A Systematic Review and Meta-Analysis on the Efficacy of Locally Delivered Adjunctive Curcumin (Curcuma longa L.) in the Treatment of Periodontitis
Abstract
1. Introduction
2. Materials and Methods
3. Results
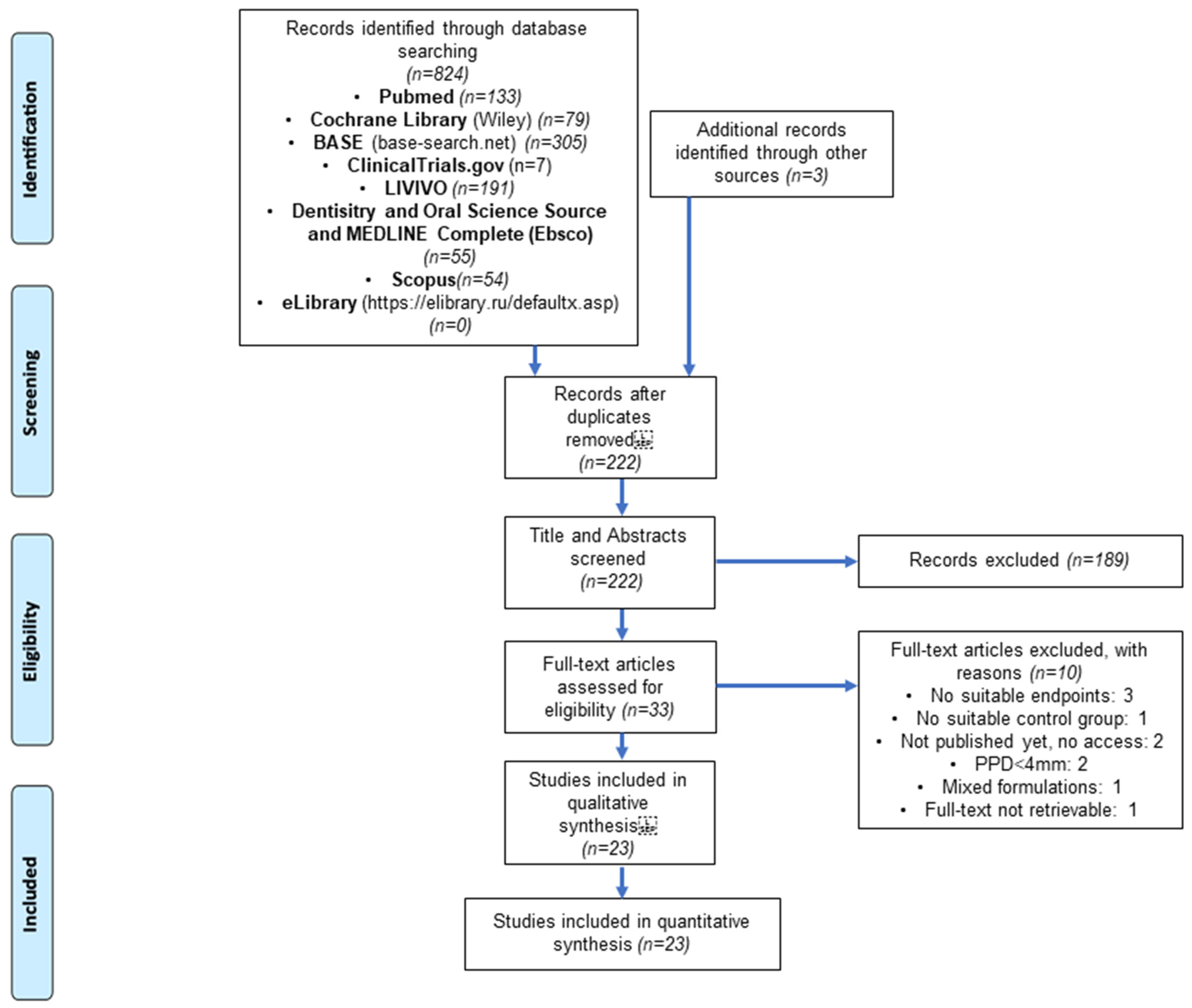
3.1. Literature Search and Screening
3.2. Study and Patient Characteristics
| Study | Sample Size | Study Design | Clinical Parameters | Intervention and Control | Stent | COE Pack | Follow-Up Periods | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies Comparing SRP alone to SRP and Curcumin | ||||||||||
| Behal et al., 2011 [31] | n = 30 PPD 5–7 mm | SMD | PI (Turesky-Gilmore-Glickman), GI (Löe and Silness), SBI (Muhlemann), PPD (William probe), CAL | Group 1: SRP alone | Group 2: SRP was followed by local application 2% turmeric gel | yes | yes | 0, 30, 45 d /0, 30 d | ||
| Bhatia et al., 2014 [32] | n = 25, (15♂, 10♀, 21–45 y.o.) PPD > 5 mm. | SMD | PI (Silness and Löe), SBI (Muhlemann), PPD, CAL | Group 1: SRP alone | Group 2: SRP was followed by local application of 1% curcumin gel | yes | yes | 0, 1, 3, 6 m /0, 1 m | ||
| Anuradha et al., 2015 [33] | n = 30 (25–60 y.o.) PPD 5–7 mm | SMD | PI (Turesky-Gilmore-Glickman), GI (Löe and Silness), PPD, CAL (UNC-15) | Group 1: SRP alone | Group 2: SRP was followed by local application of curcumin gel (10 mg of Curcuma longa extract/g) | yes | yes | 0, 30, 45 d /0, 30 d | ||
| Nagasri et al., 2015 [34] | n = 30 (12♂, 18♀, 35–60 y.o.) PPD ≥ 5 mm | SMD | PI (Silness and Löe), GI (Löe and Silness), PPD, CAL | Group 1: SRP alone | Group 2: SRP was followed by local application of curcumin gel (10 mg of Curcuma longa extract/g) | yes | no | 0, 4 w /0, 4 w | ||
| Shivanand et al., 2016 [35] | n = 14 (6♂, 8♀, 35–50 y.o.) PPD 5–7 mm | SMD | PI (Silness and Löe), GI (Löe and Silness), BOP, PPD, CAL | Group 1: upper arch received SRP alone | Group 2: lower arch received SRP and application of curcumin gel (10 mg of Curcuma longa extract/g) | no | yes | 0, 21, 30, 90 d /0, 30 d | ||
| Nasra et al., 2017 [36] | n = 10 (35–55 y.o.) PPD 5–7 mm | PGD | PPD (Glavind and Löe), SBI (Checchi), GI (Löe and Silness), PI (Silness and Löe) | Group 1: SRP alone | Group 2: SRP and curcumin gel 2%, the application was repeated once weekly over three weeks period | no | no | 0, 1 m /0, 1 m | ||
| Dave et al., 2018 [37] | n = 20 (9♂, 11♀, 20–59 y.o.) PPD 4–6 mm | PGD | PI, BOP, SBI, PPD, CAL (UNC15) | Group1: SRP alone | Group 2: SRP and curcumin gel 10%, patients were instructed to apply gel for 2–3 min once daily | no | no | 0, 2 m /0, 2 m | ||
| Raghava et al., 2019 [38] | n = 10 (5♂, 5♀, 25–40 y.o.) PPD ≥ 5 mm | SMD | PI (Silness and Löe), GI (Löe and Silness), PPD, CAL | Group 1: SRP alone | Group 2: SRP was followed by local application of curcumin gel (10 mg of Curcuma longa extract/g) | no | yes | 0, 4 w /0, 4 w | ||
| Kaur et al., 2019 [39] | n = 29 (20♂, 9♀, 20–65 y.o.) PPD ≥ 5 mm | PGD | PI (Silness and Löe), SBI (Muhlemann), PPD, CAL (UNC-15) | Group 1: SRP alone | Group 2: SRP was followed by local application 1% curcumin gel (10 mg of Curcuma longa extract/g) | yes | yes | 0, 1,3 m /0, 1 m | ||
| Perez-Pacheco et al., 2020 [40] | n = 20 (6♂, 14♀, 37–62 y.o.) PPD ≥ 5 mm | SMD | PI (O’Leary), GI (Ainamo and Bay), BOP, PPD, gingival recession, CAL (UNC-15) | Group 1: SRP and 0.05 mg/mL nano capsulated curcumin | Group 2: SRP and empty nanoparticles | no | no | 0, 1, 2, 6 m /0, 1 m | ||
| Farhood et al., 2020 [41] | n = 20 (9♂, 11♀, ≥21–45 y.o.) PPD 5–7 mm | SMD | PI, GI, BOP, PPD, CAL | Group 1: SRP alone | Group 2: SRP was followed by local application of curcumin gel (10 mg of Curcuma longa extract/g) Second application after 1 week | yes | yes | 0, 1 m /0, 1 m | ||
| Studies comparing SRP alone to SRP and Curcumin and a third or fourth control (not included in this meta-analysis) | ||||||||||
| Mohammed et al., 2020 [42] | n = 90 (35♂, 55♀, 25–54 y.o.) PPD ≥ 4 mm | PGD | PPD, CAL, GI, BOP | Group 1: healthy periodontium (control group) | Group 2: for periodontitis patients SRP and curcumin gel (C. Longa extract, 10 mg) | Group 3: periodontitis patients receiving SRP alone | no | yes | 0, 1 m /0, 1 m | |
| Rahalkar et al., 2021 [43] | n = 15 (5♂, 10♀, 37–57 y.o.) PPD ≥ 5 mm | SMD | PPD, CAL, GI (Löe and Silness), PI (Silness and Löe), SBI (Mombelli) | Group 1: SRP alone | Group 2: SRP and curcumin gel (C. Longa extract, 10 mg) | Group 3: SRP and tulsi extract | yes | yes | 0, 30 d /0, 30 d | |
| Elavarasu et al., 2016 [44] | n = 15 (35–50 y.o.) PPD 5–6 mm | SMD | PI, GI, SBI, PPD | Group 1: Healthy periodontium (control) | Group 2: SRP alone | Group 3: SRP and curcumin strip placement 0,2% loaded on to guided tissue membrane (GTR) | no | no | 0, 21 d /0, 21 d | |
| Saini et al., 2021 [45] | n = 30 (30–65 y.o.) PPD ≥ 5 mm | SMD | PI, GI, PPD, CAL (UNC-15) | Group 1: SRP and 5% neem chip | Group 2: SRP and 5% turmeric chip | Group 3: SRP and placebo chip | yes | yes | 0, 1, 3 m /0, 1 m | |
| Sreedhar et al., 2015 [46] | n = 15 (15P, 7♂, 8♀, 35–55 y.o.) PPD ≥ 5 mm | SMD | PI, SBI, PPD, CAL | Group 1: SRP alone. | Group 2: SRP anfd curcumin gel (10 mg of Curcuma longa extract/g) application for 5 min | Group 3: SRP and curcumin application for 5 min and irradiation with blue light emitting diode | Group 4: SRP and curcumin PDT | yes | no | 0, 1, 3 m /0, 3 m |
| Studies comparing SRP and CHX to SRP and Curcumin | ||||||||||
| Gottumukkala et al., 2014 [47] | n = 60 (25–55 y.o.) PPD ≥ 5 mm | SMD | PPD, CAL, GI (Löe and Silness), PI (Silness and Löe) | Group 1: SRP alone along with CHX chip (2.5 mg) | Group 2: received SRP along with curcumin chip (CU extract concentration of 50 mg/cm) | yes | no | 0, 1, 3, 6 m /0, 1 m | ||
| Anitha et al., 2015 [48] | n= 30 (20♂, 10♀, 20–50 y.o.) PPD 4–6 mm | SMD | PPD, CAL, GI (Löe and Silness), PI (Turesky-Gillmore) | Group 1: receiving SRP and curcumin gel (250 g of the powdered rhizome of Curcuma longa in 5 mL ethanol) Repeated application at day 15 | Group 2: SRP and CHX gel 0.1% Repeated application at day 15 | no | yes | 0, 15, 30 d /0, 30 d | ||
| Siddarth et al., 2020 [49] | n= 25 (20♂, 5♀, ≥30 y.o.) PPD ≥ 5 mm | SMD | GI (Löe and Silness), PI (Silness and Löe), SBI (Muhlemann), PPD, CAL | Group 1: SRP and application of 2% curcumin gel | Group 2: SRP and application of 0.2% CHX gel | no | yes | 0, 1, 3 m /0, 1 m | ||
| Studies comparing SRP alone to SRP and Curcumin and SRP and CHX | ||||||||||
| Jaswal et al., 2014 [50] | n = 15 (12♂, 3♀, 21–55 y.o.) PPD 5–7 mm | SMD | PI (Silness and Löe), GI (Löe and Silness), PPD, CAL (UNC-15) | Group 1: received SRP and 2% turmeric gel | Group 2: receiving SRP and 1% CHX | Group 3: SRP alone | no | yes | 0, 30, 45 d /0, 30 d | |
| Singh et al., 2018 [51] | n = 40 (22♂, 18♀, 30–50 y.o.) PPD 5–8 mm | SMD | PI, GI, PPD, CAL (UNC-15) | Group 1: SRP and sites treated with CHX chip (2.5 mg) | Group 2: SRP and sites treated with 5% turmeric chip | Group 3: SRP alone | yes | no | 0, 1, 3 m /0, 1 m | |
| Guru et al., 2020 [52] | n = 45 (36♂, 9♀, 25–50 y.o.) PPD 5–7 mm | PGD | PI, GI, PPD, CAL (UNC-15) | Group 1: SRP alone | Group 2: SRP, 2%curcumin with nanogel | Group 3: SRP, 1% CHX gel | yes | yes | 0, 21, 45 d /0, 21 d | |
| Gottumukkala et al., 2013 [53] | n = 26 (12♂, 14♀, 30–55 y.o.) PPD ≥ 5 mm | SMD | PI (Silness and Löe), BOP, redness, PPD (UNC15) | Group 1: SRP and saline irrigation Repeated gingival irrigation at 7,14 and 21 d | Group 2: SRP and 1% curcumin solution, repeated gingival irrigation at 7, 14 and 21 d | Group 3: SRP and 0.2% CHX Repeated gingival irrigation at 7, 14 and 21 d | yes | no | 0, 1, 3, 6 m /0, 1 m | |
3.3. Quality Assessment
3.4. Clinical Attachment Level Loss
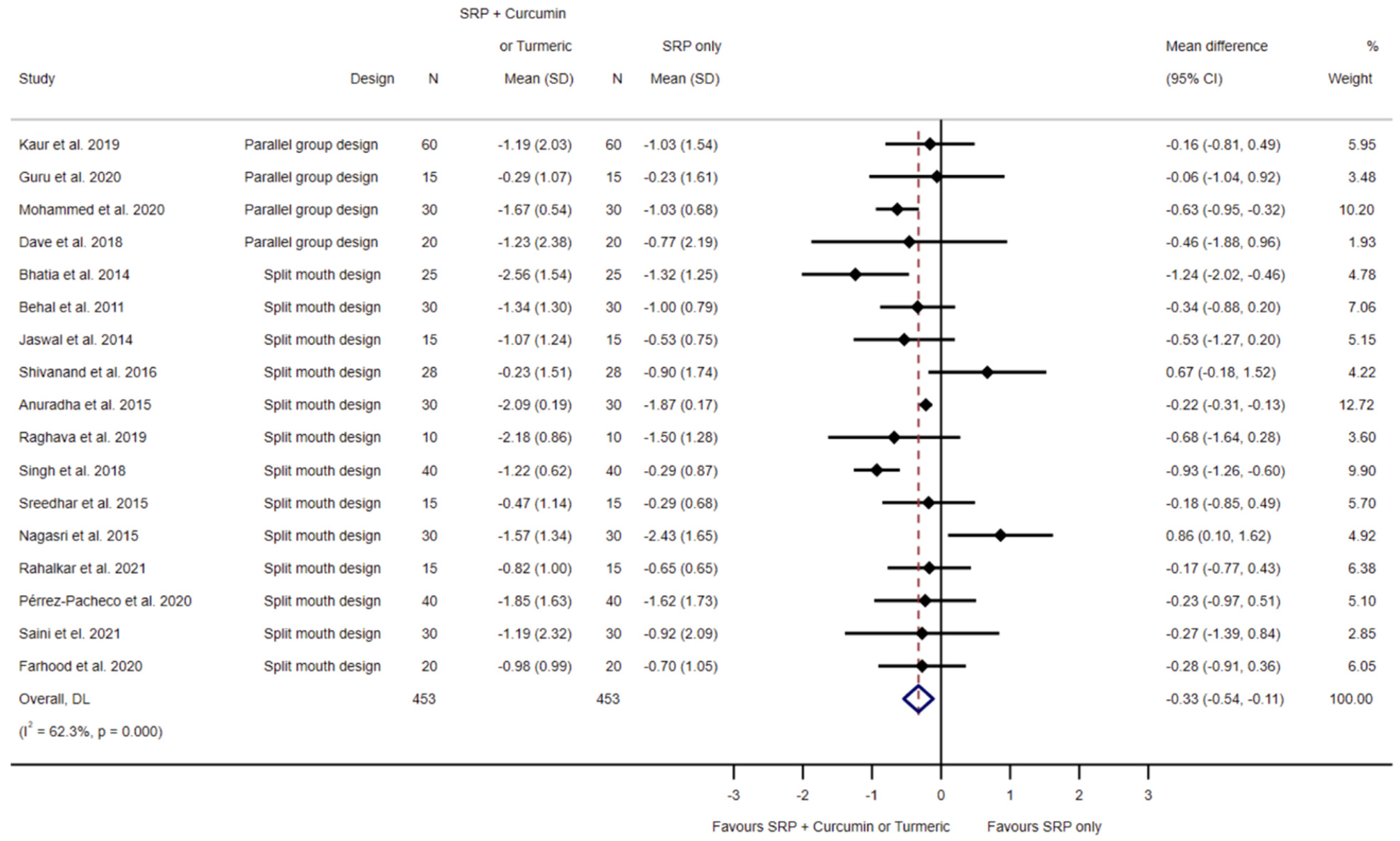
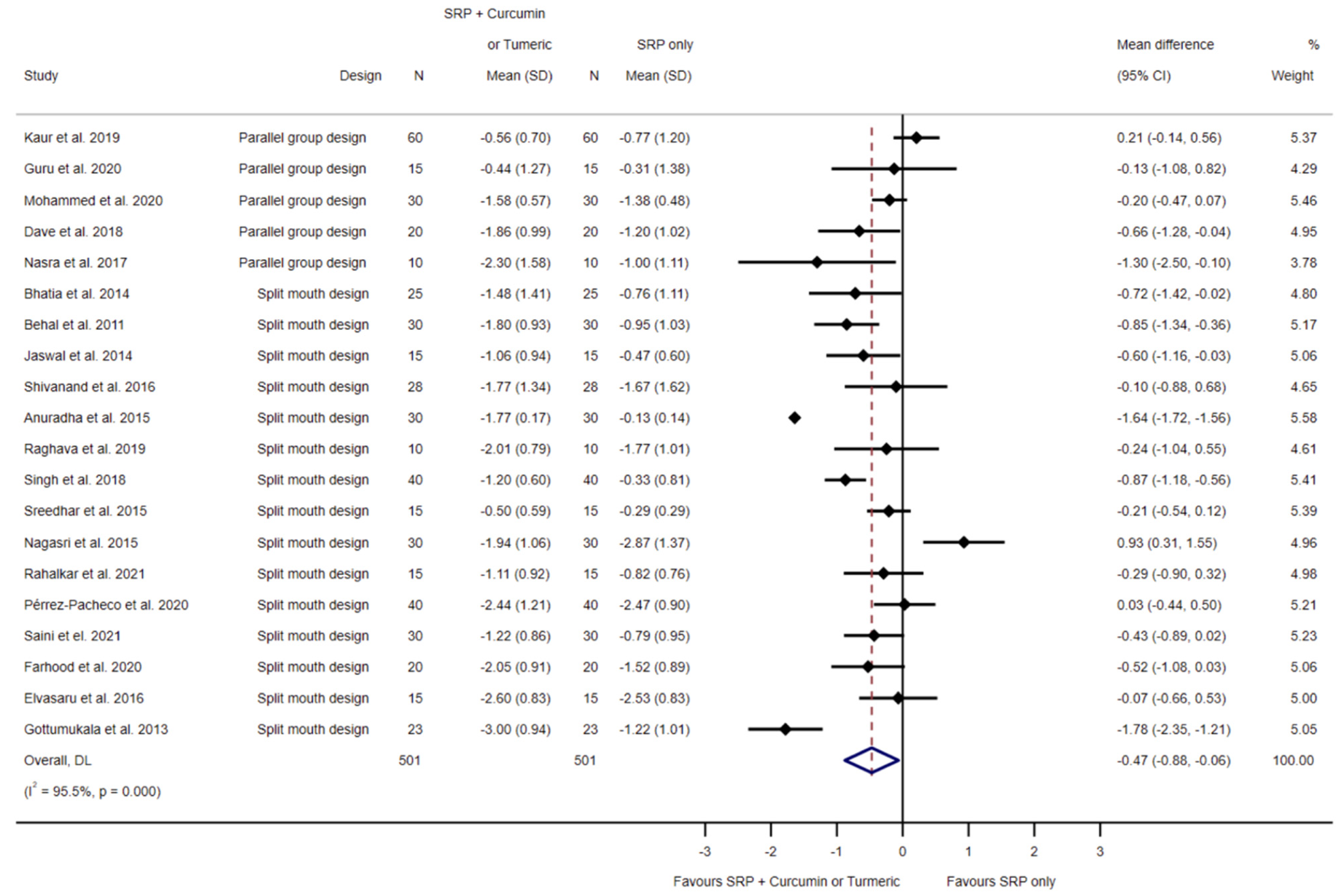
3.4.1. SRP Alone Compared to SRP and Local Curcumin
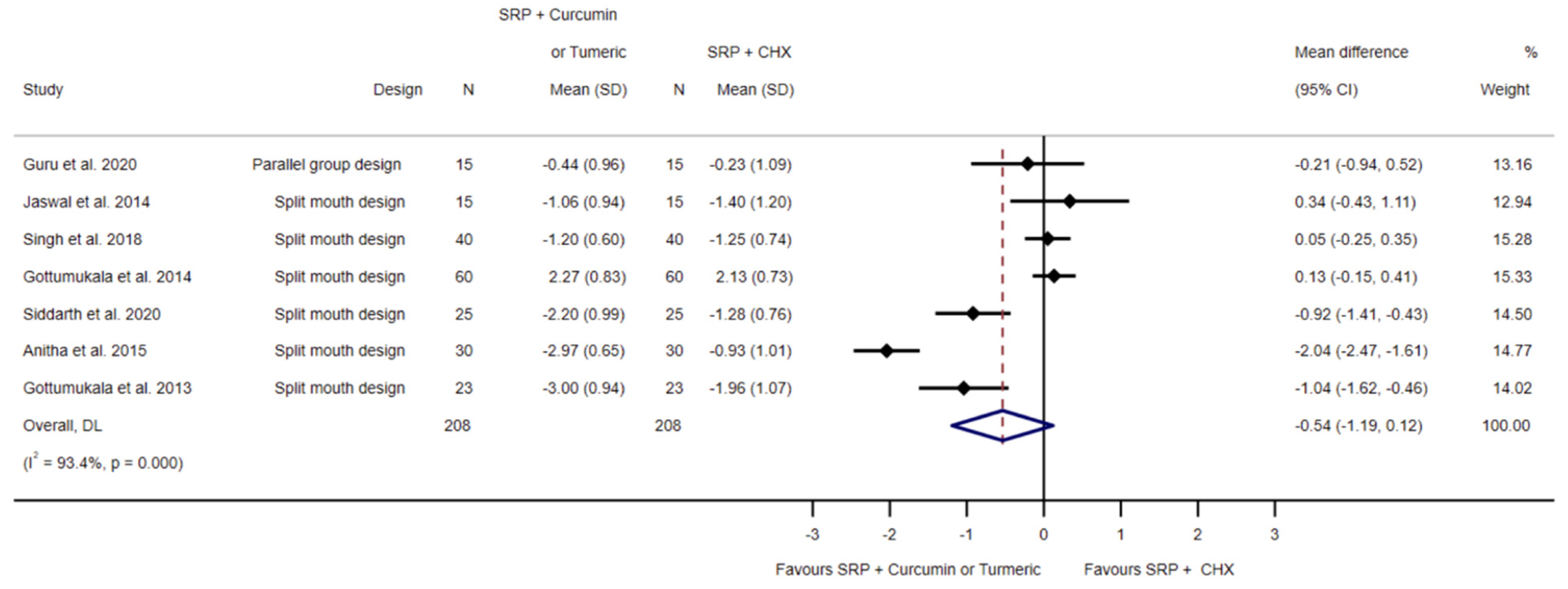
3.4.2. SRP and Chlorhexidine Compared to SRP and Local Curcumin
3.5. Probing Pocket Depth Reduction
3.5.1. SRP Alone Compared to SRP and Local Curcumin
3.5.2. SRP and Chlorhexidine Compared to SRP and Local Curcumin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, Regional, and National Burden of Severe Periodontitis, 1990–2019: An Analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef] [PubMed]
- Arigbede, A.; Babatope, B.; Bamidele, M. Periodontitis and Systemic Diseases: A Literature Review. J. Indian Soc. Periodontol. 2012, 16, 487–491. [Google Scholar] [CrossRef]
- Kuraji, R.; Sekino, S.; Kapila, Y.; Numabe, Y. Periodontal Disease–Related Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: An Emerging Concept of Oral-liver Axis. Periodontol. 2000 2021, 87, 204–240. [Google Scholar] [CrossRef] [PubMed]
- Jakubovics, N.S.; Goodman, S.D.; Mashburn-Warren, L.; Stafford, G.P.; Cieplik, F. The Dental Plaque Biofilm Matrix. Periodontol. 2000 2021, 86, 32–56. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Santonocito, S.; Dalessandri, D.; Migliorati, M.; Indelicato, F. New Frontiers on Adjuvants Drug Strategies and Treatments in Periodontitis. Sci. Pharm. 2021, 89, 46. [Google Scholar] [CrossRef]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine Mouthrinse as an Adjunctive Treatment for Gingival Health. Cochrane Database Syst. Rev. 2017, 2021, CD008676. [Google Scholar] [CrossRef]
- Marshall, M.V.; Cancro, L.P.; Fischman, S.L. Hydrogen Peroxide: A Review of Its Use in Dentistry. J. Periodontol. 1995, 66, 786–796. [Google Scholar] [CrossRef]
- Walsh, L.J. Safety Issues Relating to the Use of Hydrogen Peroxide in Dentistry. Aust. Dent. J. 2000, 45, 257–269. [Google Scholar] [CrossRef]
- Karygianni, L.; Al-Ahmad, A.; Argyropoulou, A.; Hellwig, E.; Anderson, A.C.; Skaltsounis, A.L. Natural Antimicrobials and Oral Microorganisms: A Systematic Review on Herbal Interventions for the Eradication of Multispecies Oral Biofilms. Front. Microbiol. 2016, 6, 1529. [Google Scholar] [CrossRef]
- Goudouri, O.-M.; Kontonasaki, E.; Lohbauer, U.; Boccaccini, A.R. Antibacterial Properties of Metal and Metalloid Ions in Chronic Periodontitis and Peri-Implantitis Therapy. Acta Biomater. 2014, 10, 3795–3810. [Google Scholar] [CrossRef]
- Sgolastra, F.; Petrucci, A.; Gatto, R.; Marzo, G.; Monaco, A. Photodynamic Therapy in the Treatment of Chronic Periodontitis: A Systematic Review and Meta-Analysis. Lasers Med. Sci. 2013, 28, 669–682. [Google Scholar] [CrossRef]
- Novaes, V.C.N.; Ervolino, E.; Fernandes, G.L.; Cunha, C.P.; Theodoro, L.H.; Garcia, V.G.; de Almeida, J.M. Influence of the Treatment with the Antineoplastic Agents 5-Fluorouracil and Cisplatin on the Severity of Experimental Periodontitis in Rats. Support. Care Cancer 2021, 30, 1967–1980. [Google Scholar] [CrossRef]
- Engel Naves Freire, A.; Macedo Iunes Carrera, T.; de Oliveira, G.J.P.L.; Pigossi, S.C.; Vital Ribeiro Júnior, N. Comparison between Antimicrobial Photodynamic Therapy and Low-Level Laser Therapy on Non-Surgical Periodontal Treatment: A Clinical Study. Photodiagnosis Photodyn. Ther. 2020, 31, 101756. [Google Scholar] [CrossRef]
- Zielińska, A.; Kubasiewicz, K.; Wójcicki, K.; Silva, A.M.; Nunes, F.M.; Szalata, M.; Słomski, R.; Eder, P.; Souto, E.B. Two- and Three-Dimensional Spectrofluorimetric Qualitative Analysis of Selected Vegetable Oils for Biomedical Applications. Molecules 2020, 25, 5608. [Google Scholar] [CrossRef]
- Livada, R.; Shiloah, J.; Tipton, D.A.; Dabbous, M. The Potential Role of Curcumin in Periodontal Therapy: A Review of the Literature. J. Int. Acad. Periodontol. 2017, 19, 70–79. [Google Scholar]
- Goel, A.; Boland, C.R.; Chauhan, D.P. Specific Inhibition of Cyclooxygenase-2 (COX-2) Expression by Dietary Curcumin in HT-29 Human Colon Cancer Cells. Cancer Lett. 2001, 172, 111–118. [Google Scholar] [CrossRef]
- Zhang, F.; Altorki, N.K.; Mestre, J.R.; Subbaramaiah, K.; Dannenberg, A.J. Curcumin Inhibits Cyclooxygenase-2 Transcription in Bile Acid- and Phorbol Ester-Treated Human Gastrointestinal Epithelial Cells. Carcinogenesis 1999, 20, 445–451. [Google Scholar] [CrossRef]
- Plummer, S.M.; Holloway, K.A.; Manson, M.M.; Munks, R.J.; Kaptein, A.; Farrow, S.; Howells, L. Inhibition of Cyclo-Oxygenase 2 Expression in Colon Cells by the Chemopreventive Agent Curcumin Involves Inhibition of NF-ΚB Activation via the NIK/IKK Signalling Complex. Oncogene 1999, 18, 6013–6020. [Google Scholar] [CrossRef]
- Rao, C.V.; Rivenson, A.; Simi, B. Chemoprevention of Colon Carcinogenesis by Dietary Curcumin, a Naturally Occurring Plant Phenolic Compound. Cancer Res. 1995, 55, 259–266. [Google Scholar] [PubMed]
- Yamamoto, H.; Hanada, K.; Kawasaki, K.; Nishijima, M. Inhibitory Effect of Curcumin on Mammalian Phospholipase D Activity. FEBS Lett. 1997, 417, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.T.; Safayhi, H.; Mack, T.; Sabieraj, J. Mechanism of Antiinflammatory Actions of Curcumine and Boswellic Acids. J. Ethnopharmacol. 1993, 38, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Began, G.; Sudharshan, E.; Rao, A.G.A. Inhibition of Lipoxygenase 1 by Phosphatidylcholine Micelles-Bound Curcumin. Lipids 1998, 33, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak-Jankun, E.; McCabe, N.P.; Selman, S.H.; Jankun, J. Curcumin Inhibits Lipoxygenase by Binding to Its Central Cavity: Theoretical and X-Ray Evidence. Int. J. Mol. Med. 2000, 6, 521–527. [Google Scholar] [CrossRef]
- Paris, S.; Wolgin, M.; Kielbassa, A.M.; Pries, A.; Zakrzewicz, A. Gene Expression of Human Beta-Defensins in Healthy and Inflamed Human Dental Pulps. J. Endod. 2009, 35, 520–523. [Google Scholar] [CrossRef]
- Briers, Y.; Walmagh, M.; Van Puyenbroeck, V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.-P.; et al. Engineered Endolysin-Based “Artilysins” To Combat Multidrug-Resistant Gram-Negative Pathogens. mBio 2014, 5, e01379-14. [Google Scholar] [CrossRef]
- Rudrappa, T.; Bais, H.P. Curcumin, a Known Phenolic from Curcuma Longa, Attenuates the Virulence of Pseudomonas Aeruginosa PAO1 in Whole Plant and Animal Pathogenicity Models. J. Agric. Food Chem. 2008, 56, 1955–1962. [Google Scholar] [CrossRef]
- Helalat, L.; Zarejavid, A.; Ekrami, A. The Effect of Curcumin on Growth and Adherence of Major Microorganisms Causing Tooth Decay. Middle East J. Fam. Med. 2017, 15, 214–220. [Google Scholar] [CrossRef]
- Song, J.; Choi, B.; Jin, E.-J.; Yoon, Y.; Choi, K.-H. Curcumin Suppresses Streptococcus Mutans Adherence to Human Tooth Surfaces and Extracellular Matrix Proteins. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1347–1352. [Google Scholar] [CrossRef]
- Behal, R.; Gilda, S.; Paradkar, A.; Mali, A. Evaluation of Local Drug-Delivery System Containing 2% Whole Turmeric Gel Used as an Adjunct to Scaling and Root Planing in Chronic Periodontitis: A Clinical and Microbiological Study. J. Indian Soc. Periodontol. 2011, 15, 35. [Google Scholar] [CrossRef]
- Bhatia, M.; Urolagin, S.S.; Pentyala, K.B.; Urolagin, S.B.; Menaka, K.B.; Bhoi, S. Novel Therapeutic Approach for the Treatment of Periodontitis by Curcumin. J. Clin. Diagn. Res. 2014, 8, ZC65–ZC69. [Google Scholar] [CrossRef]
- Anuradha, B.R.; Bai, Y.D.; Sailaja, S.; Sudhakar, J.; Priyanka, M.; Deepika, V. Evaluation of Anti-Inflammatory Effects of Curcumin Gel as an Adjunct to Scaling and Root Planing: A Clinical Study. J. Int. Oral Health 2015, 7, 93. [Google Scholar]
- Nagasri, M.; Madhulatha, M.; Musalaiah, S.V.V.S.; Kumar, P.M.; Krishna, C.M. Efficacy of Curcumin as an Adjunct to Scaling and Root Planning in Chronic Periodontitis Patients: A Clinical and Microbiological Study. J. Pharm. Bioallied Sci. 2015, 7, 554. [Google Scholar] [CrossRef]
- Shivanand, P.; Vandana, K.V.; Vandana, K.L.; Praksh, S. Evaluation of Curcumin 10 Mg (Curenext ®) as Local Drug Delivery Adjunct in the Treatment of Chronic Periodontitis: A Clinical Trial. CODS J. Dent. 2016, 8, 59–63. [Google Scholar] [CrossRef]
- Nasra, M.M.A.; Khiri, H.M.; Hazzah, H.A.; Abdallah, O.Y. Formulation, in-Vitro Characterization and Clinical Evaluation of Curcumin in-Situ Gel for Treatment of Periodontitis. Drug Deliv. 2017, 24, 133–142. [Google Scholar] [CrossRef]
- Dave, D.; Patel, P.; Shah, M.; Dadawala, S.; Saraiya, K.; Sant, A. Comparative Evaluation of Efficacy of Oral Curcumin Gel as an Adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis. Adv. Hum. Biol. 2018, 8, 79. [Google Scholar] [CrossRef]
- Raghava, K.V.; Sistla, K.P.; Narayan, S.J.; Yadalam, U.; Bose, A.; Mitra, K. Efficacy of Curcumin as an Adjunct to Scaling and Root Planing in Chronic Periodontitis Patients: A Randomized Controlled Clinical Trial. J. Contemp. Dent. Pract. 2019, 20, 842–846. [Google Scholar] [CrossRef]
- Kaur, H.; Malhotra, R.; Gupta, M.; Grover, D.V. Evaluation_of_Curcumin_Gel_as_Adjunct_to_Scaling_Root Planning in the Mangement of Periodontitis- Randomized Clinical & Biochemical Investigation. Infect. Disord. Drug Targets 2019, 18, 1. [Google Scholar] [CrossRef]
- Pérez-Pacheco, C.G.; Fernandes, N.A.R.; Primo, F.L.; Tedesco, A.C.; Bellile, E.; Retamal-Valdes, B.; Feres, M.; Guimarães-Stabili, M.R.; Rossa, C. Local Application of Curcumin-Loaded Nanoparticles as an Adjunct to Scaling and Root Planing in Periodontitis: Randomized, Placebo-Controlled, Double-Blind Split-Mouth Clinical Trial. Clin. Oral Investig. 2020, 25, 3217–3227. [Google Scholar] [CrossRef]
- Farhood, H.T.; Ali, B.G. Clinical and Anti-Inflammatory Effect of Curcumin Oral Gel as Adjuncts in Treatment of Periodontal Pocket. J. Res. Med. Dent. Sci. 2020, 8, 83–90. [Google Scholar]
- Mohammad, C.A. Efficacy of Curcumin Gel on Zinc, Magnesium, Copper, IL-1β, and TNF-α in Chronic Periodontitis Patients. BioMed Res. Int. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rahalkar, A.; Kumathalli, K.; Kumar, R. Determination of Efficacy of Curcumin and Tulsi Extracts as Local Drugs in Periodontal Pocket Reduction: A Clinical and Microbiological Study. J. Indian Soc. Periodontol. 2021, 25, 197. [Google Scholar] [CrossRef] [PubMed]
- Elavarasu, S.; Suthanthiran, T.; Kumar, S. Evaluation of Superoxide Dismutase Levels in Local Drug Delivery System Containing 0.2% Curcumin Strip as an Adjunct to Scaling and Root Planing in Chronic Periodontitis: A Clinical and Biochemical Study. Pharm. Bioallied Sci. 2016, 8, S48–S52. [Google Scholar] [CrossRef]
- Saini, K.; Yadav, M.; Bhardway, A.; Chopra, P.; Saini, S. Comparative Clinical Evaluation of Two Local Drug Delivery Agents (Neem Chip and Turmeric Chip) in Chronic Periodontitis: An Experimental Study. World J. Dent. 2021, 12, 138–143. [Google Scholar] [CrossRef]
- Sreedhar, A.; Sarkar, I.; Rajan, P.; Pai, J.; Malagi, S.; Kamath, V.; Barmappa, R. Comparative Evaluation of the Efficacy of Curcumin Gel with and without Photo Activation as an Adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis: A Split Mouth Clinical and Microbiological Study. J. Nat. Sci. Biol. Med. 2015, 6, 102. [Google Scholar] [CrossRef]
- Gottumukkala, S.N.V.S.; Sudarshan, S.; Mantena, S.R. Comparative Evaluation of the Efficacy of Two Controlled Release Devices: Chlorhexidine Chips and Indigenous Curcumin Based Collagen as Local Drug Delivery Systems. Contemp. Clin. Dent. 2014, 5, 175. [Google Scholar] [CrossRef]
- Anitha, V.; Rajesh, P.; Shanmugam, M.; Priya, B.M.; Prabhu, S.; Shivakumar, V. Comparative Evaluation of Natural Curcumin and Synthetic Chlorhexidine in the Management of Chronic Periodontitis as a Local Drug Delivery: A Clinical and Microbiological Study. Indian J. Dent. Res. 2015, 26, 53–56. [Google Scholar] [CrossRef]
- Siddharth, M.; Gupta, R.; Singh, P.; Sinha, A.; Shree, S.; Sharma, K. Comparative Evaluation of Subgingivally Delivered 2% Curcumin and 0.2% Chlorhexidine Gel Adjunctive to Scaling and Root Planing in Chronic Periodontitis. J. Contemp. Dent. Pract. 2020, 21, 494–499. [Google Scholar] [CrossRef]
- Jaswal, R.; Dhawan, S.; Grover, V.; Malhotra, R. Comparative Evaluation of Single Application of 2% Whole Turmeric Gel versus 1% Chlorhexidine Gel in Chronic Periodontitis Patients: A Pilot Study. J. Indian Soc. Periodontol. 2014, 18, 575–580. [Google Scholar] [CrossRef]
- Singh, A.; Sridhar, R.; Shrihatti, R.; Mandloy, A. Evaluation of Turmeric Chip Compared with Chlorhexidine Chip as a Local Drug Delivery Agent in the Treatment of Chronic Periodontitis: A Split Mouth Randomized Controlled Clinical Trial. J. Altern. Complement. Med. 2018, 24, 76–84. [Google Scholar] [CrossRef]
- Guru, S.R.; Reddy, K.A.; Rao, R.J.; Padmanabhan, S.; Guru, R.; Srinivasa, T. Comparative Evaluation of 2% Turmeric Extract with Nanocarrier and 1% Chlorhexidine Gel as an Adjunct to Scaling and Root Planing in Patients with Chronic Periodontitis: A Pilot Randomized Controlled Clinical Trial. J. Ind. Soc Periodontol. 2020, 24, 224. [Google Scholar] [CrossRef]
- Gottumukkala, S.; Koneru, S.; Mannem, S.; Mandalapu, N. Effectiveness of Sub Gingival Irrigation of an Indigenous 1% Curcumin Solution on Clinical and Microbiological Parameters in Chronic Periodontitis Patients: A Pilot Randomized Clinical Trial. Contemp. Clin. Dent. 2013, 4, 186–191. [Google Scholar] [CrossRef]
- De Oliveira, R.C.G.; Costa, C.A.; Costa, N.L.; Silva, G.C.; de Souza, J.A.C. Effects of Curcuma as an Adjunct Therapy on Periodontal Disease: A Systematic Review and Meta-Analysis. Complement. Ther. Clin. Pract. 2021, 45, 101493. [Google Scholar] [CrossRef]
- Terby, S.; Shereef, M.; Ramanarayanan, V.; Balakrishnan, B. The Effect of Curcumin as an Adjunct in the Treatment of Chronic Periodontitis: A Systematic Review and Meta-Analysis. Saudi Dent. J. 2021, 33, 375–385. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, L.; Zhang, J.; De Souza Rastelli, A.N.; Yang, J.; Deng, D. Anti-Inflammatory Efficacy of Curcumin as an Adjunct to Non-Surgical Periodontal Treatment: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 808460. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: London, UK, 2022. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Lasserson, T.; McAleenan, A.; Reeves, B.C.; Shepperd, S.; Shrier, I.; Tilling, K.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-analyses: The Prisma Statement. PLoS Med. 2019, 6, e1000097. [Google Scholar] [CrossRef]
- Gupta, N.; Rath, S.K.; Lohra, P. Comparative Evaluation of Accuracy of Periodontal Probing Depth and Attachment Levels Using a Florida Probe versus Traditional Probes. Med. J. Armed Forces India 2015, 71, 352–358. [Google Scholar] [CrossRef]
- Oringer, R.J.; Fiorellini, J.P.; Koch, G.G.; Sharp, T.J.; Nevins, M.L.; Davis, G.H.; Howell, T.H. Comparison of Manual and Automated Probing in an Untreated Periodontitis Population. J. Periodontol. 1997, 68, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.W.B.; Hovey, K.M.; Benedek, J.R.; Grossi, S.G.; Dorn, J.; Wactawski-Wende, J.; Genco, R.J.; Trevisan, M. Reproducibility of Probing Depth Measurement Using a Constant-Force Electronic Probe: Analysis of Inter- and Intraexaminer Variability. J. Periodontol. 2003, 74, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Hefti, A.F. Periodontal Probing. Crit. Rev. Oral Biol. Med. 1997, 8, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Watts, T. Constant Force Probing with and without a Stent in Untreated Periodontal Disease: The Clinical Reproducibility Problem and Possible Sources of Error. J. Clin. Periodontol. 1987, 14, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.; Barendregt, D.S.; Slot, D.E.; Van der Velden, U.; Van der Weijden, F. Probing Pressure, a Highly Undervalued Unit of Measure in Periodontal Probing: A Systematic Review on Its Effect on Probing Pocket Depth. J. Clin. Periodontol. 2009, 36, 315–322. [Google Scholar] [CrossRef]
- Niederman, R. Manual and Electronic Probes Have Similar Reliability in the Measurement of Untreated Periodontitis: Question: Do Manual or Electronic Probes Produce the Most Reproducible Measurements of Clinical Attachment Level in Periodontitis Patients? Evid. Based Dent. 2009, 10, 39. [Google Scholar] [CrossRef]
- Silva-Boghossian, C.M.; Amaral, C.S.F.; Maia, L.C.; Luiz, R.R.; Colombo, A.P.V. Manual and Electronic Probing of the Periodontal Attachment Level in Untreated Periodontitis: A Systematic Review. J. Dent. 2008, 36, 651–657. [Google Scholar] [CrossRef]
- Sholapurkar, A.; Sharma, D.; Glass, B.; Miller, C.; Nimmo, A.; Jennings, E. Professionally Delivered Local Antimicrobials in the Treatment of Patients with Periodontitis—A Narrative Review. Dent. J. 2021, 9, 2. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian Solid Gold. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Aggarwal, B.B., Surh, Y.-J., Shishodia, S., Eds.; Springer: Boston, MA, USA, 2007; Volume 595, pp. 1–75. ISBN 978-0-387-46400-8. [Google Scholar]
- Sigma-Aldrich. Curcumin. Available online: https://www.sigmaaldrich.com/AT/de/product/sigma/c1386?gclid=EAIaIQobChMI_ff86b_i9wIVBZ53Ch3R5gqrEAAYASAAEgK1YPD_BwE (accessed on 10 April 2022).
- Konark Herbals & Health Care Curcuma Longa-Haldi. Available online: https://www.kherbalhealthcare.com/commiphora-mukul-guggal.html#curcuma-longa-haldi (accessed on 10 April 2022).
- Guru, S.; Kothiwale, S.; Saroch, N.; Guru, R. Comparative Evaluation of Inhibitory Effect of Curcumin and Doxycycline on Matrix Metalloproteinase-9 Activity in Chronic Periodontitis. Indian J. Dent. Res. 2017, 28, 560. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Sohn, S.-I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin—An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Curylofo-Zotti, F.A.; Elburki, M.S.; Oliveira, P.A.; Cerri, P.S.; Santos, L.A.; Lee, H.-M.; Johnson, F.; Golub, L.M.; Rossa, C.; Guimarães-Stabili, M.R. Differential Effects of Natural Curcumin and Chemically Modified Curcumin on Inflammation and Bone Resorption in Model of Experimental Periodontitis. Arch. Oral Biol. 2018, 91, 42–50. [Google Scholar] [CrossRef]
- Smaïl-Faugeron, V.; Fron-Chabouis, H.; Courson, F.; Durieux, P. Comparison of Intervention Effects in Split-Mouth and Parallel-Arm Randomized Controlled Trials: A Meta-Epidemiological Study. BMC Med. Res. Methodol. 2014, 14, 64. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wendorff-Tobolla, L.M.; Wolgin, M.; Wagner, G.; Klerings, I.; Dvornyk, A.; Kielbassa, A.M. A Systematic Review and Meta-Analysis on the Efficacy of Locally Delivered Adjunctive Curcumin (Curcuma longa L.) in the Treatment of Periodontitis. Biomedicines 2023, 11, 481. https://doi.org/10.3390/biomedicines11020481
Wendorff-Tobolla LM, Wolgin M, Wagner G, Klerings I, Dvornyk A, Kielbassa AM. A Systematic Review and Meta-Analysis on the Efficacy of Locally Delivered Adjunctive Curcumin (Curcuma longa L.) in the Treatment of Periodontitis. Biomedicines. 2023; 11(2):481. https://doi.org/10.3390/biomedicines11020481
Chicago/Turabian StyleWendorff-Tobolla, Louisa M., Michael Wolgin, Gernot Wagner, Irma Klerings, Anna Dvornyk, and Andrej M. Kielbassa. 2023. "A Systematic Review and Meta-Analysis on the Efficacy of Locally Delivered Adjunctive Curcumin (Curcuma longa L.) in the Treatment of Periodontitis" Biomedicines 11, no. 2: 481. https://doi.org/10.3390/biomedicines11020481
APA StyleWendorff-Tobolla, L. M., Wolgin, M., Wagner, G., Klerings, I., Dvornyk, A., & Kielbassa, A. M. (2023). A Systematic Review and Meta-Analysis on the Efficacy of Locally Delivered Adjunctive Curcumin (Curcuma longa L.) in the Treatment of Periodontitis. Biomedicines, 11(2), 481. https://doi.org/10.3390/biomedicines11020481