Human Xylosyltransferase I—An Important Linker between Acute Senescence and Fibrogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Hydrogen Peroxide Treatment
2.2. Trypan Blue Dye Exclusion Assay
2.3. Bicinchoninic Acid Assay
2.4. Quantitative Senescence-Associated β-Gal Activity Assay
2.5. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.6. XT-I Selective Enzyme Activity Assay by UPLC/ESI-MS/MS
2.7. Immunoblotting
2.8. Immunofluorescence
2.9. Statistical Analysis
3. Results
3.1. H2O2-Treatment of Human Proto-Myofibroblasts Induces Acute Senescence
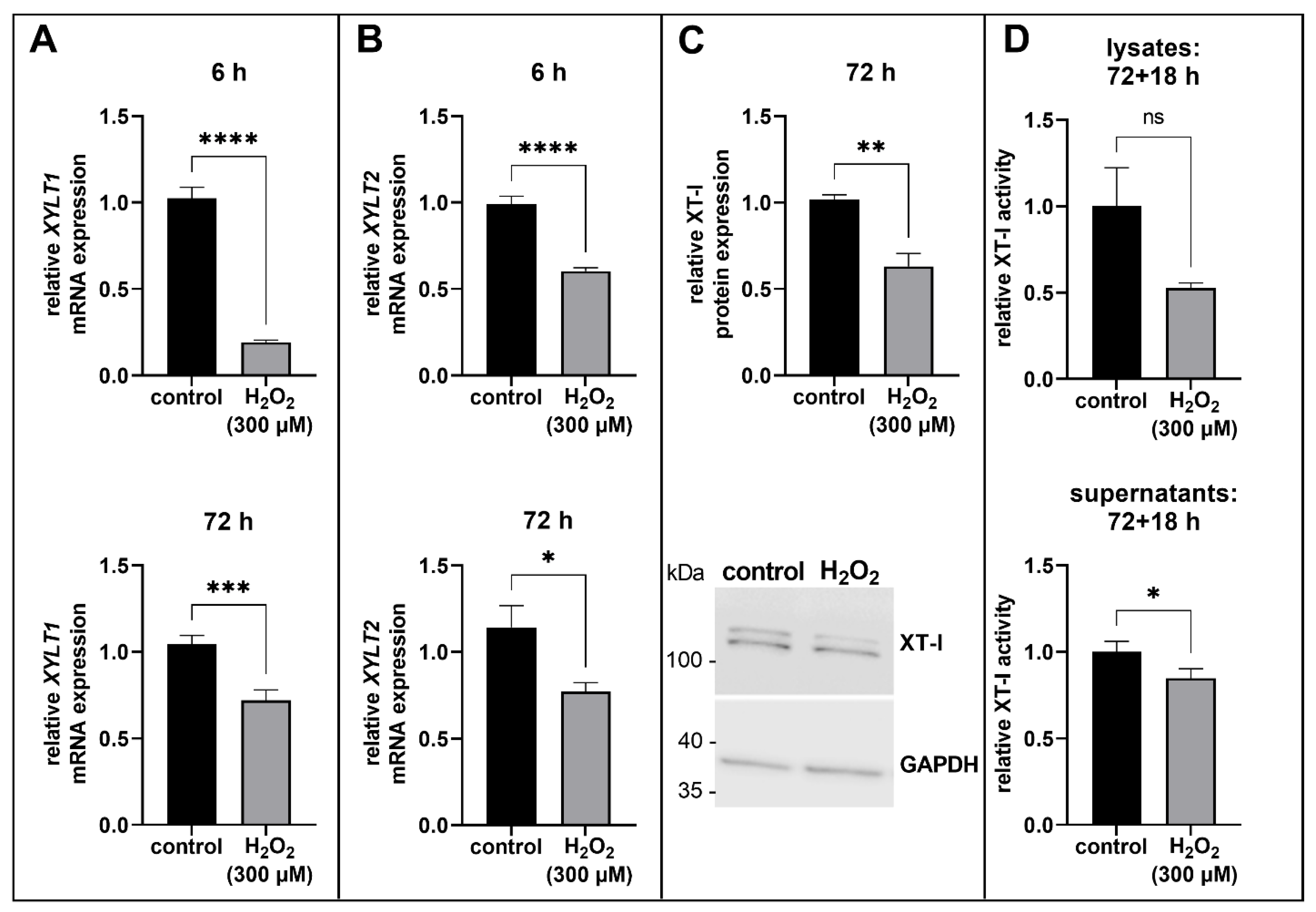
3.2. Suppression of XT Expression in Acute Senescent Proto-Myofibroblasts
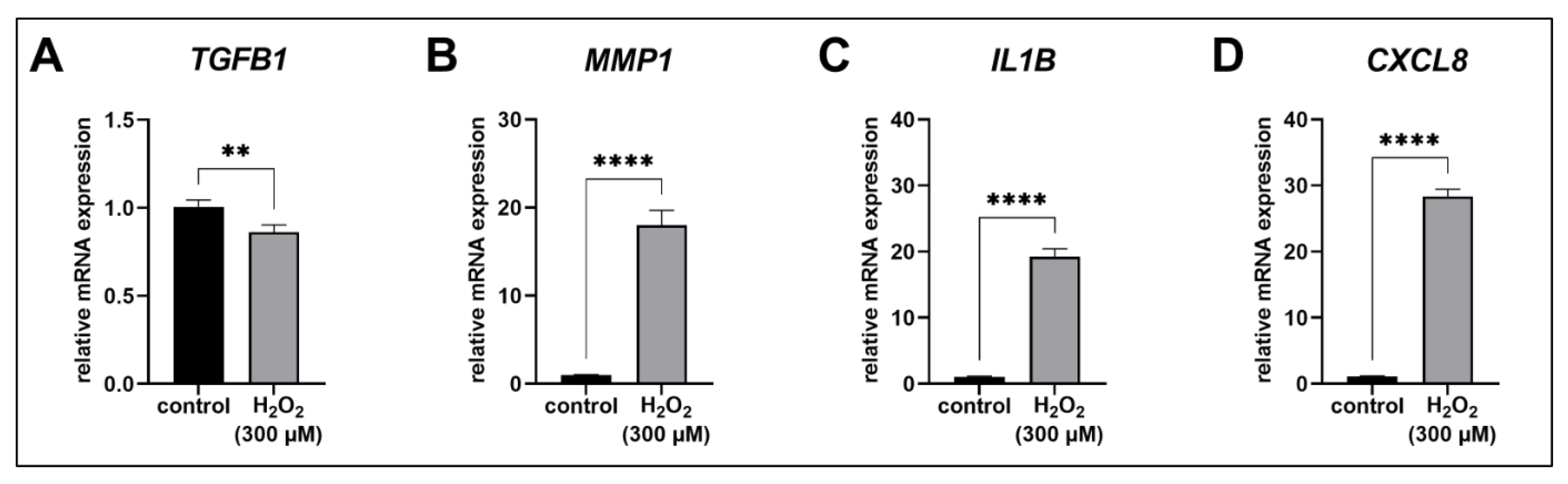
3.3. ECM Remodeling in Acute Senescent Proto-Myofibroblasts
3.4. The SASP of Acute Senescent Proto-Myofibroblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campisi, J.; Di d’Adda Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef]
- Alcorta, D.A.; Xiong, Y.; Phelps, D.; Hannon, G.; Beach, D.; Barrett, J.C. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc. Natl. Acad. Sci. USA 1996, 93, 13742–13747. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef]
- Dimri, G.P.; Testori, A.; Acosta, M.; Campisi, J. Replicative senescence, aging and growth-regulatory transcription factors. Biol. Signals 1996, 5, 154–162. [Google Scholar] [CrossRef]
- Collado, M.; Blasco, M.A.; Serrano, M. Cellular senescence in cancer and aging. Cell 2007, 130, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espín, D.; Cañamero, M.; Maraver, A.; Gómez-López, G.; Contreras, J.; Murillo-Cuesta, S.; Rodríguez-Baeza, A.; Varela-Nieto, I.; Ruberte, J.; Collado, M.; et al. Programmed cell senescence during mammalian embryonic development. Cell 2013, 155, 1104–1118. [Google Scholar] [CrossRef]
- Storer, M.; Mas, A.; Robert-Moreno, A.; Pecoraro, M.; Ortells, M.C.; Di Giacomo, V.; Yosef, R.; Pilpel, N.; Krizhanovsky, V.; Sharpe, J.; et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 2013, 155, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.-I.; Lau, L.F. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 2010, 12, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Krizhanovsky, V.; Yon, M.; Dickins, R.A.; Hearn, S.; Simon, J.; Miething, C.; Yee, H.; Zender, L.; Lowe, S.W. Senescence of activated stellate cells limits liver fibrosis. Cell 2008, 134, 657–667. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Rosenbloom, J.; Macarak, E.; Piera-Velazquez, S.; Jimenez, S.A. Human fibrotic diseases: Current challenges in fibrosis research. In Fibrosis: Methods and Protocols; Rittie, L., Ed.; Humana Press: New York, NY, USA, 2017; pp. 1–23. ISBN 978-1-4939-7112-1. [Google Scholar]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Desmoulière, A.; Darby, I.A.; Gabbiani, G. Normal and pathologic soft tissue remodeling: Role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab. Investig. 2003, 83, 1689–1707. [Google Scholar] [CrossRef]
- Hinz, B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Gressner, A.M.; Krull, N.; Bachem, M.G. Regulation of proteoglycan expression in fibrotic liver and cultured fat-storing cells. Pathol. Res. Pract. 1994, 190, 864–882. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Shinkai, H. Decorin and glycosaminoglycan synthesis in skin fibroblasts from patients with systemic sclerosis. Arch. Dermatol. Res. 1997, 289, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Bensadoun, E.S.; Burke, A.K.; Hogg, J.C.; Roberts, C.R. Proteoglycan deposition in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1996, 154, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, T.E.; Fosang, A.J. Proteoglycans: Many forms and many functions. FASEB J. 1992, 6, 861–870. [Google Scholar] [CrossRef]
- Gandhi, N.S.; Mancera, R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008, 72, 455–482. [Google Scholar] [CrossRef]
- Couchman, J.R.; Pataki, C.A. An introduction to proteoglycans and their localization. J. Histochem. Cytochem. 2012, 60, 885–897. [Google Scholar] [CrossRef]
- Götting, C.; Kuhn, J.; Zahn, R.; Brinkmann, T.; Kleesiek, K. Molecular cloning and expression of human UDP-d-Xylose:proteoglycan core protein beta-d-xylosyltransferase and its first isoform XT-II. J. Mol. Biol. 2000, 304, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Kearns, A.E.; Campbell, S.C.; Westley, J.; Schwartz, N.B. Initiation of chondroitin sulfate biosynthesis: A kinetic analysis of UDP-D-xylose: Core protein beta-D-xylosyltransferase. Biochemistry 1991, 30, 7477–7483. [Google Scholar] [CrossRef] [PubMed]
- Schön, S.; Prante, C.; Bahr, C.; Kuhn, J.; Kleesiek, K.; Götting, C. Cloning and recombinant expression of active full-length xylosyltransferase I (XT-I) and characterization of subcellular localization of XT-I and XT-II. J. Biol. Chem. 2006, 281, 14224–14231. [Google Scholar] [CrossRef] [PubMed]
- Götting, C.; Sollberg, S.; Kuhn, J.; Weilke, C.; Huerkamp, C.; Brinkmann, T.; Krieg, T.; Kleesiek, K. Serum xylosyltransferase: A new biochemical marker of the sclerotic process in systemic sclerosis. J. Investig. Dermatol. 1999, 112, 919–924. [Google Scholar] [CrossRef]
- Pönighaus, C.; Ambrosius, M.; Casanova, J.C.; Prante, C.; Kuhn, J.; Esko, J.D.; Kleesiek, K.; Götting, C. Human xylosyltransferase II is involved in the biosynthesis of the uniform tetrasaccharide linkage region in chondroitin sulfate and heparan sulfate proteoglycans. J. Biol. Chem. 2007, 282, 5201–5206. [Google Scholar] [CrossRef]
- Götting, C.; Kuhn, J.; Kleesiek, K. Human xylosyltransferases in health and disease. Cell. Mol. Life Sci. 2007, 64, 1498–1517. [Google Scholar] [CrossRef]
- Faust, I.; Roch, C.; Kuhn, J.; Prante, C.; Knabbe, C.; Hendig, D. Human xylosyltransferase-I—A new marker for myofibroblast differentiation in skin fibrosis. Biochem. Biophys. Res. Commun. 2013, 436, 449–454. [Google Scholar] [CrossRef]
- Prante, C.; Milting, H.; Kassner, A.; Farr, M.; Ambrosius, M.; Schön, S.; Seidler, D.G.; Banayosy, A.E.; Körfer, R.; Kuhn, J.; et al. Transforming growth factor beta1-regulated xylosyltransferase I activity in human cardiac fibroblasts and its impact for myocardial remodeling. J. Biol. Chem. 2007, 282, 26441–26449. [Google Scholar] [CrossRef]
- Ly, T.-D.; Kleine, A.; Plümers, R.; Fischer, B.; Schmidt, V.; Hendig, D.; Distler, J.H.W.; Kuhn, J.; Knabbe, C.; Faust, I. Cytokine-mediated induction of human xylosyltransferase-I in systemic sclerosis skin fibroblasts. Biochem. Biophys. Res. Commun. 2021, 549, 34–39. [Google Scholar] [CrossRef]
- Ly, T.-D.; Plümers, R.; Fischer, B.; Schmidt, V.; Hendig, D.; Kuhn, J.; Knabbe, C.; Faust, I. Activin A-mediated regulation of XT-I in human skin fibroblasts. Biomolecules 2020, 10, 609. [Google Scholar] [CrossRef]
- Venkatesan, N.; Barré, L.; Bourhim, M.; Magdalou, J.; Mainard, D.; Netter, P.; Fournel-Gigleux, S.; Ouzzine, M. Xylosyltransferase-I regulates glycosaminoglycan synthesis during the pathogenic process of human osteoarthritis. PLoS ONE 2012, 7, e34020. [Google Scholar] [CrossRef] [PubMed]
- Ly, T.-D.; Riedel, L.; Fischer, B.; Schmidt, V.; Hendig, D.; Distler, J.; Kuhn, J.; Knabbe, C.; Faust, I. microRNA-145 mediates xylosyltransferase-I induction in myofibroblasts via suppression of transcription factor KLF4. Biochem. Biophys. Res. Commun. 2020, 523, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Riedel, L.; Fischer, B.; Ly, T.-D.; Hendig, D.; Kuhn, J.; Knabbe, C.; Faust, I. microRNA-29b mediates fibrotic induction of human xylosyltransferase-I in human dermal fibroblasts via the Sp1 pathway. Sci. Rep. 2018, 8, 17779. [Google Scholar] [CrossRef]
- Fischer, B.; Ly, T.-D.; Schmidt, V.; Hendig, D.; Kuhn, J.; Knabbe, C.; Faust, I. Xylosyltransferase-deficient human HEK293 cells show a strongly reduced proliferation capacity and viability. Biochem. Biophys. Res. Commun. 2020, 521, 507–513. [Google Scholar] [CrossRef]
- Fischer, B.; Schmidt, V.; Ly, T.-D.; Kleine, A.; Knabbe, C.; Faust-Hinse, I. First characterization of human dermal fibroblasts showing a decreased xylosyltransferase-I expression induced by the CRISPR/Cas9 system. Int. J. Mol. Sci. 2022, 23, 5045. [Google Scholar] [CrossRef] [PubMed]
- Masur, S.K.; Dewal, H.S.; Dinh, T.T.; Erenburg, I.; Petridou, S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc. Natl. Acad. Sci. USA 1996, 93, 4219–4223. [Google Scholar] [CrossRef]
- Zdanov, S.; Remacle, J.; Toussaint, O. Establishment of H2O2-induced premature senescence in human fibroblasts concomitant with increased cellular production of H2O2. Ann. N. Y. Acad. Sci. 2006, 1067, 210–216. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, D.; Xiao, H. Methods of cellular senescence induction using oxidative stress. Methods Mol. Biol. 2013, 1048, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2015, 111, A3. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Ly, T.-D.; Kleine, A.; Fischer, B.; Schmidt, V.; Hendig, D.; Kuhn, J.; Knabbe, C.; Faust, I. Identification of putative non-substrate-based XT-I inhibitors by natural product library screening. Biomolecules 2020, 10, 1467. [Google Scholar] [CrossRef]
- Gary, R.K.; Kindell, S.M. Quantitative assay of senescence-associated beta-galactosidase activity in mammalian cell extracts. Anal. Biochem. 2005, 343, 329–334. [Google Scholar] [CrossRef]
- Fischer, B.; Kuhn, J.; Ly, T.-D.; Schmidt, V.; Kleine, A.; Hendig, D.; Knabbe, C.; Faust, I. Development of a xylosyltransferase-I-selective UPLC MS/MS activity assay using a specific acceptor peptide. Biochimie 2021, 184, 88–94. [Google Scholar] [CrossRef]
- Jun, J.-I.; Lau, L.F. Cellular senescence controls fibrosis in wound healing. Aging 2010, 2, 627–631. [Google Scholar] [CrossRef]
- Gonzalez, A.C.d.O.; Costa, T.F.; Andrade, Z.d.A.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- van de Water, L.; Varney, S.; Tomasek, J.J. Mechanoregulation of the myofibroblast in wound contraction, scarring, and fibrosis: Opportunities for new therapeutic intervention. Adv. Wound Care 2013, 2, 122–141. [Google Scholar] [CrossRef]
- Bladier, C.; Wolvetang, E.J.; Hutchinson, P.; de Haan, J.B.; Kola, I. Response of a primary human fibroblast cell line to H2O2: Senescence-like growth arrest or apoptosis? Cell Growth Differ. 1997, 8, 589–598. [Google Scholar] [PubMed]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 2000, 113 Pt 20, 3613–3622. [Google Scholar] [CrossRef]
- Zhan, H.; Suzuki, T.; Aizawa, K.; Miyagawa, K.; Nagai, R. Ataxia telangiectasia mutated (ATM)-mediated DNA damage response in oxidative stress-induced vascular endothelial cell senescence. J. Biol. Chem. 2010, 285, 29662–29670. [Google Scholar] [CrossRef]
- Nakayama, F.; Hagiwara, A.; Yamamoto, T.; Akashi, M. Hydrogen peroxide as a potential mediator of the transcriptional regulation of heparan sulphate biosynthesis in keratinocytes. Cell. Mol. Biol. Lett. 2008, 13, 475–492. [Google Scholar] [CrossRef]
- Jin, C.L.; Oh, J.-H.; Han, M.; Shin, M.K.; Yao, C.; Park, C.-H.; Jin, Z.H.; Chung, J.H. UV irradiation-induced production of monoglycosylated biglycan through downregulation of xylosyltransferase 1 in cultured human dermal fibroblasts. J. Dermatol. Sci. 2015, 79, 20–29. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell. Signal. 2012, 24, 835–845. [Google Scholar] [CrossRef]
- Karin, M.; Shaulian, E. AP-1: Linking hydrogen peroxide and oxidative stress to the control of cell proliferation and death. IUBMB Life 2001, 52, 17–24. [Google Scholar] [CrossRef]
- Khair, M.; Bourhim, M.; Barré, L.; Li, D.; Netter, P.; Magdalou, J.; Fournel-Gigleux, S.; Ouzzine, M. Regulation of xylosyltransferase I gene expression by interleukin 1β in human primary chondrocyte cells: Mechanism and impact on proteoglycan synthesis. J. Biol. Chem. 2013, 288, 1774–1784. [Google Scholar] [CrossRef]
- Müller, B.; Prante, C.; Kleesiek, K.; Götting, C. Identification and characterization of the human xylosyltransferase I gene promoter region. J. Biol. Chem. 2009, 284, 30775–30782. [Google Scholar] [CrossRef]
- Hwang, H.S.; Lee, M.H.; Kim, H.A. Fibronectin fragment inhibits xylosyltransferase-1 expression by regulating Sp1/Sp3- dependent transcription in articular chondrocytes. Osteoarthr. Cartil. 2019, 27, 833–843. [Google Scholar] [CrossRef]
- Qin, Z.; Robichaud, P.; He, T.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Oxidant exposure induces cysteine-rich protein 61 (CCN1) via c-Jun/AP-1 to reduce collagen expression in human dermal fibroblasts. PLoS ONE 2014, 9, e115402. [Google Scholar] [CrossRef]
- Qin, Z.; Fisher, G.J.; Quan, T. Cysteine-rich protein 61 (CCN1) domain-specific stimulation of matrix metalloproteinase-1 expression through αVβ3 integrin in human skin fibroblasts. J. Biol. Chem. 2013, 288, 12386–12394. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Mann, D.M.; Ruoslahti, E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 1990, 346, 281–284. [Google Scholar] [CrossRef] [PubMed]
- McCawley, L.J.; Matrisian, L.M. Matrix metalloproteinases: They’re not just for matrix anymore! Curr. Opin. Cell Biol. 2001, 13, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Mavrogonatou, E.; Papadopoulou, A.; Fotopoulou, A.; Tsimelis, S.; Bassiony, H.; Yiacoumettis, A.M.; Panagiotou, P.N.; Pratsinis, H.; Kletsas, D. Down-regulation of the proteoglycan decorin fills in the tumor-promoting phenotype of ionizing radiation-induced senescent human breast stromal fibroblasts. Cancers 2021, 13, 1987. [Google Scholar] [CrossRef]
- Chen, X.; Johns, D.C.; Geiman, D.E.; Marban, E.; Dang, D.T.; Hamlin, G.; Sun, R.; Yang, V.W. Krüppel-like factor 4 (gut-enriched Krüppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J. Biol. Chem. 2001, 276, 30423–30428. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.T.; Chen, X.; Feng, J.; Torbenson, M.; Dang, L.H.; Yang, V.W. Overexpression of Krüppel-like factor 4 in the human colon cancer cell line RKO leads to reduced tumorigenecity. Oncogene 2003, 22, 3424–3430. [Google Scholar] [CrossRef]
- Yoon, H.S.; Chen, X.; Yang, V.W. Kruppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J. Biol. Chem. 2003, 278, 2101–2105. [Google Scholar] [CrossRef]
- Zhang, W.; Geiman, D.E.; Shields, J.M.; Dang, D.T.; Mahatan, C.S.; Kaestner, K.H.; Biggs, J.R.; Kraft, A.S.; Yang, V.W. The gut-enriched Kruppel-like factor (Kruppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. J. Biol. Chem. 2000, 275, 18391–18398. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef]


| Gene | Primers | TA [°C] | Efficiency |
|---|---|---|---|
| hCDKN1A | 5′-GCTTCATGCCAGCTACTTCC-3′ 5′-CCCTTCAAAGTGCCATCTGT-3′ | 66 | 2.00 |
| hCDKN2A | 5′-ACCAGAGGCAGTAACCATGC-3′ 5′-AAGTTTCCCGAGGTTTCTCAG-3′ | 66 | 2.00 |
| hCOL1A1 | 5′-GATGTGCCACTCTGACT-3′ 5′-GGGTTCTTGCTGATG-3′ | 63 | 1.74 |
| hCXCL8 | 5′-GAACTGAGAGTGATTGAGAGTGGA-3′ 5′-CTCTTCAAAAACTTCTCCACAACC-3′ | 63 | 1.88 |
| hDCN | 5′-CCTTCCGCTGTCAATG-3′ 5′-GCAGGTCTAGCAGAGTTG-3′ | 63 | 1.76 |
| hFN | 5′-CCCAGGGAAGATGTAGA-3′ 5′-CTCTTCCCGAACCTTATG-3′ | 63 | 2.00 |
| hGAPDH | 5′-AGGTCGGAGTCAACGGAT-3′ 5′-TCCTGGAAGATGGTGATG-3′ | 59 | 1.83 |
| hIL1B | 5′-ACAGATGAAGTGCTCCTTCCA-3′ 5′-GTCGGAGATTCGTAGCTGGAT-3′ | 63 | 1.94 |
| hMMP1 | 5′-AGAAACACAAGAGCAAGATGTG-3′ 5′-TGGCGTGTAATTTTCAATCCTGT-3′ | 63 | 1.85 |
| hSDHA | 5′-AACTCGCTCTTGGACCTG-3′ 5′-GAGTCGCAGTTCCGATGT-3′ | 66 | 2.00 |
| hTGFB1 | 5′- GCGATACCTCAGCAACC-3′ 5′- ACGCAGCAGTTCTTCTCC-3′ | 59 | 1.95 |
| hXYLT1 | 5′-TGTGACCTTCTCCACAGACG-3′ 5′-CCACGATGTGCTTGTACTGG-3′ | 63 | 2.00 |
| hXYLT2 | 5′-ACACAGATGACCCGCTTGTGG-3′ 5′-TTGGTGACCCGCAGGTTGTTG-3′ | 63 | 1.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, V.; Ohmes, J.; Ly, T.-D.; Fischer, B.; Kleine, A.; Knabbe, C.; Faust-Hinse, I. Human Xylosyltransferase I—An Important Linker between Acute Senescence and Fibrogenesis. Biomedicines 2023, 11, 460. https://doi.org/10.3390/biomedicines11020460
Schmidt V, Ohmes J, Ly T-D, Fischer B, Kleine A, Knabbe C, Faust-Hinse I. Human Xylosyltransferase I—An Important Linker between Acute Senescence and Fibrogenesis. Biomedicines. 2023; 11(2):460. https://doi.org/10.3390/biomedicines11020460
Chicago/Turabian StyleSchmidt, Vanessa, Justus Ohmes, Thanh-Diep Ly, Bastian Fischer, Anika Kleine, Cornelius Knabbe, and Isabel Faust-Hinse. 2023. "Human Xylosyltransferase I—An Important Linker between Acute Senescence and Fibrogenesis" Biomedicines 11, no. 2: 460. https://doi.org/10.3390/biomedicines11020460
APA StyleSchmidt, V., Ohmes, J., Ly, T.-D., Fischer, B., Kleine, A., Knabbe, C., & Faust-Hinse, I. (2023). Human Xylosyltransferase I—An Important Linker between Acute Senescence and Fibrogenesis. Biomedicines, 11(2), 460. https://doi.org/10.3390/biomedicines11020460






