A Lymph Node Ratio Model for Prognosis of Patients with Pancreatic Neuroendocrine Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
Treatment Protocol
2.2. Statistical Methods
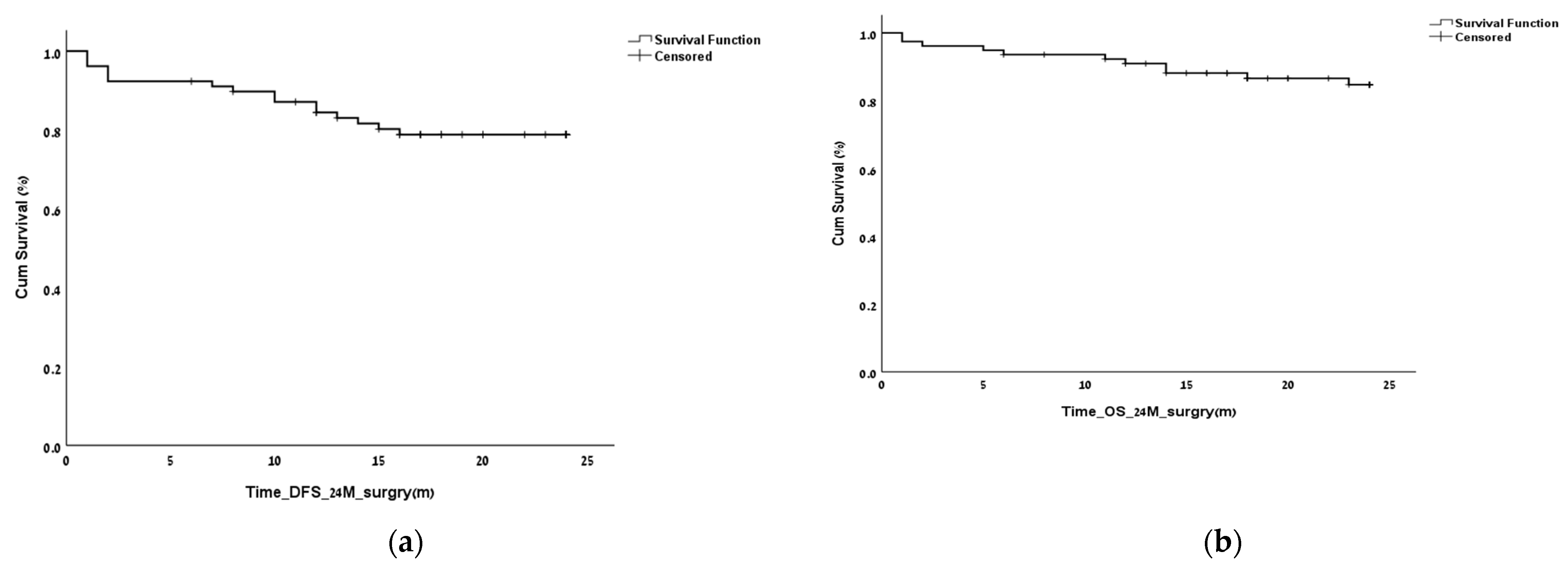
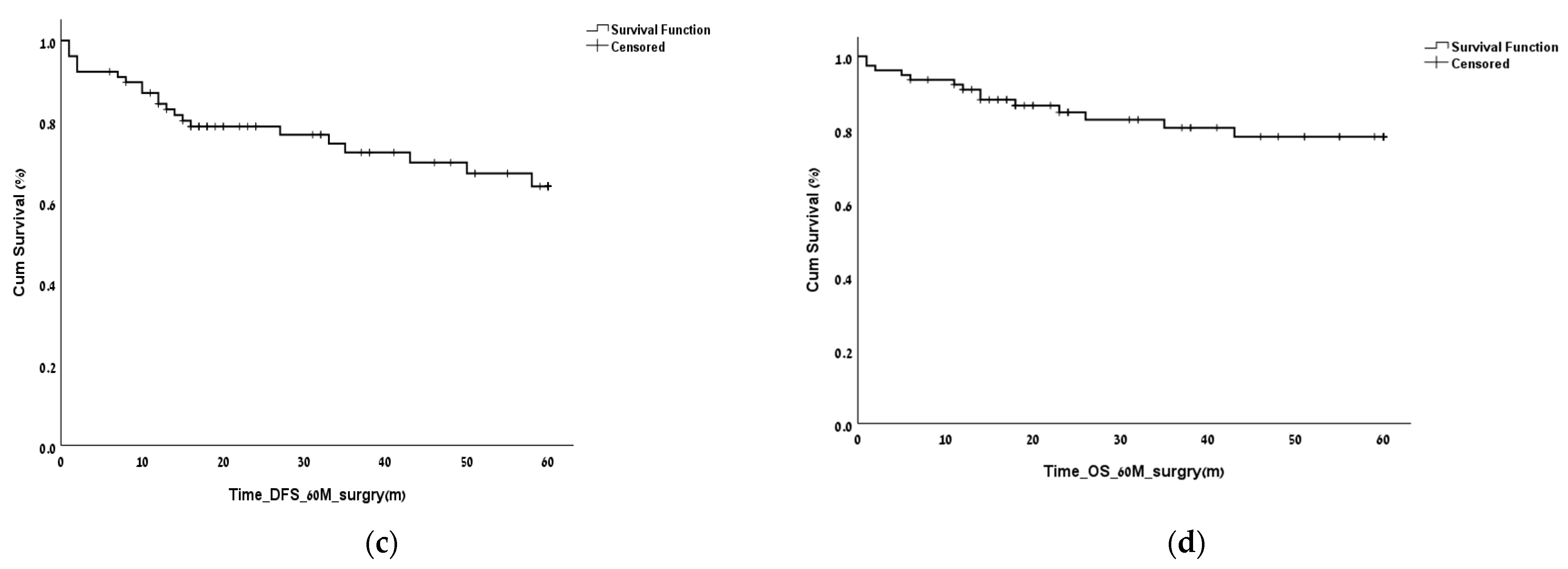
3. Results
3.1. Tumor Stage according to AJCC/ENET
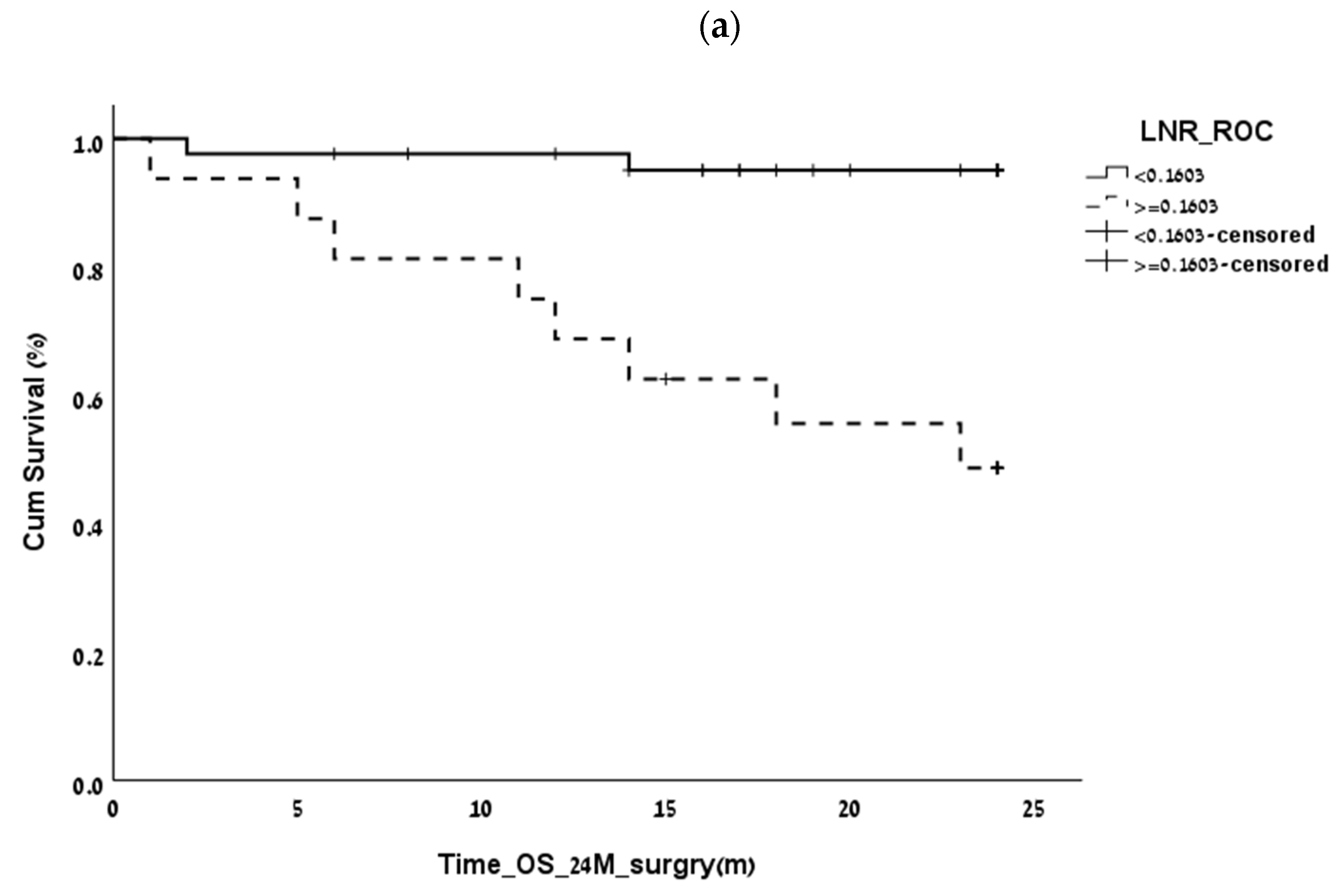
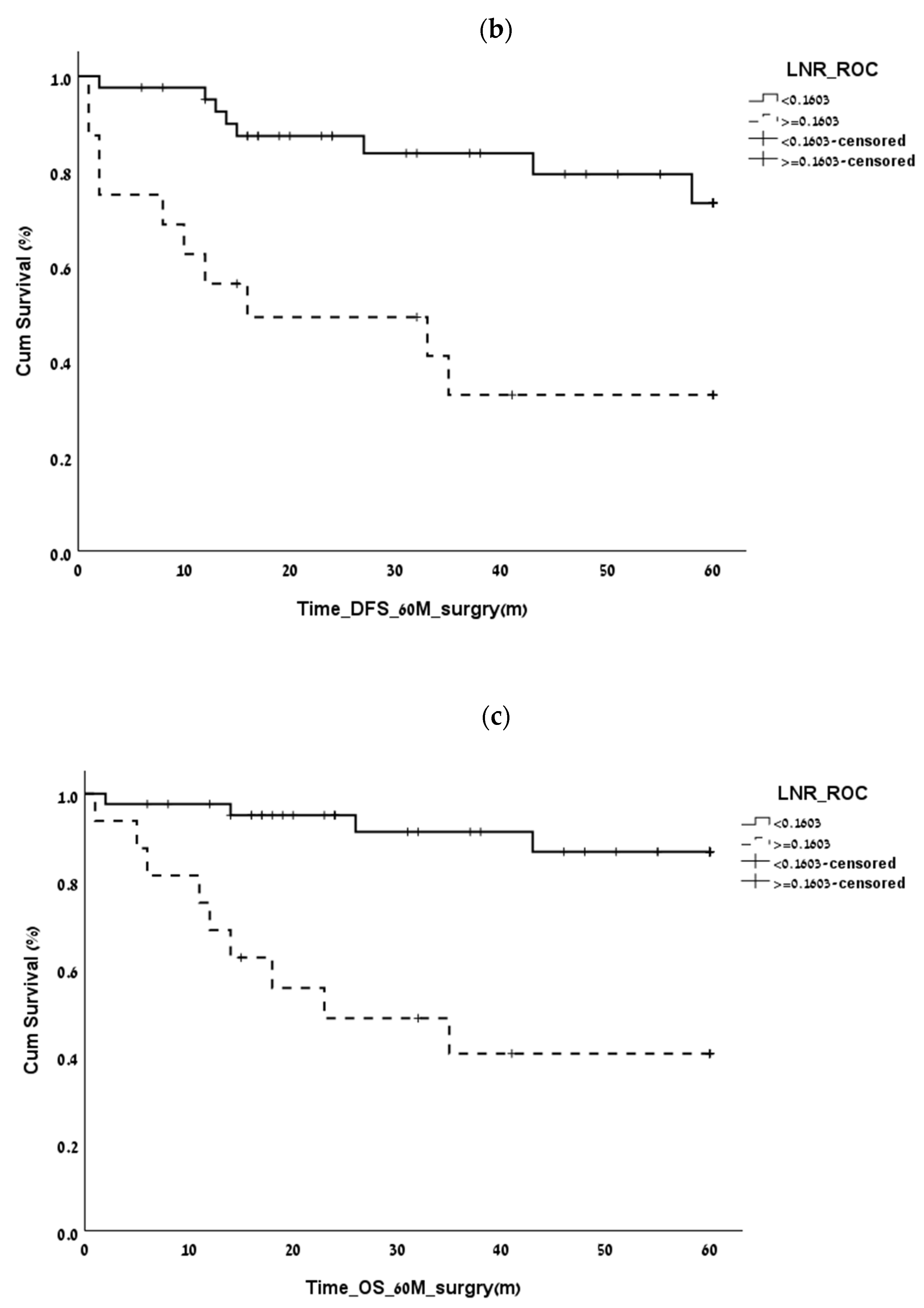
3.2. Finding the LNR Threshold
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Talamonti, M.S.; Tomlinson, J.S.; Stewart, A.K.; Winchester, D.P.; Ko, C.Y.; Bentrem, D.J. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: Analysis of 3851 patients. Ann. Surg. 2008, 247, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Klöppel, G.; et al. Vienna Consensus Conference participants ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; American Joint Committee on Cancer. AJCC Cancer Staging Manual. Available online: http://www.springer.com/us/book/9783319406176 (accessed on 5 May 2017).
- Luo, G.; Javed, A.; Strosberg, J.R.; Jin, K.; Zhang, Y.; Liu, C.; Xu, J.; Soares, K.; Weiss, M.J.; Zheng, L.; et al. Modified Staging Classification for Pancreatic Neuroendocrine Tumors on the Basis of the American Joint Committee on Cancer and European Neuroendocrine Tumor Society Systems. J. Clin. Oncol. 2017, 35, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, W.; Yu, X.; Liu, L. Patterns and predictors of pancreatic neuroendocrine tumor prognosis: Are no two leaves alike? Rev. Oncol. Hematol. 2021, 167, 103493. [Google Scholar] [CrossRef]
- Murakami, Y.; Uemura, K.; Sudo, T.; Hayashidani, Y.; Hashimoto, Y.; Nakashima, A.; Yuasa, Y.; Kondo, N.; Ohge, H.; Sueda, T. Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. J. Am. Coll. Surg. 2010, 211, 196–204. [Google Scholar] [CrossRef]
- Showalter, T.N.; Winter, K.A.; Berger, A.C.; Regine, W.F.; Abrams, R.A.; Safran, H.; Hoffman, J.P.; Benson, A.B.; MacDonald, J.S.; Willett, C.G. The influence of total nodes examined, number of positive nodes, and lymph node ratio on survival after surgical resection and adjuvant chemoradiation for pancreatic cancer: A secondary analysis of RTOG 9704. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1328–1335. [Google Scholar] [CrossRef]
- Vuarnesson, H.; Lupinacci, R.M.; Semoun, O.; Svrcek, M.; Julié, C.; Balladur, P.; Penna, C.; Bachet, J.B.; Resche-Rigon, M.; Paye, F. Number of examined lymph nodes and nodal status assessment in pancreaticoduodenectomy for pancreatic adenocarcinoma. Eur. J. Surg. Oncol. 2013, 39, 1116–1121. [Google Scholar] [CrossRef]
- Malleo, G.; Maggino, L.; Qadan, M.; Marchegiani, G.; Ferrone, C.; Paiella, S.; Luchini, C.; Mino-Kenudson, M.; Capelli, P.; Scarpa, A.; et al. Reassessment of the Optimal Number of Examined Lymph Nodes in Pancreatoduodenectomy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2022, 276, e518–e526. [Google Scholar] [CrossRef]
- Hashim, Y.M.; Trinkaus, K.M.; Linehan, D.C.; Strasberg, S.S.; Fields, R.C.; Cao, D.; Hawkins, W.G. Regional lymphade-nectomy is indicated in the surgical treatment of pancreatic neuroendocrine tumors (PNETs). Ann. Surg. 2014, 259, 197–203. [Google Scholar] [CrossRef]
- Jiang, Y.; Jin, J.-B.; Zhan, Q.; Deng, X.-X.; Shen, B.-Y. Impact and clinical predictors of lymph node metastases in non-functional pancreatic neuroendocrine tumors. Chin. Med. J. 2015, 128, 3335–3344. [Google Scholar] [CrossRef] [PubMed]
- Curran, T.; Pockaj, B.A.; Gray, R.J.; Halfdanarson, T.R.; Wasif, N. Importance of lymph node involvement in pancreatic neuroendocrine tumors: Impact on survival and implications for surgical resection. J. Gastrointest. Surg. 2015, 19, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, P.; Campana, D.; Piscitelli, L.; Casadei, R.; Santini, D.; Nori, F.; Morselli-Labate, A.M.; Pezzilli, R.; Corinaldesi, R. Endocrine pancreatic tumors: Factors correlated with survival. Ann. Oncol. 2005, 16, 1806–1810. [Google Scholar] [CrossRef]
- Bettini, R.; Boninsegna, L.; Mantovani, W.; Capelli, P.; Bassi, C.; Pederzoli, P.; Delle Fave, G.F.; Panzuto, F.; Scarpa, A.; Falconi, M. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann. Oncol. 2008, 19, 903–908. [Google Scholar] [CrossRef]
- Kazanjian, K.K.; Reber, H.A.; Hines, O.J. Resection of pancreatic neuroendocrine tumors: Results of 70 cases. Arch. Surg. 2006, 141, 765–769. [Google Scholar] [CrossRef]
- Boninsegna, L.; Panzuto, F.; Partelli, S.; Capelli, P.; Delle Fave, G.; Bettini, R.; Pederzoli, P.; Scarpa, A.; Falconi, M. Malignant pancreatic neuroendocrine tumour: Lymph node ratio and Ki67 are predictors of recurrence after curative resections. Eur. J. Cancer 2012, 48, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Gaitanidis, A.; Patel, D.; Nilubol, N.; Tirosh, A.; Kebebew, E. A Lymph Node Ratio-Based Staging Model Is Superior to the Current Staging System for Pancreatic Neuroendocrine Tumors. J. Clin. Endocrinol. Metab. 2018, 103, 187–195. [Google Scholar] [CrossRef]
- Li, J.; Lin, Y.; Wang, Y.; Lin, H.; Lin, F.; Zhuang, Q.; Wu, J. Prognostic Nomogram Based on the Metastatic Lymph Node Ratio for T1-4N0-1M0 Pancreatic Neuroendocrine Tumors after Surgery. Front. Oncol. 2022, 5, e000632. [Google Scholar] [PubMed]
- Partelli, S.; Muffatti, F.; Andreasi, V.; Giannone, F.; Rossi, R.; Palumbo, D.; Mapelli, P.; Lena, M.S.; Arcidiacono, P.G.; De Cobelli, F.; et al. Single-center Prospective Observational Study Investigating the Accuracy of Preoperative Diagnostic Procedures in the Assessment of Lymph Node Metastases in Nonfunctioning Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2022, 112, 143–152. [Google Scholar] [CrossRef]
- Tacelli, M.; Bina, N.; Crinò, S.F.; Facciorusso, A.; Celsa, C.; Vanni, A.S.; Fantin, A.; Antonini, F.; Falconi, M.; Monica, F.; et al. Reliability of grading preoperative pancreatic neuroendocrine tumors on EUS specimens: A systematic review with meta-analysis of aggregate and individual data. Gastrointest. Endosc. 2022, 96, 898–908.e23. [Google Scholar] [CrossRef]
- Paiella, P.; Landoni, L.; Tebaldi, S.; Zuffante, M.; Salgarello, M.; Cingarlini, S.; D’Onofrio, M.; Parisi, A.; Deiro, G.; Manfrin, E.; et al. Dual-Tracer (68Ga-DOTATOC and 18F-FDG-)-PET/CT Scan and G1-G2 Nonfunctioning Pancreatic Neuroendocrine Tumors: A Single-Center Retrospective Evaluation of Nonmetastatic Resected Cases. Neuroendocrinology 2022, 112, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, R.A.; Habacha, B.; Lütje, S.; Essler, M. Therapy of Patients with Neuroendocrine Neoplasia-Evidence-Based Approaches and New Horizons. J. Clin. Med. 2019, 16, 1474. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. NETTER-1 Trial Investigators. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125. [Google Scholar] [CrossRef]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Müller, H.H.; Arnold, R. PROMID Study Group Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. CLARINET Investigators Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Ruszniewski, P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Zanini, S.; Renzi, S.; Giovinazzo, F.; Bermano, G. mTOR Pathway in Gastroenteropancreatic Neuroendocrine Tumor (GEP-NETs). Front. Endocrinol. 2020, 16, 562505. [Google Scholar] [CrossRef]

| Variable | |
|---|---|
| Age, years, mean ± standard deviation Follow-up, mean ± standard deviation | 58 ± 13.4 (range 20–82) 4.2 ± 3.4 years |
| Sex, n Male Female | 50 (64.1%) 28 (35.9%) |
| Type of surgery | Whipple procedure (n = 26, 33%) Distal pancreatectomy (n = 37, 47.4%) Complete pancreatic resection (n = 4, 5.1%) Tumor excision (n = 11, 14.1%) |
| Tumor size, cm median ± standard deviation | 2.5 ± 2.75 |
| Tumor location Head (%) Body (%) Tail (%) Unknown(%) | 36 23 39.7 1.3 |
| Vascular invasion (%) Perineural invasion (%) | 17.4 13 |
| Smokers (%) Diabetes mellitus (%) | 19.2 37.8 |
| Metastatic disease at presentation (%) | 7 |
| American Joint Committee on Cancer | European Neuroendocrine Tumor Society | |
|---|---|---|
| Stage | I: 35.9% (n = 28) II: 24.4% (n = 19) III: 25.6% (n = 20) IV: 9% (n = 7) | I: 19.2% (n = 15) II: 20.5% (n = 16) III: 43.6% (n = 34) IV: 11.5% (n = 9) |
| Tumor grade | 1: 56% (n = 44) 2: 26.9% (n = 20) 3: 9% (n = 7) No data: 9% (n = 7) |
| Recurrence Site | |
|---|---|
| Liver, n | 14 (18%) |
| Lung, n | 2 (2.6%) |
| Peritoneum, n | 3 (8%) |
| Lymph nodes, n | 2 (2.6%) |
| Clinical Measure | Optimal Lymph Node Ratio | Sensitivity | Specificity | p |
|---|---|---|---|---|
| 2-year overall survival | 0.16 | 0.8 | 0.83 | 0.002 |
| 2-year disease-free survival | 0.16 | 0.62 | 0.82 | 0.023 |
| 5-year overall survival | 0.16 | 0.69 | 0.84 | 0.04 |
| 5-year disease-free survival | 0.15 | 0.6 | 0.85 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osher, E.; Shalabna, E.; Klausner, J.M.; Greenman, Y.; Stern, N.; Shibolet, O.; Scapa, E.; Yakir, O.; Shor, D.B.-A.; Bar-Yishay, I.; et al. A Lymph Node Ratio Model for Prognosis of Patients with Pancreatic Neuroendocrine Tumors. Biomedicines 2023, 11, 407. https://doi.org/10.3390/biomedicines11020407
Osher E, Shalabna E, Klausner JM, Greenman Y, Stern N, Shibolet O, Scapa E, Yakir O, Shor DB-A, Bar-Yishay I, et al. A Lymph Node Ratio Model for Prognosis of Patients with Pancreatic Neuroendocrine Tumors. Biomedicines. 2023; 11(2):407. https://doi.org/10.3390/biomedicines11020407
Chicago/Turabian StyleOsher, Esther, Eiman Shalabna, Joseph M. Klausner, Yona Greenman, Naftali Stern, Oren Shibolet, Erez Scapa, Oz Yakir, Dana Ben-Ami Shor, Iddo Bar-Yishay, and et al. 2023. "A Lymph Node Ratio Model for Prognosis of Patients with Pancreatic Neuroendocrine Tumors" Biomedicines 11, no. 2: 407. https://doi.org/10.3390/biomedicines11020407
APA StyleOsher, E., Shalabna, E., Klausner, J. M., Greenman, Y., Stern, N., Shibolet, O., Scapa, E., Yakir, O., Shor, D. B.-A., Bar-Yishay, I., Shamai, S., Sofer, Y., Lubezky, N., Goykhman, Y., Lahat, G., Wolf, I., Pelles, S., Aizic, A., Blachar, A., & Geva, R. (2023). A Lymph Node Ratio Model for Prognosis of Patients with Pancreatic Neuroendocrine Tumors. Biomedicines, 11(2), 407. https://doi.org/10.3390/biomedicines11020407






