Enamel Structure Defects in Kdf1 Missense Mutation Knock-in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Generation of Kdf1 Knock-in Mice with a Missense Mutation
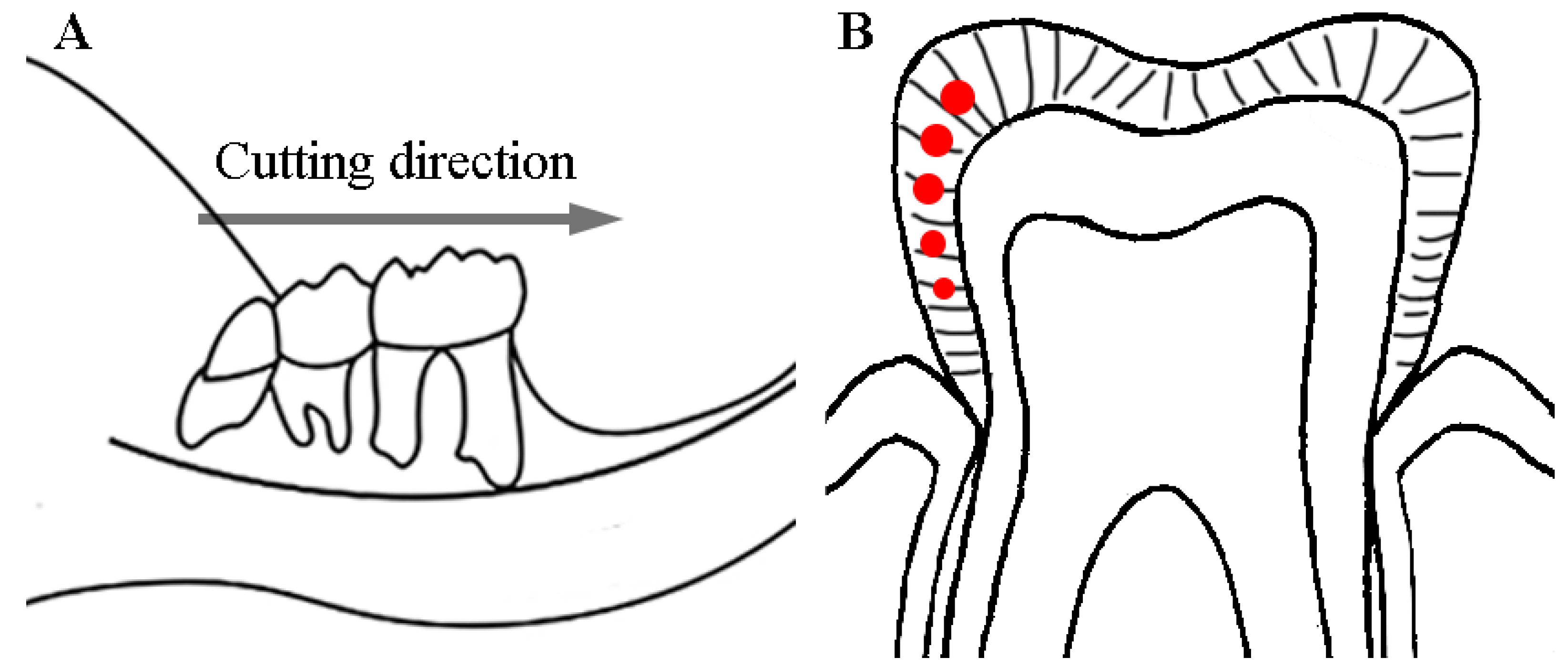
2.2. Sample Preparations and Processing
2.3. X-ray Microtomography
2.4. Scanning Electron Microscopy
2.4.1. Visualization of Prism Structures
2.4.2. Calcium-to-Phosphorus Ratio
2.5. Raman Microspectroscopy
2.6. Atomic Force Microscope Analysis
2.7. Statistics
3. Results
3.1. Generation of Kdf1 Knock-in Mice with a Pathogenic Mutation (R303P)
3.2. The Appearance of Enamel Surface in Kdf1 Mutant Mice
3.3. Enamel Prism Structure and Mechanical Properties in Kdf1 Mutant Mice
3.4. The Mineral Density and Thickness of Enamel Layer in Kdf1 Mutant Mice
3.5. Mineral Composition and Crystal Properties in Kdf1 Mutant Mice Enamel
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stifler, C.A.; Jakes, J.E.; North, J.D.; Green, D.R.; Weaver, J.C.; Gilbert, P. Crystal misorientation correlates with hardness in tooth enamels. Acta Biomater. 2021, 120, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Beniash, E.; Stifler, C.A.; Sun, C.Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Gilbert, P. The hidden structure of human enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef] [PubMed]
- Wilmers, J.; Bargmann, S. Nature’s design solutions in dental enamel: Uniting high strength and extreme damage resistance. Acta Biomater. 2020, 107, 1–24. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Wang, R.; Zhang, D. Role of crystal arrangement on the mechanical performance of enamel. Acta Biomater. 2012, 8, 3784–3793. [Google Scholar] [CrossRef]
- Simmer, J.P.; Papagerakis, P.; Smith, C.E.; Fisher, D.C.; Rountrey, A.N.; Zheng, L.; Hu, J.C. Regulation of dental enamel shape and hardness. J. Dent. Res. 2010, 89, 1024–1038. [Google Scholar] [CrossRef]
- Wright, J.T.; Carrion, I.A.; Morris, C. The molecular basis of hereditary enamel defects in humans. J. Dent. Res. 2015, 94, 52–61. [Google Scholar] [CrossRef]
- Ozdemir, D.; Hart, P.S.; Firatli, E.; Aren, G.; Ryu, O.H.; Hart, T.C. Phenotype of ENAM mutations is dosage-dependent. J. Dent. Res. 2005, 84, 1036–1041. [Google Scholar] [CrossRef]
- Hytönen, M.K.; Arumilli, M.; Sarkiala, E.; Nieminen, P.; Lohi, H. Canine models of human amelogenesis imperfecta: Identification of novel recessive ENAM and ACP4 variants. Hum. Genet. 2019, 138, 525–533. [Google Scholar] [CrossRef]
- Brookes, S.J.; Barron, M.J.; Smith, C.E.L.; Poulter, J.A.; Mighell, A.J.; Inglehearn, C.F.; Brown, C.J.; Rodd, H.; Kirkham, J.; Dixon, M.J. Amelogenesis imperfecta caused by N-terminal enamelin point mutations in mice and men is driven by endoplasmic reticulum stress. Hum. Mol. Genet. 2017, 26, 1863–1876. [Google Scholar] [CrossRef]
- Smith, C.E.; Richardson, A.S.; Hu, Y.; Bartlett, J.D.; Hu, J.C.C.; Simmer, J.P. Effect of Kallikrein 4 Loss on Enamel Mineralization: Comparison with mice lacking matrix metalloproteinase 20. J. Biol. Chem. 2011, 286, 18149–18160. [Google Scholar] [CrossRef]
- Bartlett, J.D.; Simmer, J.P. Kallikrein-related peptidase-4 (KLK4): Role in enamel formation and revelations from ablated mice. Front. Physiol. 2014, 5, 240. [Google Scholar] [CrossRef] [PubMed]
- Núñez, S.M.; Chun, Y.P.; Ganss, B.; Hu, Y.; Richardson, A.S.; Schmitz, J.E.; Fajardo, R.; Yang, J.; Hu, J.C.; Simmer, J.P. Maturation stage enamel malformations in Amtn and Klk4 null mice. Matrix Biol. 2016, 52–54, 219–233. [Google Scholar] [CrossRef]
- Simmer, J.P.; Hu, Y.; Lertlam, R.; Yamakoshi, Y.; Hu, J.C.C. Hypomaturation enamel defects in Klk4 knockout/LacZ knockin mice. J. Biol. Chem. 2009, 284, 19110–19121. [Google Scholar] [CrossRef]
- Gasse, B.; Karayigit, E.; Mathieu, E.; Jung, S.; Garret, A.; Huckert, M.; Morkmued, S.; Schneider, C.; Vidal, L.; Hemmerlé, J.; et al. Homozygous and compound heterozygous MMP20 mutations in amelogenesis imperfecta. J. Dent. Res. 2013, 92, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Skobe, Z.; Nanci, A.; Smith, C.E. Matrix metalloproteinase 20 promotes a smooth enamel surface, a strong dentino-enamel junction, and a decussating enamel rod pattern. Eur. J. Oral Sci. 2011, 119 (Suppl. 1), 199–205. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, K.; Páez, M.T.; Kurosawa, K.; Yamamoto, T. Proximal interstitial 1p36 deletion syndrome: The most proximal 3.5-Mb microdeletion identified on a dysmorphic and mentally retarded patient with inv(3)(p14.1q26.2). Brain Dev. 2009, 31, 629–633. [Google Scholar] [CrossRef]
- Zanardo, É.A.; Piazzon, F.B.; Dutra, R.L.; Dias, A.T.; Montenegro, M.M.; Novo-Filho, G.M.; Costa, T.V.; Nascimento, A.M.; Kim, C.A.; Kulikowski, L.D. Complex structural rearrangement features suggesting chromoanagenesis mechanism in a case of 1p36 deletion syndrome. Mol. Genet. Genom. 2014, 289, 1037–1043. [Google Scholar] [CrossRef]
- Lee, S.; Kong, Y.; Weatherbee, S.D. Forward genetics identifies Kdf1/1810019J16Rik as an essential regulator of the proliferation-differentiation decision in epidermal progenitor cells. Dev. Biol. 2013, 383, 201–213. [Google Scholar] [CrossRef]
- Shamseldin, H.E.; Khalifa, O.; Binamer, Y.M.; Almutawa, A.; Arold, S.T.; Zaidan, H.; Alkuraya, F.S. KDF1, encoding keratinocyte differentiation factor 1, is mutated in a multigenerational family with ectodermal dysplasia. Hum. Genet. 2017, 136, 99–105. [Google Scholar] [CrossRef]
- Manaspon, C.; Thaweesapphithak, S.; Osathanon, T.; Suphapeetiporn, K.; Porntaveetus, T.; Shotelersuk, V. A novel de novo mutation substantiates KDF1 as a gene causing ectodermal dysplasia. Br. J. Dermatol. 2019, 181, 419–420. [Google Scholar] [CrossRef]
- Zeng, B.; Lu, H.; Xiao, X.; Yu, X.; Li, S.; Zhu, L.; Yu, D.; Zhao, W. KDF1 is a novel candidate gene of non-syndromic tooth agenesis. Arch. Oral Biol. 2019, 97, 131–136. [Google Scholar] [CrossRef]
- Pan, Y.; Yi, S.; Chen, D.; Du, X.; Yao, X.; He, F.; Xiong, F. Identification of a novel missense heterozygous mutation in the KDF1 gene for non-syndromic congenital anodontia. Clin. Oral Investig. 2022, 26, 5171–5179. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Whitehouse, L.L.; Poulter, J.A.; Brookes, S.J.; Day, P.F.; Soldani, F.; Kirkham, J.; Inglehearn, C.F.; Mighell, A.J. Defects in the acid phosphatase ACPT cause recessive hypoplastic amelogenesis imperfecta. Eur. J. Hum. Genet. 2017, 25, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Brookes, S.J.; Barron, M.J.; Boot-Handford, R.; Kirkham, J.; Dixon, M.J. Endoplasmic reticulum stress in amelogenesis imperfecta and phenotypic rescue using 4-phenylbutyrate. Hum. Mol. Genet. 2014, 23, 2468–2480. [Google Scholar] [CrossRef]
- Wazen, R.M.; Moffatt, P.; Zalzal, S.F.; Yamada, Y.; Nanci, A. A mouse model expressing a truncated form of ameloblastin exhibits dental and junctional epithelium defects. Matrix Biol. 2009, 28, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Xu, Q.; Zhang, H.; Wang, S.; Diekwisch, T.G.H.; Qin, C.; Lu, Y. Enamel Defects Associated With Dentin Sialophosphoprotein Mutation in Mice. Front. Physiol. 2021, 12, 724098. [Google Scholar] [CrossRef]
- Song, W.; Wang, Y.; Chu, Q.; Qi, C.; Gao, Y.; Gao, Y.; Xiang, L.; Zhenzhen, X.; Gao, Y. Loss of transforming growth factor-β1 in epithelium cells affects enamel formation in mice. Arch. Oral Biol. 2018, 96, 146–154. [Google Scholar] [CrossRef]
- Sánchez-Quevedo, C.; Ceballos, G.; Rodríguez, I.A.; García, J.M.; Alaminos, M. Acid-etching effects in hypomineralized amelogenesis imperfecta. A microscopic and microanalytical study. Med. Oral Patol. Oral Cir. Bucal. 2006, 11, E40–E43. [Google Scholar]
- Ang, S.F.; Scholz, T.; Klocke, A.; Schneider, G.A. Determination of the elastic/plastic transition of human enamel by nanoindentation. Dent. Mater. 2009, 25, 1403–1410. [Google Scholar] [CrossRef]
- Courtois, G.; Smahi, A.; Reichenbach, J.; Döffinger, R.; Cancrini, C.; Bonnet, M.; Puel, A.; Chable-Bessia, C.; Yamaoka, S.; Feinberg, J.; et al. A hypermorphic IkappaBalpha mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T cell immunodeficiency. J. Clin. Investig. 2003, 112, 1108–1115. [Google Scholar] [CrossRef]
- Ohazama, A.; Hu, Y.; Schmidt-Ullrich, R.; Cao, Y.; Scheidereit, C.; Karin, M.; Sharpe, P.T. A dual role for Ikk alpha in tooth development. Dev. Cell 2004, 6, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, P. Genetic basis of tooth agenesis. J. Exp. Zool. B Mol. Dev. Evol. 2009, 312, 320–342. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Swain, M.V.; Hoffman, M.J. Structural integrity of enamel: Experimental and modeling. J. Dent. Res. 2009, 88, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.; Briggs, H.D.; Atkinson, P.J.; Weatherell, J.A. Matrix and mineral changes in developing enamel. J. Dent. Res. 1979, 58, 871–882. [Google Scholar] [CrossRef]
- Mohazab, L.; Koivisto, L.; Jiang, G.; Kytömäki, L.; Haapasalo, M.; Owen, G.R.; Wiebe, C.; Xie, Y.; Heikinheimo, K.; Yoshida, T.; et al. Critical role for αvβ6 integrin in enamel biomineralization. J. Cell Sci. 2013, 126, 732–744. [Google Scholar] [CrossRef]
- Gibson, C.W.; Yuan, Z.A.; Li, Y.; Daly, B.; Suggs, C.; Aragon, M.A.; Alawi, F.; Kulkarni, A.B.; Wright, J.T. Transgenic mice that express normal and mutated amelogenins. J. Dent. Res. 2007, 86, 331–335. [Google Scholar] [CrossRef]
- Xia, J.; Zheng, J.; Huang, D.; Tian, Z.R.; Chen, L.; Zhou, Z.; Ungar, P.S.; Qian, L. New model to explain tooth wear with implications for microwear formation and diet reconstruction. Proc. Natl. Acad. Sci. USA 2015, 112, 10669–10672. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Nishino, M.; Yamashita, S.; Aoba, T.; Okazaki, M.; Moriwaki, Y. The laser-Raman spectroscopic studies on human enamel and precipitated carbonate-containing apatites. J. Dent. Res. 1981, 60, 751–755. [Google Scholar] [CrossRef]
- Xie, Z.H.; Mahoney, E.K.; Kilpatrick, N.M.; Swain, M.V.; Hoffman, M. On the structure-property relationship of sound and hypomineralized enamel. Acta Biomater. 2007, 3, 865–872. [Google Scholar] [CrossRef]





| Parameters | WT | HO | Statistically Significant Difference |
|---|---|---|---|
| Ra (nm) | 20.23 ± 8.39 | 82.90 ± 20.72 | <0.01 ** |
| Rq (nm) | 23.57 ± 5.54 | 98.43 ± 14.28 | <0.01 ** |
| Rmax (nm) | 195.0 ± 34.83 | 826.73 ± 199.12 | <0.01 ** |
| Young’s Modulus (Gpa) | 75.57 ± 6.96 | 54.89 ± 3.70 | <0.05 * |
| Parameters | WT Mice Enamel | HO Mice Enamel | Statistically Significant Difference | ||
|---|---|---|---|---|---|
| Average | SD | Average | SD | ||
| Position of the Phospate ν1 band | 959.35 | 0.10 | 959.02 | 0.10 | n.s. |
| FWHM of the Phospate ν1 band | 13.83 | 0.14 | 15.11 | 0.27 | <0.001 *** |
| Position of the Carbonate band | 1070.92 | 0.38 | 1070.67 | 1.07 | n.s. |
| FWHM of the Carbonate band | 16.71 | 2.11 | 17.04 | 3.47 | n.s. |
| Ratio of Carbonate/Phosphate | 0.034 | 0.02 | 0.047 | 0.05 | <0.01 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zeng, B.; Xie, W.; Xiao, X.; Lin, L.; Yu, D.; Zhao, W. Enamel Structure Defects in Kdf1 Missense Mutation Knock-in Mice. Biomedicines 2023, 11, 482. https://doi.org/10.3390/biomedicines11020482
Li P, Zeng B, Xie W, Xiao X, Lin L, Yu D, Zhao W. Enamel Structure Defects in Kdf1 Missense Mutation Knock-in Mice. Biomedicines. 2023; 11(2):482. https://doi.org/10.3390/biomedicines11020482
Chicago/Turabian StyleLi, Pei, Binghui Zeng, Weihong Xie, Xue Xiao, Ling Lin, Dongsheng Yu, and Wei Zhao. 2023. "Enamel Structure Defects in Kdf1 Missense Mutation Knock-in Mice" Biomedicines 11, no. 2: 482. https://doi.org/10.3390/biomedicines11020482
APA StyleLi, P., Zeng, B., Xie, W., Xiao, X., Lin, L., Yu, D., & Zhao, W. (2023). Enamel Structure Defects in Kdf1 Missense Mutation Knock-in Mice. Biomedicines, 11(2), 482. https://doi.org/10.3390/biomedicines11020482








