms, central hypothyroidism |
| Sussman [102] | TSS | 6 weeks follow-up: partial recovery of lower limb paresis, persistence of upper limb paresis, full recovery of 3rd and 4th cranial nerve palsies, partial 6th cranial nerve palsy |
| Balaparameswara Rao [103] | TSS | Improvement of vision Anterior hypopituitarism |
| Grangeon [104] | C | Headache resolution |
| Humphreys [105] | TSS (n = 5) Radiotherapy (n = 2) | Hypopituitarism treated with HR (n = 4) |
| Ishigaki [204] | TSS | Resolution of nerve palsy |
| Law-Ye [106] | C | Full recovery |
| Pasha [107] | TSS | Recovery of neurological deficits |
| Patra [108] | C | Remission of acromegaly and polycythemia |
| Rais [205] | C | |
| Simsek Bagir [109] | TSS | Remission of symptoms, central hypothyroidism and hypogonadism, GH deficiency |
| Souteiro [110] | C | Resolution of hypercortisolism, disappearance of the tumor (MRI) |
| Waqar [111] | TSS (n = 36) Conservative (n = 11) | Hypopituitarism (n = 45) Diabetes insipidus (n = 3) |
| Zoli [112] | TSS (n = 75) | Anterior hypopituitarism (n = 15, 20%) Diabetes insipidus (n = 4, 5.3%) Ophthalmoplegia resolution/improvement (71%) Visual symptoms improvement (85.5%) Conscious regain in both patients |
| Abbara [113] | C (n = 33/52) Surgery (n = 19/52) | Partial resolution of visual symptoms (n = 19/19) Full resolution of visual symptoms (n = 5/16) Hypopituitarism: long-term hypocortisolism (n = 27), hypothyroidism (n = 15) Diabetes insipidus (n = 2) |
| Bettag [114] | TSS | Partial improvement of hemiparesis, persistent visual loss |
| Fan [115] | C | Complete resolution of visual disturbances after 6 months |
| Hodgson [206] | Embolization, surgical resection | Reduction in pain |
| Jang [207] | TSS | Improvement of visual disturbances |
| Joo [116] | TSS | Full recovery |
| Kuzu [118] | TSS | Recovery of symptoms, panhypopituitarism |
| Myla [119] | TSS | Hormonal replacement |
| Raj [208] | C | Remission of central adrenal insufficiency |
| Ricciuti [120] | Surgery (n = 13) C (n = 4) | Symptoms resolved in up to 6 months Hormonal substitution (n = 6) Residual tumor (n = 6) |
| Rutkowski [121] | Surgery (n = 32, 100%) | Improvement of visual symptoms (77%) Resolution of visual symptoms (38%) Resolution of oculomotor palsies (81%) Partial hormone recovery following preoperative hypopituitarism 6 (21%) of 28 |
| Salehi [209] | TSS | Complete recovery |
| Ward [210] | TSS Hormonal replacement | Stable vision, improved mental status |
| Yamada [122] | C | Panhypopituitarism |
| Almeida [123] | Surgery (n = 49) C (n = 18) | Last follow-up: Visual status: worsening (n = 1), stationary (n = 3), partial improvement (n = 12), complete recovery (n = 24) Cranial nerve palsies: partial improvement (n = 9), complete recovery (n = 17) Hormonal function: hypothyroidism (n = 3), hypocortisolism (n = 11), panhypopituitarism (n = 27), diabetes insipidus (n = 4) |
| Crisman [124] | TSS | Resolution of cranial nerve palsy |
| Dupont [125] | TSS | Slight regain of vision postoperatively |
| Ghalaenovi [126] | Lost during follow-up | The patient experienced spontaneous resolution of initial symptoms |
| Harju [127] | Craniotomy Glucocorticoid replacement | Persistent visual defects, anosmia, diabetes insipidus |
| Hosmann [128] | TSS (n = 72, 94.7%) Surgery with sub-frontal approach (n = 4, 5.3%) | Decreased visual acuity (12.3%) Visual field deficit (35.4%) Impaired eye movement (14.1%) Hormonal replacement (72.3%): hypothyroidism (61.3%), hypocortisolism (52.3%), hypogonadism (29.2%), permanent diabetes insipidus (9.2%), GH deficiency (4.6%) |
| Kirigin Biloš [129] | C | Improvement of 3rd cranial nerve deficit, diplopia, panhypopituitarism, partial empty sella |
| Krug [130] | TSS | Recovery of symptoms |
| Mittal [131] | C → TSS | |
| Naito [132] | C | Resolution of symptoms |
| Nioi [133] | Exitus | |
| Pedro [134] | C | |
| Santos [135] | C | Resolution of symptoms, hypopituitarism |
| Sanz-Sapera [136] | C (Hormonal replacement) | Acromegaly remission |
| Singhal [137] | TSS | |
| Swaid [138] | TSS | 1 month follow-up: persistence of 3rd cranial nerve palsy |
| Thomas [139] | C | Full recovery of ptosis |
| Uneda [140] | TSS | Improvement of nerve palsy |
| Wang [141] | TSS (n = 21) | Hypopituitarism requiring hormonal replacement (n = 6) Resolution of visual field defects (n = 13), improvement of visual field defects (n = 4) Resolution of visual acuity (n = 15), improvement of visual acuity (n = 2) Recovery of crania nerve palsies (n = 10/10) Residual tumor (n = 4) Hemiplegia (n = 1) |
| Waqar [142] | TSS | Resolution of nerve palsy |
| Ahn [211] | TSS | Neurological improvement; resolution of ICA stenosis |
| Brown [143] | TSS Radiotherapy | Lost to follow-up |
| Catarino [144] | TSS | Clinical improvement |
| Eichberg [145] | C → TSS 4 years later due to tumor recurrence | Resolution of symptoms No hormonal deficits |
| Elarjani [146] | TSS | Neurological improvement |
| Franzese [147] | TSS | Resolution of symptoms |
| Klimko [148] | TSS | Remission of acromegalic features |
| Lee [149] | C | Gradual improvement of headache and nerve palsies |
| Marzoughi [150] | C due to multiple comorbidities | Resolution of diplopia and 3rd cranial nerve palsy, central hypothyroidism |
| Pangal [151] | TSS (n = 50) | Headache improvement (87%) Vision loss improvement (86%) Resolution of cranial nerve palsy resolved (72%) Partial improvement of cranial nerve palsy (11%) Panhypopituitarism persistence (48%) Development of panhypopituitarism (6%) |
| Patel [152] | Stress dose corticosteroids, TSS | Resolution of symptoms, vision improvement |
| Romano [212] | TSS | Visual and functional improvement |
| Shetty [153] | TSS | |
| Siwakoti [154] | C | |
| van Boven [155] | C | |
| Yang [213] | C | |
| Alam [156] | No specific treatment | Acromegaly remission |
| Aljabri [157] | C | Tumor regression, restart of goserelin treatment without side effects, hormonal substitution (corticosteroids, levothyroxin) |
| Alkhaibary [214] | C | Visual acuity and nerve palsy improvement, hypoadrenalism, hypothyroidism |
| Ambrose [158] | C → surgical decompression | Minor neurological improvement |
| Bhogal [215] | C | Tumor size reduction |
| Bukhari [159] | Fronto-temporoparietal craniotomy for tumor excision | Panhypopituitarism |
| Cavalli [160] | Conservative n = 20 (66.7%) + 8 patients undergoing delayed elective surgery Emergency surgery n = 10 (33.3%) Hormonal replacement n = 28 (93.3%) | Resolution of visual acuity defects in 61% Resolution of visual field defects in 64% Resolution of cranial nerve palsies in 69% Poor endocrine outcomes with various degrees of hypopituitarism |
| de Silva [161] | TSS | Gradual improvement, normalization of cortisol levels |
| Elsehety [216] | TSS | Exitus |
| Falhammar [162] | Immediate pituitary surgery n = 3 Delayed pituitary surgery n = 8 | Visual defect and nerve palsies improved Hormonal deficits did not regress 3 deaths of unrelated causes during follow-up |
| Gohil [163] | C | Resolution of visual disturbances and nerve palsy; regression of adenoma Persistent hypothyroidism and hypocortisolism |
| Hanna [164] | C | Resolution of symptoms within 4 days |
| Huang [165] | TSS | Resolution of visual disturbances and nerve palsy Hormonal replacement therapy |
| Iqbal [166] | TSS (9/33) | Deterioration of pituitary function n = 5 Improvement of pituitary function n = 13 |
| Komić [167] | C (Hormonal replacement) | Resolution of symptoms after conservative treatment |
| Marx [168] | C (n = 27) TSS (n = 19) | Resolution of visual acuity defects (n = 10/11) Resolution of visual field defects (n = 16/17) Resolution of cranial nerve palsies (n = 21/23) Hormonal deficits: corticotropic (n = 17), thyrotropin (n = 24), gonadotropic (n = 20), somatotropin (n = 6) Residual tumor (n = 17) Tumor recurrence (n = 4) |
| Nakhleh [169] | C (n = 10) TSS (n = 17) | Corticotropic deficiency (n = 13) Secondary hypothyroidism (n = 8) Testosterone replacement therapy (n = 7) Central diabetes insipidus (n = 4) Cabergoline treatment (n = 2) Persistence of visual field defect (n = 2) Persistence of cranial nerve palsy (n = 2) |
| Oudghiri [170] | C (n = 3) TSS (n = 1) | Resolution of cranial nerve palsy; hormonal replacement therapy |
| Pan [171] | TSS | Central hypothyroidism and hypocortisolism |
| Pattankar [172] | C | Left hemiparesis |
| Pokhrel [217] | Emergency craniotomy and tumor excision | Significant improvement of neurological and visual disturbances |
| Rosso [173] | TSS | Resolution of the nerve palsy |
| Seaman [25] | TSS: within 24 h (n = 34) within 24–48 h (n = 3) after 7 days (n = 7) | All cranial nerve palsies were resolved Visual field disturbances resolved in 13/16 cases Visual acuity decrease resolved in 6/9 cases Hormonal deficits (unresolved/acquired): hypothyroidism (n = 11) hypocortisolism (n = 6) hypogonadism (low testosterone) (n = 3) panhypopituitarism (n = 11) transient diabetes insipidus (n = 8) permanent diabetes insipidus (n = 3) |
| Sun [174] | TSS | Total tumor resection (n = 21, 87.50%) Headache resolution in all patients Cranial nerve palsies: full recovery (n = 4); improvement (n = 3) Vision: returned to normal (n = 14); improvement (n = 3); stationary (n = 1) Resolution of hormonal deficits (n = 5) |
| Teramoto [175] | TSS | |
| Tumyan [176] | TSS | Significant improvement of neurological and visual symptoms Hormonal replacement therapy |
| Van Dong [177] | TSS | No residual tumor; transient central adrenal insufficiency Improvement of visual and neurological symptoms |
| Vargas [178] | Condition worsened, family withdrew care | |
| Yoshida [179] | TSS | Residual tumor (MRI); visual acuity improvement |
| Zhu [180] | TSS | Resolution of neurological symptoms; persistence of visual disturbances |
| Zhu [181] | TSS (n = 45) C (n = 1) | Decrease visual acuity (n = 7) Visual field deficit (n = 3) Resolution of all ocular palsy Endocrine dysfunction: hypocortisolism (n = 26) hypothyroidism (n = 19) hypogonadism (n = 24) |
| Cross [182] | TSS | Cranial nerve palsies: resolution (72%), improvement without resolution (16%) Diabetes insipidus (n = 5) Exitus due to heparin induced thrombocytopenia (n = 1) |
| Geyik [183] | TSS | |
| Hamrick [184] | TSS | Resolution of visual disturbances, central hypothyroidism |
| Hsu [185] | TSS | Hypopituitarism; hormone replacement therapy |
| Liu [186] | TSS | Remission of hypercortisolism, hypopituitarism, HRT |
| Mills [187] | TSS | 12 days postoperatively: exitus due to sepsis |
| Oldfield [188] | C | No residual tumor (MRI after 5 months) |
| Puglisi [189] | C | |
| Rai [190] | TSS | Improvement of visual symptoms |
| Shrestha [191] | TSS | Resolution of nerve palsy, residual tumor |
| Singh [192] | TSS | Resolution of 6th cranial nerve palsy; normal IGF-1 levels |
| Singh [193] | TSS | Remission of panhypopituitarism, normalization of visual field |
| Syed [194] | C | Resolution of pituitary adenoma and abducens palsy |
| Viola [195] | C | Panhypopituitarism requiring hormonal replacement; resolution of ophthalmologic and neurologic symptoms |
| Author Reference Number/ Year of Publication | Population | Underlying Pituitary Condition |
|---|---|---|
| Bujawansa [66] 2014 | 55 patients (35 males and 20 females) Mean age: 58.4 years | Nonfunctioning pituitary adenomas in 45 cases (82%), lactotroph PitNETs in 6 cases (11.5%), somatotroph PitNETs in 4 cases (7.2%), multiple endocrine neoplasia syndrome in 2 cases |
| Jho [70] 2014 | 109 patients (69 males and 40 females) mean age: 51 years | PitNET (n = 98) Rathke’s cyst (n = 8) Primitive neuroectodermal tumor (n = 1) Craniofaringioma (n = 1) Metastatic lung carcinoma (n = 1) |
| Singh [90] 2015 | 87 patients (55 males and 30 females) mean age: 50.9 years | Null cell (n = 18) Lactotroph PitNET (n = 8) |
| Ogawa [100] 2016 | 43 patients (30 males and 13 females) mean age: 56.67 years | Nonfunctioning pituitary adenoma (n = 29) Lactosomatotroph PitNET (n = 5) Lactotroph PitNET (n = 4) Thyrotroph PitNET (n = 3) Somatotroph PitNET (n = 1) Corticotroph PitNET (n = 1) |
| Waqar [111] 2017 | 47 patients with pituitary apoplexy (33 males and 14 females) mean age: 54 ± 15 years Patients were compared with 50 surgically treated patients with nonfunctioning pituitary adenomas | Pituitary adenoma |
| Zoli [112] 2017 | 75 patients (45 males and 30 females) mean age: 52.4 ± 16.2 years | Pituitary adenoma |
| Abbara [113] 2018 | 52 patients (25 males and 27 females) mean age: 46.7 years | Nonfunctioning adenoma or gonadotroph PitNET (n = 47) Lactotroph PitNET (n = 5) |
| Ricciuti [120] 2018 | 17 patients (12 males and 5 females) mean age: 58.76 years | |
| Rutkowski [121] 2018 | 32 patients (21 males and 11 females) mean age: 49 years | Nonfunctional adenoma (70%) Clinically hypersecreting adenoma (15%) Lactotroph PitNET Somatotroph PitNET Corticotroph PitNET |
| Almeida [123] 2019 | 67 patients (41 males and 26 females) mean age: 57.4 +/− 16.2 years | Pituitary adenoma |
| Hosmann [128] 2019 | 76 patients (53 males and 23 females) mean age: 53.7 +/-14.3 years | Clinically nonfunctioning PitNETs (81%): gonadotroph PitNET (37.9%), null-cell (29.3%), plurihormonal (8.6%), corticotroph (3.5%), somatotroph (1.7%) Clinically functioning PitNETs: lactotroph (10.4%), corticotroph (6.9%), somatotroph (1.7%) |
| Wang [141] 2019 | 21 patients (15 males and 6 females), with a mean age of 50.7 ± 15.0 years | Nonfunctioning pituitary adenoma (n = 18) Lactotroph PitNET (n = 2) Somatotroph PitNET (n = 1) |
| Pangal [151] 2020 | 50 patients (31 males, 19 females) mean age: 53 years | Pituitary adenoma |
| Cavalli [160] 2021 | 30 patients (22 males, 8 females) mean age: 54 years | PitNET |
| Falhammar [162] 2021 | 33 patients (18 male and 15 female) mean age: 46.5 years | PitNET |
| Iqbal [166] 2021 | 55 patients (26 males and 29 females) mean age: 50 years | Somatotroph PitNET Lactotroph PitNET |
| Marx [168] 2021 | 46 patients (29 males and 17 females) mean age: 47.3 years | Nonfunctioning adenoma (n = 31) Corticotroph PitNET (n = 1) Lactotroph PitNET (n = 12) Somatotroph PitNET (n = 1) |
| Nakhleh [169] 2021 | 27 patients (14 males and 13 females) mean age: 40.7 ± 12.5 years | Nonfunctioning pituitary adenoma (n = 21) Lactotroph PitNET (n = 5) Somatotroph PitNET (n = 1) |
| Seaman [25] 2021 | 44 patients (24 males and 20 females) median age: 55 years | Nonfunctioning adenoma (n = 38) Functioning PitNET (n = 6) |
| Sun Z [174] 2021 | 24 patients (13 males and 11 females) mean age: 46.46 ± 14.95 years | Nonfunctioning adenomas (n = 7) Lactotroph PitNET (n = 3) Corticotroph PitNET (n = 1) Gondadotroph PitNET (n = 1) Lactocorticotroph PitNET (n = 1) |
| Teramoto [175] 2021 | 45 patients (33 males and 12 females) mean age: 56 years | Nonfunctioning adenoma (n = 37) Somatotroph PitNET (n = 5) Lactotroph PitNET (n = 3) |
| Zhu Q [181] 2021 | 46 patients (35 males and 11 females) mean age:46.78 ± 12.32 | PitNET |
| Cross [182] 2022 | 59 patients (40 males and 19 females) median age: 54 years | PitNET |
| Geyik [183] 2022 | 143 patients with pituitary adenoma, out of which 8 patients with PA (4 males and 4 females, mean age: 26.75 years) | Lactotroph PitNET (n = 5) Nonfunctioning adenoma (n = 3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, A.-M.; Trandafir, A.I.; Ionovici, N.; Carsote, M.; Nistor, C.; Popa, F.L.; Stanciu, M. Pituitary Apoplexy in Patients with Pituitary Neuroendocrine Tumors (PitNET). Biomedicines 2023, 11, 680. https://doi.org/10.3390/biomedicines11030680
Gheorghe A-M, Trandafir AI, Ionovici N, Carsote M, Nistor C, Popa FL, Stanciu M. Pituitary Apoplexy in Patients with Pituitary Neuroendocrine Tumors (PitNET). Biomedicines. 2023; 11(3):680. https://doi.org/10.3390/biomedicines11030680
Chicago/Turabian StyleGheorghe, Ana-Maria, Alexandra Ioana Trandafir, Nina Ionovici, Mara Carsote, Claudiu Nistor, Florina Ligia Popa, and Mihaela Stanciu. 2023. "Pituitary Apoplexy in Patients with Pituitary Neuroendocrine Tumors (PitNET)" Biomedicines 11, no. 3: 680. https://doi.org/10.3390/biomedicines11030680
APA StyleGheorghe, A.-M., Trandafir, A. I., Ionovici, N., Carsote, M., Nistor, C., Popa, F. L., & Stanciu, M. (2023). Pituitary Apoplexy in Patients with Pituitary Neuroendocrine Tumors (PitNET). Biomedicines, 11(3), 680. https://doi.org/10.3390/biomedicines11030680








