Brain Degeneration in Synucleinopathies Based on Analysis of Cognition and Other Nonmotor Features: A Multimodal Imaging Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Demographic and PD-Related Features Assessment
2.3. Nonmotor Assessment
2.3.1. Cognitive and Clinical Assessment
2.3.2. Dysautonomia, Olfaction, and Visual Assessment
2.4. Selection of Variables and Clustering Analysis
2.5. Neuroimaging Preprocessing and Analysis
2.5.1. Structural and Diffusion MRI Preprocessing and Analysis
2.5.2. Resting-State Functional MRI Preprocessing and Analysis
2.6. Data Analysis
3. Results
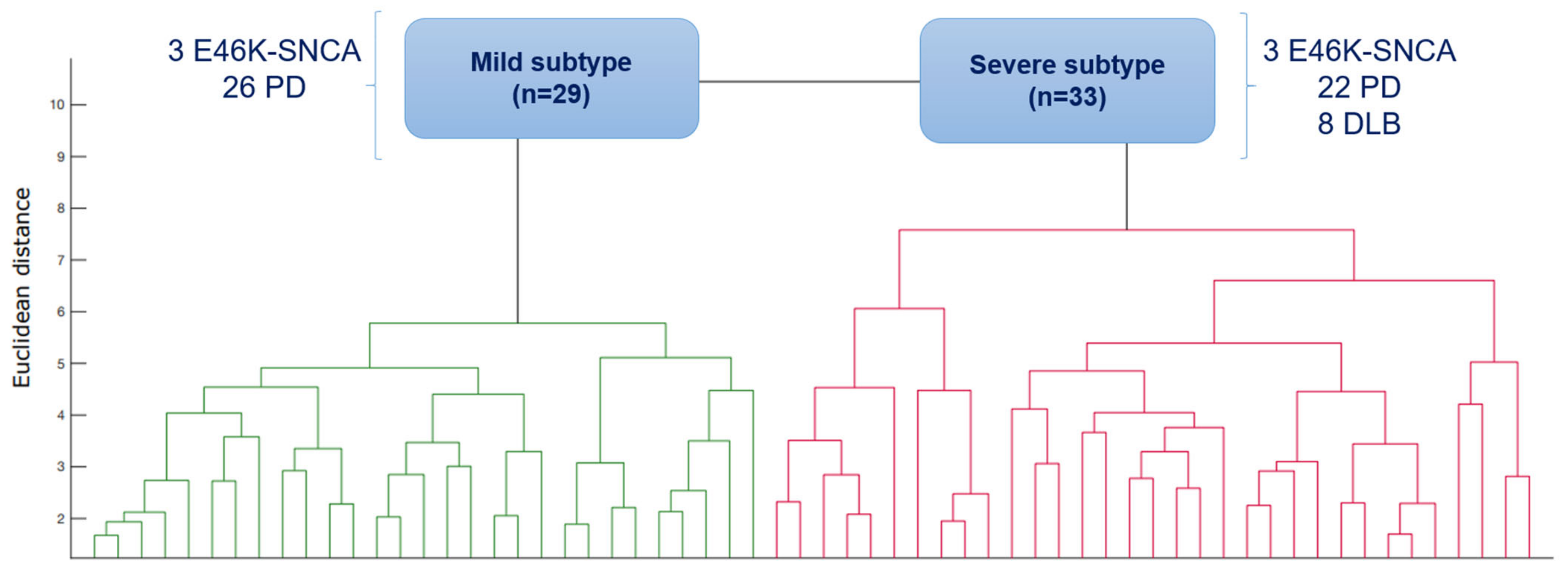
3.1. Subtypes of Synucleinopathies Based on Cluster Analysis
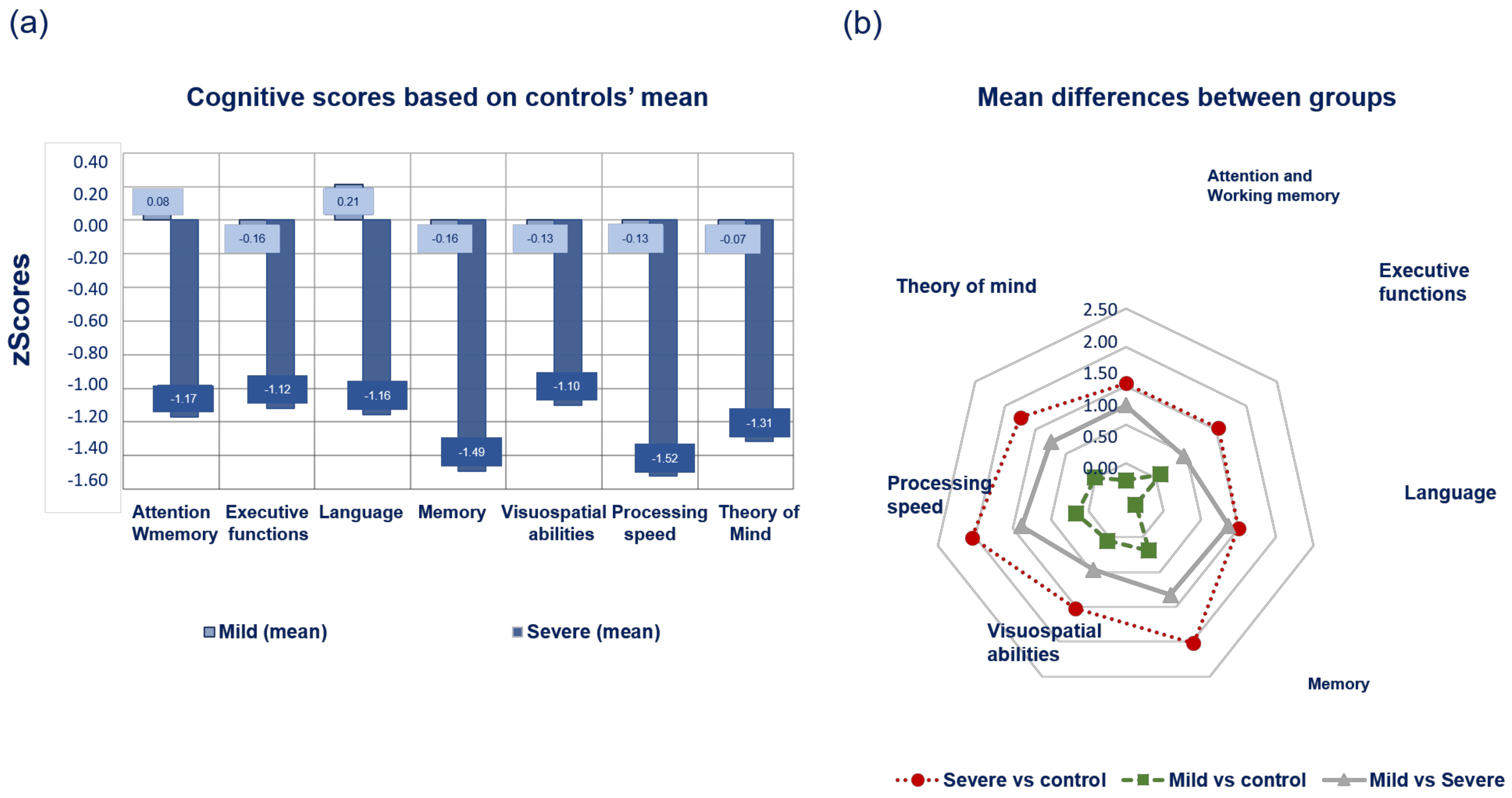
3.2. Cognitive Profile of Subtypes According to PDMCI Level II Criteria
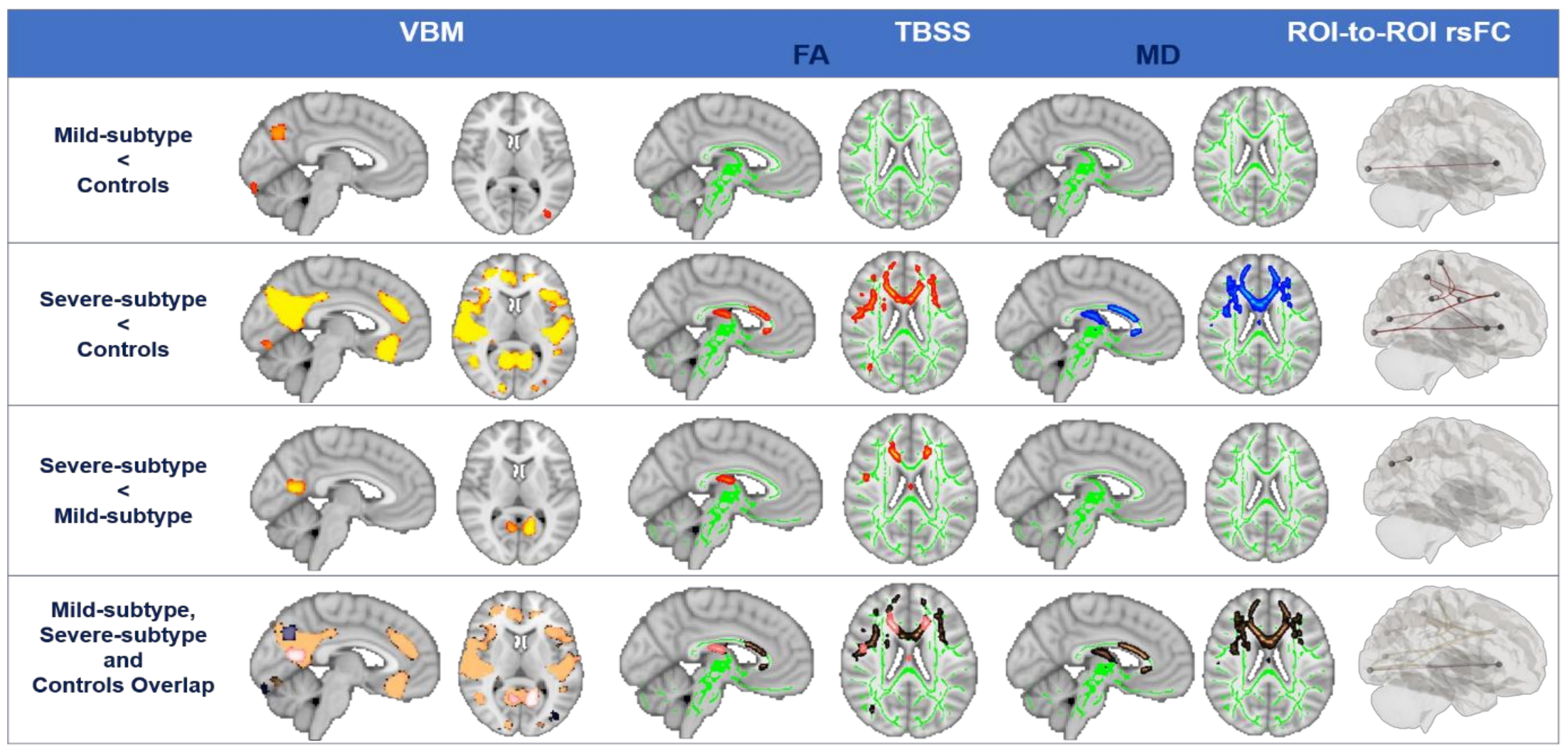
3.3. Structural and Functional Brain Degeneration in Synucleinopathies Based on Clusters
4. Discussion
4.1. Distinct Clinically Relevant Patterns in Synucleinopathies
4.2. Distinct Cognitive Profiles in Synucleinopathies
4.3. Brain Degeneration in Synucleinopathies Based on Neuroimaging: Towards a Pathophysiological Explanation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Regions | Cluster Size (Voxels) | Stats | p Value (FWE-Corrected) | MNI Coordinates X Y Z |
|---|---|---|---|---|
| Mild < Controls | ||||
| Inferior Temporooccipital Left | 522 | 3.98 | 0.020 | −50 −54 −10 |
| Precuneus Left | 318 | 4.29 | 0.020 | −2 −70 −34 |
| Lateral Occipital Left | 223 | 3.96 | 0.031 | −36 −82 −4 |
| Occipital Pole Right | 105 | 4.21 | 0.033 | 2 −90 −24 |
| Severe < Controls | ||||
| Superior Temporal Right | 3972 | 4.47 | <0.001 | 52 −22 0 |
| Superior Temporal Left | 2408 | 4.56 | 0.005 | 54 −4 −12 |
| Superior Lateral Occipital Right | 2151 | 5.10 | 0.007 | 14 −84 24 |
| Temporal Fusiform Right | 1769 | 4.54 | 0.011 | 40 −34 −16 |
| Hippocampus Left | 430 | 4.58 | 0.022 | −28 −32 −14 |
| Occipital Pole Left | 313 | 3.84 | 0.020 | −22 −90 6 |
| Occipital Pole Right | 185 | 3.86 | 0.025 | 18 −88 4 |
| Frontal Orbital Left | 174 | 4.31 | 0.036 | −32 30 −2 |
| Frontal Medial Left | 147 | 4.73 | 0.038 | −2 30 −20 |
| Temporal Pole Left | 113 | 3.91 | 0.037 | −22 6 −24 |
| Frontal Orbital Right | 106 | 4.76 | 0.032 | 32 28 −6 |
| Severe < Mild | ||||
| Precuneus Left | 1036 | 4.91 | 0.002 | −12 −64 8 |
| Frontal Orbital Right | 82 | 4.34 | 0.035 | 36 30 4 |
| Occipital Left | 32 | 3.69 | 0.044 | −18 −84 −14 |
| Hippocampus Left | 16 | 4.45 | 0.044 | −36 −32 −6 |
| Regions | Cluster Size (Voxels) | Stats | p Value (FWE-Corrected) | MNI Coordinates X Y Z |
|---|---|---|---|---|
| Severe < Controls | ||||
| FA | ||||
| Anterior Thalamic Radiation Right | 13,377 | 3.51 | <0.001 | 19 42 −4 |
| Anterior Thalamic Radiation Left | 366 | 5.51 | 0.007 | −7 −24 14 |
| Inferior Longitudinal Right | 65 | 5.41 | 0.009 | 47 −21 1 |
| Superior Longitudinal Right | 56 | 4.39 | 0.010 | 33 4 40 |
| Severe > Controls | ||||
| MD | ||||
| Cingulum Left | 8361 | 3.52 | 0.003 | −16 32 16 |
| Anterior Thalamic Radiation Right | 764 | 4.24 | 0.009 | 21 12 10 |
| Superior Longitudinal Right | 719 | 2.31 | 0.010 | 35 2 21 |
| Anterior Thalamic Radiation Left | 657 | 6.24 | 0.005 | −11 −31 12 |
| Body of Corpus Callosum | 545 | 3.57 | 0.009 | 14 −29 28 |
| Cingulum Left | 239 | 4.71 | 0.009 | −9 25 −5 |
| Inferior Fronto-Occipital Right | 127 | 3.43 | 0.010 | 22 51 −9 |
| Longitudinal Right | 103 | 2.29 | 0.010 | 25 −47 25 |
| Severe < Mild | ||||
| FA | ||||
| Body of Corpus Callosum | 1866 | 4.21 | <0.010 * | 19 −17 38 |
| Anterior Thalamic Radiation Right | 515 | 5.68 | 0.046 | 12 −29 13 |
| Cingulum Left | 482 | 4.29 | <0.010 * | −16 31 21 |
| Superior Longitudinal Right | 66 | 3.59 | <0.010 * | 47 0 25 |
| Seed | Target | Stats | p Value (FDR-Corrected) |
|---|---|---|---|
| Mild < Controls | |||
| Visual Occipital (Visual) | IFG Left (Language) | 3.75 | <0.001 |
| Severe < Controls | |||
| IPS Left (DAN) | SMG Right (Salience) | 4.19 | 0.024 |
| Visual Lateral Left (Visual) | ACC (Salience) | 3.93 | 0.028 |
| Visual Occipital (Visual) | ACC (Salience) | 3.61 | 0.031 |
| Visual Occipital (Visual) | IFG Left (Language) | 3.58 | 0.031 |
| IPS Left (DAN) | ACC (Salience) | 3.57 | 0.031 |
| Sensorimotor Superior (Sensorimotor) | Sensorimotor Lateral Right (Sensorimotor) | 3.55 | 0.031 |
| Sensorimotor Superior (Sensorimotor) | Sensorimotor Lateral Left (Sensorimotor) | 3.48 | 0.033 |
| SMG Left (Salience) | aInsula Left (Salience) | 3.43 | 0.034 |
| SMG Left (Salience) | ACC (Salience) | 3.33 | 0.041 |
| Visual Occipital (Visual) | aInsula Left (Salience) | 3.24 | 0.048 |
| Severe < Mild | |||
| PCC (Default Mode) | LP Left (Default Mode) | 4.19 | 0.028 |
References
- Aarsland, D.; Brønnick, K.; Larsen, J.P.; Tysnes, O.B.; Alves, G. Cognitive impairment in incident, untreated parkinson disease: The norwegian parkwest study. Neurology 2009, 72, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Muslimović, D.; Post, B.; Speelman, J.D.; Schmand, B. Cognitive profile of patients with newly diagnosed Parkinson’s disease. Neurology 2005, 65, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Bronnick, K.; Williams-Gray, C.; Weintraub, D.; Marder, K.; Kulisevsky, J.; Burn, D.; Barone, P.; Pagonabarraga, J.; Allcock, L.; et al. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology 2010, 75, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Litvan, I.; Goldman, J.G.; Tröster, A.I.; Ben, A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-gray, C.H. Diangostic Criteria for Mild Cognitive Impairment in Parkinson’s disease:Movement Disorder Society Task Force Guidelines. Mov. Disord. 2012, 27, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Hely, M.A.; Reid, W.G.J.; Adena, M.A.; Halliday, G.M.; Morris, J.G.L. The Sydney Multicenter Study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007, 22, 1689–1707. [Google Scholar] [CrossRef]
- Foltynie, T.; Brayne, C.; Barker, R.A. The heterogeneity of idiopathic Parkinson’s disease. J. Neurol. 2002, 249, 138–145. [Google Scholar] [CrossRef]
- Van Rooden, S.M.; Heiser, W.J.; Kok, J.N.; Verbaan, D.; Van Hilten, J.J.; Marinus, J. The identification of Parkinson’s disease subtypes using cluster analysis: A systematic review. Mov. Disord. 2010, 25, 969–978. [Google Scholar] [CrossRef]
- Erro, R.; Picillo, M.; Vitale, C.; Palladino, R.; Amboni, M.; Moccia, M.; Pellecchia, M.T.; Barone, P. Clinical clusters and dopa-minergic dysfunction in de-novo Parkinson disease. Park. Relat. Disord. 2016, 28, 137–140. [Google Scholar] [CrossRef]
- Lawton, M.; Ben-shlomo, Y.; May, M.T.; Baig, F.; Barber, T.R.; Klein, J.C.; Swallow, D.M.A.; Malek, N.; Grosset, K.A.; Bajaj, N.; et al. Developing and validating Parkinson’s disease subtypes and their motor and cognitive progression. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1279–1287. [Google Scholar] [CrossRef]
- Campbell, M.C.; Myers, P.S.; Weigand, A.J.; Foster, E.R.; Cairns, N.J.; Jackson, J.J.; Lessov-schlaggar, C.N.; Perlmutter, J.S. Parkinson disease clinical subtypes: Key features & clinical milestones. Ann. Clin. Transl. Neurol. 2020, 7, 1272–1283. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.-M.; Romenets, S.R.; Anang, J.B.M.; Latreille, V.; Gagnon, J.-F.; Postuma, R.B. New Clinical Subtypes of Parkinson Disease and Their Longitudinal Progression. JAMA Neurol. 2015, 72, 863. [Google Scholar] [CrossRef] [PubMed]
- Uribe, C.; Segura, B.; Cesar, H.; Abos, A.; Garcia-diaz, A.I.; Campabadal, A.; Jose, M. Parkinsonism and Related Disorders Cortical atrophy patterns in early Parkinson’s disease patients using hierarchical cluster analysis. Park. Relat. Disord. 2018, 50, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Inguanzo, A.; Sala-llonch, R.; Segura, B.; Erostarbe, H.; Abos, A. Parkinsonism and Related Disorders Hierarchical cluster analysis of multimodal imaging data identifies brain atrophy and cognitive patterns in Parkinson’s disease. Park. Relat. Disord. 2021, 82, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Cigdem, O.; Demirel, H.; Unay, D. The Performance of Local-Learning Based Clustering Feature Selection Method on the Diagnosis of Parkinson’s Disease Using Structural MRI. In Proceedings of the 2019 IEEE International Conference on Systems, Man and Cybernetics (SMC), Bari, Italy, 6–9 October 2019; pp. 1286–1291. [Google Scholar]
- Abbasi, N.; Fereshtehnejad, S.; Zeighami, Y.; Larcher, K.M.; Postuma, R.B.; Dagher, A. Predicting severity and prognosis in Parkinson’s disease from brain microstructure and connectivity. NeuroImage Clin. 2019, 25, 102111. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Guan, X.; Zhou, C.; Gao, T.; Wu, J.; Song, Z.; Xuan, M.; Gu, Q.; Huang, P.; Pu, J.; et al. Clinically relevant connectivity features define three subtypes of Parkinson’s disease patients. Hum. Brain Mapp. 2020, 41, 4077–4092. [Google Scholar] [CrossRef]
- Taylor, J.; Colloby, S.J.; Mckeith, I.G.; Brien, J.T.O. Covariant perfusion patterns provide clues to the origin of cognitive fluctuations and attentional dysfunction in Dementia with Lewy bodies. Int. Psychogeriatr. 2013, 25, 1917–1928. [Google Scholar] [CrossRef]
- Somme, J.H.; Gomez-Esteban, J.C.; Molano, A.; Tijero, B.; Lezcano, E.; Zarranz, J.J. Initial neuropsychological impairments in patients with the E46K mutation of the α-synuclein gene (PARK 1). J. Neurol. Sci. 2011, 310, 86–89. [Google Scholar] [CrossRef]
- Zarranz, J.J.; Alegre, J.; Gómez-Esteban, J.C.; Lezcano, E.; Ros, R.; Ampuero, I.; Vidal, L.; Hoenicka, J.; Rodriguez, O.; Atarés, B.; et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004, 55, 164–173. [Google Scholar] [CrossRef]
- Tröster, A.I. Neuropsychological Characteristics of Dementia with Lewy Bodies and Parkinson’s Disease with Dementia: Differentiation, Early Detection, and Implications for “Mild Cognitive Impairment” and Biomarkers. Neuropsychol. Rev. 2008, 18, 103–119. [Google Scholar] [CrossRef]
- Peraza, L.R.; Kaiser, M.; Firbank, M.; Graziadio, S.; Bonanni, L.; Onofrj, M.; Colloby, S.J.; Blamire, A.; Brien, J.O.; Taylor, J. NeuroImage: Clinical fMRI resting state networks and their association with cognitive fluctuations in dementia with Lewy bodies. NeuroImage Clin. 2014, 4, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Cagnin, A.; Gardini, S.; Guzzo, C.; Gnoato, F.; Mitolo, M.; Caffarra, P.; Jelcic, N.; Ermani, M. Clinical and Cognitive Phenotype of Mild Cognitive Impairment Evolving to Dementia with Lewy Bodies. Dement. Geriatr. Cogn. Disord. Extra 2015, 5, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Murueta-Goyena, A.; Del Pino, R.; Galdós, M.; Arana, B.; Acera, M.; Carmona-Abellán, M.; Fernández-Valle, T.; Tijero, B.; Lucas-Jiménez, O.; Ojeda, N.; et al. Retinal Thickness Predicts the Risk of Cognitive Decline in Parkinson Disease. Ann. Neurol. 2021, 89, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Del Pino, R.; Murueta-Goyena, A.; Acera, M.; Carmona-Abellan, M.; Tijero, B.; Lucas-Jiménez, O.; Ojeda, N.; Ibarretxe-Bilbao, N.; Peña, J.; Gabilondo, I.; et al. Autonomic dysfunction is associated with neuropsychological impairment in Lewy body disease. J. Neurol. 2020, 267, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Smith, S.; Jenkinson, M.; Behrens, T.; Johansen-Berg, H.; Vickers, J.; James, S.; Voets, N.; Watkins, K.; Matthews, P.M. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 2007, 130, 2375–2386. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.J.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23, S208–S219. [Google Scholar] [CrossRef]
- Jones, D.K.; Cercignani, M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010, 23, 803–820. [Google Scholar] [CrossRef]
- Weissenbacher, A.; Kasess, C.; Gerstl, F.; Lanzenberger, R.; Moser, E.; Windischberger, C. Correlations and anticorrelations in resting-state functional connectivity MRI: A quantitative comparison of preprocessing strategies. Neuroimage 2009, 47, 1408–1416. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef]
- Mattay, V.S.; Tessitore, A.; Callicott, J.H.; Bertolino, A.; Goldberg, T.E.; Chase, T.N.; Hyde, T.M.; Weinberger, D.R. Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Ann. Neurol. 2002, 51, 156–164. [Google Scholar] [CrossRef]
- Belvisi, D.; Fabbrini, A.; De Bartolo, M.I.; Costanzo, M.; Manzo, N.; Fabbrini, G.; Defazio, G.; Conte, A.; Berardelli, A. The Pathophysiological Correlates of Parkinson’s Disease Clinical Subtypes. Mov. Disord. 2021, 36, 370–379. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, Y.E.; Yun, J.Y.; Kim, H.J.; Jeon, B.S. Identifying the clusters within nonmotor manifestations in early Parkinson’s disease by using unsupervised cluster analysis. PLoS ONE 2014, 9, e91906. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Chaudhuri, K.R.; Bielza, C.; De Pedro-Cuesta, J.; Larrañaga, P.; Martinez-Martin, P. Parkinson’s disease subtypes identified from cluster analysis of motor and non-motor symptoms. Front. Aging Neurosci. 2017, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. 2017, 3, 17013. [Google Scholar] [CrossRef]
- Pont-Sunyer, C.; Hotter, A.; Gaig, C.; Seppi, K.; Compta, Y.; Katzenschlager, R.; Mas, N.; Hofeneder, D.; Brücke, T.; Bayés, A.; et al. The Onset of Nonmotor Symptoms in Parkinson’s disease (The ONSET PDStudy). Mov. Disord. 2015, 30, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Fereshtehnejad, S.M.; Zeighami, Y.; Dagher, A.; Postuma, R.B. Clinical criteria for subtyping Parkinson’s disease: Biomarkers and longitudinal progression. Brain 2017, 140, 1959–1976. [Google Scholar] [CrossRef] [PubMed]
- Deng, I.; Corrigan, F.; Zhai, G.; Zhou, X.; Bobrovskaya, L. Brain, Behavior, & Immunity—Health Lipopolysaccharide animal models of Parkinson’s disease: Recent progress and relevance to clinical disease. Brain Behav. Immun. Health 2020, 4, 100060. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; De Vos, R.A.I.; Steur, E.N.H.J.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Del Tredici, K.; Rüb, U.; De Vos, R.A.I.; Bohl, J.R.E.; Braak, H. Where does Parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 2002, 61, 413–426. [Google Scholar] [CrossRef]
- Ponsen, M.M.; Stoffers, D.; Booij, J.; Van Eck-Smit, B.L.F.; Wolters, E.C.; Berendse, H.W. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann. Neurol. 2004, 56, 173–181. [Google Scholar] [CrossRef]
- Sui, X.; Zhou, C.; Li, J.; Chen, L.; Yang, X.; Li, F. Hyposmia as a predictive marker of Parkinson’s disease: A systematic review and meta-analysis. Biomed Res. Int. 2019, 2019, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Coon, E.A.; Cutsforth-Gregory, J.K.; Benarroch, E.E. Neuropathology of autonomic dysfunction in synucleinopathies. Mov. Disord. 2018, 33, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Jakes, R.; Spillantini, M.G. The Synucleinopathies: Twenty Years on. J. Parkinsons. Dis. 2017, 7, S53–S71. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Non-motor symptoms in Parkinson’s disease. Park. Relat. Disord. 2016, 22, S119–S122. [Google Scholar] [CrossRef]
- Murueta-Goyena, A.; Del Pino, R.; Reyero, P.; Galdós, M.; Arana, B.; Lucas-Jiménez, O.; Acera, M.; Tijero, B.; Ibarretxe-Bilbao, N.; Ojeda, N.; et al. Parafoveal thinning of inner retina is associated with visual dysfunction in Lewy body diseases. Mov. Disord. 2019, 34, 1315–1324. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Marras, C.; Chaudhuri, K.R. Nonmotor features of Parkinson’s disease subtypes. Mov. Disord. 2016, 31, 1095–1102. [Google Scholar] [CrossRef]
- Weintraub, D.; Chahine, L.M.; Hawkins, K.A.; Siderowf, A.; Eberly, S.; Oakes, D.; Seibyl, J.; Stern, M.B.; Marek, K. Cognition and the Course of Prodromal Parkinson’s Disease Methods Study Description. Mov. Disord. 2017, 32, 1640–1645. [Google Scholar] [CrossRef]
- Williams-Gray, C.H.; Foltynie, T.; Brayne, C.E.G.; Robbins, T.W.; Barker, R.A. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain 2007, 130, 1787–1798. [Google Scholar] [CrossRef]
- Rosenthal, E.; Brennan, L.; Xie, S.; Hurtig, H.; Milber, J.; Weintraub, D.; Karlawish, J.; Siderowf, A. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov. Disord. 2010, 25, 1170–1176. [Google Scholar] [CrossRef]
- Kluger, B.M.; Freddy, K.; Tysnes, O.; Ongre, S.O.; Øygarden, B.; Herlofson, K. Is fatigue associated with cognitive dysfunction in early Parkinson’s disease? Park. Relat. Disord. 2017, 37, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, J.C.; Duffy, S.L.; Mckinnon, A.C.; Naismith, S.L.; Mowszowski, L.; Guastella, A. Theory of Mind in mild cognitive impairment: Relationship with the default mode network (DMN). Alzheimer’s Dement. 2020, 16, 39164. [Google Scholar] [CrossRef]
- Díez-Cirarda, M.; Ojeda, N.; Peña, J.; Cabrera-Zubizarreta, A.; Gómez-Beldarrain, M.Á.; Gómez-Esteban, J.C.; Ibarretxe-Bilbao, N. Neuroanatomical correlates of theory of mind deficit in Parkinson’s disease: A multimodal imaging study. PLoS ONE 2015, 10, e0142234. [Google Scholar] [CrossRef]
- Rektor, I.; Svátková, A.; Vojtíšek, L.; Zikmundová, I.; Vaníček, J.; Király, A.; Szabó, N. White matter alterations in Parkinson’s disease with normal cognition precede grey matter atrophy. PLoS ONE 2018, 13, e0187939. [Google Scholar] [CrossRef] [PubMed]
- Auning, E.; Kjærvik, V.K.; Selnes, P.; Aarsland, D.; Haram, A.; Bjørnerud, A.; Hessen, E.; Esnaashari, A. White matter integrity and cognition in Parkinson’s disease: A cross-sectional study. BMJ Open 2014, 4, e003976. [Google Scholar] [CrossRef]
- Zhang, J.; Bi, W.; Zhang, Y.; Zhu, M.; Zhang, Y.; Hua, F.; Wang, J.; Zhang, Y.; Jiang, T. Abnormal functional connectivity density in Parkinson’s disease. Behav. Brain Res. 2015, 280, 113–118. [Google Scholar] [CrossRef]
- Su, M.; Wang, S.; Fang, W.; Zhu, Y.; Li, R.; Sheng, K.; Zou, D.; Han, Y.; Wang, X.; Cheng, O. Parkinsonism and Related Disorders Alterations in the limbic/paralimbic cortices of Parkinson’s disease patients with hyposmia under resting-state functional MRI by regional homogeneity and functional connectivity analysis. Park. Relat. Disord. 2015, 21, 698–703. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.H.; Kim, Y.; Park, S.; Byeon, G.H. Depressive symptoms are associated with worse cognitive prognosis in patients with newly diagnosed idiopathic Parkinson disease. Psychogeriatrics 2020, 20, 880–890. [Google Scholar] [CrossRef]

| Variables | Synucleinopathies (n:62) | Controls (n:37) | Comparisons | |||
|---|---|---|---|---|---|---|
Mild (n:29) | Severe (n:33) | Among Subtypes (p Value) | Subtypes vs. Controls (p Value) | |||
| Mean (SD) | Mean (SD) | Mean (SD) | Mild vs. Controls | Severe vs. Controls | ||
| Demographics | ||||||
| Age, years | 53.60 (8.78) | 67.55 (6.73) | 53.48 (12.74) | 0.000 | 0.960 | 0.000 |
| Sex (m/f) | 18/11 | 22/11 | 21/16 | 0.793 | 0.802 | 0.465 |
| Education, years | 13.24 (3.45) | 8.61 (4.03) | 13.84 (5.05) | 0.000 | 0.577 | 0.000 |
| Cognition | ||||||
| MoCA | 26.51 (1.76) | 20.54 (5.02) | 27.24 (3.14) | 0.000 | 0.419 | 0.000 |
| Attention and WM | 0.44 (0.52) | −0.81 (0.56) | 0.36 (0.78) | 0.000 | 0.601 | 0.000 |
| Memory | 0.37 (0.62) | −0.96 (0.64) | 0.53 (0.58) | 0.000 | 0.302 | 0.000 |
| Executive functions | 0.39 (0.56) | −0.92 (0.87) | 0.48 (0.44) | 0.000 | 0.375 | 0.000 |
| Language | 0.41 (0.49) | −0.94 (0.48) | 0.46 (0.82) | 0.000 | 0.161 | 0.000 |
| Visuospatial abilities | 0.29 (0.55) | −0.76 (0.87) | 0.47 (0.55) | 0.000 | 0.439 | 0.000 |
| Processing speed | 0.40 (0.51) | −0.99 (0.50) | 0.53 (0.80) | 0.000 | 0.423 | 0.000 |
| Theory of mind | 0.37 (0.74) | −0.87 (0.96) | 0.44 (0.70) | 0.000 | 0.720 | 0.000 |
| PD-related features | ||||||
| Disease duration, years | 5.80 (3.52) | 7.85 (4.86) | - | 0.078 | - | - |
| Age of onset, years | 48.34 (7.29) | 59.29 (8.05) | - | 0.000 | - | - |
| UPDRS III R no midline | 7.14 (5.27) | 11.28 (3.89) | - | 0.001 | - | - |
| UPDRS III L no midline | 8.61 (4.49) | 12.21 (5.11) | - | 0.007 | - | - |
| Bradykinesia R | 4.30 (2.83) | 7.07 (1.98) | - | 0.000 | - | - |
| Bradykinesia L | 5.52 (2.46) | 7.66 (2.72) | - | 0.003 | - | - |
| Rigidity | 2.11 (1.55) | 2.93 (1.71) | - | 0.066 | - | - |
| Rigidity L | 2.44 (1.58) | 3.14 (1.66) | - | 0.116 | - | - |
| Tremor R | 0.89 (2.23) | 1.45 (1.76) | - | 0.300 | - | - |
| Tremor L | 0.93 (1.47) | 1.59 (1.97) | - | 0.159 | - | - |
| LEDD | 618.76 (369.41) | 736.22 (454.39) | - | 0.295 | - | - |
| PDQ-39 Mobility | 9.40 (8.88) | 11.79 (9.08) | - | 0.325 | - | - |
| PDQ-39 AVDL | 4.51 (5.42) | 8.10 (6.93) | - | 0.035 | - | - |
| PDQ-39 EW | 7.11 (5.43) | 6.31 (6.89) | - | 0.633 | - | - |
| PDQ-39 Stigma | 2.59 (2.91) | 2.20 (4.91) | - | 0.725 | - | - |
| PDQ-39 SS | 1.25 (2.56) | 1.59 (2.18) | - | 0.609 | - | - |
| PDQ-39 Cognition | 3.25 (2.83) | 5.38 (4.73) | - | 0.046 | - | - |
| PDQ-39 Com | 1.22 (1.88) | 3.20 (3.57) | - | 0.012 | - | - |
| PDQ-39 BD | 4 (3.44) | 5.41 (3.81) | - | 0.152 | - | - |
| Dysautonomia | ||||||
| OHT | 0.66 (0.87) | 0.58 (0.84) | 0.12 (0.33) | 0.663 | 0.012 | 0.028 |
| Valsalva PRT | 3.69 (2.05) | 7.61 (4.28) | 2.64 (1.74) | 0.000 | 0.177 | 0.000 |
| HRVdb | 1.01 (0.09) | 0.90 (0.05) | 1.03 (0.09) | 0.000 | 0.334 | 0.000 |
| Vision | ||||||
| Binocular LCVA | 32.93 (6.11) | 18.19 (13.21) | 37.10 (6.38) | 0.000 | 0.031 | 0.000 |
| Photopic CS | 2.0 (0.11) | 1.85 (0.14) | 2.05 (0.13) | 0.000 | 0.086 | 0.000 |
| VFQ-25 | 92.22 (11.99) | 84.32 (15.01) | 96.08 (4.66) | 0.008 | 0.187 | 0.000 |
| Clinical | ||||||
| Apathy | 27.89 (5.13) | 22.62 (8.61) | 29.5 (4.04) | 0.057 | 0.551 | 0.004 |
| Fatigue | 29.76 (17.25) | 34.50 (16.57) | 20.81 (10.96) | 0.218 | 0.017 | 0.000 |
| Depression | 2.59 (2.33) | 3.75 (3.52) | 1.11 (1.62) | 0.082 | 0.023 | 0.000 |
| Olfaction | 7.68 (2.30) | 5.48 (2.61) | 10.48 (1.21) | 0.000 | 0.000 | 0.000 |
| AVDL | 7.83 (0.75) | 6.19 (2.28) | 8 (0) | 0.000 | 0.612 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucas-Jiménez, O.; Ibarretxe-Bilbao, N.; Diez, I.; Peña, J.; Tijero, B.; Galdós, M.; Murueta-Goyena, A.; Del Pino, R.; Acera, M.; Gómez-Esteban, J.C.; et al. Brain Degeneration in Synucleinopathies Based on Analysis of Cognition and Other Nonmotor Features: A Multimodal Imaging Study. Biomedicines 2023, 11, 573. https://doi.org/10.3390/biomedicines11020573
Lucas-Jiménez O, Ibarretxe-Bilbao N, Diez I, Peña J, Tijero B, Galdós M, Murueta-Goyena A, Del Pino R, Acera M, Gómez-Esteban JC, et al. Brain Degeneration in Synucleinopathies Based on Analysis of Cognition and Other Nonmotor Features: A Multimodal Imaging Study. Biomedicines. 2023; 11(2):573. https://doi.org/10.3390/biomedicines11020573
Chicago/Turabian StyleLucas-Jiménez, Olaia, Naroa Ibarretxe-Bilbao, Ibai Diez, Javier Peña, Beatriz Tijero, Marta Galdós, Ane Murueta-Goyena, Rocío Del Pino, Marian Acera, Juan Carlos Gómez-Esteban, and et al. 2023. "Brain Degeneration in Synucleinopathies Based on Analysis of Cognition and Other Nonmotor Features: A Multimodal Imaging Study" Biomedicines 11, no. 2: 573. https://doi.org/10.3390/biomedicines11020573
APA StyleLucas-Jiménez, O., Ibarretxe-Bilbao, N., Diez, I., Peña, J., Tijero, B., Galdós, M., Murueta-Goyena, A., Del Pino, R., Acera, M., Gómez-Esteban, J. C., Gabilondo, I., & Ojeda, N. (2023). Brain Degeneration in Synucleinopathies Based on Analysis of Cognition and Other Nonmotor Features: A Multimodal Imaging Study. Biomedicines, 11(2), 573. https://doi.org/10.3390/biomedicines11020573






