Emerging Therapy for Diabetic Cardiomyopathy: From Molecular Mechanism to Clinical Practice
Abstract
1. Introduction: Diabetic Cardiomyopathy as a Distinct Disease Entity
1.1. Definition of Diabetic Cardiomyopathy
1.2. Evolution of Diabetic Cardiomyopathy to Clinical Heart Failure
1.3. Epidemiology of Diabetic Cardiomyopathy
1.4. Initial and Serial Evaluation
2. Current Treatment Strategies for Heart Failure in Patients with Diabetes
2.1. Sodium–Glucose Cotransporter 2 Inhibitors (SGLT2i)
2.2. Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs)
2.3. Metformin
2.4. Dipeptidyl Peptidase 4 Inhibitors (DPP-4i)
2.5. Sulfonylurea
2.6. Insulin
2.7. Thiazolidinediones (TZDs)
3. Molecular Mechanism of Diabetic Cardiomyopathy
3.1. Impaired Nutrient-Sensing Signaling, Dysregulated Autophagy, Impaired Mitochondrial Energetics, Metabolic Alteration, and Altered Fuel Utilization
3.2. Oxidative Stress, Lipid Peroxidation, Advanced Glycation End-Products (AGEs), and Maladaptive Immune Responses
3.3. Impaired Calcium Homeostasis
3.4. Abnormal Endothelial Function and Nitric Oxide (NO) Production
3.5. Aberrant Epidermal Growth Factor Receptor (EGFR)-v-erb-b2 Avian Erythroblastic Leukemia Viral Oncogene Homolog 2(ErbB2/4) Receptor Signaling
3.6. Activation of Renin–Angiotensin–Aldosterone System (RAAS) and Autonomic Dysfunction
3.7. Extracellular Matrix (ECM) Accumulation and Fibrosis
3.8. Epigenetic Changes in Diabetic Cardiomyopathy
4. Staging and Diagnostic Algorithm of Diabetic Cardiomyopathy
5. Emerging Treatment Targeting Specific Mechanisms of Diabetic Cardiomyopathy
5.1. Modulators of Energy Utilization
5.2. Improving Calcium Homeostasis
5.2.1. Istaroxime
5.2.2. Ranolazine
5.3. ROS Scavenging
5.3.1. MitoQ
5.3.2. Coenzyme Q10
5.3.3. Elamipretide
5.3.4. GKT137831
5.4. Inhibition of Advanced Glycation End-Products (AGEs)
5.4.1. Aminoguanidine
5.4.2. Alagebrium(ALT-711)
5.4.3. MitoGamide
5.5. Inhibition of Aldose Reductase
5.6. Activation of the Neuregulin–ErbB Pathway
5.7. Activation of Apelin and the Apelin Receptor Pathway
5.8. Targeting the Soluble Guanylate Cyclase–cGMP Pathway and Endothelial Dysfunction
5.8.1. Cinaciguat
5.8.2. Vericiguat
5.9. Reducing Toxic Aldehydes
5.10. Suppression of Inflammation
5.10.1. Phosphoinositide 3-kinaseγ (PI3Kγ) Inhibitor
5.10.2. Canakinumab and HMG-CoA Reductase Inhibitors (Statins)
5.11. Ameliorating RAAS Axis Activation
5.11.1. Angiotensin 1–7
5.11.2. Dipeptidyl Peptidase III
5.12. Inhibition of Fibrosis
5.12.1. Glucose-Dependent Insulinotropic Polypeptide
5.12.2. Cathelicidin-Related Antimicrobial Peptide
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability Statement
Acknowledgments
Conflicts of Interest
References
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972, 30, 595–602. [Google Scholar] [CrossRef]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef]
- Bando, Y.K.; Murohara, T. Diabetes-related heart failure. Circ. J. 2014, 78, 576–583. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef]
- Ryden, L.; Grant, P.J.; Anker, S.D.; Berne, C.; Cosentino, F.; Danchin, N.; Deaton, C.; Escaned, J.; Hammes, H.P.; Huikuri, H.; et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2013, 34, 3035–3087. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; DeMarco, V.G.; Sowers, J.R. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat. Rev. Endocrinol. 2016, 12, 144–153. [Google Scholar] [CrossRef]
- Westermeier, F.; Riquelme, J.A.; Pavez, M.; Garrido, V.; Díaz, A.; Verdejo, H.E.; Castro, P.F.; García, L.; Lavandero, S. New molecular insights ofinsulin in diabeticcardiomyopathy. Front. Physiol. 2016, 7, 125. [Google Scholar] [CrossRef]
- Jia, G.; Habibi, J.; DeMarco, V.G.; Martinez-Lemus, L.A.; Ma, L.; Whaley-Connell, A.T.; Aroor, A.R.; Domeier, T.L.; Zhu, Y.; Meininger, G.A.; et al. Endothelial mineralocorticoid receptor deletion prevents diet-induced cardiac diastolic dysfunction in females. Hypertension 2015, 66, 1159–1167. [Google Scholar] [CrossRef]
- Paolillo, S.; Marsico, F.; Prastaro, M.; Renga, F.; Esposito, L.; De Martino, F.; Di Napoli, P.; Esposito, I.; Ambrosio, A.; Ianniruberto, M.; et al. Diabetic Cardiomyopathy: Definition, Diagnosis, and Therapeutic Implications. Heart Fail. Clin. 2019, 15, 341–347. [Google Scholar] [CrossRef]
- Bahrami, H.; Bluemke, D.A.; Kronmal, R.; Bertoni, A.G.; Lloyd-Jones, D.M.; Shahar, E.; Szklo, M.; Lima, J.A. Novel metabolic risk factors for incident heart failure and their relationship with obesity: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J. Am. Coll. Cardiol. 2008, 51, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Gardin, J.M.; Lynch, J.C.; Smith, V.E.; Tracy, R.P.; Savage, P.J.; Szklo, M.; Ward, B.J. Diabetes mellitus and echocardiographic left ventricular function in free-living elderly men and women: The Cardiovascular Health Study. Am. Heart J. 1997, 133, 36–43. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Segar, W.; Khan, M.S.; Patel, K.V.; Butler, J.; Tang, W.H.W.; Vaduganathan, M.; Lam, C.S.P.; Verma, S.; McGuire, D.K.; Pandey, A. Prevalence and Prognostic Implications of Diabetes With Cardiomyopathy in Community-Dwelling Adults. J. Am. Coll. Cardiol. 2021, 78, 1587–1598. [Google Scholar] [CrossRef]
- Liu, J.E.; Palmieri, V.; Roman, M.J.; Bella, J.N.; Fabsitz, R.; Howard, B.V.; Welty, T.K.; Lee, E.T.; Devereux, R.B. The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: The Strong Heart Study. J. Am. Coll. Cardiol. 2001, 37, 1943–1949. [Google Scholar] [CrossRef]
- Bertoni, A.G.; Goff, D.C.; D’Agostino, R.B.; Liu, K.; Hundley, W.G.; Lima, J.A.; Polak, J.F.; Saad, M.F.; Szklo, M.; Tracy, R.P.; et al. Diabetic cardiomyopathy and subclinical cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2006, 29, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Aronow, W.S.; Ahn, C. Incidence of heart failure in 2737 older persons with and without diabetes mellitus. Chest 1999, 115, 867–868. [Google Scholar] [CrossRef]
- Lind, M.; Bounias, I.; Olsson, M.; Gudbjörnsdottir, S.; Svensson, A.M.; Rosengren, A. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: An observational study. Lancet 2011, 378, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Dandamudi, S.; Slusser, J.; Mahoney, D.W.; Redfield, M.M.; Rodeheffer, R.J.; Chen, H.H. The prevalence of diabetic cardiomyopathy: A population-based study in Olmsted County, Minnesota. J. Card. Fail. 2014, 20, 304–309. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 6. Glycemic Targets: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S97–S110. [Google Scholar] [CrossRef]
- Gulsin, G.S.; Athithan, L.; McCann, G.P. Diabetic cardiomyopathy: Prevalence, determinants and potential treatments. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819834869. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S140–S157. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018, 61, 2461–2498. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Das, S.R.; Hilliard, M.E.; Diana Isaacs, D.; et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S158–S190. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Braunwald, E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 422–434. [Google Scholar] [CrossRef]
- Cong, S.; Chan, X.; Ping, E.; Yap, E.P.; Ramachandra, C.J.; Hausenloy, D.J. Insights into the potential cardioprotective mechanisms of SGLT2 inhibitors. Cond. Med. 2020, 5, 1–10. [Google Scholar]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes–State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Nordisk, N. Prescribing Information for RYBELSUS. Plainsboro, NJ, Novo Nordisk. 2019. Available online: www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf (accessed on 20 June 2022).
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- McGuire, D.K.; Alexander, J.H.; Johansen, O.E.; Perkovic, V.; Rosenstock, J.; Cooper, M.E.; Wanner, C.; Kahn, S.E.; Toto, R.D.; Zinman, B.; et al. Linagliptin Effects on Heart Failure and Related Outcomes in Individuals With Type 2 Diabetes Mellitus at High Cardiovascular and Renal Risk in CARMELINA. Circulation 2019, 139, 351–361. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jodar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Køber, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B., Sr.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar]
- Andersson, C.; Olesen, J.B.; Hansen, P.R.; Weeke, P.; Norgaard, M.L.; Jørgensen, C.H.; Lange, T.; Abildstrøm, S.Z.; Schramm, T.K.; Vaag, A.; et al. Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: A retrospective nationwide cohort study. Diabetologia 2010, 53, 2546–2553. [Google Scholar] [CrossRef]
- Boussageon, R.; Supper, I.; Bejan-Angoulvant, T.; Kellou, N.; Cucherat, M.; Boissel, J.P.; Kassai, B.; Moreau, A.; Gueyffier, F.; Cornu, C. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: A meta-analysis of randomised controlled trials. PLoS Med. 2012, 9, e1001204. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes–2022. Diabetes Care 2022, 45 (Suppl. S1), S144–S174. [Google Scholar] [CrossRef]
- Scirica, B.M.; Braunwald, E.; Raz, I.; Cavender, M.A.; Morrow, D.A.; Jarolim, P.; Udell, J.A.; Mosenzon, O.; Im, K.; Umez-Eronini, A.A.; et al. Heart failure, saxagliptin, and diabetes mellitus: Observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014, 130, 1579–1588. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Januzzi, J.L.; Bruemmer, D.; Butalia, S.; Green, J.B.; Horton, W.B.; Knight, C.; Levi, M.; Rasouli, N.; Richardson, C.R. Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care 2022, 45, 1670–1690. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853, reprinted in Lancet 1999, 354, 602. [Google Scholar] [CrossRef]
- Hougen, I.; Whitlock, R.H.; Komenda, P.; Rigatto, C.; Clemens, K.K.; Tangri, N. Safety of add-on sulfonylurea therapy in patients with type 2 diabetes using metformin: A population-based real-world study. BMJ Open Diab. Res. Care 2021, 9, e002352. [Google Scholar] [CrossRef]
- Khan, M.S.; Solomon, N.; DeVore, A.D.; Sharma, A.; Felker, G.M.; Hernandez, A.F.; Heidenreich, P.A.; Matsouaka, R.A.; Green, J.B.; Butler, J. Clinical Outcomes With Metformin and Sulfonylurea Therapies Among Patients With Heart Failure and Diabetes. J. Am. Coll. Cardiol. HF 2022, 10, 198–210. [Google Scholar] [CrossRef]
- Richardson, T.L., Jr.; Hackstadt, A.J.; Hung, A.M.; Greevy, R.A.; Grijalva, C.G.; Griffin, M.R.; Elasy, T.A.; Roumie, C.L. Hospitalization for Heart Failure Among Patients with Diabetes Mellitus and Reduced Kidney Function Treated with Metformin Versus Sulfonylureas: A Retrospective Cohort Study. J. Am. Heart Assoc. 2021, 10, e019211. [Google Scholar] [CrossRef]
- Nathan, D.M.; Cleary, P.A.; Backlund, J.Y.; Genuth, S.M.; Lachin, J.M.; Orchard, T.J.; Raskin, P.; Zinman, B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 2005, 22, 2643–2653. [Google Scholar] [CrossRef]
- Cosmi, F.; Shen, L.; Magnoli, M.; Abraham, W.T.; Anand, I.S.; Cleland, J.G.; Cohn, J.N.; Cosmi, D.; De Berardis, G.; Dickstein, K.; et al. Treatment with insulin is associated with worse outcome in patients with chronic heart failure and diabetes. Eur. J. Heart Fail. 2018, 20, 888–895. [Google Scholar] [CrossRef]
- Erdmann, E.; Charbonnel, B.; Wilcox, R.G.; Skene, A.M.; Massi-Benedetti, M.; Yates, J.; Tan, M.; Spanheimer, R.; Standl, E.; Dormandy, J.A.; et al. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: Data from the PROactive study (PROactive 08). Diabetes Care 2007, 30, 2773–2778. [Google Scholar] [CrossRef]
- Lago, R.M.; Singh, P.P.; Nesto, R.W. Congestiveheartfailure and cardiovasculardeath in patientswith prediabetes and type 2 diabetes given thiazolidinediones: A meta-analysisofrandomizedclinical trials. Lancet 2007, 370, 1129–1136. [Google Scholar] [CrossRef]
- Franssen, C.; Gonzalez Miqueo, A. The role of titin and extracellular matrix remodeling in heart failure with preserved ejection fraction. Neth. Heart J. 2016, 24, 259–267. [Google Scholar] [CrossRef]
- Malhowski, A.J.; Hira, H.; Bashiruddin, S.; Warburton, R.; Goto, J.; Robert, B.; Kwiatkowski, D.J.; Finaly, G.A. Smooth muscle protein-22-mediated deletion of Tsc1 results in cardiac hypertrophy that is mTORC1-mediated and reversed by rapamycin. Hum. Mol. Genet. 2011, 20, 1290–1305. [Google Scholar] [CrossRef]
- Proud, C.G. Ras, PI3-kinase and mTOR signaling in cardiac hypertrophy. Cardiovasc. Res. 2004, 63, 403–413. [Google Scholar] [CrossRef]
- Völkers, M.; Doroudgar, S.; Nguyen, N.; Konstandin, M.H.; Quijada, P.; Din, S.; Ornelas, L.; Thuerauf, D.J.; Gude, N.; Friedrich, K.; et al. PRAS40 prevents development of diabetic cardiomyopathy and improves hepatic insulin sensitivity in obesity. EMBO Mol. Med. 2014, 6, 57–65. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Wu, S.; Lu, Q.; Ding, Y.; Wu, Y.; Qiu, Y.; Wang, P.; Mao, X.; Huang, K.; Xie, Z.; Zou, M.H. Hyperglycemia-Driven Inhibition of AMP-Activated Protein Kinase α2 Induces Diabetic Cardiomyopathy by Promoting Mitochondria-Associated Endoplasmic Reticulum Membranes In Vivo. Circulation 2019, 139, 1913–1936. [Google Scholar] [CrossRef]
- Xie, Z.; He, C.; Zou, M.H. AMP-activated protein kinase modulates cardiac autophagy in diabetic cardiomyopathy. Autophagy 2011, 7, 1254–1255. [Google Scholar] [CrossRef]
- Xie, Z.; Lau, K.; Eby, B.; Lozano, P.; He, C.; Pennington, B.; Li, H.; Rathi, S.; Dong, Y.; Tian, R.; et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 2011, 60, 1770–1778. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Yao, F.; Su, Q.; Liu, D.; Xue, R.; Dai, G.; Fang, R.; Zeng, J.; Chen, Y.; et al. AMPK inhibits cardiachypertrophy by promotingautophagy via mTORC1. Arch. Biochem. Biophys. 2014, 558, 79–86. [Google Scholar] [CrossRef]
- McCandless, M.G.; Altara, R.; Booz, G.W.; Kurdi, M. What Role do Mitochondria Have in Diastolic Dysfunction? Implications for Diabetic Cardiomyopathy and Heart FailureWith Preserved Ejection Function. J. Cardiovasc. Pharmacol. 2022, 79, 399–406. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Huynh, K.; McMullen, J.R.; Julius, T.L.; Tan, J.W.; Love, J.E.; Cemerlang, N.; Kiriazis, H.; Du, X.J.; Ritchie, R.H. Cardiac-specific IGF-1 receptor transgenic expression protects against cardiac fibrosis and diastolic dysfunction in a mouse model of diabetic cardiomyopathy. Diabetes 2010, 59, 1512–1520. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, X.D.; Hao, Z.H.; Xu, D. Insulin-like growth factor-1 improves diabetic cardiomyopathy through antioxidative and anti-inflammatory processes along with modulation of Akt/GSK-3β signaling in rats. Korean J. Physiol. Pharmacol. 2016, 20, 613–619. [Google Scholar] [CrossRef]
- Rodrigues, B.; Cam, M.C.; McNeill, J.H. Metabolic disturbances in diabetic cardiomyopathy. Mol. Cell. Biochem. 1998, 180, 53–57. [Google Scholar] [CrossRef]
- Ritchie, R.H.; Abel, E.D. Basic Mechanisms of Diabetic Heart Disease. Circ. Res. 2020, 126, 1501–1525. [Google Scholar] [CrossRef]
- Maisch, B.; Alter, P.; Pankuweit, S. Diabetic cardiomyopathy—Fact or fiction? Herz 2011, 36, 102–115. [Google Scholar] [CrossRef]
- Selvaraj, S.; Kelly, D.P.; Margulies, K.B. Implications of Altered Ketone Metabolism and Therapeutic Ketosis in Heart Failure. Circulation 2020, 141, 1800–1812. [Google Scholar] [CrossRef]
- Schugar, R.C.; Moll, A.R.; André d’Avignon, D.; Weinheimer, C.J.; Kovacs, A.; Crawford, P.A. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol. Metab. 2014, 3, 754–769. [Google Scholar] [CrossRef]
- Mudaliar, S.; Alloju, S.; Henry, R.R. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME Study? A unifying hypothesis. Diabetes Care 2016, 39, 1115–1122. [Google Scholar] [CrossRef]
- Wakasaki, H.; Koya, D.; Schoen, F.J.; Jirousek, M.R.; Ways, D.K.; Hoit, B.D.; Walsh, R.A.; King, G.L. Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy. Proc. Natl. Acad. Sci. USA 1997, 94, 9320–9325. [Google Scholar] [CrossRef]
- Song, M.; Matkovich, S.J.; Zhang, Y.; Hammer, D.J.; Dorn, G.W. Combined cardiomyocyte PKCδ and PKCε gene deletion uncovers their central role in restraining developmental and reactive heart growth. Sci. Signal. 2015, 8, ra39. [Google Scholar] [CrossRef]
- Pan, G.; Deshpande, M.; Pang, H.; Palaniyandi, S.S. Precision medicine approach: Empagliflozin for diabetic cardiomyopathy in mice with aldehyde dehydrogenase (ALDH) 2*2 mutation, a specific genetic mutation in millions of East Asians. Eur. J. Pharmacol. 2018, 839, 76–81. [Google Scholar] [CrossRef]
- Chen, C.H.; Budas, G.R.; Churchill, E.N.; Disatnik, M.H.; Hurley, T.D.; Mochly-Rosen, D. Activation of Aldehyde Dehydrogenase-2 Reduces Ischemic Damage to the Heart. Science 2008, 321, 1493–1495. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Hao, P.; Xue, L.; Wei, S.; Zhang, Y.; Chen, Y. Inhibition of Aldehyde Dehydrogenase 2 by Oxidative Stress Is Associated with Cardiac Dysfunction in Diabetic Rats. Mol. Med. 2011, 17, 172–179. [Google Scholar] [CrossRef]
- Lee, H.L.; Hee, S.W.; Hsuan, C.F.; Yang, W.; Huang, J.Y.; Lin, Y.L.; Hsu, C.N.; Hwang, J.J.; Chen, S.M.; Ding, Z.Z.; et al. A Novel ALDH2 Activator AD-9308 Improves Diastolic and Systolic Myocardial Functions in Streptozotocin-Induced Diabetic Mice. Antioxidants 2021, 10, 450. [Google Scholar] [CrossRef]
- Wan, Z.; Fan, Y.; Liu, X.; Xue, J.; Han, Z.; Zhu, C.; Wang, X. NLRP3 inflammasome promotes diabetes-induced endothelial inflammation and atherosclerosis. Diabetes Metab. Syndr. Obes. 2019, 12, 1931–1942. [Google Scholar] [CrossRef]
- Yokoyama, T.; Nakano, M.; Bednarczyk, J.L.; McIntyre, B.W.; Entman, M.L.; Mann, D.L. Tumor necrosis factor-α provokes a hypertrophic growth response in adult cardiac myocytes. Circulation 1997, 95, 1247–1252. [Google Scholar] [CrossRef]
- Mano, Y.; Anzai, T.; Kaneko, H.; Nagatomo, Y.; Nagai, T.; Anzai, A.; Maekawa, Y.; Takahashi, T.; Meguro, T.; Yoshikawa, T.; et al. Overexpression of human C-reactive protein exacerbates left ventricular remodeling in diabetic cardiomyopathy. Circ. J. 2011, 75, 1717–1727. [Google Scholar] [CrossRef]
- Westermann, D.; Rutschow, S.; Van Linthout, S.; Linderer, A.; Bücker-Gärtner, C.; Sobirey, M.; Riad, A.; Pauschinger, M.; Schultheiss, H.P.; Tschöpe, C. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia 2006, 49, 2507–2513. [Google Scholar] [CrossRef]
- Smail, M.M.; Qureshi, M.A.; Shmygol, A.; Oz, M.; Singh, J.; Sydorenko, V.; Arabi, A.; Howarth, F.C.; Al Kury, L. Regional effects of streptozotocin-induced diabetes on shortening and calcium transport in epicardial and endocardial myocytes from rat left ventricle. Physiol. Rep. 2016, 4, 13034. [Google Scholar] [CrossRef]
- Lacombe, V.A.; Viatchenko-Karpinski, S.; Terentyev, D.; Sridhar, A.; Emani, S.; Bonagura, J.D.; Feldman, D.S.; Györke, S.; Carnes, C.A. Mechanisms of impaired calcium handling underlying subclinical diastolic dysfunction in diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1787–R1797. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Zhong, Y.; Hoit, B.D.; Grupp, I.L.; Hahn, H.; Dilly, K.W.; Guatimosim, S.; Lederer, W.J.; Matlib, M.A. Defective intracellular Ca(2+) signaling contributes to cardiomyopathy in Type 1 diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1398–H1408. [Google Scholar] [CrossRef]
- Teshima, Y.; Takahashi, N.; Saikawa, T.; Hara, M.; Yasunaga, S.; Hidaka, S.; Sakata, T. Diminished expression of sarcoplasmic reticulum Ca(2+)-ATPase and ryanodine sensitive Ca(2+)Channel mRNA in streptozotocin-induced diabetic rat heart. J. Mol. Cell Cardiol. 2000, 32, 655–664. [Google Scholar] [CrossRef]
- Hamouda, N.N.; Sydorenko, V.; Qureshi, M.A.; Alkaabi, J.M.; Oz, M.; Howarth, F.C. Dapagliflozin reduces the amplitude of shortening and Ca(2+) transient in ventricular myocytes from streptozotocin-induced diabetic rats. Mol. Cell Biochem. 2015, 400, 57–68. [Google Scholar] [CrossRef]
- Wang, D.W.; Kiyosue, T.; Shigematsu, S.; Arita, M. Abnormalities of K+ and Ca2+ currents in ventricular myocytes from rats with chronic diabetes. Am. J. Physiol. 1995, 269, H1288–H1296. [Google Scholar] [CrossRef]
- Woodall, A.; Bracken, N.; Qureshi, A.; Howarth, F.C.; Singh, J. Halothane alters contractility and Ca2+ transport in ventricular myocytes from streptozotocin-induced diabetic rats. Mol. Cell Biochem. 2004, 261, 251–261. [Google Scholar] [CrossRef]
- Chattou, S.; Diacono, J.; Feuvray, D. Decrease in sodium-calcium exchange and calcium currents in diabetic rat ventricular myocytes. Acta Physiol. Scand. 1999, 166, 137–144. [Google Scholar] [CrossRef]
- Lu, Z.; Jiang, Y.P.; Xu, X.H.; Ballou, L.M.; Cohen, I.S.; Lin, R.Z. Decreased L-type Ca2+ current in cardiac myocytes of type 1 diabetic Akita mice due to reduced phosphatidylinositol 3-kinase signaling. Diabetes 2007, 56, 2780–2789. [Google Scholar] [CrossRef]
- Bracken, N.; Howarth, F.C.; Singh, J. Effects of streptozotocin-induced diabetes on contraction and calcium transport in rat ventricular cardiomyocytes. Ann. N. Y. Acad. Sci. 2006, 1084, 208–222. [Google Scholar] [CrossRef]
- Stølen, T.O.; Høydal, M.A.; Kemi, O.J.; Catalucci, D.; Ceci, M.; Aasum, E.; Larsen, T.; Rolim, N.; Condorelli, G.; Smith, G.L.; et al. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ. Res. 2009, 105, 527–536. [Google Scholar] [CrossRef]
- Howarth, F.C.; Qureshi, M.A.; Hassan, Z.; Al Kury, L.T.; Isaev, D.; Parekh, K.; Yammahi, S.R.; Oz, M.; Adrian, T.E.; Adeghate, E. Changing pattern of gene expression is associated with ventricular myocyte dysfunction and altered mechanisms of Ca2+ signalling in young type 2 Zucker diabetic fatty rat heart. Exp. Physiol. 2011, 96, 325–337. [Google Scholar] [CrossRef]
- van Oort, R.J.; Respress, J.L.; Li, N.; Reynolds, C.; De Almeida, A.C.; Skapura, D.G.; De Windt, L.J.; Wehrens, X.H. Accelerated development of pressure overload-induced cardiac hypertrophy and dysfunction in an RyR2-R176Q knockin mouse model. Hypertension 2010, 55, 932–938. [Google Scholar] [CrossRef]
- Jiang, D.; Xiao, B.; Yang, D.; Wang, R.; Choi, P.; Zhang, L.; Cheng, H.; Chen, S.R. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR). Proc. Natl. Acad. Sci. USA 2004, 101, 13062–13067. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Shi, L.; Chen, X.; Cui, C.; Huang, J.; Chen, B.; Hall, D.D.; Pan, Z.; Lu, M.; et al. Integrin β1D Deficiency-Mediated RyR2 Dysfunction Contributes to Catecholamine-Sensitive Ventricular Tachycardia in Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation 2020, 141, 1477–1493. [Google Scholar] [CrossRef]
- Rampazzo, A. Genetic bases of arrhythmogenic right ventricular Cardiomyopathy. Heart Int. 2006, 2, 17. [Google Scholar] [CrossRef]
- Yu, Z.; Tibbits, G.F.; McNeill, J.H. Cellular functions of diabetic cardiomyocytes: Contractility, rapid-cooling contracture, and ryanodine binding. Am. J. Physiol. 1994, 266, H2082–H2089. [Google Scholar] [CrossRef]
- Zhao, S.M.; Wang, Y.L.; Guo, C.Y.; Chen, J.L.; Wu, Y.Q. Progressive decay of Ca2+ homeostasis in the development of diabetic cardiomyopathy. Cardiovasc. Diabetol. 2014, 13, 75. [Google Scholar] [CrossRef]
- Shao, C.H.; Wehrens, X.H.; Wyatt, T.A.; Parbhu, S.; Rozanski, G.J.; Patel, K.P.; Bidasee, K.R. Exercise training during diabetes attenuates cardiac ryanodine receptor dysregulation. J. Appl. Physiol. 2009, 106, 1280–1292. [Google Scholar] [CrossRef]
- Tuncay, E.; Okatan, E.N.; Toy, A.; Turan, B. Enhancement of cellular antioxidant-defence preserves diastolic dysfunction via regulation of both diastolic Zn2+ and Ca2+ and prevention of RyR2-leak in hyperglycemic cardiomyocytes. Oxid. Med. Cell Longev. 2014, 14, 290381. [Google Scholar] [CrossRef]
- Shao, C.H.; Rozanski, G.J.; Patel, K.P.; Bidasee, K.R. Dyssynchronous (non-uniform) Ca2+ release in myocytes from streptozotocin-induced diabetic rats. J. Mol. Cell Cardiol. 2007, 42, 234–246. [Google Scholar] [CrossRef]
- Sánchez, G.; Araneda, F.; Peña, J.P.; Finkelstein, J.P.; Riquelme, J.A.; Montecinos, L.; Barrientos, G.; Llanos, P.; Pedrozo, Z.; Said, M.; et al. High-Fat-Diet-Induced Obesity Produces Spontaneous Ventricular Arrhythmias and Increases the Activity of Ryanodine Receptors in Mice. Int. J. Mol. Sci. 2018, 19, 533. [Google Scholar] [CrossRef]
- Talukder, M.A.; Kalyanasundaram, A.; Zuo, L.; Velayutham, M.; Nishijima, Y.; Periasamy, M.; Zweier, J.L. Is reduced SERCA2a expression detrimental or beneficial to postischemic cardiac function and injury? Evidence from heterozygous SERCA2a knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1426–H1434. [Google Scholar] [CrossRef]
- Torre, E.; Arici, M.; Lodrini, A.M.; Ferrandi, M.; Barassi, P.; Hsu, S.C.; Chang, G.J.; Boz, E.; Sala, E.; Vagni, S.; et al. SERCA2a stimulation by istaroxime improves intracellular Ca2+ handling and diastolic dysfunction in a model of diabetic cardiomyopathy. Cardiovasc. Res. 2022, 118, 1020–1032. [Google Scholar] [CrossRef]
- Alvarez, B.V.; Villa-Abrille, M.C. Mitochondrial NHE1: A newly identified target to prevent heart disease. Front. Physiol. 2013, 4, 152. [Google Scholar] [CrossRef]
- Landstrom, A.P.; Dobrev, D.; Wehrens, X.H.T. Calcium Signaling and Cardiac Arrhythmias. Circ. Res. 2017, 120, 1969–1993. [Google Scholar] [CrossRef]
- Nakamura, T.Y.; Iwata, Y.; Arai, Y.; Komamura, K.; Wakabayashi, S. Activation of Na+/H+ exchanger 1 is sufficient to generate Ca2+ signals that induce cardiac hypertrophy and heart failure. Circ. Res. 2008, 103, 891–899. [Google Scholar] [CrossRef]
- Xue, J.; Mraiche, F.; Zhou, D.; Karmazyn, M.; Oka, T.; Fliegel, L.; Haddad, G.G. Elevated myocardial Na+/H+ exchanger isoform 1 activity elicits gene expression that leads to cardiac hypertrophy. Physiol. Genomics 2010, 42, 374–383. [Google Scholar] [CrossRef]
- Molkentin, J.D.; Lu, J.R.; Antos, C.L.; Markham, B.; Richardson, J.; Robbins, J.; Grant, S.R.; Olson, E.N. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 1998, 93, 215–228. [Google Scholar] [CrossRef]
- Anderson, M.E.; Brown, J.H.; Bers, D.M. CaMKII in myocardial hypertrophy and heart failure. J. Mol. Cell. Cardiol. 2011, 51, 468–473. [Google Scholar] [CrossRef]
- JaquenodDe Giusti, C.; Blanco, P.G.; Lamas, P.A.; Carrizo Velasquez, F.; Lofeudo, J.M.; Portiansky, E.L.; Alvarez, B.V. Carbonic anhydrase II/sodium-proton exchanger 1 metabolon complex in cardiomyopathy of ob-/- type 2 diabetic mice. J. Mol. Cell. Cardiol. 2019, 136, 53–63. [Google Scholar] [CrossRef]
- Darmellah, A.; Baetz, D.; Prunier, F.; Tamareille, S.; Rücker-Martin, C.; Feuvray, D. Enhanced activity of the myocardial Na+/H+ exchanger contributes to left ventricular hypertrophy in the Goto-Kakizaki rat model of type 2 diabetes: Critical role of Akt. Diabetologia 2007, 50, 1335–1344. [Google Scholar] [CrossRef]
- Uthman, L.; Baartscheer, A.; Bleijlevens, B.; Schumacher, C.A.; Fiolet, J.W.T.; Koeman, A.; Jancev, M.; Hollmann, M.W.; Weber, N.C.; Coronel, R.; et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: Inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 2018, 61, 722–726. [Google Scholar] [CrossRef]
- Miller, L.M.; Gal, A. Chapter 10—Cardiovascular System and Lymphatic Vessels1. In Pathologic Basis of Veterinary Disease, 6th ed.; Zachary, J.F., Ed.; Mosby: Maryland Heights, MI, USA, 2017; pp. 561–616.e1. ISBN 9780323357753. [Google Scholar] [CrossRef]
- Mohan, P.; Brutsaert, D.L.; Paulus, W.J.; Sys, S.U. Myocardial contractile response to nitric oxide and cGMP. Circulation 1996, 93, 1223–1229. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Kuboki, K.; Jiang, Z.Y.; Takahara, N.; Ha, S.W.; Igarashi, M.; Yamauchi, T.; Feener, E.P.; Herbert, T.P.; Rhodes, C.J.; King, G.L. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: A specific vascular action of insulin. Circulation 2000, 101, 676–681. [Google Scholar] [CrossRef]
- Fisslthaler, B.; Benzing, T.; Busse, R.; Fleming, I. Insulin enhances the expression of the endothelial nitric oxide synthase in native endothelial cells: A dual role for Akt and AP-1. Nitric Oxide 2003, 8, 253–261. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Hayes, R.G.; Stitt, A.W.; McAuley, E.; Archer, D.B. Constitutive nitric oxide synthase expression in retinal vascular endothelial cells is suppressed by high glucose and advanced glycation end products. Diabetes 1998, 47, 945–952. [Google Scholar] [CrossRef]
- Naruse, K.; Rask-Madsen, C.; Takahara, N.; Ha, S.W.; Suzuma, K.; Way, K.J.; Jacobs, J.R.; Clermont, A.C.; Ueki, K.; Ohshiro, Y.; et al. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes 2006, 55, 691–698. [Google Scholar] [CrossRef]
- Flaherty, M.P.; Brown, M.; Grupp, I.L.; Schultz, J.E.; Murphree, S.S.; Jones, W.K. eNOS deficient mice develop progressive cardiac hypertrophy with altered cytokine and calcium handling protein expression. Cardiovasc. Toxicol. 2007, 7, 165–177. [Google Scholar] [CrossRef]
- Fleming, I.; Busse, R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1–R12. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Li, S.; Lv, J. Endothelial Dysfunction and Diabetic Cardiomyopathy. Front. Endocrinol. 2022, 13, 851941. [Google Scholar] [CrossRef]
- Widyantoro, B.; Emoto, N.; Nakayama, K.; Anggrahini, D.W.; Adiarto, S.; Iwasa, N.; Yagi, K.; Miyagawa, K.; Rikitake, Y.; Suzuki, T.; et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation 2010, 121, 2407–2418. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbBsignalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Gui, C.; Zhu, L.; Hu, M.; Lei, L.; Long, Q. Neuregulin-1/ErbB signaling is impaired in the rat model of diabetic cardiomyopathy. Cardiovasc. Pathol. 2012, 21, 414–420. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Feron, O.; Dessy, C.; Han, X.; Marchionni, M.A.; Kelly, R.A. Neuregulin signaling in the heart. Dynamic targeting of erbB4 to caveolar microdomains in cardiac myocytes. Circ. Res. 1999, 84, 1380–1387. [Google Scholar] [CrossRef]
- Fukazawa, R.; Miller, T.A.; Kuramochi, Y.; Frantz, S.; Kim, Y.D.; Marchionni, M.A.; Kelly, R.A.; Sawyer, D.B. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J. Mol. Cell Cardiol. 2003, 35, 1473–1479. [Google Scholar] [CrossRef]
- De Keulenaer, G.W.; Doggen, K.; Lemmens, K. The vulnerability of the heart as a pluricellular paracrine organ: Lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ. Res. 2010, 106, 35–46. [Google Scholar] [CrossRef]
- Crone, S.A.; Zhao, Y.Y.; Fan, L.; Gu, Y.; Minamisawa, S.; Liu, Y.; Peterson, K.L.; Chen, J.; Kahn, R.; Condorelli, G.; et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat. Med. 2002, 8, 459–465. [Google Scholar] [CrossRef]
- García-Rivello, H.; Taranda, J.; Said, M.; Cabeza-Meckert, P.; Vila-Petroff, M.; Scaglione, J.; Ghio, S.; Chen, J.; Lai, C.; Laguens, R.P.; et al. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1153–H1160. [Google Scholar] [CrossRef]
- Ozcelik, C.; Erdmann, B.; Pilz, B.; Wettschureck, N.; Britsch, S.; Hübner, N.; Chien, K.R.; Birchmeier, C.; Garratt, A.N. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc. Natl. Acad. Sci. USA 2002, 99, 8880–8885. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, F.; Forrester, S.J.; Eguchi, S.; Zhang, M.Z.; Harris, R.C. Expression and Function of the Epidermal Growth Factor Receptor in Physiology and Disease. Physiol. Rev. 2016, 96, 1025–1069. [Google Scholar] [CrossRef]
- Leri, A.; Claudio, P.P.; Li, Q.; Wang, X.; Reiss, K.; Wang, S.; Malhotra, A.; Kajstura, J.; Anversa, P. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin angiotensin system and decreases the Bel-2 to Bax protein ratio in the cell. J. Clin. Investig. 1998, 101, 1326–1342. [Google Scholar] [CrossRef]
- Leri, A.; Fiordaliso, F.; Setoguchi, M.; Limana, F.; Bishopric, N.H.; Kajstura, J.; Webster, K.; Anversa, P. Inhibition of p53 function prevents renin angiotensin system activation and stretch-mediated myocyte apoptosis. Am. J. Pathol. 2000, 157, 843–857. [Google Scholar] [CrossRef]
- Fiordaliso, F.; Leri, A.; Cesselli, D.; Limana, F.; Safai, B.; Nadal-Ginard, B.; Anversa, P.; Kajstura, J. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes 2001, 50, 2363–2375. [Google Scholar] [CrossRef]
- Xu, Y.Z.; Zhang, X.; Wang, L.; Zhang, F.; Qiu, Q.; Liu, M.L.; Zhang, G.R.; Wu, X.L. An increased circulating angiotensin II concentration is associated with hypoadiponectinemia and postprandial hyperglycemia in men with nonalcoholic fatty liver disease. Intern. Med. 2013, 52, 855–861. [Google Scholar] [CrossRef]
- Nguyen Dinh Cat, A.; Montezano, A.C.; Burger, D.; Touyz, R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal. 2013, 19, 1110–1120. [Google Scholar] [CrossRef]
- Giacchetti, G.; Sechi, L.A.; Rilli, S.; Carey, R.M. The renin-angiotensin-aldosterone system, glucose metabolism and diabetes. Trends Endocrinol. Metab. 2005, 16, 120–126. [Google Scholar] [CrossRef]
- Brilla, C. Aldosterone and Myocardial Fibrosis in Heart Failure. Herz 2000, 25, 299–306. [Google Scholar] [CrossRef]
- Mori, J.; Patel, V.B.; Abo Alrob, O.; Basu, R.; Altamimi, T.; Desaulniers, J.; Wagg, C.S.; Kassiri, Z.; Lopaschuk, G.D.; Oudit, G.Y. Angiotensin 1-7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ. Heart Fail. 2014, 7, 327–339. [Google Scholar] [CrossRef]
- Mustonen, J.; Uusitupa, M.; Lansimies, E.; Vainio, P.; Laakso, M.; Pyörälä, K. Autonomic nervous function and its relationship to cardiac performancein middle-aged diabetic patients without clinically evident cardiovascular disease. J. Intern. Med. 1992, 232, 65–72. [Google Scholar] [CrossRef]
- Didangelos, T.P.; Arsos, G.A.; Karamitsos, D.T.; Athyros, V.G.; Karatzas, N.D. Left ventricular systolic and diastolic function in normotensive type 1diabetic patients with or without autonomic neuropathy: A radionuclideventriculography study. Diabetes Care 2003, 26, 1955–1960. [Google Scholar] [CrossRef]
- Vidal, M.; Wieland, T.; Lohse, M.J.; Lorenz, K. β-Adrenergic receptor stimulation causes cardiac hypertrophy via a Gβγ/Erk-dependent pathway. Cardiovasc. Res. 2012, 96, 255–264. [Google Scholar] [CrossRef]
- Stevens, M.J.; Dayanikli, F.; Raffel, D.M.; Allman, K.C.; Sandford, T.; Feldman, E.L.; Wieland, D.M.; Corbett, J.; Schwaiger, M. Scintigraphic assessment of regionalized defects in myocardial sympathetic innervation and blood flow regulation in diabetic patients with autonomic neuropathy. J. Am. Coll. Cardiol. 1998, 31, 1575–1584. [Google Scholar] [CrossRef]
- Pop-Busui, R. What do we know and we do not know about cardiovascular autonomic neuropathy in diabetes. J. Cardiovasc. Transl. Res. 2012, 5, 463–478. [Google Scholar] [CrossRef]
- Symons, J.D.; Hu, P.; Yang, Y.; Wang, X.; Zhang, Q.J.; Wende, A.R.; Sloan, C.L.; Sena, S.; Abel, E.D.; Litwin, S.E. Knockout of insulin receptors in cardiomyocytes attenuates coronary arterial dysfunction induced by pressure overload. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H374–H381. [Google Scholar] [CrossRef]
- Riehle, C.; Weatherford, E.T.; Wende, A.R.; Jaishy, B.P.; Seei, A.W.; McCarty, N.S.; Rech, M.; Shi, Q.; Reddy, G.R.; Kutschke, W.J.; et al. Insulin receptor substrates differentially exacerbate insulin-mediated left ventricular remodeling. JCI Insight 2020, 5, e134920. [Google Scholar] [CrossRef]
- Song, R.; Peng, W.; Zhang, Y.; Lv, F.; Wu, H.K.; Guo, J.; Cao, Y.; Pi, Y.; Zhang, X.; Jin, L.; et al. Central role of E3 ubiquitin ligase MG53 in insulin resistance and metabolic disorders. Nature 2013, 494, 375–379. [Google Scholar] [CrossRef]
- Wu, H.K.; Zhang, Y.; Cao, C.M.; Hu, X.; Fang, M.; Yao, Y.; Jin, L.; Chen, G.; Jiang, P.; Zhang, S.; et al. Glucose-Sensitive Myokine/Cardiokine MG53 Regulates Systemic Insulin Response and Metabolic Homeostasis. Circulation 2019, 139, 901–914. [Google Scholar] [CrossRef]
- Liu, F.; Song, R.; Feng, Y.; Guo, J.; Chen, Y.; Zhang, Y.; Chen, T.; Wang, Y.; Huang, Y.; Li, C.Y.; et al. Upregulation of MG53 induces diabetic cardiomyopathy through transcriptional activation of peroxisome proliferation-activated receptor α. Circulation 2015, 131, 795–804. [Google Scholar] [CrossRef]
- Feng, H.; Shen, H.; Robeson, M.J.; Wu, Y.H.; Wu, H.K.; Chen, G.J.; Zhang, S.; Xie, P.; Jin, L.; He, Y.; et al. MG53 E3 Ligase-Dead Mutant Protects Diabetic Hearts From Acute Ischemic/Reperfusion Injury and Ameliorates Diet-Induced Cardiometabolic Damage. Diabetes 2022, 71, 298–314. [Google Scholar] [CrossRef]
- Aronson, D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J. Hypertens. 2003, 21, 3–12. [Google Scholar] [CrossRef]
- Ducheix, S.; Magré, J.; Cariou, B.; Prieur, X. Chronic O-GlcNAcylation and Diabetic Cardiomyopathy: The Bitterness of Glucose. Front. Endocrinol. 2018, 9, 642. [Google Scholar] [CrossRef]
- Joubert, M.; Jagu, B.; Montaigne, D.; Marechal, X.; Tesse, A.; Ayer, A.; Dollet, L.; Le May, C.; Toumaniantz, G.; Manrique, A.; et al. The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Cardiomyopathy in a Diabetic Lipodystrophic Mouse Model. Diabetes 2017, 66, 1030–1040. [Google Scholar] [CrossRef]
- Way, K.J.; Isshiki, K.; Suzuma, K.; Yokota, T.; Zvagelsky, D.; Schoen, F.J.; Sandusky, G.E.; Pechous, P.A.; Vlahos, C.J.; Wakasaki, H.; et al. Expression of connective tissue growth factor is increased in injured myocardium associated with protein kinase C beta2 activation and diabetes. Diabetes 2002, 51, 2709–2718. [Google Scholar] [CrossRef]
- Murdoch, C.E.; Chaubey, S.; Zeng, L.; Yu, B.; Ivetic, A.; Walker, S.J.; Vanhoutte, D.; Heymans, S.; Grieve, D.J.; Cave, A.C.; et al. Endothelial NADPH oxidase-2 promotesinterstitial cardiac fibrosis and diastolic dysfunction through proinflammatory effects and endothelial-mesenchymal transition. J. Am. Coll. Cardiol. 2014, 63, 2734–2741. [Google Scholar] [CrossRef]
- Zhao, X.; Hua, Y.; Chen, H.; Yang, H.; Zhang, T.; Huang, G.; Fan, H.; Tan, Z.; Huang, X.; Liu, B.; et al. Aldehyde dehydrogenase-2 protects against myocardial infarction-related cardiac fibrosis through modulation of the Wnt/β-catenin signaling pathway. Ther. Clin. Risk Manag. 2015, 11, 1371–1381. [Google Scholar] [CrossRef]
- Granzier, H.; Labeit, S. Cardiac titin: An adjustable multi-functional spring. J. Physiol. 2002, 541, 335–342. [Google Scholar] [CrossRef]
- Jandu, S.K.; Webb, A.K.; Pak, A.; Sevinc, B.; Nyhan, D.; Belkin, A.M.; Flavahan, N.A.; Berkowitz, D.E.; Santhanam, L. Nitric oxide regulates tissue transglutaminase localization and function in the vasculature. Amino Acids 2013, 44, 261–269. [Google Scholar] [CrossRef]
- Steppan, J.; Bergman, Y.; Viegas, K.; Armstrong, D.; Tan, S.; Wang, H.; Melucci, S.; Hori, D.; Park, S.Y.; Barreto, S.F.; et al. Tissue Transglutaminase Modulates Vascular Stiffness and Function Through Crosslinking-Dependent and Crosslinking-Independent Functions. J. Am. Heart Assoc. 2017, 6, e004161. [Google Scholar] [CrossRef]
- Sane, D.C.; Kontos, J.L.; Greenberg, C.S. Roles of transglutaminases in cardiac and vascular diseases. Front. Biosci. 2007, 12, 2530–2545. [Google Scholar] [CrossRef]
- Small, K.; Feng, J.F.; Lorenz, J.; Donnelly, E.T.; Yu, A.; Im, M.J.; Dorn, G.W., 2nd; Liggett, S.B. Cardiac specific overexpression of transglutaminase II (G(h)) results in a unique hypertrophy phenotype independent of phospholipase C activation. J. Biol. Chem. 1999, 274, 21291–21296. [Google Scholar] [CrossRef]
- Singh, G.B.; Sharma, R.; Khullar, M. Epigenetics and diabetic cardiomyopathy. Diabetes Res. Clin. Pract. 2011, 94, 14–21. [Google Scholar] [CrossRef]
- Deng, J.; Liao, Y.; Liu, J.; Liu, W.; Yan, D. Research Progress on Epigenetics of Diabetic Cardiomyopathy in Type 2 Diabetes. Front. Cell Dev. Biol. 2021, 9, 777258. [Google Scholar] [CrossRef]
- Guo, R.; Nair, S. Role of microRNA in diabetic cardiomyopathy: From mechanism to intervention. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2070–2077. [Google Scholar] [CrossRef]
- Costantino, S.; Paneni, F.; Mitchell, K.; Mohammed, S.A.; Hussain, S.; Gkolfos, C.; Berrino, L.; Volpe, M.; Schwarzwald, C.; Lüscher, T.F.; et al. Hyperglycaemia-induced epigenetic changes drive persistent cardiac dysfunction via the adaptor p66Shc. Int. J. Cardiol. 2018, 268, 179–186. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, A.W.; Akhmedov, A.; Suades, R.; Costantino, S.; Paneni, F.; Caidahl, K.; Mohammed, S.A.; Hage, C.; Gkolfos, C.; et al. Hyperglycemia Induces Myocardial Dysfunction via Epigenetic Regulation of JunD. Circ. Res. 2020, 127, 1261–1273. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Horie, T.; Baba, O.; Watanabe, S.; Nishiga, M.; Usami, S.; Izuhara, M.; Nakao, T.; Nishino, T.; Otsu, K.; et al. MicroRNA-451 exacerbates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy in mice through suppression of the LKB1/AMPK pathway. Circ. Res. 2015, 116, 279–288. [Google Scholar] [CrossRef]
- Li, H.; Fan, J.; Zhao, Y.; Zhang, X.; Dai, B.; Zhan, J.; Yin, Z.; Nie, X.; Fu, X.D.; Chen, C.; et al. Nuclear miR-320 Mediates Diabetes-Induced Cardiac Dysfunction by Activating Transcription of Fatty Acid Metabolic Genes to Cause Lipotoxicity in the Heart. Circ. Res. 2019, 125, 1106–1120. [Google Scholar] [CrossRef]
- Fang, Z.Y.; Prins, J.B.; Marwick, T.H. Diabetic cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Endocr. Rev. 2004, 25, 543–567. [Google Scholar] [CrossRef]
- Kumric, M.; Kurir, T.T.; Borovac, J.A.; Bozic, J. Role of novel biomarkers in diabetic cardiomyopathy. World J. Diabetes 2021, 12, 685–705. [Google Scholar] [CrossRef]
- Dahlström, U. Can natriuretic peptides be used for the diagnosis of diastolic heart failure? Eur. J. Heart Fail. 2004, 6, 281–287. [Google Scholar] [CrossRef]
- Fousteris, E.; Melidonis, A.; Panoutsopoulos, G.; Tzirogiannis, K.; Foussas, S.; Theodosis-Georgilas, A.; Tzerefos, S.; Matsagos, S.; Boutati, E.; Economopoulos, T.; et al. Toll/interleukin-1 receptor member ST2 exhibits higher soluble levels in type 2 diabetes, especially when accompanied with left ventricular diastolic dysfunction. Cardiovasc. Diabetol. 2011, 10, 101. [Google Scholar] [CrossRef]
- Kiencke, S.; Handschin, R.; von Dahlen, R.; Muser, J.; Brunner-Larocca, H.P.; Schumann, J.; Felix, B.; Berneis, K.; Rickenbacher, P. Pre-clinical diabetic cardiomyopathy: Prevalence, screening, and outcome. Eur. J. Heart Fail. 2010, 12, 951–957. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; Ho, J.E.; Liu, F.T.; de Boer, R.A. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef]
- Luís, C.; Costa, R.; Rodrigues, I.; Castela, Â.; Coelho, P.; Guerreiro, S.; Gomes, J.; Reis, C.; Soares, R. Xanthohumol and 8-prenylnaringenin reduce type 2 diabetes-associated oxidative stress by downregulating galectin-3. Porto Biomed. J. 2019, 4, e23. [Google Scholar] [CrossRef]
- Yingchoncharoen, T.; Agarwal, S.; Popović, Z.B.; Marwick, T.H. Normal ranges of left ventricular strain: A meta-analysis. J. Am. Soc. Echocardiogr. 2013, 26, 185–191. [Google Scholar] [CrossRef]
- Keng, B.M.H.; Gao, F.; Ewe, S.H.; Tan, R.S.; Teo, L.L.Y.; Xie, B.Q.; Koh, W.P.; Koh, A.S. Galectin-3 as a candidate upstream biomarker for quantifying risks of myocardial aging. ESC Heart Fail. 2019, 6, 1068–1076. [Google Scholar] [CrossRef]
- Devaux, Y.; Zangrando, J.; Schroen, B.; Creemers, E.E.; Pedrazzini, T.; Chang, C.P.; Dorn, G.W., 2nd; Thum, T.; Heymans, S.; Cardiolinc Network. Long noncoding RNAs in cardiac development and ageing. Nat. Rev. Cardiol. 2015, 12, 415–425. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Bauters, C.; Volkmann, I.; Maury, F.; Fetisch, J.; Holzmann, A.; Lemesle, G.; de Groote, P.; Pinet, F.; Thum, T. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ. Res. 2014, 114, 1569–1575. [Google Scholar] [CrossRef]
- Berezin, A.E. Diabetes mellitus related biomarker: The predictive role of growth-differentiation factor-15. Diabetes Metab. Syndr. 2016, 10, S154–S157. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Avanzas, P. Usefulness of growth differentiation factor-15 Levels to predict diabetic cardiomyopathy in asymptomatic patients with type 2 diabetesmellitus. Am. J. Cardiol. 2014, 114, 890–894. [Google Scholar] [CrossRef]
- Ziyadeh, F.N.; Hoffman, B.B.; Han, D.C.; Iglesias-De La Cruz, M.C.; Hong, S.W.; Isono, M.; Chen, S.; McGowan, T.A. Sharma K Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal anti-transforming growth factor-beta antibody in db/db diabetic mice. Proc. Natl. Acad. Sci. USA 2000, 97, 8015–8020. [Google Scholar] [CrossRef]
- Asbun, J.; Villarreal, F.J. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2006, 47, 693–700. [Google Scholar] [CrossRef]
- Shaver, A.; Nichols, A.; Thompson, E.; Mallick, A.; Payne, K.; Jones, C.; Manne, N.D.; Sundaram, S.; Shapiro, J.I.; Sodhi, K. Role of Serum Biomarkers in Early Detection of Diabetic Cardiomyopathy in the West Virginian Population. Int. J. Med. Sci. 2016, 13, 161–168. [Google Scholar] [CrossRef]
- Gandhi, P.U.; Gaggin, H.K.; Sheftel, A.D.; Belcher, A.M.; Weiner, R.B.; Baggish, A.L.; Motiwala, S.R.; Liu, P.P.; Januzzi, J.L., Jr. Prognostic usefulness of insulin-like growth factor-binding protein 7 in heart failure with reduced ejection fraction: A novel biomarker of myocardial diastolic function? Am. J. Cardiol. 2014, 114, 1543–1549. [Google Scholar] [CrossRef]
- Greulich, S.; Maxhera, B.; Vandenplas, G.; de Wiza, D.H.; Smiris, K.; Mueller, H.; Heinrichs, J.; Blumensatt, M.; Cuvelier, C.; Akhyari, P.; et al. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation 2012, 126, 2324–2334. [Google Scholar] [CrossRef]
- Chen, W.J.; Greulich, S.; van der Meer, R.W.; Rijzewijk, L.J.; Lamb, H.J.; de Roos, A.; Smit, J.W.; Romijn, J.A.; Ruige, J.B.; Lammertsma, A.A.; et al. Activin A is associated with impaired myocardial glucose metabolism and left ventricular remodeling in patients with uncomplicated type 2 diabetes. Cardiovasc. Diabetol. 2013, 12, 150. [Google Scholar] [CrossRef]
- Akbal, E.; Özbek, M.; Güneş, F.; Akyürek, Ö.; Üreten, K.; Delibaşı, T. Serum heart type fatty acid binding protein levels in metabolic syndrome. Endocrine 2009, 36, 433–437. [Google Scholar] [CrossRef]
- Hoffmann, U.; Espeter, F.; Weiß, C.; Ahmad-Nejad, P.; Lang, S.; Brueckmann, M.; Akin, I.; Neumaier, M.; Borggrefe, M.; Behnes, M. Ischemic biomarker heart-type fatty acid binding protein (hFABP) in acute heart failure—Diagnostic and prognostic insights compared to NT-proBNP and troponin I. BMC Cardiovasc. Disord. 2015, 15, 50. [Google Scholar] [CrossRef]
- Rhman, M.A.; Owira, P. The role of microRNAs in the pathophysiology, diagnosis, and treatment of diabetic cardiomyopathy. J. Pharm. Pharmacol. 2022, 74, 1663–1676. [Google Scholar] [CrossRef]
- Haugen, E.; Chen, J.; Wikström, J.; Grönros, J.; Gan, L.M.; Fu, L.X. Parallel gene expressions of IL-6 and BNP during cardiac hypertrophy complicated with diastolic dysfunction in spontaneously hypertensive rats. Int. J. Cardiol. 2007, 115, 24–28. [Google Scholar] [CrossRef]
- Duprez, D.A.; Gross, M.D.; Kizer, J.R.; Ix, J.H.; Hundley, W.G.; Jacobs, D.R., Jr. Predictive Value of Collagen Biomarkers for Heart Failure With and Without Preserved Ejection Fraction: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Heart Assoc. 2018, 7, e007885. [Google Scholar] [CrossRef]
- Barasch, E.; Gottdiener, J.S.; Aurigemma, G.; Kitzman, D.W.; Han, J.; Kop, W.J.; Tracy, R.P. The Relationship Between Serum Markers of Collagen Turnover and Cardiovascular Outcome in the Elderly. Circ. Heart Fail. 2011, 4, 733–739. [Google Scholar] [CrossRef]
- Lopez-Andres, N.; Fortuno, M.A.; Diez, J.; Zannad, F.; Lacolley, P.; Rossignol, P. Vascular effects of cardiotrophin-1: A role in hypertension? J. Hypertens. 2010, 28, 1261–1272. [Google Scholar] [CrossRef]
- Gamella-Pozuelo, L.; Fuentes-Calvo, I.; Gómez-Marcos, M.A.; Recio-Rodriguez, J.I.; Agudo-Conde, C.; Fernández-Martín, J.L.; Cannata-Andía, J.B.; López-Novoa, J.M.; García-Ortiz, L.; Martínez-Salgado, C. Plasma Cardiotrophin-1 as a Marker of Hypertension and Diabetes-Induced Target Organ Damage and Cardiovascular Risk. Medicine 2015, 94, e1218. [Google Scholar] [CrossRef]
- McCarthy, C.P.; Mullins, K.V.; Kerins, D.M. The role of trimetazidine in cardiovascular disease: Beyond an anti-anginal agent. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 266–272. [Google Scholar] [CrossRef]
- Shu, H.; Peng, Y.; Hang, W.; Zhou, N.; Wang, D.W. Trimetazidine in Heart Failure. Front. Pharmacol. 2021, 11, 569132. [Google Scholar] [CrossRef]
- Marzilli, M.; Vinereanu, D.; Lopaschuk, G.; Chen, Y.; Dalal, J.J.; Danchin, N.; Etriby, E.; Ferrari, R.; Gowdak, L.H.; Lopatin, Y.; et al. Trimetazidine in cardiovascular medicine. Int. J. Cardiol. 2019, 293, 39–44. [Google Scholar] [CrossRef]
- Ferraro, E.; Giammarioli, A.M.; Caldarola, S.; Lista, P.; Feraco, A.; Tinari, A.; Salvatore, A.M.; Malorni, W.; Berghella, L.; Rosano, G. The metabolic modulator trimetazidine triggers autophagy and counteracts stress-induced atrophy in skeletal muscle myotubes. FEBS J. 2013, 280, 5094–5108. [Google Scholar] [CrossRef]
- Liu, X.; Gai, Y.; Liu, F.; Gao, W.; Zhang, Y.; Xu, M.; Li, Z. Trimetazidine inhibits pressure overload-induced cardiac fibrosis through NADPH oxidase-ROS-CTGF pathway. Cardiovasc. Res. 2010, 88, 150–158. [Google Scholar] [CrossRef]
- Zhou, X.; Li, C.; Xu, W.; Chen, J. Trimetazidine protects against smoking-induced left ventricular remodeling via attenuating oxidative stress, apoptosis, and inflammation. PLoS ONE 2012, 7, e40424. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, C. The cystathionine γ-lyase/hydrogen sulfide pathway mediates the trimetazidine-induced protection of H9c2 cells against hypoxia/reoxygenation-induced apoptosis and oxidative stress. Anatol. J. Cardiol. 2019, 22, 102–111. [Google Scholar] [CrossRef]
- Gao, D.; Ning, N.; Niu, X.; Hao, G.; Meng, Z. Trimetazidine: A meta-analysis of randomised controlled trials in heart failure. Heart 2011, 97, 278–286. [Google Scholar] [CrossRef]
- Fragasso, G.; Salerno, A.; Lattuada, G.; Cuko, A.; Calori, G.; Scollo, A.; Ragogna, F.; Arioli, F.; Bassanelli, G.; Spoladore, R.; et al. Effect of partial inhibition of fatty acid oxidation by trimetazidine on whole body energy metabolism in patients with chronic heart failure. Heart 2011, 97, 1495–1500. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, W.Y.; Wang, Z.H.; Tang, M.X.; Wang, F.; Li, Y.; Zhong, M.; Zhang, Y.; Zhang, W. Early administration of trimetazidine attenuates diabetic cardiomyopathy in rats by alleviating fibrosis, reducing apoptosis and enhancing autophagy. J. Transl. Med. 2016, 14, 109. [Google Scholar] [CrossRef]
- Tang, S.G.; Liu, X.Y.; Wang, S.P.; Wang, H.H.; Jovanović, A.; Tan, W. Trimetazidine prevents diabetic cardiomyopathy by inhibiting Nox2/TRPC3-induced oxidative stress. J. Pharmacol. Sci. 2019, 139, 311–318. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Quan, E.; Zhang, H.; Wu, Y.; Luo, Y.; Peng, L.; Wang, J.; Zhu, J.; Liu, J. Trimetazidine inhibits cardiac fibrosis by reducing reactive oxygen species and downregulating connective tissue growth factor in streptozotocin-induced diabetic rats. Exp. Ther. Med. 2019, 18, 1477–1485. [Google Scholar] [CrossRef]
- Khan, H.; Metra, M.; Blair, J.E.; Vogel, M.; Harinstein, M.E.; Filippatos, G.S.; Sabbah, H.N.; Porchet, H.; Valentini, G.; Gheorghiade, M. Istaroxime, a first in class new chemical entity exhibiting SERCA-2 activation and Na-K-ATPase inhibition: A new promising treatment for acute heart failure syndromes? Heart Fail. Rev. 2009, 14, 277–287. [Google Scholar] [CrossRef]
- Micheletti, R.; Palazzo, F.; Barassi, P.; Giacalone, G.; Ferrandi, M.; Schiavone, A.; Moro, B.; Parodi, O.; Ferrari, P.; Bianchi, G. Istaroxime, a stimulator of sarcoplasmic reticulum calcium adenosine triphosphatase isoform 2a activity, as a novel therapeutic approach to heart failure. Am. J. Cardiol. 2007, 99, 24A–32A. [Google Scholar] [CrossRef]
- Sabbah, H.N.; Imai, M.; Cowart, D.; Amato, A.; Carminati, P.; Gheorghiade, M. Hemodynamic properties of a new-generation positive luso-inotropic agent for the acute treatment of advanced heart failure. Am. J. Cardiol. 2007, 9, 41A–46A. [Google Scholar] [CrossRef]
- Shah, S.J.; Blair, J.E.; Filippatos, G.S.; Macarie, C.; Ruzyllo, W.; Korewicki, J.; Bubenek-Turconi, S.I.; Ceracchi, M.; Bianchetti, M.; Carminati, P.; et al. Effects of istaroxime on diastolic stiffness in acute heart failure syndromes: Results from the Hemodynamic, Echocardiographic, and Neurohormonal Effects of Istaroxime, a Novel Intravenous Inotropic and Lusitropic Agent: A Randomized Controlled Trial in Patients Hospitalized with Heart Failure (HORIZON-HF) trial. Am. Heart J. 2009, 157, 1035–1041. [Google Scholar] [CrossRef]
- Carubelli, V.; Zhang, Y.; Metra, M.; Lombardi, C.; Felker, G.M.; Filippatos, G.; O’Connor, C.M.; Teerlink, J.R.; Simmons, P.; Segal, R.; et al. Treatment with 24 houristaroxime infusion in patients hospitalised for acute heart failure: A randomised, placebo-controlled trial. Eur. J. Heart Fail. 2020, 22, 1684–1693. [Google Scholar] [CrossRef]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Dallas, A.P.; Douglas, P.S.; Foody, J.M.; Gerber, T.C.; Hinderliter, A.L.; et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2012, 60, 2564–2603. [Google Scholar] [CrossRef]
- Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; Di Mario, C.; et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 2013, 34, 2949–3003. [Google Scholar] [CrossRef]
- Kaplan, A.; Amin, G.; Abidi, E.; Altara, R.; Booz, G.W.; Zouein, F.A. Role of ranolazine in heart failure: From cellular to clinic perspective. Eur. J. Pharmacol. 2022, 919, 174787. [Google Scholar] [CrossRef]
- Mezincescu, A.; Karthikeyan, V.J.; Nadar, S.K. Ranolazine: A true pluripotent cardiovascular drug or jack of all trades, master of none? Sultan Qaboos Univ. Med. J. 2018, 18, e13–e23. [Google Scholar] [CrossRef]
- Nie, J.; Duan, Q.; He, M.; Li, X.; Wang, B.; Zhou, C.; Wu, L.; Wen, Z.; Chen, C.; Wang, D.W.; et al. Ranolazine prevents pressure overload-induced cardiac hypertrophy and heart failure by restoring aberrant Na+ and Ca2+ handling. J. Cell. Physiol. 2019, 234, 11587–11601. [Google Scholar] [CrossRef]
- Maier, L.S.; Layug, B.; Karwatowska-Prokopczuk, E.; Belardinelli, L.; Lee, S.; Sander, J.; Lang, C.; Wachter, R.; Edelmann, F.; Hasenfuss, G.; et al. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: The RALI-DHF proof-of-concept study. JACC Heart Fail. 2013, 1, 115–122. [Google Scholar] [CrossRef]
- Murray, G.L.; Colombo, J. Ranolazine preserves and improves left ventricular ejection fraction and autonomic measures when added to guideline-driven therapy in chronic heart failure. Heart Int. 2014, 9, 66–73. [Google Scholar] [CrossRef]
- Chen, X.; Ren, L.; Liu, X.; Sun, X.; Dong, C.; Jiang, Y.; Qin, Y.; Qu, H.; Jiao, J.; Wang, S.; et al. Ranolazine protects against diabetic cardiomyopathy by activating the NOTCH1/NRG1 pathway. Life Sci. 2020, 261, 118306. [Google Scholar] [CrossRef]
- Hamblin, M.; Smith, H.M.; Hill, M.F. Dietary supplementation with vitamin E ameliorates cardiac failure in type I diabetic cardiomyopathy by suppressing myocardial generation of 8-iso-prostaglandin F2alpha and oxidized glutathione. J. Card. Fail. 2007, 13, 884–892. [Google Scholar] [CrossRef]
- Shirpoor, A.; Salami, S.; Khadem-Ansari, M.H.; Ilkhanizadeh, B.; Pakdel, F.G.; Khademvatani, K. Cardioprotective effect of vitamin E: Rescues of diabetes-induced cardiac malfunction, oxidative stress, and apoptosis in rat. J. Diabetes Complicat. 2009, 23, 310–316. [Google Scholar] [CrossRef]
- Yusuf, S.; Dagenais, G.; Pogue, J.; Bosch, J.; Sleight, P. Heart Outcomes Prevention Evaluation Study Investigators. Vitamin E supplementation and cardiovascular events in high-risk patients. N. Engl. J. Med. 2000, 342, 154–160. [Google Scholar] [CrossRef]
- Keith, M.E.; Jeejeebhoy, K.N.; Langer, A.; Kurian, R.; Barr, A.; O’Kelly, B.; Sole, M.J. A controlled clinical trial of vitamin E supplementation in patients with congestive heart failure. Am. J. Clin. Nutr. 2001, 73, 219–224. [Google Scholar] [CrossRef]
- Morris, C.D.; Carson, S. Routine vitamin supplementation to prevent cardiovascular disease: A summary of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2003, 139, 56–70. [Google Scholar] [CrossRef]
- Vivekananthan, D.P.; Penn, M.S.; Sapp, S.K.; Hsu, A.; Topol, E.J. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet 2003, 361, 2017–2023. [Google Scholar] [CrossRef]
- Lonn, E.; Bosch, J.; Yusuf, S.; Sheridan, P.; Pogue, J.; Arnold, J.M.; Ross, C.; Arnold, A.; Sleight, P.; Probstfield, J.; et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA 2005, 293, 1338–1347. [Google Scholar] [CrossRef]
- Marchioli, R.; Levantesi, G.; Macchia, A.; Marfisi, R.M.; Nicolosi, G.L.; Tavazzi, L.; Tognoni, G.; Valagussa, F. Vitamin E increases the risk of developing heart failure after myocardial infarction: Results from the GISSI-Prevenzione trial. J. Cardiovasc. Med. 2006, 7, 347–350. [Google Scholar] [CrossRef]
- Anderson, E.J.; Kypson, A.P.; Rodriguez, E.; Anderson, C.A.; Lehr, E.J.; Neufer, P.D. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J. Am. Coll. Cardiol. 2009, 54, 1891–1898. [Google Scholar] [CrossRef]
- Schwemmlein, J.; Maack, C.; Bertero, E. Mitochondria as Therapeutic Targets in Heart Failure. Curr. Heart Fail. Rep. 2022, 19, 27–37. [Google Scholar] [CrossRef]
- Peugnet, V.; Chwastyniak, M.; Mulder, P.; Lancel, S.; Bultot, L.; Fourny, N.; Renguet, E.; Bugger, H.; Beseme, O.; Loyens, A.; et al. Mitochondrial-Targeted Therapies Require Mitophagy to Prevent Oxidative Stress Induced by SOD2 Inactivation in Hypertrophied Cardiomyocytes. Antioxidants 2022, 11, 723. [Google Scholar] [CrossRef]
- Ribeiro Junior, R.F.; Dabkowski, E.R.; Shekar, K.C.; O Connell, K.A.; Hecker, P.A.; Murphy, M.P. MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic. Biol. Med. 2018, 117, 18–29. [Google Scholar] [CrossRef]
- Goh, K.Y.; He, L.; Song, J.; Jinno, M.; Rogers, A.J.; Sethu, P.; Halade, G.V.; Rajasekaran, N.S.; Liu, X.; Prabhu, S.D.; et al. Mitoquinone ameliorates pressure overload-induced cardiac fibrosis and left ventricular dysfunction in mice. Redox Biol. 2019, 21, 101100. [Google Scholar] [CrossRef]
- Kim, S.; Song, J.; Ernst, P.; Latimer, M.N.; Ha, C.M.; Goh, K.Y.; Ma, W.; Rajasekaran, N.S.; Zhang, J.; Liu, X.; et al. MitoQ regulates redox-related noncoding RNAs to preserve mitochondrial network integrity in pressure-overload heart failure. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H682–H695. [Google Scholar] [CrossRef]
- Raizner, A.E.; Quiñones, M.A. Coenzyme Q10 for Patients With Cardiovascular Disease: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 609–619. [Google Scholar] [CrossRef]
- Rabanal-Ruiz, Y.; Llanos-González, E.; Alcain, F.J. The Use of Coenzyme Q10 in Cardiovascular Diseases. Antioxidants 2021, 10, 755. [Google Scholar] [CrossRef]
- Molyneux, S.L.; Florkowski, C.M.; George, P.M.; Pilbrow, A.P.; Frampton, C.M.; Lever, M.; Richards, A.M. Coenzyme Q10: An independent predictor of mortality in chronic heart failure. J. Am. Coll. Cardiol. 2008, 52, 1435–1441. [Google Scholar] [CrossRef]
- Fotino, A.D.; Thompson-Paul, A.M.; Bazzano, L.A. Effect of coenzyme Q10 supplementation on heart failure: A meta-analysis. Am. J. Clin. Nutr. 2013, 97, 268–275. [Google Scholar] [CrossRef]
- Jafari, M.; Mousavi, S.M.; Asgharzadeh, A.; Yazdani, N. Coenzyme Q10 in the treatment of heart failure: A systematic review of systematic reviews. Indian Heart J. 2018, 70 (Suppl. S1), S111–S117. [Google Scholar] [CrossRef]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. Q-SYMBIO Study Investigators. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef]
- De Blasio, M.J.; Huynh, K.; Qin, C.; Rosli, S.; Kiriazis, H.; Ayer, A.; Cemerlang, N.; Stocker, R.; Du, X.J.; McMullen, J.R.; et al. Therapeutic targeting of oxidative stress with coenzyme Q10 counteracts exaggerated diabetic cardiomyopathy in a mouse model of diabetes with diminished PI3K(p110α) signaling. Free Radic. Biol. Med. 2015, 87, 137–147. [Google Scholar] [CrossRef]
- Huynh, K.; Kiriazis, H.; Du, X.J.; Love, J.E.; Jandeleit-Dahm, K.A.; Forbes, J.M.; McMullen, J.R.; Ritchie, R.H. Coenzyme Q10 attenuates diastolic dysfunction, cardiomyocyte hypertrophy and cardiac fibrosis in the db/db mouse model of type 2 diabetes. Diabetologia 2012, 55, 1544–1553. [Google Scholar] [CrossRef]
- Huynh, K.; Kiriazis, H.; Du, X.J.; Love, J.E.; Gray, S.P.; Jandeleit-Dahm, K.A.; McMullen, J.R.; Ritchie, R.H. Targeting the upregulation of reactive oxygen species subsequent to hyperglycemia prevents type 1 diabetic cardiomyopathy in mice. Free Radic. Biol. Med. 2013, 60, 307–317. [Google Scholar] [CrossRef]
- Candido, R.; Forbes, J.M.; Thomas, M.C.; Thallas, V.; Dean, R.G.; Burns, W.C.; Tikellis, C.; Ritchie, R.H.; Twigg, S.M.; Cooper, M.E.; et al. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ. Res. 2003, 92, 785–792. [Google Scholar] [CrossRef]
- Kranstuber, A.L.; Del Rio, C.; Biesiadecki, B.J.; Hamlin, R.L.; Ottobre, J.; Gyorke, S.; Lacombe, V.A. Advanced glycation end product cross-link breaker attenuates diabetes-induced cardiac dysfunction by improving sarcoplasmic reticulum calcium handling. Front. Physiol. 2012, 3, 292. [Google Scholar] [CrossRef]
- Tate, M.; Higgins, G.C.; De Blasio, M.J.; Lindblom, R.; Prakoso, D.; Deo, M.; Kiriazis, H.; Park, M.; Baeza-Garza, C.D.; Caldwell, S.T.; et al. The Mitochondria-Targeted Methylglyoxal Sequestering Compound, MitoGamide, Is Cardioprotective in the Diabetic Heart. Cardiovasc. Drugs Ther. 2019, 33, 669–674. [Google Scholar] [CrossRef]
- Li, B.; Zheng, Z.; Wei, Y.; Wang, M.; Peng, J.; Kang, T.; Huang, X.; Xiao, J.; Li, Y.; Li, Z. Therapeutic effects of neuregulin-1 in diabetic cardiomyopathy rats. Cardiovasc. Diabetol. 2011, 10, 69. [Google Scholar] [CrossRef]
- Gui, C.; Zeng, Z.Y.; Chen, Q.; Luo, Y.W.; Li, L.; Chen, L.L. Neuregulin-1 Promotes Myocardial Angiogenesis in the Rat Model of Diabetic Cardiomyopathy. Cell Physiol. Biochem. 2018, 46, 2325–2334. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Hu, F.; Wang, P.; Xie, Y.; Li, F.; Guo, B. Neuregulin-4 attenuates diabetic cardiomyopathy by regulating autophagy via the AMPK/mTOR signalling pathway. Cardiovasc. Diabetol. 2022, 21, 205. [Google Scholar] [CrossRef]
- Li, B.; Yin, J.; Chang, J.; Zhang, J.; Wang, Y.; Huang, H.; Wang, W.; Zeng, X. Apelin/APJ relieve diabetic cardiomyopathy by reducing microvascular dysfunction. J. Endocrinol. 2021, 249, 1–18. [Google Scholar] [CrossRef]
- Mátyás, C.; Németh, B.T.; Oláh, A.; Hidi, L.; Birtalan, E.; Kellermayer, D.; Ruppert, M.; Korkmaz-Icöz, S.; Kökény, G.; Horváth, E.M.; et al. The soluble guanylate cyclase activator cinaciguat prevents cardiac dysfunction in a rat model of type-1 diabetes mellitus. Cardiovasc. Diabetol. 2015, 14, 145. [Google Scholar] [CrossRef]
- Maffei, A.; Cifelli, G.; Carnevale, R.; Iacobucci, R.; Pallante, F.; Fardella, V.; Fardella, S.; Hirsch, E.; Lembo, G.; Carnevale, D. PI3Kγ Inhibition Protects Against Diabetic Cardiomyopathy in Mice. Rev. Esp. Cardiol. (Engl. Ed.) 2017, 70, 16–24. [Google Scholar] [CrossRef]
- Komeno, M.; Pang, X.; Shimizu, A.; Molla, M.R.; Yasuda-Yamahara, M.; Kume, S.; Rahman, N.I.A.; Soh, J.E.C.; Nguyen, L.K.C.; Ahmat Amin, M.K.B.; et al. Cardio- and reno-protective effects of dipeptidyl peptidase III in diabetic mice. J. Biol. Chem. 2021, 296, 100761. [Google Scholar] [CrossRef]
- Hiromura, M.; Mori, Y.; Terasaki, M.; Kushima, H.; Saito, T.; Osaka, N.; Yashima, H.; Ohara, M.; Fukui, T.; Matsui, T.; et al. Glucose-dependent insulinotropic polypeptide inhibits cardiac hypertrophy and fibrosis in diabetic mice via suppression of TGF-β2. Diab. Vasc. Dis. Res. 2021, 18, 1479164121999034. [Google Scholar] [CrossRef]
- Zheng, X.; Peng, M.; Li, Y.; Wang, X.; Lu, W.; Wang, X.; Shan, Y.; Li, R.; Gao, L.; Qiu, C. Cathelicidin-related antimicrobial peptide protects against cardiac fibrosis in diabetic mice heart by regulating endothelial-mesenchymal transition. Int. J. Biol. Sci. 2019, 15, 2393–2407. [Google Scholar] [CrossRef]
- Dai, W.; Shi, J.; Gupta, R.C.; Sabbah, H.N.; Hale, S.L.; Kloner, R.A. Bendavia, a mitochondria-targeting peptide, improves postinfarction cardiac function, prevents adverse left ventricular remodeling, and restores mitochondria-related gene expression in rats. J. Cardiovasc. Pharmacol. 2014, 64, 543–553. [Google Scholar] [CrossRef]
- Sabbah, H.N.; Gupta, R.C.; Kohli, S.; Wang, M.; Hachem, S.; Zhang, K. Chronic Therapy WithElamipretide (MTP-131), a Novel Mitochondria-Targeting Peptide, Improves Left Ventricular and Mitochondrial Function in Dogs With Advanced Heart Failure. Circ. Heart Fail. 2016, 9, e002206. [Google Scholar] [CrossRef]
- Daubert, M.A.; Yow, E.; Dunn, G.; Marchev, S.; Barnhart, H.; Douglas, P.S.; O’Connor, C.; Goldstein, S.; Udelson, J.E.; Sabbah, H.N. Novel Mitochondria-Targeting Peptide in Heart Failure Treatment: A Randomized, Placebo-Controlled Trial of Elamipretide. Circ. Heart Fail. 2017, 10, e004389. [Google Scholar] [CrossRef]
- Butler, J.; Khan, M.S.; Anker, S.D.; Fonarow, G.C.; Kim, R.J.; Nodari, S.; O’Connor, C.M.; Pieske, B.; Pieske-Kraigher, E.; Sabbah, H.N.; et al. Effects of Elamipretide on Left Ventricular Function in Patients With Heart Failure With Reduced Ejection Fraction: The PROGRESS-HF Phase 2 Trial. J. Card. Fail. 2020, 26, 429–437. [Google Scholar] [CrossRef]
- Gray, S.P.; Jha, J.C.; Kennedy, K.; van Bommel, E.; Chew, P.; Szyndralewiez, C.; Touyz, R.M.; Schmidt, H.H.H.W.; Cooper, M.E.; Jandeleit-Dahm, K.A.M. Combined NOX1/4 inhibition with GKT137831 in mice provides dose-dependent reno- and atheroprotectioneven in established micro- and macrovascular disease. Diabetologia 2017, 60, 927–937. [Google Scholar] [CrossRef]
- Zeng, S.Y.; Yang, L.; Yan, Q.J.; Gao, L.; Lu, H.Q.; Yan, P.K. Nox1/4 dual inhibitor GKT137831 attenuates hypertensive cardiac remodelling associating with the inhibition of ADAM17-dependent proinflammatory cytokines-induced signalling pathways in the rats with abdominal artery constriction. Biomed. Pharmacother. 2019, 109, 1907–1914. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, N.; Zhang, Z.; Wang, F.; Xiao, J.; Ji, X. Setanaxib (GKT137831) Ameliorates Doxorubicin-Induced Cardiotoxicity by Inhibiting the NOX1/NOX4/Reactive Oxygen Species/MAPK Pathway. Front. Pharmacol. 2022, 13, 823975. [Google Scholar] [CrossRef]
- Jha, J.C.; Gray, S.P.; Barit, D.; Okabe, J.; El-Osta, A.; Namikoshi, T.; Thallas-Bonke, V.; Wingler, K.; Szyndralewiez, C.; Heitz, F.; et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J. Am. Soc. Nephrol. 2014, 25, 1237–1254. [Google Scholar] [CrossRef]
- Goh, S.Y.; Cooper, M.E. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef]
- Ma, H.; Li, S.Y.; Xu, P.; Babcock, S.A.; Dolence, E.K.; Brownlee, M.; Li, J.; Ren, J. Advanced glycation endproduct (AGE) accumulation and AGE receptor (RAGE) up-regulation contribute to the onset of diabetic cardiomyopathy. J. Cell Mol. Med. 2009, 13, 1751–1764. [Google Scholar] [CrossRef]
- Huynh, K.; Bernardo, B.C.; McMullen, J.R.; Ritchie, R.H. Diabetic cardiomyopathy: Mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol. Ther. 2014, 142, 375–415. [Google Scholar] [CrossRef]
- Piperi, C.; Goumenos, A.; Adamopoulos, C.; Papavassiliou, A.G. AGE/RAGE signalling regulation by miRNAs: Associations with diabetic complications and therapeutic potential. Int. J. Biochem. Cell Biol. 2015, 60, 197–201. [Google Scholar] [CrossRef]
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus. Circ. Res. 2019, 124, 121–141. [Google Scholar] [CrossRef]
- Bidasee, K.R.; Zhang, Y.; Shao, C.H.; Wang, M.; Patel, K.P.; Dincer, U.D.; Besch, H.R., Jr. Diabetes increases formation of advanced glycation end products on Sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes 2004, 53, 463–473. [Google Scholar] [CrossRef]
- Pei, Z.; Deng, Q.; Babcock, S.A.; He, E.Y.; Ren, J.; Zhang, Y. Inhibition of advanced glycation endproduct (AGE) rescues against streptozotocin-induced diabetic cardiomyopathy: Role of autophagy and ER stress. Toxicol. Lett. 2018, 284, 10–20. [Google Scholar] [CrossRef]
- Norton, G.R.; Candy, G.; Woodiwiss, A.J. Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation 1996, 93, 1905–1912. [Google Scholar] [CrossRef]
- Freedman, B.I.; Wuerth, J.P.; Cartwright, K.; Bain, R.P.; Dippe, S.; Hershon, K.; Mooradian, A.D.; Spinowitz, B.S. Design and baseline characteristics for the aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II). Control Clin. Trials. 1999, 20, 493–510. [Google Scholar] [CrossRef]
- Little, W.C.; Zile, M.R.; Kitzman, D.W.; Hundley, W.G.; O’Brien, T.X.; Degroof, R.C. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J. Card. Fail. 2005, 11, 191–195. [Google Scholar] [CrossRef]
- Hartog, J.W.; Willemsen, S.; van Veldhuisen, D.J.; Posma, J.L.; van Wijk, L.M.; Hummel, Y.M.; Hillege, H.L.; Voors, A.A.; BENEFICIAL investigators. Effects of alagebrium, an advanced glycation endproduct breaker, on exercise tolerance and cardiac function in patients with chronic heart failure. Eur. J. Heart Fail. 2011, 13, 899–908. [Google Scholar] [CrossRef]
- Borg, D.J.; Forbes, J.M. Targeting advanced glycation with pharmaceutical agents: Where are we now? Glycoconj. J. 2016, 33, 653–670. [Google Scholar] [CrossRef]
- De Geest, B.; Mishra, M. Role of Oxidative Stress in Diabetic Cardiomyopathy. Antioxidants 2022, 11, 784. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Butler, J.; Prato, S.D.; Ezekowitz, J.A.; Ibrahim, N.E.; Lam, C.S.; Lewis, G.D.; Marwick, T.H.; Rosenstock, J.; Tang, W.W.; et al. Rationale and Design of the Aldose Reductase Inhibition for Stabilization of Exercise Capacity in Heart Failure Trial (ARISE-HF) in Patients with High-Risk Diabetic Cardiomyopathy. Am. Heart J. 2023, 256, 25–36. [Google Scholar] [CrossRef]
- Jannapureddy, S.; Sharma, M.; Yepuri, G.; Schmidt, A.M.; Ramasamy, R. Aldose Reductase: An Emerging Target for Development of Interventions for Diabetic Cardiovascular Complications. Front. Endocrinol. 2021, 12, 636267. [Google Scholar] [CrossRef]
- Ramasamy, R.; Oates, P.J.; Schaefer, S. Aldose reductase inhibition protects diabetic and nondiabetic rat hearts from ischemic injury. Diabetes 1997, 46, 292–300. [Google Scholar] [CrossRef]
- Tracey, W.R.; Magee, W.P.; Ellery, C.A.; MacAndrew, J.T.; Smith, A.H.; Knight, D.R.; Oates, P.J. Aldose reductase inhibition alone or combined with an adenosine A(3) agonist reduces ischemic myocardial injury. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1447–H1452. [Google Scholar] [CrossRef]
- Johnson, B.F.; Nesto, R.W.; Pfeifer, M.A.; Slater, W.R.; Vinik, A.I.; Chyun, D.A.; Law, G.; Wackers, F.J.; Young, L.H. Cardiac abnormalities in diabetic patients with neuropathy: Effects of aldose reductase inhibitor administration. Diabetes Care 2004, 27, 448–454. [Google Scholar] [CrossRef]
- Quattrini, L.; La Motta, C. Aldose reductase inhibitors: 2013-present. Expert Opin. Ther. Pat. 2019, 29, 199–213. [Google Scholar] [CrossRef]
- Perfetti, R.; Yepuri, G.; Quadri, N.A.; Ghannam, A.F.; Ramasamy, R.; Shendelman, S. 1080-P: Addressing the Safety Challenges of Aldose Reductase Inhibition: Development of AT-001 for Diabetic Cardiomyopathy, a Potent and Selective Next Generation Aldose Reductase Inhibitor. Diabetes 2020, 69 (Suppl. S1), 1080-P. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, H.; Wang, X. Neuregulin-1, a microvascular endothelial derived protein, protects against myocardial ischemia-reperfusion injury (Review). Int. J. Mol. Med. 2020, 46, 925–935. [Google Scholar] [CrossRef]
- Gu, X.; Liu, X.; Xu, D.; Li, X.; Yan, M.; Qi, Y.; Yan, W.; Wang, W.; Pan, J.; Xu, Y.; et al. Cardiac functional improvement in rats with myocardial infarction by up-regulating cardiac myosin light chain kinase with neuregulin. Cardiovasc. Res. 2010, 88, 334–343. [Google Scholar] [CrossRef]
- Galindo, C.L.; Kasasbeh, E.; Murphy, A.; Ryzhov, S.; Lenihan, S.; Ahmad, F.A.; Williams, P.; Nunnally, A.; Adcock, J.; Song, Y.; et al. Anti-remodeling and anti-fibrotic effects of the neuregulin-1β glial growth factor 2 in a large animal model of heart failure. J. Am. Heart Assoc. 2014, 3, e000773. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, J.; Cheng, L.; Wu, X.; Dong, W.; Yang, X.; Li, T.; Liu, X.; Xu, Y.; Li, X.; et al. A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J. Am. Coll. Cardiol. 2010, 5, 1907–1914. [Google Scholar] [CrossRef]
- Jabbour, A.; Hayward, C.S.; Keogh, A.M.; Kotlyar, E.; McCrohon, J.A.; England, J.F.; Amor, R.; Liu, X.; Li, X.Y.; Zhou, M.D.; et al. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur. J. Heart Fail. 2011, 13, 83–92. [Google Scholar] [CrossRef]
- Lenihan, D.J.; Anderson, S.A.; Lenneman, C.G.; Brittain, E.; Muldowney, J.A.S., 3rd; Mendes, L.; Zhao, P.Z.; Iaci, J.; Frohwein, S.; Zolty, R.; et al. A Phase I, Single Ascending Dose Study of Cimaglermin Alfa (Neuregulin 1β3) in Patients With Systolic Dysfunction and Heart Failure. JACC: Basic Transl. Sci. 2016, 1, 576–586. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, Z.; Miao, Y.; Xu, Y.; Zhu, J.; Wan, Q. Changes of circulating neuregulin 4 and its relationship with 25-hydroxy vitamin D and other diabetic vascular complications in patients with diabetic peripheral neuropathy. Diabetol. Metab. Syndr. 2020, 12, 42. [Google Scholar] [CrossRef]
- Kocak, M.Z.; Aktas, G.; Atak, B.M.; Duman, T.T.; Yis, O.M.; Erkus, E.; Savli, H. Is Neuregulin-4 a predictive marker of microvascular complications in type 2 diabetes mellitus? Eur. J. Clin. Investig. 2020, 50, e13206. [Google Scholar] [CrossRef]
- Castro-Torres, Y.; Katholi, R.E. Recently Approved and Under Investigation Drugs for Treating Patients with Heart Failure. Curr. Cardiol. Rev. 2020, 16, 202–211. [Google Scholar] [CrossRef]
- Castan-Laurell, I.; Dray, C.; Valet, P. The therapeutic potentials of apelin in obesity-associated diseases. Mol. Cell Endocrinol. 2021, 529, 111278. [Google Scholar] [CrossRef]
- Wang, M.; Gupta, R.C.; Rastogi, S.; Kohli, S.; Sabbah, M.S.; Zhang, K.; Mohyi, P.; Hogie, M.; Fischer, Y.; Sabbah, H.N. Effects of acute intravenous infusion of apelin on left ventricular function in dogs with advanced heart failure. J. Card. Fail. 2013, 19, 509–516. [Google Scholar] [CrossRef]
- Barnes, G.D.; Alam, S.; Carter, G.; Pedersen, C.M.; Lee, K.M.; Hubbard, T.J.; Veitch, S.; Jeong, H.; White, A.; Cruden, N.L.; et al. Sustained cardiovascular actions of APJ agonism during renin-angiotensin system activation and in patients with heart failure. Circ. Heart Fail. 2013, 6, 482–491. [Google Scholar] [CrossRef]
- Zeng, H.; He, X.; Hou, X.; Li, L.; Chen, J.X. Apelin gene therapy increases myocardial vascular density and ameliorates diabetic cardiomyopathy via upregulation of sirtuin. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H585–H597. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Marti, C.N.; Sabbah, H.N.; Roessig, L.; Greene, S.J.; Böhm, M.; Burnett, J.C.; Campia, U.; Cleland, J.G.; Collins, S.P.; et al. Soluble guanylate cyclase: A potential therapeutic target for heart failure. Heart Fail. Rev. 2013, 18, 123–134. [Google Scholar] [CrossRef]
- Korkmaz, S.; Loganathan, S.; Mikles, B.; Radovits, T.; Barnucz, E.; Hirschberg, K.; Li, S.; Hegedüs, P.; Páli, S.; Weymann, A.; et al. Nitric oxide- and heme-independent activation of soluble guanylate cyclase attenuates peroxynitrite-induced endothelial dysfunction in rat aorta. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 70–77. [Google Scholar] [CrossRef]
- Irvine, J.C.; Ganthavee, V.; Love, J.E.; Alexander, A.E.; Horowitz, J.D.; Stasch, J.P.; Kemp-Harper, B.K.; Ritchie, R.H. The soluble guanylyl cyclase activator bay 58-2667 selectively limits cardiomyocyte hypertrophy. PLoS ONE 2012, 7, e44481. [Google Scholar] [CrossRef]
- Erdmann, E.; Semigran, M.J.; Nieminen, M.S.; Gheorghiade, M.; Agrawal, R.; Mitrovic, V.; Mebazaa, A. Cinaciguat, a soluble guanylate cyclase activator, unloads the heart but also causes hypotension in acute decompensated heart failure. Eur. Heart J. 2013, 34, 57–67. [Google Scholar] [CrossRef]
- Evgenov, O.V.; Pacher, P.; Schmidt, P.M.; Haskó, G.; Schmidt, H.H.; Stasch, J.P. NO-independent stimulators and activators of soluble guanylate cyclase: Discovery and therapeutic potential. Nat. Rev. Drug Discov. 2006, 5, 755–768. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Greene, S.J.; Butler, J.; Filippatos, G.; Lam, C.S.; Maggioni, A.P.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Kraigher-Krainer, E.; et al. Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA 2015, 314, 2251–2262. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008, 153, 6–20. [Google Scholar] [CrossRef]
- Dham, D.; Roy, B.; Gowda, A.; Pan, G.; Sridhar, A.; Zeng, X.; Thandavarayan, R.A.; Palaniyandi, S.S. 4-Hydroxy-2-nonenal, a lipid peroxidation product, as a biomarker in diabetes and its complications: Challenges and opportunities. Free Radic. Res. 2021, 55, 547–561. [Google Scholar] [CrossRef]
- Mali, V.R.; Ning, R.; Chen, J.; Yang, X.P.; Xu, J.; Palaniyandi, S.S. Impairment of aldehyde dehydrogenase-2 by 4-hydroxy-2-nonenal adduct formation and cardiomyocyte hypertrophy in mice fed a high-fat diet and injected with low-dose streptozotocin. Exp. Biol. Med. 2014, 239, 610–618. [Google Scholar] [CrossRef]
- Kang, P.; Wang, J.; Fang, D.; Fang, T.; Yu, Y.; Zhang, W.; Shen, L.; Li, Z.; Wang, H.; Ye, H.; et al. Activation of ALDH2 attenuates high glucose induced rat cardiomyocyte fibrosis and necroptosis. Free Radic. Biol. Med. 2020, 146, 198–210. [Google Scholar] [CrossRef]
- Cao, R.; Fang, D.; Wang, J.; Yu, Y.; Ye, H.; Kang, P.; Li, Z.; Wang, H.; Gao, Q. ALDH2 Overexpression Alleviates High Glucose-Induced Cardiotoxicity by Inhibiting NLRP3 Inflammasome Activation. J. Diabetes Res. 2019, 2019, 4857921. [Google Scholar] [CrossRef]
- Yang, W.; Yu, Y.T.; Jiang, C. Mitochondrial Aldehyde Dehydrogenase-2 Binding Compounds and Methods of Use Thereof. U.S. Patent 9,879,036 B2, 30 January 2018. [Google Scholar]
- Patrucco, E.; Notte, A.; Barberis, L.; Selvetella, G.; Maffei, A.; Brancaccio, M.; Marengo, S.; Russo, G.; Azzolino, O.; Rybalkin, S.D.; et al. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell 2004, 118, 375–387. [Google Scholar] [CrossRef]
- Hirsch, E.; Katanaev, V.L.; Garlanda, C.; Azzolino, O.; Pirola, L.; Silengo, L.; Sozzani, S.; Mantovani, A.; Altruda, F.; Wymann, M.P. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 2000, 287, 1049–1053. [Google Scholar] [CrossRef]
- Damilano, F.; Franco, I.; Perrino, C.; Schaefer, K.; Azzolino, O.; Carnevale, D.; Cifelli, G.; Carullo, P.; Ragona, R.; Ghigo, A.; et al. Distinct effects of leukocyte and cardiac phosphoinositide 3-kinase γ activity in pressure overload-induced cardiac failure. Circulation 2011, 123, 391–399. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Everett, B.M.; Cornel, J.H.; Lainscak, M.; Anker, S.D.; Abbate, A.; Thuren, T.; Ridker, P.M. Anti-Inflammatory Therapy With Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation 2019, 139, 1289–1299. [Google Scholar] [CrossRef]
- Everett, B.M.; Donath, M.Y.; Pradhan, A.D.; Thuren, T.; Pais, P.; Nicolau, J.C.; Glynn, R.J.; Libby, P.; Ridker, P.M. Anti-Inflammatory Therapy With Canakinumab for the Prevention and Management of Diabetes. J. Am. Coll. Cardiol. 2018, 71, 2392–2401. [Google Scholar] [CrossRef]
- Afilalo, J.; Majdan, A.A.; Eisenberg, M.J. Intensive statin therapy in acute coronary syndromes and stable coronary heart disease: A comparative meta-analysis of randomized controlled trials. Heart 2007, 93, 914–921. [Google Scholar] [CrossRef]
- Liberale, L.; Carbone, F.; Camici, G.G.; Montecucco, F. IL-1β and Statin Treatment in Patients with Myocardial Infarction and Diabetic Cardiomyopathy. J. Clin. Med. 2019, 8, 1764. [Google Scholar] [CrossRef]
- Peiro, C.; Lorenzo, O.; Carraro, R.; Sanchez-Ferrer, C.F. IL-1beta inhibition in cardiovascular complications associated to diabetes mellitus. Front. Pharmacol. 2017, 8, 363. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Liu, G.C.; Zhong, J.; Basu, R.; Chow, F.L.; Zhou, J.; Loibner, H.; Janzek, E.; Schuster, M.; Penninger, J.M.; et al. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes 2010, 59, 529–538. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Penninger, J.M. Recombinant human angiotensin-converting enzyme 2 as a new renin-angiotensin system peptidase for heart failure therapy. Curr. Heart Fail. Rep. 2011, 8, 176–183. [Google Scholar] [CrossRef]
- Medina, D.; Arnold, A.C. Angiotensin-(1-7): Translational Avenues in Cardiovascular Control. Am. J. Hypertens. 2019, 32, 1133–1142. [Google Scholar] [CrossRef]
- Zhong, J.; Basu, R.; Guo, D.; Chow, F.L.; Byrns, S.; Schuster, M.; Loibner, H.; Wang, X.H.; Penninger, J.M.; Kassiri, Z.; et al. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation 2010, 122, 717–728, 18 p following 728. [Google Scholar] [CrossRef]
- Dong, B.; Yu, Q.T.; Dai, H.Y.; Gao, Y.Y.; Zhou, Z.L.; Zhang, L.; Jiang, H.; Gao, F.; Li, S.Y.; Zhang, Y.H.; et al. Angiotensin-converting enzyme-2 overexpression improves left ventricular remodeling and function in a rat model of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2012, 59, 739–747. [Google Scholar] [CrossRef]
- Prajapati, S.C.; Chauhan, S.S. Dipeptidyl peptidase III: A multifaceted oligopeptide N-end cutter. FEBS J. 2011, 278, 3256–3276. [Google Scholar] [CrossRef]
- Pang, X.; Shimizu, A.; Kurita, S.; Zankov, D.P.; Takeuchi, K.; Yasuda-Yamahara, M.; Kume, S.; Ishida, T.; Ogita, H. Novel Therapeutic Role for Dipeptidyl Peptidase III in the Treatment of Hypertension. Hypertension 2016, 68, 630–641. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Mori, Y.; Matsui, T.; Hirano, T.; Yamagishi, S.I. GIP as a Potential Therapeutic Target for Atherosclerotic Cardiovascular Disease-A Systematic Review. Int. J. Mol. Sci. 2020, 21, 1509. [Google Scholar] [CrossRef]
- Hiromura, M.; Mori, Y.; Kohashi, K.; Terasaki, M.; Shinmura, K.; Negoro, T.; Kawashima, H.; Kogure, M.; Wachi, T.; Watanabe, R.; et al. Suppressive Effects of Glucose-Dependent Insulinotropic Polypeptide on Cardiac Hypertrophy and Fibrosis in Angiotensin II-Infused Mouse Models. Circ. J. 2016, 80, 1988–1997. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Liu, X.; Mujahid, H.; Rong, B.; Lu, Q.H.; Zhang, W.; Li, P.; Li, N.; Liang, E.S.; Wang, Q.; Tang, D.Q.; et al. Irisin inhibits high glucose-induced endothelial-to-mesenchymal transition and exerts a dose-dependent bidirectional effect on diabetic cardiomyopathy. J. Cell Mol. Med. 2018, 22, 808–822. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Ovchinnikova, T.V. Immunomodulatory and Allergenic Properties of Antimicrobial Peptides. Int. J. Mol. Sci. 2022, 23, 2499. [Google Scholar] [CrossRef]
- Zanetti, M.; Gennaro, R.; Romeo, D. Cathelicidins: A novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995, 374, 1–5. [Google Scholar] [CrossRef]
- Klyachkin, Y.M.; Idris, A.; Rodell, C.B.; Tripathi, H.; Ye, S.; Nagareddy, P.; Asfour, A.; Gao, E.; Annabathula, R.; Ratajczak, M.; et al. Cathelicidin Related Antimicrobial Peptide (CRAMP) Enhances Bone Marrow Cell Retention and Attenuates Cardiac Dysfunction in a Mouse Model of Myocardial Infarction. Stem Cell Rev. Rep. 2018, 14, 702–714. [Google Scholar] [CrossRef]
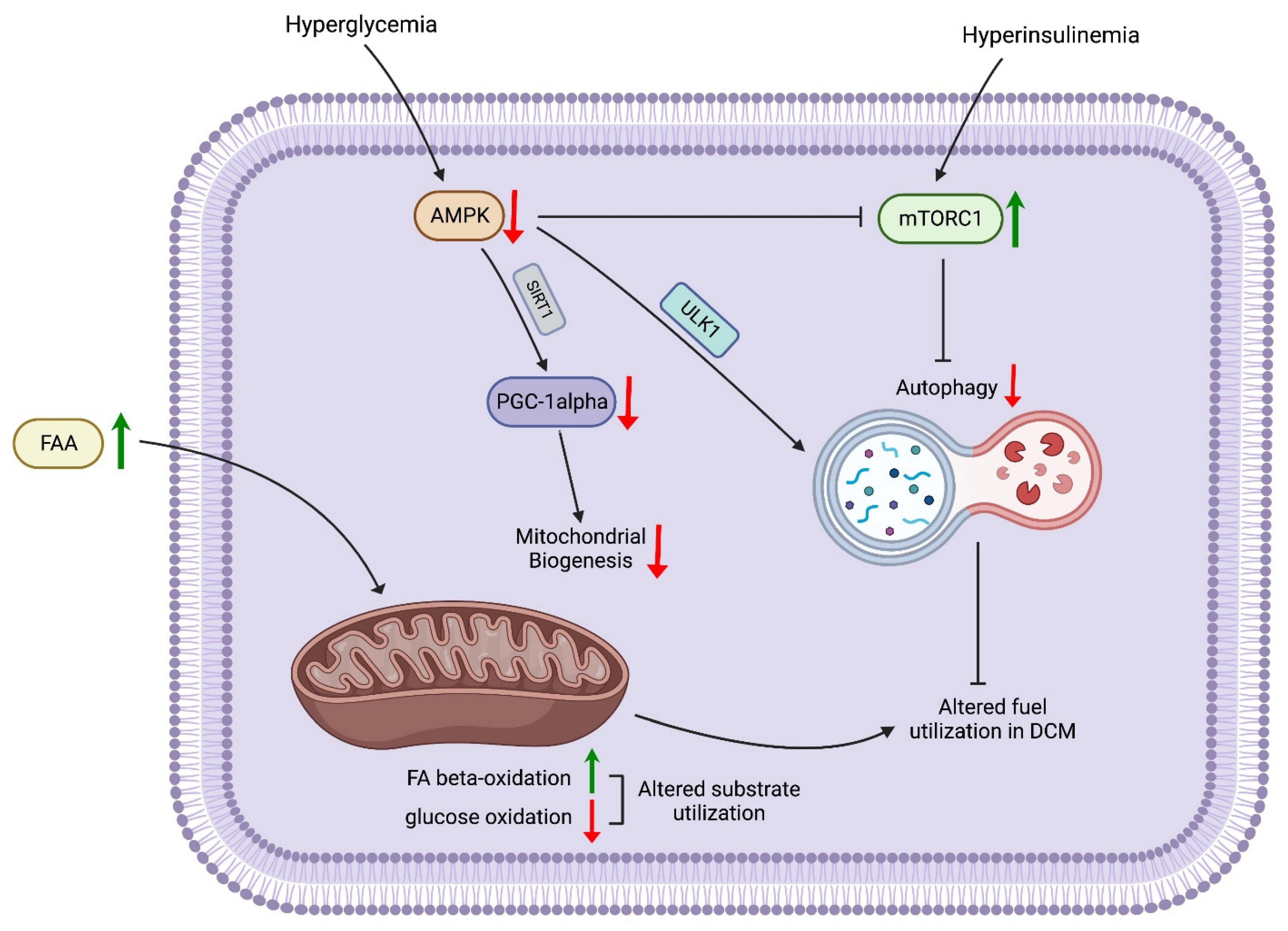
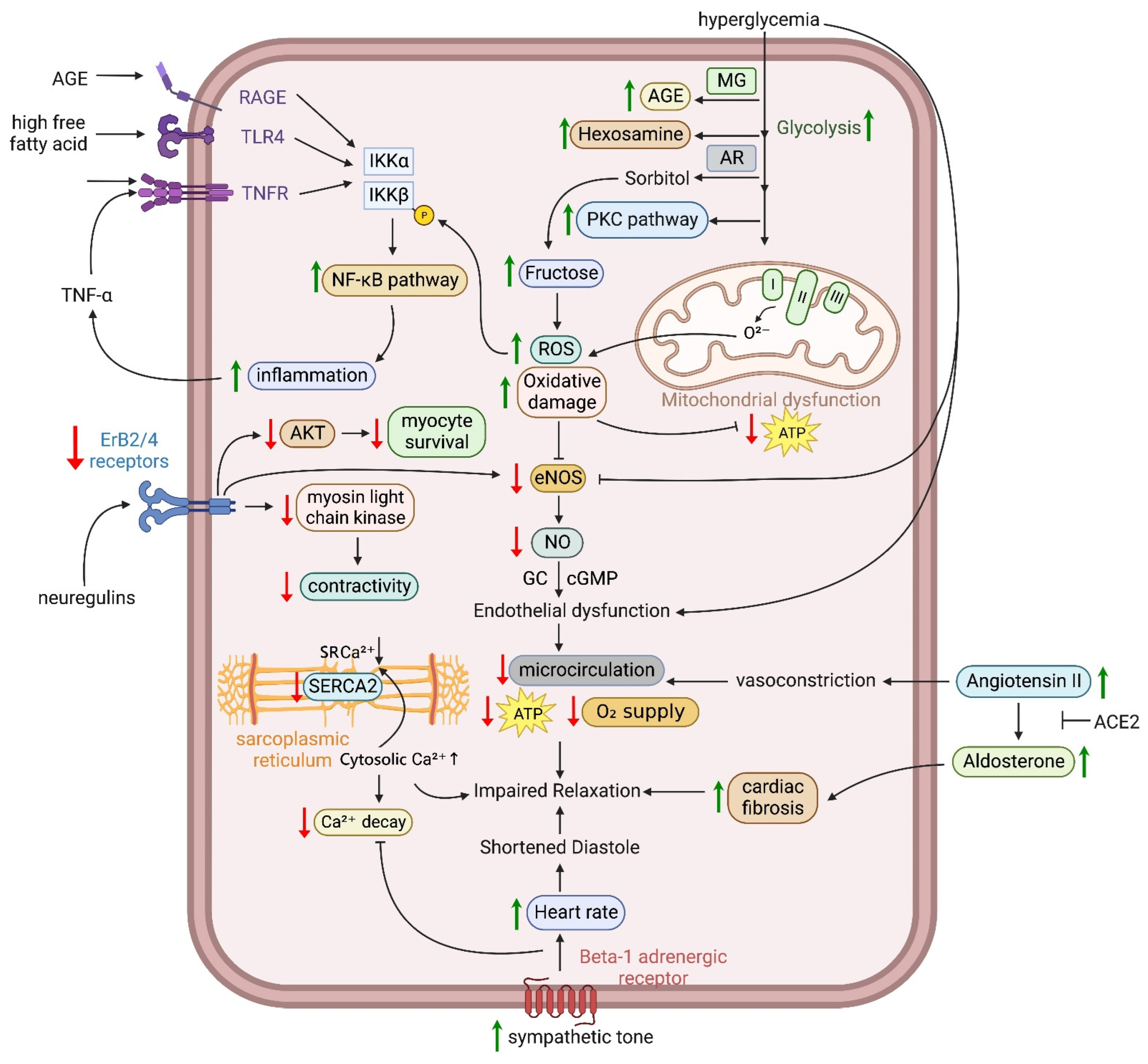
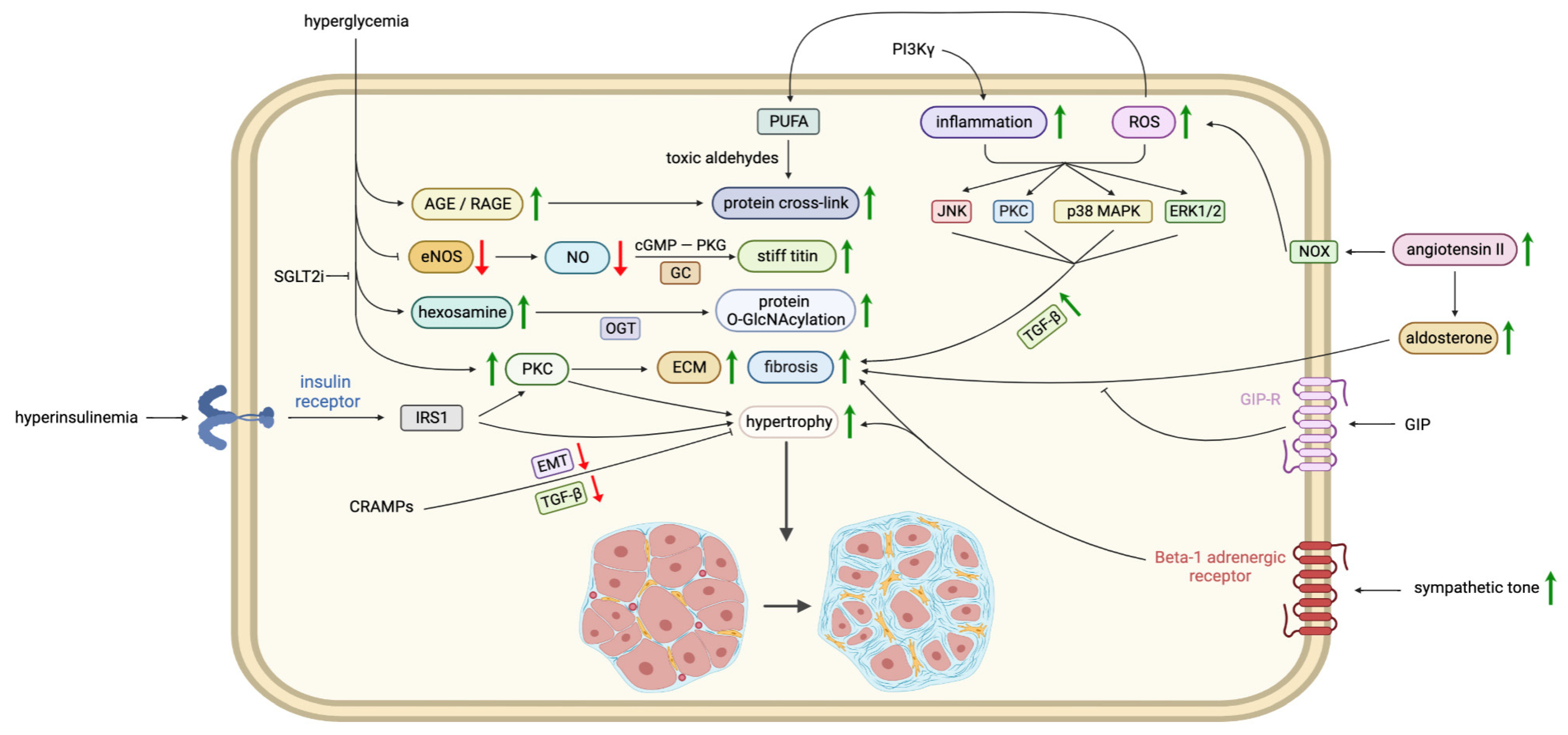
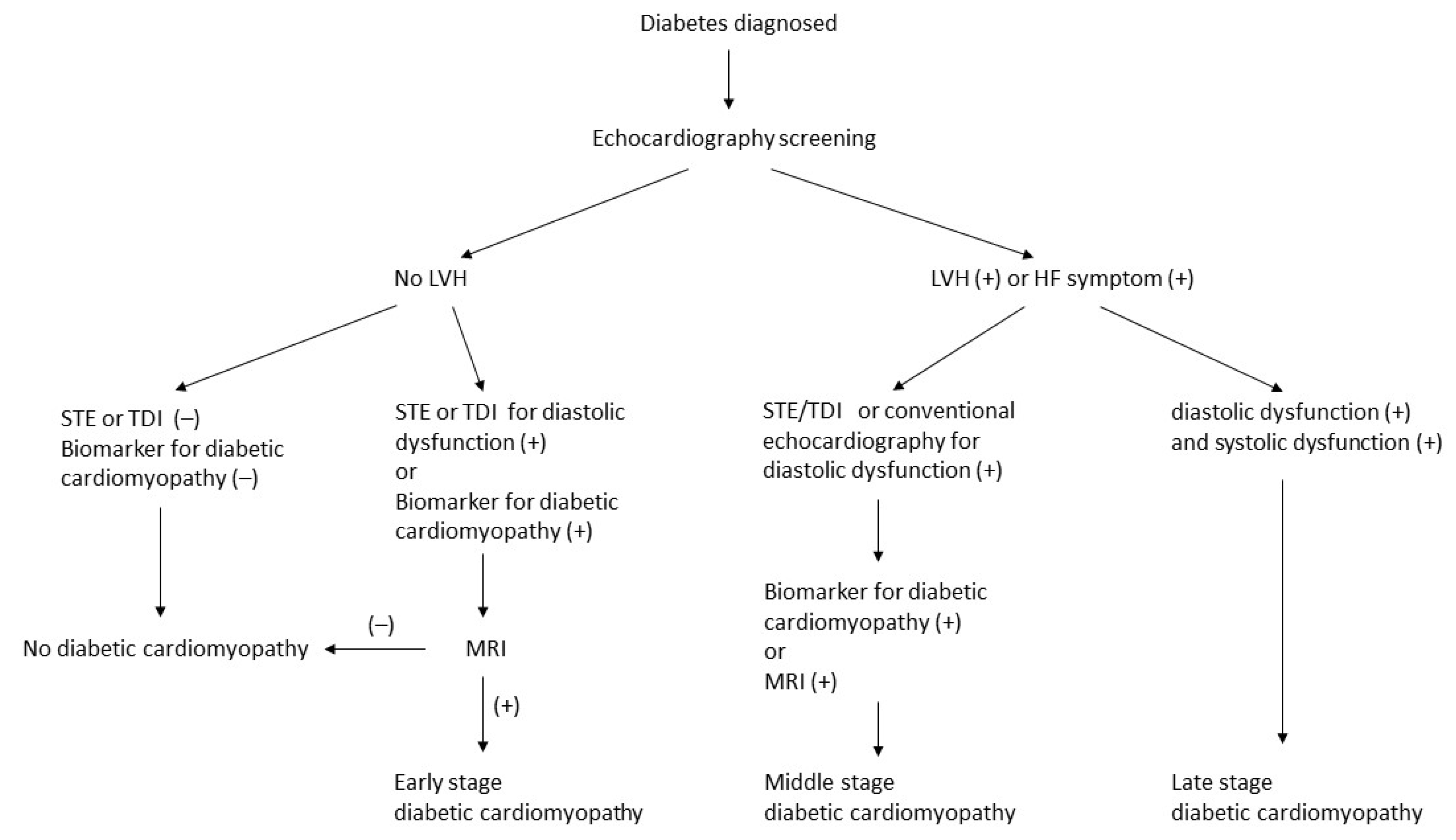
| Stages | Stage 1 (Early) | Stage 2 (Middle) | Stage 3 (Late) | Stage 4 (Late) |
|---|---|---|---|---|
| Morphological features | Normal LV wall thickness, size, and mass | Slightly increased LV wall thickness, size, and mass | Significantly increased LV wall thickness, size, and mass | Significantly increased LV wall thickness, size, and mass |
| Functional changes | Normal or slightly diastolic dysfunction | Diastolic dysfunction with normal or slightly systolic dysfunction | Diastolic and systolic dysfunction | Diastolic and systolic dysfunction |
| Cellular and metabolic abnormalities | Insulin resistance with reduced glucose uptake Increased circulating free fatty acids and fatty acid utilization Ca2+ homeostasis impairment | AGE formation Cardiomyocyte death Myocardial fibrosis Increased oxidative stress and inflammation Reduced nitric oxide Activated RAAS Mild CAN | Extensive myocardial fibrosis Microvascular changes Severe CAN | Extensive myocardial fibrosis Microvascular changes Severe CAN CAD |
| Echocardiographic methods for diagnosis | Sensitive echocardiographic techniques such as speckle-tracking echocardiography and tissue Doppler imaging | Conventional echocardiography with sensitive echocardiographic techniques | Conventional echocardiography | Conventional echocardiography |
| Promising Biomarkers | References | |
|---|---|---|
| sST2 | An interleukin-33 (IL-33) decoy receptor that ameliorates the Th2 inflammatory response. Increased plasma levels of sST2 have been linked to a worse prognosis of HF. | [179,180,181] |
| Galectin-3 | A lectin family protein that has been associated with fibrosis and inflammation in heart failure. It is elevated in diabetic patients with a mild depressed ejection fraction and is associated with a diminished global longitudinal strain. It is an easy and reproducible tool for the evaluation and follow-up of diabetic cardiomyopathy. It was approved by the FDA as a novel biomarker for predicting adverse cardiovascular events. | [182,183,184,185] |
| lncRNAs | Long noncoding RNAs (lncRNAs)are a diverse subgroup of noncoding RNAs that are epigenetic regulators of gene expression. Circulating lncRNA serum levels are associated with cardiac remodeling and are positively correlated with cardiac diastolic dysfunction. | [7,186,187] |
| GDF-15 | Expressed in response to oxidative stress and inflammation. GDF-15 levels predict diabetic cardiomyopathy. | [188,189] |
| TGF-β | A fibrogenic cytokine that promotes extracellular matrix accumulation. Higher plasma levels of TGF-β are associated with both DM and diastolic dysfunction. | [190,191,192] |
| IGFBP7 | Regulates the IGF signaling pathway and is related to insulin resistance. IGFBP7 is correlated with DM with diastolic dysfunction. | [192,193] |
| Other candidate biomarkers | ||
| Activin A | A protein produced by epicardial adipose tissue. It is correlated with both myocardial glucose metabolism abnormality and left ventricular remodeling. | [194,195] |
| H-FABP | It has an independent association with outcomes in patients with HF. It enables the detection of early myocardial injuries in patients with metabolic syndrome or DM. | [196,197,198] |
| TNF-α | The level was increased in diabetic patients with diastolic dysfunction. It might be used for the early detection of diabetic cardiomyopathy. | [192,199] |
| ICTP and PIIINP | Circulating collagen turnover biomarkers are associated with adverse cardiac outcomes and can be used to predict HF progression. | [200,201] |
| Cardiotrophin-1 | A glycoprotein 130 and a potent inducer of myocardial hypertrophy. It is correlated with blood glucose and ventricular hypertrophy. It is less specific for dilated cardiomyopathy. | [202,203] |
| Drug | Diabetic Animal Model | Mechanism | Effects | Reference |
|---|---|---|---|---|
| Trimetazidine | High-fat diet and streptozotocin induced type 2 diabetes in rats | 3-ketoacyl coenzyme A thiolase inhibitor (inhibition of β-oxidation of fatty acid) | Ameliorated metabolic disturbance and insulin resistance, ↓LVEDP, ↓cardiomyocyte apoptosis, ↓myocardial fibrosis, restored cardiac autophagy | [213] |
| Trimetazidine | Streptozotocin-induced diabetes in rats | 3-ketoacyl coenzyme A thiolase inhibitor | ↓cardiac hypertrophy and fibrosis, ↑dP/dtmax, ↓LVEDP, ↓inflammation and oxidative stress, ↓Nox2 and TRPC3 | [214] |
| Trimetazidine | Streptozotocin-induced diabetes in rats | 3-ketoacyl coenzyme A thiolase inhibitor | ↓malondialdehyde level, ↓LVEDP, ↓myocardial fibrosis, ↓ROS production | [215] |
| Istaroxime | Streptozotocin-induced diabetes in rats | Increases SERCA2a pump activity and inhibits Na+/K+ ATPase | Improved LV diastolic dysfunction, ↑SERCA2 protein level and activity, improved intracellular Ca2+ handling anomalies | [109] |
| Ranolazine | Streptozotocin-induced diabetes in rats | Inhibits the late inward sodium current | ↓cardiac hypertrophy, ↑LVEF, ↓cardiomyocyte apoptosis, ↑NOTCH1/NRG1 signaling pathway | [228] |
| Coenzyme Q10 | Streptozotocin-induced diabetes in mice | Mitochondrial ROS scavenger | ↓oxidative stress, improved LV diastolic dysfunction, ↓cardiomyocyte hypertrophy and fibrosis, ↓connective tissue growth factor and β-myosin heavy chain | [249] |
| Coenzyme Q10 | db/db mice | Mitochondrial ROS scavenger | ↓oxidative stress, improved LV diastolic dysfunction, ↓cardiomyocyte hypertrophy and fibrosis | [250] |
| Coenzyme Q10 | Streptozotocin-induced diabetes in mice | Mitochondrial ROS scavenger | ↓oxidative stress, improved LV diastolic dysfunction, improved LV remodeling (↓cardiomyocyte hypertrophy, cardiac fibrosis, and apoptosis), ↓proinflammatory mediators | [251] |
| Alagebrium | Streptozotocin-induced diabetes in rats | AGE crosslink breaker | Restored LV collagen solubility and ↓crosslinked collagen, ↓cardiac BNP, ↓cardiac AGE levels and AGE receptor, ↓connective tissue growth factor and collagen III expression | [252] |
| Alagebrium | Streptozotocin-induced diabetes in rats | AGE crosslink breaker | Partially alleviated diastolic dysfunction, ↑SERCA2a and RyR2 protein content, improved SR Ca2+ reuptake | [253] |
| MitoGamide | Akita mice | Mitochondria-targeted methylglyoxal scavenger (preventing AGE formation) | Improved LV diastolic dysfunction | [254] |
| Recombinant human neuregulin-1 | Streptozotocin-induced diabetes in rats | Signaling molecule binding to epidermal growth factor (ErbB) receptor | ↑dP/dtmax, ↓LVEDP, ↓cardiomyocyte apoptosis, ↓cardiac fibrosis, ↓collagen type I and III | [255] |
| Recombinant human neuregulin-1 | Streptozotocin-induced diabetes in rats | Signaling molecule binding to epidermal growth factor (ErbB) receptor | ↑dP/dtmax, ↑myocardial angiogenesis, ↑expression of vascular endothelial growth factor and angiopoietin-1 | [256] |
| Neuregulin-4 | Streptozotocin-induced diabetes in mice | Signaling molecule binding to epidermal growth factor (ErbB) receptor | ↓cardiac hypertrophy, ↑LVEF, ↓cardiac fibrosis and apoptosis, activated autophagy via the AMPK/mTOR signaling pathway | [257] |
| Apelin | Streptozotocin-induced diabetes in kkAy mice | Binding to APJ receptor | ↑LVEF, ↓cardiac hypertrophy and fibrosis, ↑myocardial angiogenesis, ↓microvascular dysfunction, ↓expression of adhesion molecules | [258] |
| Cinaciguat | Streptozotocin-induced diabetes in rats | Soluble guanylate cyclase activator | ↑plasma and myocardial cGMP;↓the expression of PDE-5;↑PKG activity;↑systolic function (preload recruitable stroke work) and diastolic function (tau);↓myocardial hypertrophy, apoptosis, and fibrosis;↓oxidative stress | [259] |
| AD-9308 | Streptozotocin-induced diabetes in mice | Mitochondrial aldehyde dehydrogenase 2 activator | ↓serum 4-HNE levels and 4-HNE protein adducts in cardiac tissue; improved LV diastolic and systolic function;↓myocardial fibrosis, inflammation, and apoptosis; improved mitochondrial function, SR Ca2+ handling, and autophagy regulation | [81] |
| GE21 | Streptozotocin-induced diabetes in mice | Inhibitor of PI3Kγ | Improved systolic and diastolic dysfunction on echocardiography, ↓cardiac inflammation and fibrosis | [260] |
| Angiotensin 1–7 | db/db mice | Negative regulators of the renin–angiotensin system | Improved diastolic dysfunction, ↓myocardial hypertrophy and fibrosis, ↓lipotoxicity, ↓adipose inflammation, ↓myocardial oxidative stress, ↑phosphorylation of myocardial Erk1/2, ↓expression of myocardial protein kinase Cα and PKCβ1, ↑adipose triacylglycerol lipase | [146] |
| Dipeptidyl peptidase III | db/db mice | Cleavage of a cytotoxic peptide that is a fragment of complement component 3 | Improved diastolic dysfunction, ↓cardiac inflammation and fibrosis | [261] |
| GIP | db/db mice | Suppression of TGF-β2 | ↓cardiac hypertrophy and fibrosis, ↓TGF-β pathway, ↓myosin heavy chain β and TGF-β2 | [262] |
| CRAMP | Streptozotcin induced diabetes in mice | Inhibits endothelial-to-mesenchymal transition | Improved systolic and diastolic dysfunction, ↓cardiac fibrosis, ↓endothelial-to-mesenchymal transition, ↓TGF-β/Smad signaling, ↓AMPKa1 signaling | [263] |
| Drug | Mechanism | Phase of Development | ClinicalTrials.gov Identifier |
|---|---|---|---|
| AT-001 | Aldose reductase inhibitor | Phase III | NCT04083339 |
| Trimetazidine | 3-ketoacyl coenzyme A thiolase inhibitor | Phase I | NCT05556005 |
| Dapagliflozin | Sodium–glucose transporter-2 inhibitor | Phase IV | NCT04200586 |
| Dapagliflozin | Sodium–glucose transporter-2 inhibitor | Not applicable | NCT04591639 |
| Dapagliflozin | Sodium–glucose transporter-2 inhibitor | Phase II | NCT04304560 |
| Eplerenone | Mineralocorticoid receptor antagonist | Phase I | NCT01794091 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsuan, C.-F.; Teng, S.I.F.; Hsu, C.-N.; Liao, D.; Chang, A.J.-W.; Lee, H.-L.; Hee, S.-W.; Chang, Y.-C.; Chuang, L.-M. Emerging Therapy for Diabetic Cardiomyopathy: From Molecular Mechanism to Clinical Practice. Biomedicines 2023, 11, 662. https://doi.org/10.3390/biomedicines11030662
Hsuan C-F, Teng SIF, Hsu C-N, Liao D, Chang AJ-W, Lee H-L, Hee S-W, Chang Y-C, Chuang L-M. Emerging Therapy for Diabetic Cardiomyopathy: From Molecular Mechanism to Clinical Practice. Biomedicines. 2023; 11(3):662. https://doi.org/10.3390/biomedicines11030662
Chicago/Turabian StyleHsuan, Chin-Feng, Sean I. F. Teng, Chih-Neng Hsu, Daniel Liao, Allen Jiun-Wei Chang, Hsiao-Lin Lee, Siow-Wey Hee, Yi-Cheng Chang, and Lee-Ming Chuang. 2023. "Emerging Therapy for Diabetic Cardiomyopathy: From Molecular Mechanism to Clinical Practice" Biomedicines 11, no. 3: 662. https://doi.org/10.3390/biomedicines11030662
APA StyleHsuan, C.-F., Teng, S. I. F., Hsu, C.-N., Liao, D., Chang, A. J.-W., Lee, H.-L., Hee, S.-W., Chang, Y.-C., & Chuang, L.-M. (2023). Emerging Therapy for Diabetic Cardiomyopathy: From Molecular Mechanism to Clinical Practice. Biomedicines, 11(3), 662. https://doi.org/10.3390/biomedicines11030662





