A Preliminary Study on Microbiota Characteristics of Bronchoalveolar Lavage Fluid in Patients with Pulmonary Nodules Based on Metagenomic Next-Generation Sequencing
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Sample Collection and DNA Extraction
2.3. Library Preparation and Sequencing
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Subjects
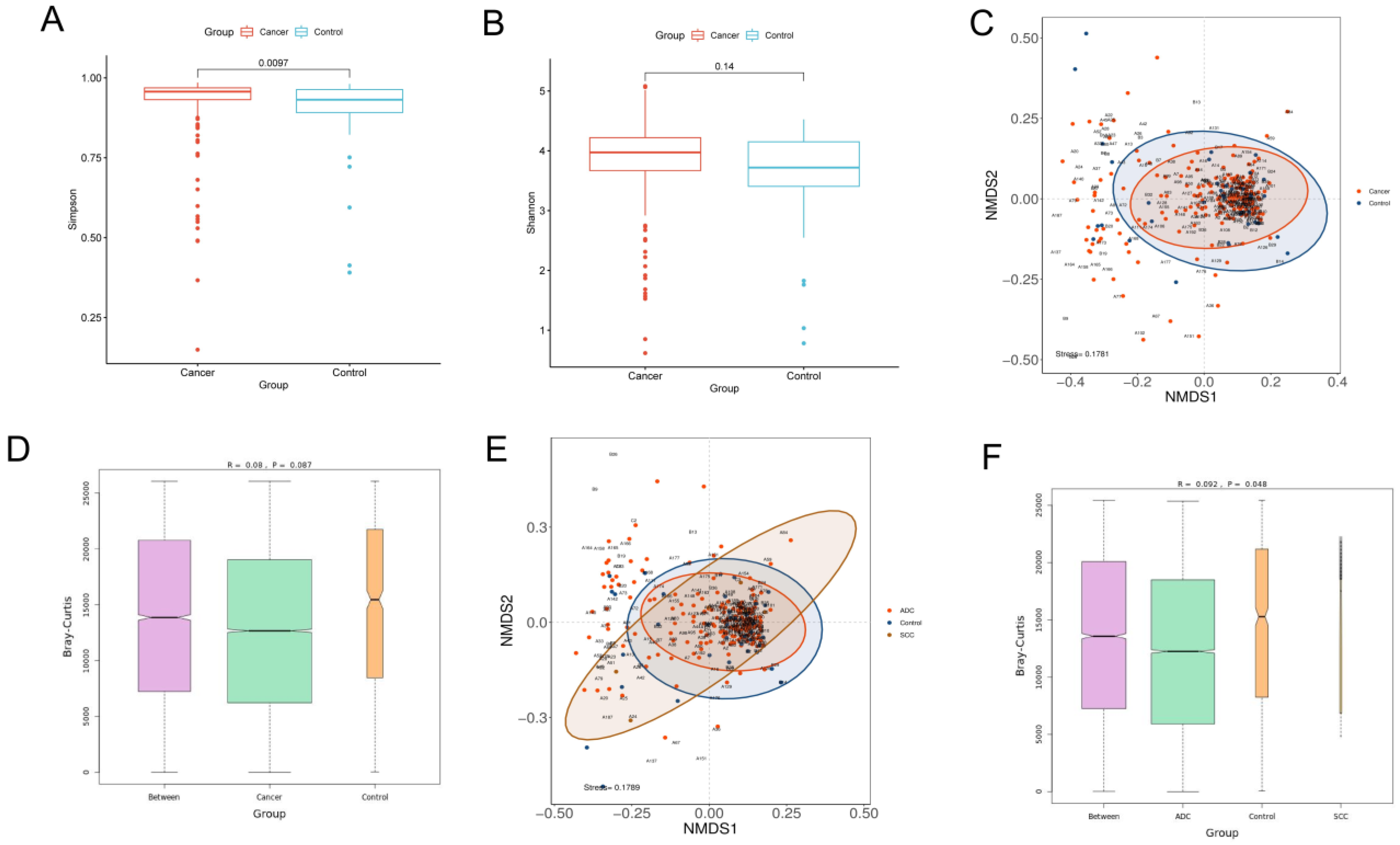
3.2. Biodiversity between Lung Cancer and Benign Lesions
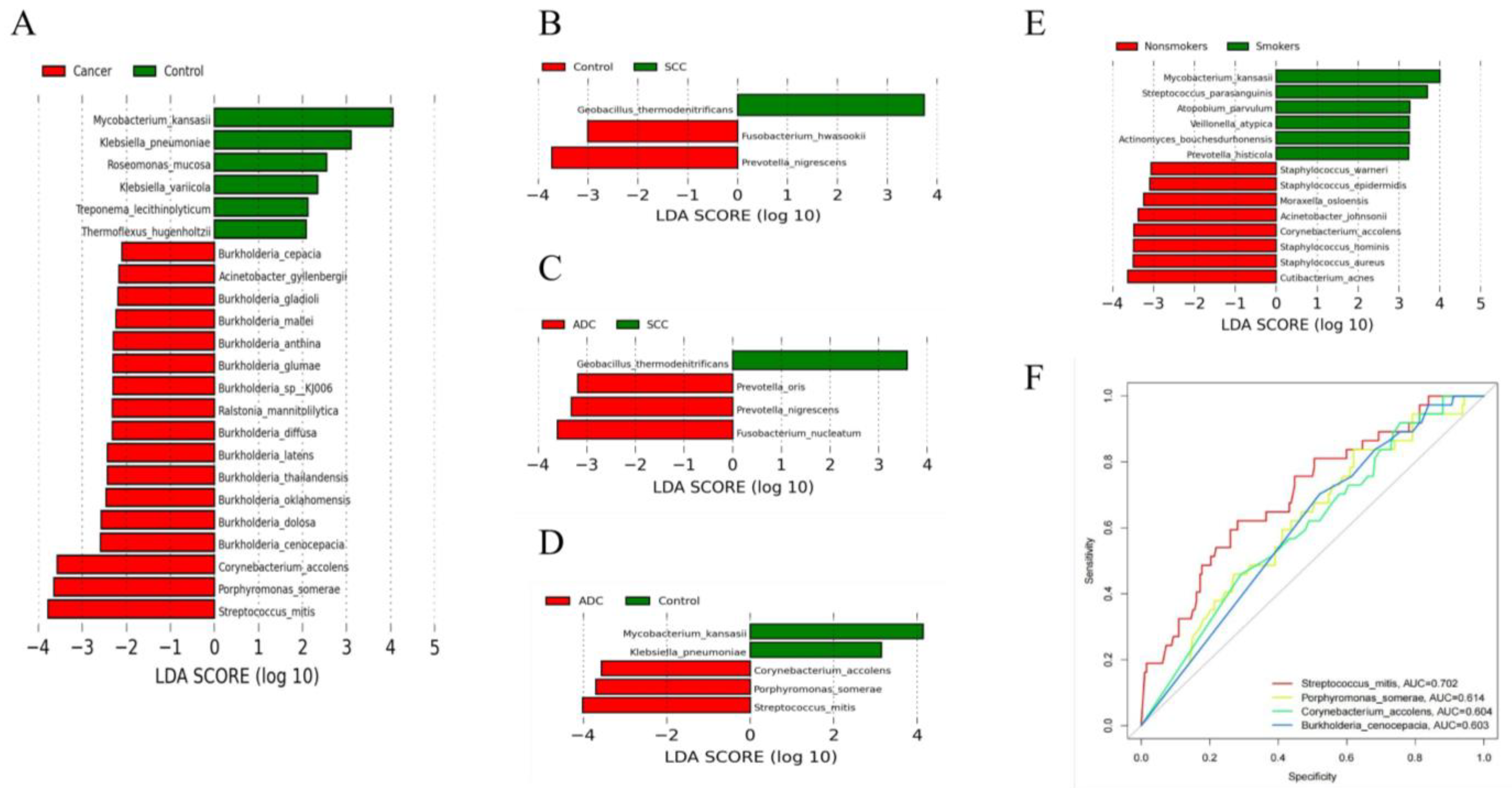
3.3. Analysis of Differences in the Microbiota Community between Lung Cancer and Benign Lesions
3.4. Potential Microbe Biomarkers for Lung Cancer
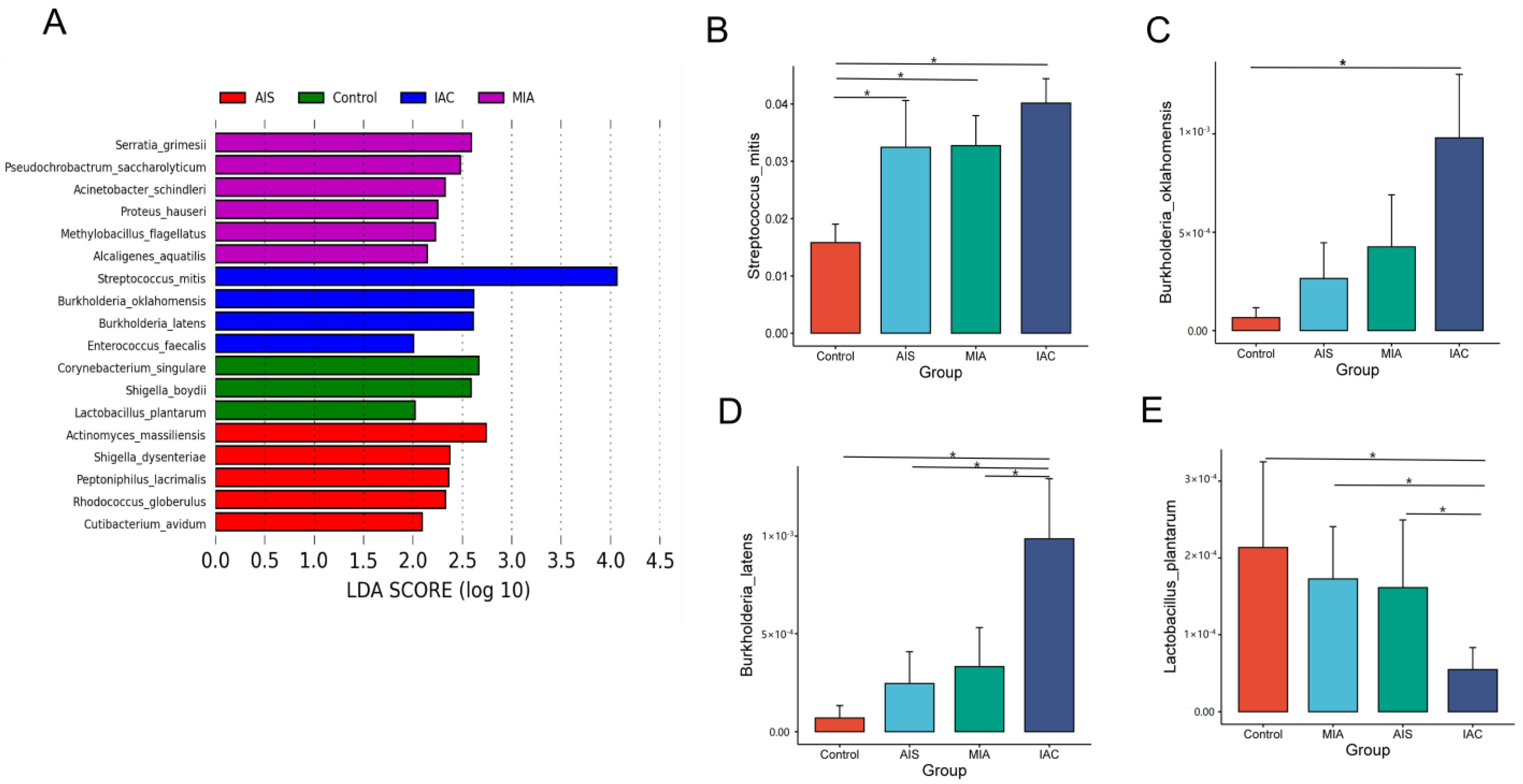
3.5. Differences in Microbiota According to Different Histopathological Lung Adenocarcinomas
3.5.1. Biodiversity Analysis
3.5.2. Significant Differential Microbiota Compositions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Brownlee, A.R.; Donington, J.S. Update on Lung Cancer Screening. Semin. Respir. Crit. Care Med. 2020, 41, 447–452. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Wagner, G.; Stollenwerk, H.K.; Klerings, I.; Pecherstorfer, M.; Gartlehner, G.; Singer, J. Efficacy and safety of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer (NSCLC): A systematic literature review. Oncoimmunology 2020, 9, 1774314. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef]
- Garrett, W.S. The gut microbiota and colon cancer. Science 2019, 364, 1133–1135. [Google Scholar] [CrossRef]
- Khatoon, J.; Rai, R.P.; Prasad, K.N. Role of Helicobacter pylori in gastric cancer: Updates. World J. Gastrointest. Oncol. 2016, 8, 147–158. [Google Scholar] [CrossRef]
- Perrone, F.; Belluomini, L.; Mazzotta, M.; Bianconi, M.; Di Noia, V.; Meacci, F.; Montrone, M.; Pignataro, D.; Prelaj, A.; Rinaldi, S.; et al. Exploring the role of respiratory microbiome in lung cancer: A systematic review. Crit. Rev. Oncol. 2021, 164, 103404. [Google Scholar] [CrossRef]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef]
- Jin, J.; Gan, Y.; Liu, H.; Wang, Z.; Yuan, J.; Deng, T.; Zhou, Y.; Zhu, Y.; Zhu, H.; Yang, S.; et al. Diminishing microbiome richness and distinction in the lower respiratory tract of lung cancer patients: A multiple comparative study design with independent validation. Lung Cancer 2019, 136, 129–135. [Google Scholar] [CrossRef]
- Lee, S.H.; Sung, J.Y.; Yong, D.; Chun, J.; Kim, S.Y.; Song, J.H.; Chung, K.S.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; et al. Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer comparing with benign mass like lesions. Lung Cancer 2016, 102, 89–95. [Google Scholar] [CrossRef]
- Zhuo, M.; An, T.; Zhang, C.; Wang, Z. Characterization of Microbiota in Cancerous Lung and the Contralateral Non-Cancerous Lung Within Lung Cancer Patients. Front. Oncol. 2020, 10, 1584. [Google Scholar] [CrossRef]
- Huang, D.; Su, X.; Yuan, M.; Zhang, S.; He, J.; Deng, Q.; Qiu, W.; Dong, H.; Cai, S. The characterization of lung microbiome in lung cancer patients with different clinicopathology. Am. J. Cancer Res. 2019, 9, 2047–2063. [Google Scholar]
- Li, Y.; Sun, B.; Tang, X.; Liu, Y.-L.; He, H.-Y.; Li, X.-Y.; Wang, R.; Guo, F.; Tong, Z.-H. Application of metagenomic next-generation sequencing for bronchoalveolar lavage diagnostics in critically ill patients. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 369–374. [Google Scholar] [CrossRef]
- Garzoni, C.; Brugger, S.D.; Qi, W.; Wasmer, S.; Cusini, A.; Dumont, P.; Gorgievski-Hrisoho, M.; Mühlemann, K.; von Garnier, C.; Hilty, M. Microbial communities in the respiratory tract of patients with interstitial lung disease. Thorax 2013, 68, 1150–1156. [Google Scholar] [CrossRef]
- Miao, Q.; Ma, Y.; Wang, Q.; Pan, J.; Zhang, Y.; Jin, W.; Yao, Y.; Su, Y.; Huang, Y.; Wang, M.; et al. Microbiological Diagnostic Performance of Metagenomic Next-generation Sequencing When Applied to Clinical Practice. Clin. Infect. Dis. 2018, 67, S231–S240. [Google Scholar] [CrossRef]
- Goto, T. Airway Microbiota as a Modulator of Lung Cancer. Int. J. Mol. Sci. 2020, 21, 3044. [Google Scholar] [CrossRef]
- Zeng, W.; Zhao, C.; Yu, M.; Chen, H.; Pan, Y.; Wang, Y.; Bao, H.; Ma, H.; Ma, S. Alterations of lung microbiota in patients with non-small cell lung cancer. Bioengineered 2022, 13, 6665–6677. [Google Scholar] [CrossRef]
- Shimizu, M.; Miyanaga, A.; Seike, M.; Matsuda, K.; Matsumoto, M.; Noro, R.; Fujita, K.; Mano, Y.; Furuya, N.; Kubota, K.; et al. The respiratory microbiome associated with chronic obstructive pulmonary disease comorbidity in non-small cell lung cancer. Thorac. Cancer 2022, 13, 1940–1947. [Google Scholar] [CrossRef]
- Wang, K.; Huang, Y.; Zhang, Z.; Liao, J.; Ding, Y.; Fang, X.; Liu, L.; Luo, J.; Kong, J. A Preliminary Study of Microbiota Diversity in Saliva and Bronchoalveolar Lavage Fluid from Patients with Primary Bronchogenic Carcinoma. Med. Sci. Monit. 2019, 25, 2819–2834. [Google Scholar] [CrossRef]
- Cameron, S.J.S.; Lewis, K.E.; Huws, S.A.; Hegarty, M.J.; Lewis, P.D.; Pachebat, J.A.; Mur, L.A.J. A pilot study using metagenomic sequencing of the sputum microbiome suggests potential bacterial biomarkers for lung cancer. PLoS ONE 2017, 12, e0177062. [Google Scholar] [CrossRef]
- Hosgood, H.D., 3rd; Sapkota, A.R.; Rothman, N.; Rohan, T.; Hu, W.; Xu, J.; Vermeulen, R.; He, X.; White, J.R.; Wu, G.; et al. The potential role of lung microbiota in lung cancer attributed to household coal burning exposures. Environ. Mol. Mutagen. 2014, 55, 643–651. [Google Scholar] [CrossRef]
- Liu, Y.; O’Brien, J.L.; Ajami, N.J.; Scheurer, M.E.; Amirian, E.S.; Armstrong, G.; Tsavachidis, S.; Thrift, A.P.; Jiao, L.; Wong, M.C.; et al. Lung tissue microbial profile in lung cancer is distinct from emphysema. Am. J. Cancer Res. 2018, 8, 1775–1787. [Google Scholar]
- Gomes, S.; Cavadas, B.; Ferreira, J.C.; Marques, P.I.; Monteiro, C.; Sucena, M.; Sousa, C.; Vaz Rodrigues, L.; Teixeira, G.; Pinto, P.; et al. Profiling of lung microbiota discloses differences in adenocarcinoma and squamous cell carcinoma. Sci. Rep. 2019, 9, 12838. [Google Scholar] [CrossRef]
- Cheng, M.; Hu, S. Lung-resident gammadelta T cells and their roles in lung diseases. Immunology 2017, 151, 375–384. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, E.S.; Zhao, C.; Jin, C. Host-Microbiome Interaction in Lung Cancer. Front. Immunol. 2021, 12, 679829. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J. Thorac. Oncol. 2015, 10, 1240–1242. [Google Scholar] [CrossRef]
- Aviles-Jimenez, F.; Vazquez-Jimenez, F.; Medrano-Guzman, R.; Mantilla, A.; Torres, J. Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Sci. Rep. 2014, 4, 4202. [Google Scholar] [CrossRef]
- Flemer, B.; Lynch, D.B.; Brown, J.M.R.; Jeffery, I.B.; Ryan, F.J.; Claesson, M.J.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 2017, 66, 633–643. [Google Scholar] [CrossRef]
- Liu, H.-X.; Tao, L.-L.; Zhang, J.; Zhu, Y.-G.; Zheng, Y.; Liu, D.; Zhou, M.; Ke, H.; Shi, M.-M.; Qu, J.-M. Difference of lower airway microbiome in bilateral protected specimen brush between lung cancer patients with unilateral lobar masses and control subjects. Int. J. Cancer 2018, 142, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Toller, I.M.; Neelsen, K.J.; Steger, M.; Hartung, M.L.; Hottiger, M.O.; Stucki, M.; Kalali, B.; Gerhard, M.; Sartori, A.A.; Lopes, M.; et al. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc. Natl. Acad. Sci. USA 2011, 108, 14944–14949. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, R.; de Lima, S.G.; Liu, Y.; Mihara, K.; Saifeddine, M.; Noorbakhsh, F.; Scarisbrick, I.A.; Hollenberg, M.D. Microenvironment proteinases, proteinase-activated receptor regulation, cancer and inflammation. Biol. Chem. 2018, 399, 1023–1039. [Google Scholar] [CrossRef] [PubMed]


| Variables | Lung Cancer (n = 192) | Benign Lesions (n = 37) | p |
|---|---|---|---|
| Age | 55.24 (12.94) | 49.32 (12.30) | 0.014 |
| Sex | 0.131 | ||
| Male | 78 (40.6) | 20 (54.1) | |
| Female | 114 (59.4) | 17 (45.9) | |
| BMI | 23.55 (3.63) | 24.95 (12.09) | 0.489 |
| Pulmonary function test | (n = 80) | (n = 14) | |
| FVC (%) | 98.70 (18.67) | 105.02 (12.22) | 0.226 |
| FEV1 (%) | 97.17 (8.6) | 98.75 (11.52) | 0.55 |
| DLCO (%) | 86.8 (14.14) | 79.1 (22.65) | 0.095 |
| Smoking status | |||
| Never | 153 (79.7) | 30 (81.1) | 0.846 |
| Ever | 39 (20.3) | 7 (18.9) | |
| Histology | |||
| ADC | 183 (95.3) | - | - |
| SCC | 6 (3) | - | - |
| Others | 3 (1.6) | - | - |
| Tumor stage | |||
| 0 | 31 (16.15) | - | - |
| I | 135 (70.31) | - | - |
| II | 6 (3.13) | - | - |
| III | 11 (5.7) | - | - |
| IV | 7 (3.6) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Q.; Wang, X.; Li, Z.; Guo, W.; Cheng, H.; Cao, Q. A Preliminary Study on Microbiota Characteristics of Bronchoalveolar Lavage Fluid in Patients with Pulmonary Nodules Based on Metagenomic Next-Generation Sequencing. Biomedicines 2023, 11, 631. https://doi.org/10.3390/biomedicines11020631
Yuan Q, Wang X, Li Z, Guo W, Cheng H, Cao Q. A Preliminary Study on Microbiota Characteristics of Bronchoalveolar Lavage Fluid in Patients with Pulmonary Nodules Based on Metagenomic Next-Generation Sequencing. Biomedicines. 2023; 11(2):631. https://doi.org/10.3390/biomedicines11020631
Chicago/Turabian StyleYuan, Qian, Xiaojin Wang, Zhanglin Li, Wenzhuo Guo, Hua Cheng, and Qingdong Cao. 2023. "A Preliminary Study on Microbiota Characteristics of Bronchoalveolar Lavage Fluid in Patients with Pulmonary Nodules Based on Metagenomic Next-Generation Sequencing" Biomedicines 11, no. 2: 631. https://doi.org/10.3390/biomedicines11020631
APA StyleYuan, Q., Wang, X., Li, Z., Guo, W., Cheng, H., & Cao, Q. (2023). A Preliminary Study on Microbiota Characteristics of Bronchoalveolar Lavage Fluid in Patients with Pulmonary Nodules Based on Metagenomic Next-Generation Sequencing. Biomedicines, 11(2), 631. https://doi.org/10.3390/biomedicines11020631







