An Overview of Hepatocellular Carcinoma Surveillance Focusing on Non-Cirrhotic NAFLD Patients: A Challenge for Physicians
Abstract
1. Introduction
2. Research Strategy and Study Selection
3. Differences between HCC across Aetiologies
3.1. HCC in Patients with Viral Hepatitis
3.2. HCC in Patients with Alcoholic and Non-Alcoholic Liver Disease
3.3. HCC Clinical Features and Survival across Different Aetiologies
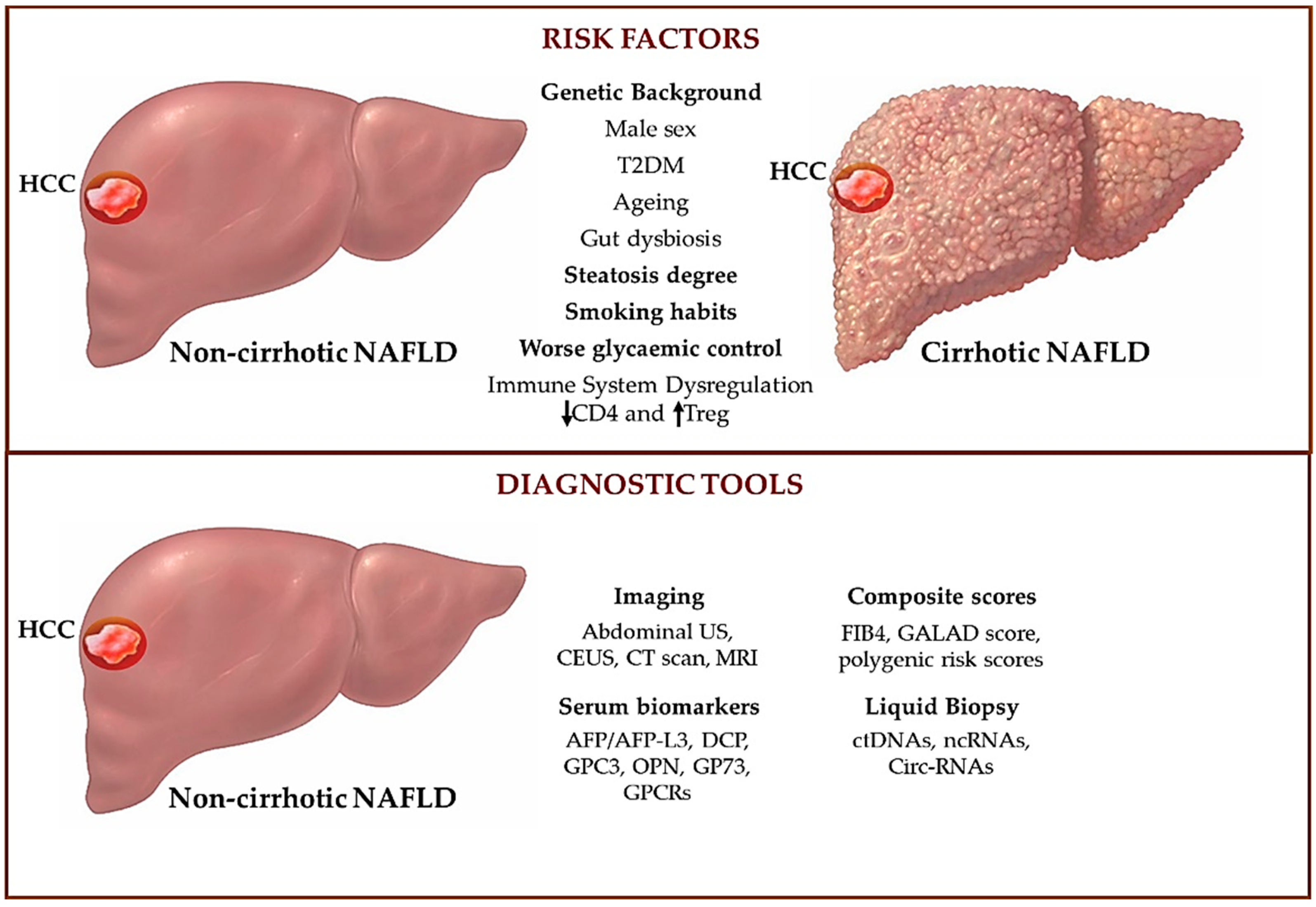
4. HCC Onset in Cirrhotic and Non-Cirrhotic NAFLD Patients
4.1. Shared Predisposing Factors Triggering NAFLD-HCC in Cirrhotic and Non-Cirrhotic Patients
4.2. Risk Factors That Discriminate HCC Onset in Cirrhotic and Non-Cirrhotic NAFLD Patients
5. Tumor Surveillance in HCC
5.1. Abdominal Ultrasound for the Screening of HCC
5.2. Alternative Imaging Approaches for the Screening of NAFLD-HCC
5.3. Alpha-Fetoprotein Assessment for the Screening of HCC
5.4. Novel Strategies to Non-Invasively Assess NAFLD-HCC: Proteins and Receptors
5.5. Novel Strategies to Non-Invasively Assess NAFLD-HCC: Cell-Circulating Tumor DNA and Non-Coding RNAs
6. New Proposed Scores to Estimate the Risk of NAFLD-HCC
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AASLD | American Association for the Study of Liver Disease |
| AFP | alpha-fetoprotein |
| ALD | alcoholic liver disease |
| ALT | alanine aminotransferase |
| APOB | apolipoprotein B |
| AST | aspartate aminotransferase |
| AUROC | area under receiver operating characteristic curve |
| BMI | body mass index |
| CAP | controlled attenuation parameter |
| CCRK | cycle-related kinase |
| ctDNAs | cell-circulating tumor DNAs |
| CLD | chronic liver disease |
| CEUS | contrast-enhanced ultrasound |
| Circ-RNAs | circular non-coding RNAs |
| CT | computed tomography |
| DAAs | direct antiviral agents |
| DCP | des-gamma carboxyprothrombin |
| DPP4 | dipeptidyl peptidase 4 |
| EASL | European Association for the Study of the Liver |
| ECM | extracellular matrix |
| ELF | enhanced liver fibrosis |
| ER | endoplasmic reticulum |
| FFAs | free fatty acids |
| FIB-4 | fibrosis-4 index |
| GCKR | glucokinase regulator |
| GP73 | Golgi protein-73 |
| GPC3 | glypican-3 |
| GPCRs | G protein-coupled receptors |
| HA | hyaluronic acid |
| HBV | hepatitis B virus |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| HIF1α | hypoxia-inducible factor-1a |
| HIV | human immunodeficiency virus |
| HR | hazard ratio |
| HSCs | hepatic stellate cells |
| HSD17B13 | 17beta-hydroxysteroid dehydrogenase type 13 |
| ICB | immune checkpoint blockade |
| IFG | impaired fasting glucose |
| IFN-γ | interferon-gamma |
| IL | interleukin |
| IR | insulin resistance |
| Li-RADS | Liver Imaging Reporting and Data System |
| lncRNAs | long non-coding RNAs |
| LSM | liver stiffness measurement |
| MBOAT7 | membrane-bound O-acyltransferase domain-containing 7 |
| miRNA | microRNA |
| MRE | miRNA response elements |
| MRI | magnetic resonance imaging |
| MTA1 | metastasis-associated protein 1 |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic fatty liver disease |
| ncRNAs | non-coding RNAs |
| NFS | NAFLD fibrosis score |
| NITs | non-invasive tests |
| NLR | neutrophil-lymphocyte ratio |
| NTS | neurotensin |
| OPN | osteopontin |
| PD-1 | programmed cell death-1 |
| PDC1 | programmed cell death 1 |
| PDL | programmed death ligand |
| PIVKA II | pro-thrombin induced by vitamin K absence-II |
| PLT | platelets |
| PNPLA3 | patatin-like phospholipase domain-containing 3 |
| PRS | polygenic risk score |
| PSRC1 | proline/serine-rich coiled-coil protein 1 |
| P3NP | pro-peptide of type 3 procollagen |
| RCT | randomized clinical trial |
| ROS | reactive oxygen species |
| SGLT2 | sodium-glucose cotransporter-2 |
| SNP | single-nucleotide polymorphisms |
| SVR | sustained virological response |
| TE | transient elastography |
| TERT | telomerase reverse transcriptase |
| TIMP1 | tissue inhibitor of matrix metalloproteinase type 1 |
| TM6SF2 | transmembrane 6 superfamily member 2 |
| T2DM | type 2 diabetes mellitus |
| TNFs | tumor necrosis factor superfamily |
| Tregs | T regulatory |
| US | ultrasound |
| VCTE | vibration-controlled transient elastography |
| VEGF | vascular endothelial growth factor |
References
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes. Facts 2016, 9, 65–90. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 20, 2809–2817.e2828. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef]
- Pais, R.; Maurel, T. Natural History of NAFLD. J. Clin. Med. 2021, 10, 1161. [Google Scholar] [CrossRef]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Sharma, R. Cancer Risk in Patients With Biopsy-Confirmed Nonalcoholic Fatty Liver Disease: A Population-Based Cohort Study. Hepatology 2021, 74, 2410–2423. [Google Scholar] [CrossRef]
- Mittal, S.; El-Serag, H.B.; Sada, Y.H.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2016, 14, 124–131.e121. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Meroni, M. Genetics, Immunity and Nutrition Boost the Switching from NASH to HCC. Biomedicines 2021, 9, 1524. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Paolini, E.; Tria, G.; Ripolone, M.; Napoli, L.; Moggio, M.; Fracanzani, A.L.; Dongiovanni, P. Expanding the phenotypic spectrum of non-alcoholic fatty liver disease and hypertriglyceridemia. Front. Nutr. 2022, 9, 967899. [Google Scholar] [CrossRef] [PubMed]
- Hirsova, P.; Ibrabim, S.H.; Gores, G.J.; Malhi, H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: Relevance to NASH pathogenesis. J. Lipid Res. 2016, 57, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lee, G.; Heo, S.Y.; Roh, Y.S. Oxidative Stress Is a Key Modulator in the Development of Nonalcoholic Fatty Liver Disease. Antioxidants 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. 2019, 76, 99–128. [Google Scholar] [CrossRef] [PubMed]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression From NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Yang, Y.M.; Kim, S.Y.; Seki, E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin. Liver Dis. 2019, 39, 26–42. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M. Genetics Is of the Essence to Face NAFLD. Biomedicines 2021, 9, 1359. [Google Scholar] [CrossRef]
- Gellert-Kristensen, H.; Richardson, T.G. Combined Effect of PNPLA3, TM6SF2, and HSD17B13 Variants on Risk of Cirrhosis and Hepatocellular Carcinoma in the General Population. Hepatology 2020, 72, 845–856. [Google Scholar] [CrossRef]
- Pinsky, P.F. Principles of Cancer Screening. Surg. Clin. N. Am. 2015, 95, 953–966. [Google Scholar] [CrossRef]
- Loomba, R.; Lim, J.K.; Patton, H.; El-Serag, H.B. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020, 158, 1822–1830. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e315. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Florio, A.A.; Znaor, A. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global epidemiology of hepatocellular carcinoma: An emphasis on demographic and regional variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef]
- El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012, 142, 1264–1273.e1261. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Kanwal, F. Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology 2014, 60, 1767–1775. [Google Scholar] [CrossRef]
- Zhao, P.; Malik, S.; Xing, S. Epigenetic Mechanisms Involved in HCV-Induced Hepatocellular Carcinoma (HCC). Front. Oncol. 2021, 11, 677926. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Kramer, J.; Duan, Z.; Kanwal, F. Racial differences in the progression to cirrhosis and hepatocellular carcinoma in HCV-infected veterans. Am. J. Gastroenterol. 2014, 109, 1427–1435. [Google Scholar] [CrossRef]
- Singal, A.G.; Rich, N.E.; Mehta, N.; Branch, A.D.; Pillai, A.; Hoteit, M.; Volk, M.; Odewole, M.; Scaglione, S.; Guy, J.; et al. Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection Is Associated With Increased Survival in Patients With a History of Hepatocellular Carcinoma. Gastroenterology 2019, 157, 1253–1263.e1252. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N. HCC surveillance after SVR in patients with F3/F4 fibrosis. J. Hepatol. 2021, 74, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Galeone, C.; Rota, M.; Pelucchi, C.; Negri, E.; Bagnardi, V.; Corrao, G.; Boffetta, P.; La Vecchia, C. Alcohol and liver cancer: A systematic review and meta-analysis of prospective studies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Bucci, L.; Garuti, F.; Camelli, V.; Lenzi, B.; Farinati, F.; Giannini, E.G.; Ciccarese, F.; Piscaglia, F.; Rapaccini, G.L.; Di Marco, M.; et al. Comparison between alcohol- and hepatitis C virus-related hepatocellular carcinoma: Clinical presentation, treatment and outcome. Aliment. Pharmacol. Ther. 2016, 43, 385–399. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M. Genetic and Epigenetic Modifiers of Alcoholic Liver Disease. Int. J. Mol. Sci. 2018, 19, 3857. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef]
- Ganne-Carrié, N.; Chaffaut, C.; Bourcier, V.; Archambeaud, I.; Perarnau, J.M.; Oberti, F.; Roulot, D.; Moreno, C.; Louvet, A.; Dao, T.; et al. Estimate of hepatocellular carcinoma incidence in patients with alcoholic cirrhosis. J. Hepatol. 2018, 69, 1274–1283. [Google Scholar] [CrossRef]
- Huang, D.Q.; Tan, D.J.H.; Ng, C.H.; Amangurbanova, M.; Sutter, N.; Lin Tay, P.W.; Lim, W.H.; Yong, J.N.; Tang, A.; Syn, N.; et al. Hepatocellular Carcinoma Incidence in Alcohol-Associated Cirrhosis: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022; in press. [Google Scholar] [CrossRef]
- Legaz, I.; Bolarin, J.M.; Campillo, J.A.; Moya, R.M.; Luna, A.; Osuna, E.; Minguela, A.; Sanchez-Bueno, F.; Alvarez, M.R.; Muro, M. Pretransplant ascites and encephalopathy and their influence on survival and liver graft rejection in alcoholic cirrhosis disease. Arch. Med. Sci. 2021, 17, 682–693. [Google Scholar] [CrossRef]
- Legaz, I.; Navarro-Noguera, E.; Bolarín, J.M.; García-Alonso, A.M.; Luna Maldonado, A.; Mrowiec, A.; Campillo, J.A.; Gimeno, L.; Moya-Quiles, R.; Álvarez-López Mdel, R.; et al. Epidemiology, Evolution, and Long-Term Survival of Alcoholic Cirrhosis Patients Submitted to Liver Transplantation in Southeastern Spain. Alcohol. Clin. Exp. Res. 2016, 40, 794–805. [Google Scholar] [CrossRef]
- Legaz, I.; Navarro Noguera, E.; Bolarín, J.M.; Campillo, J.A.; Moya, R.; Luna, A.; Miras, M.; Minguela, A.; Álvarez-López, M.R.; Muro, M. Patient Sex in the Setting of Liver Transplant in Alcoholic Liver Disease. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ 2019, 17, 355–362. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828–1837.e1822. [Google Scholar] [CrossRef]
- Liew, Z.H.; Goh, G.B.; Hao, Y.; Chang, P.E.; Tan, C.K. Comparison of Hepatocellular Carcinoma in Patients with Cryptogenic Versus Hepatitis B Etiology: A Study of 1079 Cases Over 3 Decades. Dig. Dis. Sci. 2019, 64, 585–590. [Google Scholar] [CrossRef]
- Jung, Y.B.; Yoo, J.E.; Han, D.H.; Kim, K.S.; Choi, J.S.; Kim, D.Y.; Park, Y.N.; Choi, G.H. Clinical and survival outcomes after hepatectomy in patients with non-alcoholic fatty liver and hepatitis B-related hepatocellular carcinoma. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2021, 23, 1113–1122. [Google Scholar] [CrossRef]
- Lin, B.Z.; Lin, T.J.; Lin, C.L.; Liao, L.Y.; Chang, T.A.; Lu, B.J.; Chen, K.Y. Differentiation of clinical patterns and survival outcomes of hepatocellular carcinoma on hepatitis B and nonalcoholic fatty liver disease. J. Chin. Med. Assoc. 2021, 84, 606–613. [Google Scholar] [CrossRef]
- D’Silva, M.; Na, H.Y.; Cho, J.Y.; Han, H.S.; Yoon, Y.S.; Lee, H.W.; Lee, J.S.; Lee, B.; Kim, M. Pathological prognostic factors for post-resection survival in patients with hepatocellular carcinoma associated with non-alcoholic fatty liver disease. Transl. Cancer Res. 2021, 10, 3345–3355. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Piscaglia, F.; Svegliati-Baroni, G.; Barchetti, A.; Pecorelli, A.; Marinelli, S.; Tiribelli, C.; Bellentani, S. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 2016, 63, 827–838. [Google Scholar] [CrossRef]
- Than, N.N.; Ghazanfar, A.; Hodson, J.; Tehami, N.; Coldham, C.; Mergental, H.; Manas, D.; Shah, T.; Newsome, P.N.; Reeves, H.; et al. Comparing clinical presentations, treatments and outcomes of hepatocellular carcinoma due to hepatitis C and non-alcoholic fatty liver disease. QJM Mon. J. Assoc. Physicians 2017, 110, 73–81. [Google Scholar] [CrossRef]
- Nguyen, N.; Rode, A.; Trillaud, H.; Aubé, C. Percutaneous radiofrequency ablation for hepatocellular carcinoma developed on non-alcoholic fatty liver disease. Liver Int. 2022, 42, 905–917. [Google Scholar] [CrossRef]
- Benhammou, J.N.; Aby, E.S.; Shirvanian, G.; Manansala, K.; Hussain, S.K.; Tong, M.J. Improved survival after treatments of patients with nonalcoholic fatty liver disease associated hepatocellular carcinoma. Sci. Rep. 2020, 10, 9902. [Google Scholar] [CrossRef]
- Hernandez-Alejandro, R.; Croome, K.P.; Drage, M.; Sela, N.; Parfitt, J.; Chandok, N.; Marotta, P.; Dale, C.; Wall, W.; Quan, D. A comparison of survival and pathologic features of non-alcoholic steatohepatitis and hepatitis C virus patients with hepatocellular carcinoma. World J. Gastroenterol. 2012, 18, 4145–4149. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Kim, S.B.; Song, I.H. Clinical Patterns and Outcome of Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease. Can. J. Gastroenterol. Hepatol. 2020, 2020, 4873875. [Google Scholar] [CrossRef]
- Kumar, R.; Goh, B.G.; Kam, J.W.; Chang, P.E.; Tan, C.K. Comparisons between non-alcoholic steatohepatitis and alcohol-related hepatocellular carcinoma. Clin. Mol. Hepatol. 2020, 26, 196–208. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef]
- Turati, F.; Trichopoulos, D.; Polesel, J.; Bravi, F.; Rossi, M.; Talamini, R.; Franceschi, S.; Montella, M.; Trichopoulou, A.; La Vecchia, C.; et al. Mediterranean diet and hepatocellular carcinoma. J. Hepatol. 2014, 60, 606–611. [Google Scholar] [CrossRef]
- Moussa, I.; Day, R.S.; Li, R.; Du, X.L.; Kaseb, A.O. Dietary Patterns and Hepatocellular Carcinoma Risk among US Adults. Nutrients 2021, 13, 2011. [Google Scholar] [CrossRef]
- Duarte-Salles, T.; Fedirko, V.; Stepien, M.; Aleksandrova, K.; Bamia, C.; Lagiou, P.; Laursen, A.S.; Hansen, L.; Overvad, K.; Tjønneland, A.; et al. Dietary fat, fat subtypes and hepatocellular carcinoma in a large European cohort. Int. J. Cancer 2015, 137, 2715–2728. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Z.; Liang, D.; Li, J.; Ma, S.; Wang, G.; Chen, W. Meat Intake and the Risk of Hepatocellular Carcinoma: A Meta-Analysis of Observational Studies. Nutr. Cancer 2022, 74, 3340–3350. [Google Scholar] [CrossRef]
- Baumeister, S.E.; Schlesinger, S.; Aleksandrova, K.; Jochem, C.; Jenab, M.; Gunter, M.J.; Overvad, K.; Tjønneland, A.; Boutron-Ruault, M.C.; Carbonnel, F.; et al. Association between physical activity and risk of hepatobiliary cancers: A multinational cohort study. J. Hepatol. 2019, 70, 885–892. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005.e1001. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Rich, N.E.; Mehta, N.; Branch, A.; Pillai, A.; Hoteit, M.; Volk, M.; Odewole, M.; Scaglione, S.; Guy, J.; et al. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology 2019, 156, 1683–1692.e1681. [Google Scholar] [CrossRef] [PubMed]
- Schütte, K.; Schulz, C.; Poranzke, J.; Antweiler, K.; Bornschein, J.; Bretschneider, T.; Arend, J.; Ricke, J.; Malfertheiner, P. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, B.; Shah, V.; Onyshchenko, M.; Elshamy, M.; Aucejo, F.; Lopez, R.; Hanouneh, I.A.; Alhaddad, R.; Alkhouri, N. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol. Int. 2016, 10, 632–639. [Google Scholar] [CrossRef]
- Pinyopornpanish, K.; Khoudari, G.; Saleh, M.A.; Angkurawaranon, C.; Pinyopornpanish, K.; Mansoor, E.; Dasarathy, S.; McCullough, A. Hepatocellular carcinoma in nonalcoholic fatty liver disease with or without cirrhosis: A population-based study. BMC Gastroenterol. 2021, 21, 394. [Google Scholar] [CrossRef]
- Jiang, L.; Shan, J.; Shen, J.; Wang, Y.; Yan, P.; Liu, L.; Zhao, W.; Xu, Y.; Zhu, W.; Su, L.; et al. Androgen/androgen receptor axis maintains and promotes cancer cell stemness through direct activation of Nanog transcription in hepatocellular carcinoma. Oncotarget 2016, 7, 36814–36828. [Google Scholar] [CrossRef]
- Kur, P.; Kolasa-Wołosiuk, A. Sex Hormone-Dependent Physiology and Diseases of Liver. Int. J. Environ. Res. Public Health 2020, 17, 2620. [Google Scholar] [CrossRef]
- Ryu, S.H.; Jang, M.K.; Kim, W.J.; Lee, D.; Chung, Y.H. Metastatic tumor antigen in hepatocellular carcinoma: Golden roads toward personalized medicine. Cancer Metastasis Rev. 2014, 33, 965–980. [Google Scholar] [CrossRef]
- Arnold, M.; Pandeya, N.; Byrnes, G.; Renehan, P.A.G.; Stevens, G.A.; Ezzati, P.M.; Ferlay, J.; Miranda, J.J.; Romieu, I.; Dikshit, R.; et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet. Oncol. 2015, 16, 36–46. [Google Scholar] [CrossRef]
- Myers, S.; Neyroud-Caspar, I.; Spahr, L.; Gkouvatsos, K.; Fournier, E.; Giostra, E.; Magini, G.; Frossard, J.L.; Bascaron, M.E.; Vernaz, N.; et al. NAFLD and MAFLD as emerging causes of HCC: A populational study. JHEP Rep. Innov. Hepatol. 2021, 3, 100231. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Helbling, D.; Schöb, O.; Eltobgy, M.; Mohamed, H.; Schmidt, J.; Giryes, A.; Mehrabi, A.; Iype, S.; John, H.; et al. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: An updated systematic review of 81 epidemiological studies. J. Evid. -Based Med. 2017, 10, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, M.; Anand, A.C. Tobacco, Cigarettes, and the Liver: The Smoking Gun. J. Clin. Exp. Hepatol. 2021, 11, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Doycheva, I.; Zhang, T.; Amjad, W.; Thuluvath, P.J. Diabetes and Hepatocellular Carcinoma: Incidence Trends and Impact of Liver Disease Etiology. J. Clin. Exp. Hepatol. 2020, 10, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, Z.; Teng, F.; Feng, J.; Wu, X.; Chang, Q. Role of Asxl2 in non-alcoholic steatohepatitis-related hepatocellular carcinoma developed from diabetes. Int. J. Mol. Med. 2021, 47, 101–112. [Google Scholar] [CrossRef]
- Gao, C.; Fang, L.; Zhang, H. Metformin Induces Autophagy via the AMPK-mTOR Signaling Pathway in Human Hepatocellular Carcinoma Cells. Cancer Manag. Res. 2020, 12, 5803–5811. [Google Scholar] [CrossRef]
- de Oliveira, S.; Houseright, R.A.; Graves, A.L.; Golenberg, N.; Korte, B.G.; Miskolci, V.; Huttenlocher, A. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J. Hepatol. 2019, 70, 710–721. [Google Scholar] [CrossRef]
- Kramer, J.R.; Natarajan, Y. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Hepatology 2022, 75, 1420–1428. [Google Scholar] [CrossRef]
- Nishina, S.; Yamauchi, A.; Kawaguchi, T.; Kaku, K.; Goto, M.; Sasaki, K.; Hara, Y.; Tomiyama, Y.; Kuribayashi, F.; Torimura, T.; et al. Dipeptidyl Peptidase 4 Inhibitors Reduce Hepatocellular Carcinoma by Activating Lymphocyte Chemotaxis in Mice. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 115–134. [Google Scholar] [CrossRef]
- Yoshioka, N.; Tanaka, M.; Ochi, K.; Watanabe, A.; Ono, K.; Sawada, M.; Ogi, T.; Itoh, M.; Ito, A.; Shiraki, Y.; et al. The sodium-glucose cotransporter-2 inhibitor Tofogliflozin prevents the progression of nonalcoholic steatohepatitis-associated liver tumors in a novel murine model. Biomed. Pharmacother. 2021, 140, 111738. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease. Hepatology 2020, 71, 808–819. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Donati, B.; Fares, R.; Lombardi, R.; Mancina, R.M.; Romeo, S.; Valenti, L. PNPLA3 I148M polymorphism and progressive liver disease. World J. Gastroenterol. 2013, 19, 6969–6978. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Meroni, M.; Paolini, E.; Erconi, V.; Carli, F.; Fortunato, F.; Ronchi, D.; Piciotti, R.; Sabatini, S.; Macchi, C.; et al. TM6SF2/PNPLA3/MBOAT7 Loss-of-Function Genetic Variants Impact on NAFLD Development and Progression Both in Patients and in In Vitro Models. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 759–788. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Darlay, R.; Cockell, S.; Meroni, M.; Govaere, O.; Tiniakos, D.; Burt, A.D.; Bedossa, P.; Palmer, J.; Liu, Y.L.; et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. J. Hepatol. 2020, 73, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Meroni, M.; Longo, M.; Fracanzani, A.L.; Dongiovanni, P. MBOAT7 down-regulation by genetic and environmental factors predisposes to MAFLD. EBioMedicine 2020, 57, 102866. [Google Scholar] [CrossRef] [PubMed]
- Abul-Husn, N.S.; Cheng, X.; Li, A.H.; Xin, Y.; Schurmann, C.; Stevis, P.; Liu, Y.; Kozlitina, J.; Stender, S.; Wood, G.C.; et al. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N. Engl. J. Med. 2018, 378, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Kesarwala, A.H.; Eggert, T.; Medina-Echeverz, J.; Kleiner, D.E.; Jin, P.; Stroncek, D.F.; Terabe, M.; Kapoor, V.; ElGindi, M.; et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 2016, 531, 253–257. [Google Scholar] [CrossRef]
- Kreiter, S.; Vormehr, M.; van de Roemer, N.; Diken, M.; Löwer, M.; Diekmann, J.; Boegel, S.; Schrörs, B.; Vascotto, F.; Castle, J.C.; et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 2015, 520, 692–696. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Wang, Y.; Brown, Z.J.; Xia, Y.; Huang, Z.; Shen, C.; Hu, Z.; Beane, J.; Ansa-Addo, E.A.; et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J. Hepatol. 2021, 75, 1271–1283. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef]
- Singh, V.; Yeoh, B.S.; Chassaing, B.; Xiao, X.; Saha, P.; Aguilera Olvera, R.; Lapek, J.D., Jr.; Zhang, L.; Wang, W.B.; Hao, S.; et al. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell 2018, 175, 679–694.e622. [Google Scholar] [CrossRef]
- Behary, J.; Amorim, N.; Jiang, X.T.; Raposo, A.; Gong, L. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Paroni Sterbini, F.; Petito, V.; et al. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 107–120. [Google Scholar] [CrossRef]
- Perumpail, R.B.; Liu, A.; Wong, R.J.; Ahmed, A.; Harrison, S.A. Pathogenesis of hepatocarcinogenesis in non-cirrhotic nonalcoholic fatty liver disease: Potential mechanistic pathways. World J. Hepatol. 2015, 7, 2384–2388. [Google Scholar] [CrossRef]
- Alexander, J.; Torbenson, M.; Wu, T.T.; Yeh, M.M. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: A clinical and pathological study. J. Gastroenterol. Hepatol. 2013, 28, 848–854. [Google Scholar] [CrossRef]
- Zaki, M.Y.W.; Mahdi, A.K.; Patman, G.L.; Whitehead, A.; Maurício, J.P.; McCain, M.V.; Televantou, D.; Abou-Beih, S.; Ramon-Gil, E.; Watson, R.; et al. Key features of the environment promoting liver cancer in the absence of cirrhosis. Sci. Rep. 2021, 11, 16727. [Google Scholar] [CrossRef]
- Lee, T.Y.; Wu, J.C.; Yu, S.H.; Lin, J.T.; Wu, M.S.; Wu, C.Y. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int. J. Cancer 2017, 141, 1307–1314. [Google Scholar] [CrossRef]
- Donati, B.; Dongiovanni, P.; Romeo, S.; Meroni, M.; McCain, M.; Miele, L.; Petta, S.; Maier, S.; Rosso, C.; De Luca, L.; et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci. Rep. 2017, 7, 4492. [Google Scholar] [CrossRef]
- Meroni, M.; Dongiovanni, P.; Longo, M.; Carli, F.; Baselli, G.; Rametta, R.; Pelusi, S.; Badiali, S.; Maggioni, M.; Gaggini, M.; et al. Mboat7 down-regulation by hyper-insulinemia induces fat accumulation in hepatocytes. EBioMedicine 2020, 52, 102658. [Google Scholar] [CrossRef]
- Ki Kim, S.; Ueda, Y.; Hatano, E.; Kakiuchi, N.; Takeda, H.; Goto, T.; Shimizu, T.; Yoshida, K.; Ikura, Y.; Shiraishi, Y.; et al. TERT promoter mutations and chromosome 8p loss are characteristic of nonalcoholic fatty liver disease-related hepatocellular carcinoma. Int. J. Cancer 2016, 139, 2512–2518. [Google Scholar] [CrossRef]
- Pelusi, S.; Baselli, G. Rare Pathogenic Variants Predispose to Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease. Sci. Rep. 2019, 9, 3682. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M. The rs599839 A>G Variant Disentangles Cardiovascular Risk and Hepatocellular Carcinoma in NAFLD Patients. Cancers 2021, 13, 1783. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Meroni, M.; Petta, S.; Longo, M.; Alisi, A.; Soardo, G.; Valenti, L.; Miele, L.; Grimaudo, S.; Pennisi, G.; et al. Neurotensin up-regulation is associated with advanced fibrosis and hepatocellular carcinoma in patients with MAFLD. Biochim. Et Biophys. Acta. Mol. Cell Biol. Lipids 2020, 1865, 158765. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Stender, S.; Pietrelli, A.; Mancina, R.M.; Cespiati, A.; Petta, S.; Pelusi, S.; Pingitore, P.; Badiali, S.; Maggioni, M.; et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J. Intern. Med. 2018, 283, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Meroni, M.; Longo, M.; Paolini, E.; Lombardi, R.; Piciotti, R.; Francione, P.; Badiali, S.; Maggioni, M.; Fracanzani, A.L. MAFLD definition underestimates the risk to develop HCC in genetically predisposed patients. J. Intern. Med. 2022, 291, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Eldafashi, N.; Darlay, R.; Shukla, R.; McCain, M.V. A PDCD1 Role in the Genetic Predisposition to NAFLD-HCC? Cancers 2021, 13, 1412. [Google Scholar] [CrossRef] [PubMed]
- Gok Yavuz, B.; Hasanov, E.; Lee, S.S.; Mohamed, Y.I. Current Landscape and Future Directions of Biomarkers for Immunotherapy in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 1195–1207. [Google Scholar] [CrossRef]
- Pinter, M.; Jain, R.K.; Duda, D.G. The Current Landscape of Immune Checkpoint Blockade in Hepatocellular Carcinoma: A Review. JAMA Oncol. 2021, 7, 113–123. [Google Scholar] [CrossRef]
- Leung, C.; Yeoh, S.W.; Patrick, D.; Ket, S.; Marion, K.; Gow, P.; Angus, P.W. Characteristics of hepatocellular carcinoma in cirrhotic and non-cirrhotic non-alcoholic fatty liver disease. World J. Gastroenterol. 2015, 21, 1189–1196. [Google Scholar] [CrossRef]
- Gaddikeri, S.; McNeeley, M.F.; Wang, C.L.; Bhargava, P.; Dighe, M.K.; Yeh, M.M.; Dubinsky, T.J.; Kolokythas, O.; Lalwani, N. Hepatocellular carcinoma in the noncirrhotic liver. AJR. Am. J. Roentgenol. 2014, 203, W34–W47. [Google Scholar] [CrossRef]
- Jamwal, R.; Krishnan, V. Hepatocellular carcinoma in non-cirrhotic versus cirrhotic liver: A clinico-radiological comparative analysis. Abdom. Imaging 2020, 45, 2378–2387. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.P.; Lee, W.J.; Meloni, M.F.; Clevert, D.A.; Chammas, M.C.; Tannapfel, A.; Forgione, A.; Piscaglia, F.; Dietrich, C.F. Contrast-Enhanced Ultrasound Features of Histopathologically Proven Hepatocellular Carcinoma in the Non-cirrhotic Liver: A Multicenter Study. Ultrasound Med. Biol. 2022, 48, 1797–1805. [Google Scholar] [CrossRef]
- Konyn, P.; Ahmed, A. Current epidemiology in hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1295–1307. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Trinchet, J.C.; Chaffaut, C.; Bourcier, V.; Degos, F.; Henrion, J.; Fontaine, H.; Roulot, D.; Mallat, A.; Hillaire, S.; Cales, P.; et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: A randomized trial comparing 3- and 6-month periodicities. Hepatology 2011, 54, 1987–1997. [Google Scholar] [CrossRef]
- Tanaka, H. Current role of ultrasound in the diagnosis of hepatocellular carcinoma. J. Med. Ultrason. (2001) 2020, 47, 239–255. [Google Scholar] [CrossRef]
- Ultrasound diagnostic criteria for hepatic tumors. J. Med. Ultrason. (2001) 2014, 41, 113–123. [CrossRef]
- Schacherer, D.; Schoelmerich, J.; Zuber-Jerger, I. The diagnostic approach to hepatocellular carcinoma. Z. Fur Gastroenterol. 2007, 45, 1067–1074. [Google Scholar] [CrossRef]
- Kutami, R.; Nakashima, Y.; Nakashima, O.; Shiota, K.; Kojiro, M. Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J. Hepatol. 2000, 33, 282–289. [Google Scholar] [CrossRef]
- Kanno, T.; Kurioka, N.; Kim, S.; Tamori, A.; Kim, K.; Oka, H.; Kuroki, T.; Mizoguchi, Y.; Kobayashi, K. Implications of hyperechoic lesions in small hepatocellular carcinoma. Gastroenterol. Jpn. 1989, 24, 528–534. [Google Scholar] [CrossRef]
- Stevens, W.R.; Gulino, S.P.; Batts, K.P.; Stephens, D.H.; Johnson, C.D. Mosaic pattern of hepatocellular carcinoma: Histologic basis for a characteristic CT appearance. J. Comput. Assist. Tomogr. 1996, 20, 337–342. [Google Scholar] [CrossRef]
- Wernecke, K.; Henke, L.; Vassallo, P.; von Bassewitz, D.B.; Diederich, S.; Peters, P.E.; Edel, G. Pathologic explanation for hypoechoic halo seen on sonograms of malignant liver tumors: An in vitro correlative study. AJR. Am. J. Roentgenol. 1992, 159, 1011–1016. [Google Scholar] [CrossRef]
- Shibata, T.; Sakahara, H.; Kawakami, S.; Konishi, J. Sonographic characteristics of recurrent hepatocellular carcinoma. Eur. Radiol. 1996, 6, 443–447. [Google Scholar] [CrossRef]
- Maturen, K.E.; Wasnik, A.P.; Bailey, J.E.; Higgins, E.G.; Rubin, J.M. Posterior acoustic enhancement in hepatocellular carcinoma. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2011, 30, 495–499. [Google Scholar] [CrossRef]
- Sun, H.C.; Tang, Z.Y. Angiogenesis in hepatocellular carcinoma: The retrospectives and perspectives. J. Cancer Res. Clin. Oncol. 2004, 130, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Tochio, H.; Kudo, M. Afferent and efferent vessels of premalignant and overt hepatocellular carcinoma: Observation by color Doppler imaging. Intervirology 2004, 47, 144–153. [Google Scholar] [CrossRef]
- Zhang, B.H.; Yang, B.H.; Tang, Z.Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef]
- Singal, A.G.; Pillai, A.; Tiro, J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: A meta-analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef]
- van Meer, S.; de Man, R.A.; Coenraad, M.J.; Sprengers, D.; van Nieuwkerk, K.M.; Klümpen, H.J.; Jansen, P.L.; JN, I.J.; van Oijen, M.G.; Siersema, P.D.; et al. Surveillance for hepatocellular carcinoma is associated with increased survival: Results from a large cohort in the Netherlands. J. Hepatol. 2015, 63, 1156–1163. [Google Scholar] [CrossRef]
- Wu, C.Y.; Hsu, Y.C.; Ho, H.J.; Chen, Y.J.; Lee, T.Y.; Lin, J.T. Association between ultrasonography screening and mortality in patients with hepatocellular carcinoma: A nationwide cohort study. Gut 2016, 65, 693–701. [Google Scholar] [CrossRef]
- Mittal, S.; Kanwal, F.; Ying, J.; Chung, R.; Sada, Y.H.; Temple, S.; Davila, J.A.; El-Serag, H.B. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: A United States cohort. J. Hepatol. 2016, 65, 1148–1154. [Google Scholar] [CrossRef]
- Choi, D.T.; Kum, H.C.; Park, S.; Ohsfeldt, R.L.; Shen, Y.; Parikh, N.D.; Singal, A.G. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 976–987.e974. [Google Scholar] [CrossRef]
- Barbara, L.; Benzi, G.; Gaiani, S.; Fusconi, F.; Zironi, G.; Siringo, S.; Rigamonti, A.; Barbara, C.; Grigioni, W.; Mazziotti, A.; et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: A multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology 1992, 16, 132–137. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Trevisani, F.; De Notariis, S.; Rapaccini, G.; Farinati, F.; Benvegnù, L.; Zoli, M.; Grazi, G.L.; Del, P.P.; Di, N.; Bernardi, M. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: Effects on cancer stage and patient survival (Italian experience). Am. J. Gastroenterol. 2002, 97, 734–744. [Google Scholar] [CrossRef]
- Santi, V.; Trevisani, F.; Gramenzi, A.; Grignaschi, A.; Mirici-Cappa, F.; Del Poggio, P.; Di Nolfo, M.A.; Benvegnù, L.; Farinati, F.; Zoli, M.; et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J. Hepatol. 2010, 53, 291–297. [Google Scholar] [CrossRef]
- Kudo, M.; Izumi, N.; Kokudo, N.; Matsui, O.; Sakamoto, M.; Nakashima, O.; Kojiro, M.; Makuuchi, M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig. Dis. 2011, 29, 339–364. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1701. [Google Scholar] [CrossRef]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef]
- Simmons, O.; Fetzer, D.T.; Yokoo, T.; Marrero, J.A.; Yopp, A.; Kono, Y.; Parikh, N.D.; Browning, T.; Singal, A.G. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment. Pharmacol. Ther. 2017, 45, 169–177. [Google Scholar] [CrossRef]
- Huang, D.Q.; Fowler, K.J.; Liau, J.; Cunha, G.M.; Louie, A.L.; An, J.Y.; Bettencourt, R.; Jung, J. Comparative efficacy of an optimal exam between ultrasound versus abbreviated MRI for HCC screening in NAFLD cirrhosis: A prospective study. Aliment. Pharmacol. Ther. 2022, 55, 820–827. [Google Scholar] [CrossRef]
- Samoylova, M.L.; Mehta, N.; Roberts, J.P.; Yao, F.Y. Predictors of Ultrasound Failure to Detect Hepatocellular Carcinoma. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2018, 24, 1171–1177. [Google Scholar] [CrossRef]
- Schoenberger, H.; Chong, N.; Fetzer, D.T.; Rich, N.E.; Yokoo, T.; Khatri, G.; Olivares, J.; Parikh, N.D.; Yopp, A.C.; Marrero, J.A.; et al. Dynamic Changes in Ultrasound Quality for Hepatocellular Carcinoma Screening in Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 20, 1561–1569.e1564. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metab. Clin. Exp. 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Uppot, R.N.; Sahani, D.V.; Hahn, P.F.; Kalra, M.K.; Saini, S.S.; Mueller, P.R. Effect of obesity on image quality: Fifteen-year longitudinal study for evaluation of dictated radiology reports. Radiology 2006, 240, 435–439. [Google Scholar] [CrossRef]
- Del Poggio, P.; Olmi, S.; Ciccarese, F.; Di Marco, M.; Rapaccini, G.L.; Benvegnù, L.; Borzio, F.; Farinati, F.; Zoli, M.; Giannini, E.G.; et al. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2014, 12, 1927–1933.e1922. [Google Scholar] [CrossRef]
- Tchelepi, H.; Ralls, P.W.; Radin, R.; Grant, E. Sonography of diffuse liver disease. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2002, 21, 1023–1032. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, J.I. Current status of image-based surveillance in hepatocellular carcinoma. Ultrasonography (Seoul, Korea) 2021, 40, 45–56. [Google Scholar] [CrossRef]
- Ahn, J.C.; Lee, Y.-T.; Agopian, V.G.; Zhu, Y.; You, S.; Tseng, H.-R.; Yang, J.D. Hepatocellular carcinoma surveillance: Current practice and future directions. Hepatoma Res. 2022, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Pocha, C.; Dieperink, E.; McMaken, K.A.; Knott, A.; Thuras, P.; Ho, S.B. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography—A randomised study. Aliment. Pharmacol. Ther. 2013, 38, 303–312. [Google Scholar] [CrossRef]
- Forner, A.; Vilana, R.; Ayuso, C.; Bianchi, L.; Solé, M.; Ayuso, J.R.; Boix, L.; Sala, M.; Varela, M.; Llovet, J.M.; et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008, 47, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Rimola, J.; Forner, A.; Tremosini, S.; Reig, M.; Vilana, R.; Bianchi, L.; Rodríguez-Lope, C.; Solé, M.; Ayuso, C.; Bruix, J. Non-invasive diagnosis of hepatocellular carcinoma ≤ 2 cm in cirrhosis. Diagnostic accuracy assessing fat, capsule and signal intensity at dynamic MRI. J. Hepatol. 2012, 56, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Matsui, O.; Kobayashi, S.; Sanada, J.; Kouda, W.; Ryu, Y.; Kozaka, K.; Kitao, A.; Nakamura, K.; Gabata, T. Hepatocelluar nodules in liver cirrhosis: Hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom. Imaging 2011, 36, 264–272. [Google Scholar] [CrossRef]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; Saba, L.; Bosco, S.; Rossi, M.; Miles, K.A.; Di Miscio, R.; Lombardo, C.V.; Tamponi, E.; Piga, M.; Catalano, C. Hepatocellular carcinoma (HCC) in non-cirrhotic liver: Clinical, radiological and pathological findings. Eur. Radiol. 2014, 24, 1446–1454. [Google Scholar] [CrossRef]
- Al-Sharhan, F.; Dohan, A.; Barat, M.; Feddal, A.; Terris, B.; Pol, S.; Mallet, V.; Soyer, P. MRI presentation of hepatocellular carcinoma in non-alcoholic steatohepatitis (NASH). Eur. J. Radiol. 2019, 119, 108648. [Google Scholar] [CrossRef]
- Fischer, M.A.; Raptis, D.A.; Donati, O.F.; Hunziker, R.; Schade, E.; Sotiropoulos, G.C.; McCall, J.; Bartlett, A.; Bachellier, P.; Frilling, A.; et al. MR imaging features for improved diagnosis of hepatocellular carcinoma in the non-cirrhotic liver: Multi-center evaluation. Eur. J. Radiol. Radiol. 2015, 84, 1879–1887. [Google Scholar] [CrossRef]
- Barat, M.; Nguyen, T.T.L.; Hollande, C.; Coty, J.B.; Hoeffel, C.; Terris, B.; Dohan, A.; Mallet, V.; Pol, S.; Soyer, P. LI-RADS v2018 major criteria: Do hepatocellular carcinomas in non-alcoholic steatohepatitis differ from those in virus-induced chronic liver disease on MRI? Eur. J. Radiol. 2021, 138, 109651. [Google Scholar] [CrossRef]
- Thompson, S.M.; Garg, I.; Ehman, E.C.; Sheedy, S.P.; Bookwalter, C.A.; Carter, R.E.; Roberts, L.R.; Venkatesh, S.K. Non-alcoholic fatty liver disease-associated hepatocellular carcinoma: Effect of hepatic steatosis on major hepatocellular carcinoma features at MRI. Br. J. Radiol. 2018, 91, 20180345. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Hwang, J.A.; Shin, H.C.; Choi, S.Y.; Kang, T.W.; Jou, S.S.; Lee, W.H.; Park, S.; Heo, N.H. LI-RADS v2017 categorisation of HCC using CT: Does moderate to severe fatty liver affect accuracy? Eur. Radiol. 2019, 29, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, H. Biomarkers in Hepatocellular Carcinoma: Current Status and Future Perspectives. Biomedicines 2020, 8, 576. [Google Scholar] [CrossRef] [PubMed]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Reviews. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef]
- Schütte, K.; Schulz, C.; Link, A.; Malfertheiner, P. Current biomarkers for hepatocellular carcinoma: Surveillance, diagnosis and prediction of prognosis. World J. Hepatol. 2015, 7, 139–149. [Google Scholar] [CrossRef]
- Lersritwimanmaen, P.; Nimanong, S. Hepatocellular Carcinoma Surveillance: Benefit of Serum Alfa-fetoprotein in Real-world Practice. Euroasian J. Hepato-Gastroenterol. 2018, 8, 83–87. [Google Scholar] [CrossRef]
- Chew, V.; Tow, C.; Teo, M.; Wong, H.L.; Chan, J.; Gehring, A.; Loh, M.; Bolze, A.; Quek, R.; Lee, V.K.; et al. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J. Hepatol. 2010, 52, 370–379. [Google Scholar] [CrossRef]
- Wong, L.; Bozhilov, K.; Hernandez, B.; Kwee, S.; Chan, O.; Ellis, L.; LeMarchand, L. Underlying liver disease and advanced stage liver cancer are associated with elevated neutrophil-lymphocyte ratio. Clin. Mol. Hepatol. 2019, 25, 305–316. [Google Scholar] [CrossRef]
- Lim, T.S.; Rhee, H.; Kim, G.M.; Kim, S.U.; Kim, B.K.; Park, J.Y.; Ahn, S.H.; Han, K.H.; Choi, J.Y.; Kim, D.Y. Alpha-Fetoprotein, Des-Gamma-Carboxy Prothrombin, and Modified RECIST Response as Predictors of Survival after Transarterial Radioembolization for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. JVIR 2019, 30, 1194–1200.e1191. [Google Scholar] [CrossRef]
- Lee, S.; Rhim, H.; Kim, Y.S.; Kang, T.W.; Song, K.D. Post-ablation des-gamma-carboxy prothrombin level predicts prognosis in hepatitis B-related hepatocellular carcinoma. Liver Int. Off. J. Int. Assoc. Study Liver 2016, 36, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.S.; Shyu, Y.C.; Turner, R.; Chen, H.Y.; Chen, P.J. Diagnostic performance of alpha-fetoprotein, lens culinaris agglutinin-reactive alpha-fetoprotein, des-gamma carboxyprothrombin, and glypican-3 for the detection of hepatocellular carcinoma: A systematic review and meta-analysis protocol. Syst. Rev. 2013, 2, 37. [Google Scholar] [CrossRef]
- Piciotti, R.; Longo, M.; Agresta, A.; Paolini, E.; Cespiati, A.; Meroni, M.; Dongiovanni, P. Old-fashioned and newly discovered biomarkers: The future of NAFLD-related HCC screening and monitoring. Hepatoma Res. 2022, 8, 37. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.K.; Zhang, K.; Zhang, H.; Pan, Q.; Liu, Z.; Pan, H.; Xue, L.; Yen, Y.; Chu, P.G. Expression of glypican 3 enriches hepatocellular carcinoma development-related genes and associates with carcinogenesis in cirrhotic livers. Carcinogenesis 2015, 36, 232–242. [Google Scholar] [CrossRef]
- Sun, B.; Huang, Z.; Wang, B.; Yu, Y.; Lin, S.; Luo, L.; Wang, Y. Significance of Glypican-3 (GPC3) Expression in Hepatocellular Cancer Diagnosis. Med. Sci. Monit. 2017, 23, 850–855. [Google Scholar] [CrossRef]
- Tsuchiya, N.; Sawada, Y.; Endo, I.; Saito, K.; Uemura, Y.; Nakatsura, T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 10573–10583. [Google Scholar] [CrossRef]
- Shang, S.; Plymoth, A.; Ge, S.; Feng, Z.; Rosen, H.R.; Sangrajrang, S.; Hainaut, P.; Marrero, J.A.; Beretta, L. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology 2012, 55, 483–490. [Google Scholar] [CrossRef]
- Wan, H.G.; Xu, H.; Gu, Y.M.; Wang, H.; Xu, W.; Zu, M.H. Comparison osteopontin vs. AFP for the diagnosis of HCC: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 706–714. [Google Scholar] [CrossRef]
- Jin, D.; Tao, J.; Li, D.; Wang, Y.; Li, L.; Hu, Z.; Zhou, Z.; Chang, X.; Qu, C.; Zhang, H. Golgi protein 73 activation of MMP-13 promotes hepatocellular carcinoma cell invasion. Oncotarget 2015, 6, 33523–33533. [Google Scholar] [CrossRef]
- Witjes, C.D.; van Aalten, S.M.; Steyerberg, E.W.; Borsboom, G.J.; de Man, R.A.; Verhoef, C.; Ijzermans, J.N. Recently introduced biomarkers for screening of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatol. Int. 2013, 7, 59–64. [Google Scholar] [CrossRef]
- Chen, D.; Xing, W.; Hong, J.; Wang, M.; Huang, Y.; Zhu, C.; Yuan, Y.; Zeng, W. The beta2-adrenergic receptor is a potential prognostic biomarker for human hepatocellular carcinoma after curative resection. Ann. Surg. Oncol. 2012, 19, 3556–3565. [Google Scholar] [CrossRef]
- Underhill, H.R.; Kitzman, J.O.; Hellwig, S.; Welker, N.C.; Daza, R.; Baker, D.N.; Gligorich, K.M.; Rostomily, R.C.; Bronner, M.P.; Shendure, J. Fragment Length of Circulating Tumor DNA. PLoS Genet. 2016, 12, e1006162. [Google Scholar] [CrossRef]
- Chan, K.C.; Lai, P.B.; Mok, T.S.; Chan, H.L.; Ding, C.; Yeung, S.W.; Lo, Y.M. Quantitative analysis of circulating methylated DNA as a biomarker for hepatocellular carcinoma. Clin. Chem. 2008, 54, 1528–1536. [Google Scholar] [CrossRef]
- Chan, K.C.; Jiang, P.; Zheng, Y.W.; Liao, G.J.; Sun, H.; Wong, J.; Siu, S.S.; Chan, W.C.; Chan, S.L.; Chan, A.T.; et al. Cancer genome scanning in plasma: Detection of tumor-associated copy number aberrations, single-nucleotide variants, and tumoral heterogeneity by massively parallel sequencing. Clin. Chem. 2013, 59, 211–224. [Google Scholar] [CrossRef]
- Pessoa, L.S.; Heringer, M.; Ferrer, V.P. ctDNA as a cancer biomarker: A broad overview. Crit. Rev. Oncol. Hematol. 2020, 155, 103109. [Google Scholar] [CrossRef]
- Stroun, M.; Lyautey, J.; Lederrey, C.; Olson-Sand, A.; Anker, P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin. Chim. Acta. 2001, 313, 139–142. [Google Scholar] [CrossRef]
- Mezzalira, S.; De Mattia, E.; Guardascione, M.; Dalle Fratte, C.; Cecchin, E.; Toffoli, G. Circulating-Free DNA Analysis in Hepatocellular Carcinoma: A Promising Strategy to Improve Patients’ Management and Therapy Outcomes. Int. J. Mol. Sci. 2019, 20, 5498. [Google Scholar] [CrossRef]
- Xu, R.H.; Wei, W.; Krawczyk, M.; Wang, W.; Luo, H.; Flagg, K.; Yi, S.; Shi, W.; Quan, Q.; Li, K.; et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat. Mater. 2017, 16, 1155–1161. [Google Scholar] [CrossRef]
- Huang, Z.; Hua, D.; Hu, Y.; Cheng, Z.; Zhou, X.; Xie, Q.; Wang, Q.; Wang, F.; Du, X.; Zeng, Y. Quantitation of plasma circulating DNA using quantitative PCR for the detection of hepatocellular carcinoma. Pathol. Oncol. Res. POR 2012, 18, 271–276. [Google Scholar] [CrossRef]
- Ye, Q.; Ling, S.; Zheng, S.; Xu, X. Liquid biopsy in hepatocellular carcinoma: Circulating tumor cells and circulating tumor DNA. Mol. Cancer 2019, 18, 114. [Google Scholar] [CrossRef]
- Bratman, S.V.; Yang, S.Y.C.; Iafolla, M.A.J.; Liu, Z.; Hansen, A.R.; Bedard, P.L.; Lheureux, S.; Spreafico, A.; Razak, A.A.; Shchegrova, S.; et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat. Cancer 2020, 1, 873–881. [Google Scholar] [CrossRef]
- Cao, S.Q.; Zheng, H.; Sun, B.C.; Wang, Z.L.; Liu, T.; Guo, D.H.; Shen, Z.Y. Long non-coding RNA highly up-regulated in liver cancer promotes exosome secretion. World J. Gastroenterol. 2019, 25, 5283–5299. [Google Scholar] [CrossRef]
- Klingenberg, M.; Matsuda, A.; Diederichs, S.; Patel, T. Non-coding RNA in hepatocellular carcinoma: Mechanisms, biomarkers and therapeutic targets. J. Hepatol. 2017, 67, 603–618. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, J.-K.; Peng, Y.; He, W.; Huang, C. The role of long noncoding RNAs in hepatocellular carcinoma. Mol. Cancer 2020, 19, 77. [Google Scholar] [CrossRef]
- Ling, H.; Fabbri, M.; Calin, G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013, 12, 847–865. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Fang, Z.; Dou, G.; Wang, L. MicroRNAs in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Int. J. Biol. Sci. 2021, 17, 1851–1863. [Google Scholar] [CrossRef]
- Lou, G.; Liu, Y.; Wu, S.; Xue, J.; Yang, F.; Fu, H.; Zheng, M.; Chen, Z. The p53/miR-34a/SIRT1 Positive Feedback Loop in Quercetin-Induced Apoptosis. Cell. Physiol. Biochem. 2015, 35, 2192–2202. [Google Scholar] [CrossRef]
- Oura, K.; Morishita, A.; Masaki, T. Molecular and Functional Roles of MicroRNAs in the Progression of Hepatocellular Carcinoma-A Review. Int. J. Mol. Sci. 2020, 21, 8362. [Google Scholar] [CrossRef]
- Ilott, N.E.; Heward, J.A.; Roux, B.; Tsitsiou, E.; Fenwick, P.S.; Lenzi, L.; Goodhead, I.; Hertz-Fowler, C.; Heger, A.; Hall, N.; et al. Long non-coding RNAs and enhancer RNAs regulate the lipopolysaccharide-induced inflammatory response in human monocytes. Nat. Commun. 2014, 5, 3979. [Google Scholar] [CrossRef]
- Huang, R.; Duan, X.; Fan, J.; Li, G.; Wang, B. Role of Noncoding RNA in Development of Nonalcoholic Fatty Liver Disease. BioMed. Res. Int. 2019, 2019, 8690592. [Google Scholar] [CrossRef]
- Chen, L.L.; Yang, L. Regulation of circRNA biogenesis. RNA Biol. 2015, 12, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cai, Y.; Xu, J. Circular RNAs: Biogenesis, Mechanism, and Function in Human Cancers. Int. J. Mol. Sci. 2019, 20, 3926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y. Circular RNAs in Hepatocellular Carcinoma: Emerging Functions to Clinical Significances. Front. Oncol. 2021, 11, 667428. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.A.; Lee, H.C.; Choe, J.; Kim, M.J.; Lee, M.J.; Chang, H.S.; Bae, I.Y.; Kim, H.K.; An, J.; Shim, J.H.; et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 2018, 68, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Kostev, K.; Keitel, V.; Tacke, F.; Roderburg, C.; Luedde, T. An elevated FIB-4 score predicts liver cancer development: A longitudinal analysis from 29,999 patients with NAFLD. J. Hepatol. 2022, 76, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Izumi, T.; Sho, T.; Morikawa, K. Assessing the risk of hepatocellular carcinoma by combining liver stiffness and the controlled attenuation parameter. Hepatol. Res. 2019, 49, 1207–1217. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Felix, S.; Jeffers, T.; Younossi, E.; Nader, F.; Pham, H.; Afendy, A.; Cable, R.; Racila, A.; Younoszai, Z.; et al. Performance of the Enhanced Liver Fibrosis Test to Estimate Advanced Fibrosis Among Patients With Nonalcoholic Fatty Liver Disease. JAMA Netw. Open 2021, 4, e2123923. [Google Scholar] [CrossRef]
- Sanyal, A.; Shankar, S.; Yates, K.; Bolognese, J.; Daly, E.; Dehn, C.; Neuschwander-Tetri, B.; Kowdley, K.; Vuppalanchi, R.; Behling, C.A.; et al. The Nimble Stage 1 Study Validates Diagnostic Circulating Biomarkers for Nonalcoholic Steatohepatitis. Res. Sq. 2023, rs.3.rs-2492725. [Google Scholar] [CrossRef]
- Best, J.; Bechmann, L.P.; Sowa, J.P.; Sydor, S.; Dechêne, A.; Pflanz, K.; Bedreli, S.; Schotten, C.; Geier, A.; Berg, T.; et al. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients With Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 728–735.e724. [Google Scholar] [CrossRef]
- Yang, J.D.; Addissie, B.D.; Mara, K.C.; Harmsen, W.S.; Dai, J.; Zhang, N.; Wongjarupong, N. GALAD Score for Hepatocellular Carcinoma Detection in Comparison with Liver Ultrasound and Proposal of GALADUS Score. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Trépo, E.; Valenti, L. Update on NAFLD genetics: From new variants to the clinic. J. Hepatol. 2020, 72, 1196–1209. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Jamialahmadi, O.; Pelusi, S.; Baselli, G.; Dongiovanni, P.; Zanoni, I.; Santoro, L.; Maier, S.; Liguori, A.; Meroni, M.; et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J. Hepatol. 2021, 74, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Yoneda, M.; Seko, Y.; Ishiba, H.; Hara, T. Surveillance of Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease. Diagnostics 2020, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Zhou, P.; Chen, Y. Machine-learning algorithms based on personalized pathways for a novel predictive model for the diagnosis of hepatocellular carcinoma. BMC Bioinform. 2022, 23, 248. [Google Scholar] [CrossRef]
- Singal, A.G.; Mukherjee, A.; Elmunzer, B.J.; Higgins, P.D.; Lok, A.S.; Zhu, J.; Marrero, J.A.; Waljee, A.K. Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Am. J. Gastroenterol. 2013, 108, 1723–1730. [Google Scholar] [CrossRef]
| HCV | HBV | ALD | NAFLD | |
|---|---|---|---|---|
| HCC prevalence | 15–20% | 10–25% | 46–66% | 0.5–2.6% |
| Predisposing background |
|
|
|
|
| Risk factors |
|
|
|
|
| Preventing factors |
|
|
|
|
| Variant | Gene | Global MAF | Function | Effect | Impact | Phenotype |
|---|---|---|---|---|---|---|
| rs738409 C > G | PNPLA3 | 0.26 (G) | Lipid remodeling | p.I148M | Loss-of-function | ↑ NAFLD, NASH, fibrosis, and HCC |
| rs58542926 C > T | TM6SF2 | 0.07 (T) | VLDL secretion | p.E167K | Loss-of-function | ↑ NAFLD, NASH, and fibrosis |
| rs641738 C > T | TMC4/MBOAT7 | 0.37 (T) | Lipid remodeling | p.G17E | Loss-of-function | ↑ NAFLD, NASH, and fibrosis, HCC |
| rs1260326 C > T | GCKR | 0.29 (T) | Regulation of DNL | p.P446L | Loss-of-function | ↑ NAFLD, NASH, and fibrosis |
| rs72613567 T > TA | HSD17B13 | 0.18 (TA) | Lipid remodeling | Truncated protein | Loss-of-function | ↓ NASH, fibrosis, and HCC |
| Several | APOB | NA | VLDL secretion | Protein change | Loss-of-function | ↑ NAFLD NASH, fibrosis, and HCC |
| Several | TERT | NA | Telomere maintenance | Protein change | Loss-of-function | ↑ Fibrosis and HCC |
| Several | TP53 | NA | Genomic stability maintenance | Deletion | Loss-of-function | ↑ HCC |
| rs1800832 A > G | NTS | 0.11 (G) | Lipid metabolism | Overexpression of circulating Pro-NTS | Gain-of-function | ↑ Fibrosis and HCC |
| rs599839 A > G | PSRC1 | 0.24 (G) | Microtubule destabilization and spindle assembly | Overexpression of CELSR2-PSRC1-SORT1 gene cluster | Gain-of-function | ↑ HCC |
| Non-Cirrhotic vs. Cirrhotic NAFLD | |
|---|---|
| Common risk factors |
|
| Mechanisms involved in carcinogenesis |
|
| Macroscopic features |
|
| NIT | Formula | Higher Risk |
|---|---|---|
| VCTE | NA | >18 KPa |
| MRE | NA | >3.63 KPa |
| BARD score | BMI ≥ 28 kg/m2 = 1 AST/ALT ratio ≥ 0.8 = 2 T2DM = 1 | ≥2 |
| FIB-4 index | age (years) × AST (U/L)/[PLT (109/L) × ALT1/2 (U/L)] | >2.67 |
| NFS | −1.675 + 0.037 × age (year) + 0.094 × BMI (kg/m2) + 1.13 × IFG/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × PLT count (×109/L) − 0.66 × albumin (g/dL) | >0.676 |
| ELF test | 2.494 + 0.846 × ln(HA) + 0.735 × ln(P3NP) + 0.391 × ln(TIMP1) | >9.89 |
| GALAD score | −10.08 + 1.67 × [Gender (1 for male, 0 for female)] + 0.09 × [Age] + 0.04 × [AFP-L3%] + 2.34 × log[AFP] + 1.33 × log[DCP] | ≥−0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cespiati, A.; Cinque, F.; Meroni, M.; Lombardi, R.; Dongiovanni, P.; Fracanzani, A.L. An Overview of Hepatocellular Carcinoma Surveillance Focusing on Non-Cirrhotic NAFLD Patients: A Challenge for Physicians. Biomedicines 2023, 11, 586. https://doi.org/10.3390/biomedicines11020586
Cespiati A, Cinque F, Meroni M, Lombardi R, Dongiovanni P, Fracanzani AL. An Overview of Hepatocellular Carcinoma Surveillance Focusing on Non-Cirrhotic NAFLD Patients: A Challenge for Physicians. Biomedicines. 2023; 11(2):586. https://doi.org/10.3390/biomedicines11020586
Chicago/Turabian StyleCespiati, Annalisa, Felice Cinque, Marica Meroni, Rosa Lombardi, Paola Dongiovanni, and Anna Ludovica Fracanzani. 2023. "An Overview of Hepatocellular Carcinoma Surveillance Focusing on Non-Cirrhotic NAFLD Patients: A Challenge for Physicians" Biomedicines 11, no. 2: 586. https://doi.org/10.3390/biomedicines11020586
APA StyleCespiati, A., Cinque, F., Meroni, M., Lombardi, R., Dongiovanni, P., & Fracanzani, A. L. (2023). An Overview of Hepatocellular Carcinoma Surveillance Focusing on Non-Cirrhotic NAFLD Patients: A Challenge for Physicians. Biomedicines, 11(2), 586. https://doi.org/10.3390/biomedicines11020586







